Abstract
Objective
With bilateral cochlear implant (CI) users there is typically a place mismatch between the locations stimulated by the left and right electrode arrays. This mismatch can affect performance, potentially limiting binaural benefits. One way to address this is by perceptually realigning the arrays such that a given frequency in the input stimulates perceptually matched locations in the two ears. A clinically feasible technique is needed that can determine the appropriate perceptual alignment. A pitch matching task can potentially be used for this, but only if it can be performed in a clinically feasible amount of time. The objective of this study was to determine the minimal number of electrodes that need to be pitch matched to accurately determine pitch matches across the entire array.
Design
A retrospective analysis of pitch matching data was conducted. Subsets of pitch matches were selected and the predicted pitch matching across the array was compared to that predicted by the full dataset.
Study Sample
16 bilateral CI users.
Results
The results indicated that nine pitch matches are sufficient, which can typically be obtained in approximately seven minutes.
Conclusion
The results reveal a clinically feasible method for determining pitch matches across the array.
Keywords: Bilateral Cochlear Implants, Pitch Matching
Introduction
Having two cochlear implants (CIs) improves speech perception in noise and localization abilities compared to having only one CI (Ricketts et al., 2006; Dunn et al., 2008; Litovsky et al., 2009; Dunn et al., 2010). However, although bilateral CI users receive some binaural benefits, they do not receive the same benefits that normal hearing (NH) listeners do (Loizou et al., 2009; Poon et al., 2009; Aronoff et al., 2012). For example, CI users have more difficulty localizing sounds (Aronoff, Freed et al., 2012; Kerber & Seeber, 2012) and more difficulty fusing sounds from the two ears into a unitary coherent percept (Fitzgerald et al., 2015).
The reduced binaural benefits that CI users receive may partly reflect the presence of interaural mismatches in terms of place of stimulation. For bilateral CI users, interaural mismatches can occur because of insertion depth differences (Marsh et al., 1993; Aschendorff et al., 2005) and differences in the distribution of neural survival in the two cochleae (Fayad et al., 1991). When interaural place mismatches occur, they can result in poor interaural time difference (ITD) sensitivity (Long et al., 2003; Poon, Eddington et al., 2009), difficulty lateralizing sounds (Kan et al., 2013), a lateral shift in the perceived location of a sound source (Goupell et al., 2013; Kan, Stoelb et al., 2013), poor speech recognition in noisy environments (Li & Fu, 2010), and difficulty fusing sounds from the two ears into a unitary percept (Goupell, Stoelb et al., 2013; Kan, Stoelb et al., 2013; Aronoff et al., 2015).
Although not the only method for reducing the effects of interaural mismatches (c.f., auditory image centering; Kan et al., 2015), a popular method is to perceptually realign the arrays such that a given frequency in the input stimulates perceptually matched locations in the two ears. However, to do this, perceptually matched locations must first be identified. Pitch matching tasks, where participants are asked to identify the bilateral pair of stimulation sites that yield the same perceived pitch (Litovsky et al., 2012; Kan, Stoelb et al., 2013; Aronoff et al., 2016), can be used to identify the perceptually matched locations. In the laboratory, pitch matches are typically obtained for each electrode (e.g., Litovsky et al., 2012; Aronoff et al., 2016) but such an approach would generally be too time consuming to be used in a clinical setting. In order for pitch matching to be clinically useful, it must be fast and reliable. Although there are alternative approaches to align the array such as measuring ITD sensitivity (Long, Eddington et al., 2003; Poon, Eddington et al., 2009) or the binaural interaction component (Hu & Dietz, 2015), the differences across these approaches in terms of which electrodes are best matched across ears is typically one electrode or less (Long, Eddington et al., 2003; Poon, Eddington et al., 2009; Hu & Dietz, 2015). Pitch matching, given its relatively minimal time requirements, has the greatest potential to be modified for use within the time constraints of the clinic. The primary goal of this study was to determine the minimal number of electrodes that need to be pitch matched to accurately determine pitch matches across the entire array.
Materials & Methods
Pitch Matching Method
The current study analyzes pitch matching data collected as a preliminary step in a number of different experiments in the laboratory. Data was obtained in the process of creating maps where the programming of the frequency allocations for the left and right processors were adjusted to create bilateral pitch matched maps (e.g., Aronoff, Stelmach et al., 2016). The following section describes the methods used to collect that pitch matching data.
Inclusion criteria were that the participants were adults and used Advanced Bionics (AB) bilateral CIs (CII or later generations). Testing was done with the Bionic Ear Data Collection System (BEDCS) and/or HRStream research interfaces (Litvak, 2003; Nogueira & Buechner, 2012), both of which allowed direct control of stimulation parameters for each electrode. The electric stimulation parameters consisted of biphasic monopolar pulses with a phase duration of 32 μs and a pulse rate of 1000 pulses per second, which is within the range of clinical settings. The maximum comfort level was found for each individual electrode in both ears. Additionally, loudness balancing was conducted within and across arrays to ensure the electrodes had the same loudness level. Loudness balancing within arrays was completed by sweeping in groups of four adjacent electrodes at the most comfortable loudness level. The stimulation level was adjusted for any electrode that was louder or softer than the first electrode in the group. After all electrodes in that group were loudness balanced, a new group of four adjacent electrodes were chosen with the first electrode for the new group being the same as the last electrode from the previous group (i.e., Group 1: Electrodes 1–4; Group 2: Electrodes 4–7). Loudness balancing across arrays was conducted for Electrode 9 and the stimulation levels for all electrodes were then globally adjusted accordingly.
For each pitch matching run, a pseudo-randomly selected electrode was initially chosen in the reference ear. This reference stimulation could either be an individual electrode or it could be a stimulation location in between electrodes (i.e., a virtual channel). Typically, data were acquired with each electrode (i.e., non-virtual channels) being a reference stimulation location prior to acquiring data with virtual channel reference locations. Virtual channels were used for the target locations whether or not the reference locations were virtual channels. These allowed stimulation location changes of 0.1 electrodes. The patient used a knob (Powermate, Griffin Technology) to change the stimulation location in the target ear. This process of reference presentation followed by target adjustment was repeated until the participant indicated that the left and right ear had the same pitch. Both stimuli, reference and target, were presented using 500 ms pulse trains, with an interstimulus interval of approximately 500 ms. The task was self-paced, with the next trial starting after the participant entered in their response. If the participant did not perceive an exact match, they were instructed to select the closest perceived response.
At the start of each pitch matching run, the target stimulation location was randomly selected. Based on time constraints as well as variations in the protocol across experiments, the number of references used for pitch matches obtained for each patient ranged from 22 to 38. In most cases each reference was used one time. In the event that two trials were run for one reference location, the average was found between the two target responses; however, this rarely occurred. I02 and C03 were the only two participants where stimulation locations were tested twice. With I02, one stimulation location was tested twice and for C03, six stimulation locations were tested twice. In the cases where a reference location was tested twice, the repeated measures yielded similar results, with an average test-retest difference of 0.33 mm for C03 and 0 mm for I02.
Analysis of Pitch Matching Data
Patients
The data for the current analysis consisted of pitch matching data from 16 bilateral CI patients, representing all participants tested with the conditions and parameters previously described. Patient details are provided in Table 1.
Table 1.
Patient demographics.
| Patient | Age | Gender | Hearing loss onset | Cause | Implant experience | Number of pitch matches collected |
|---|---|---|---|---|---|---|
| 101 | 62 | Female | Birth | Unknown | 8 years (L) 5 years (R) |
31 |
| 102 | 60 | female | 2 years old | Meningitis | 2 years (L) 5 years (R) |
38 |
| 103 | 70 | Female | Birth | Unknown | 12 years (L) 7 years (R) |
22 |
| 104 | 57 | Female | 36 years old | Progressive/Autoimmune | <1 month (L) 1.5 years (R) |
31 |
| 105 | 56 | Male | 5 years old | Unknown (Injury or Genetic) | 12 years <L) 12 years (R) |
31 |
| 106 | 56 | Female | 36 years old | Genetic-Maternal | 1 year (L) 3 years (R) |
31 |
| 107 | 53 | Male | 30 years old | Familial | 1 week (L) 2 week (R) |
30 |
| 109 | 56 | Male | 28 years old (L) 44 years old (R) |
Unknown | 1.5 years (L) 6 months (R) |
31 |
| 110 | 49 | Female | 29 years old | Autoimmune | 1 year (L) 2 years (R) |
31 |
| 111 | 67 | Male | 9–10 years old (L) 57 years old (R) |
Sudden, Unknown | 2 years (L) 10 years (R) |
31 |
| 113 | 33 | Mule | 3 years old | High fever/Viral | 6 months (L) 1 year (R) |
38 |
| 114 | 65 | Male | <25 years old | Menieres Progressive | 2 years (L) 3 years (R) |
31 |
| 115 | 46 | Female | 6 months old | Measles | 0 days (L) 10 years (R) |
31 |
| 126 | 45 | Female | Birth | Hereditary | 2 years (L) 2 years (R) |
31 |
| C03 | 57 | Female | 29 years old | Hereditary | 7 years (L) 4 years (R) |
38 |
| C143 | 48 | Male | 4.5 months | Maternal Rubella | 4 years (L) 8 years (R) |
31 |
The set of all pitch-matched stimulation sites for a given patient are referred to as the full pitch matching data set. To determine which stimulation sites would be paired across ears in a speech processor (i.e. assigned the same frequency allocation) based on the full pitch matching data set, a slope and intercept was calculated based on all of an individual’s pitch matches. This was done using a least trimmed squares regression, a robust regression method that minimizes the effect of outliers. All patients had a slope greater than 0.5 and all slopes were significantly different from zero (adjusted for familywise error based on (Rom, 1990)) except for the four subjects with the largest slope confidence intervals (I02, I03, I13, and I15). The points on the best fitting line for the full pitch matching data set were considered the most accurate representation of the participant’s pitch match. Based on this slope and intercept, 16 bilateral electrode pairs (corresponding to the number of electrodes for the implants used by the participants in this study) were generated.
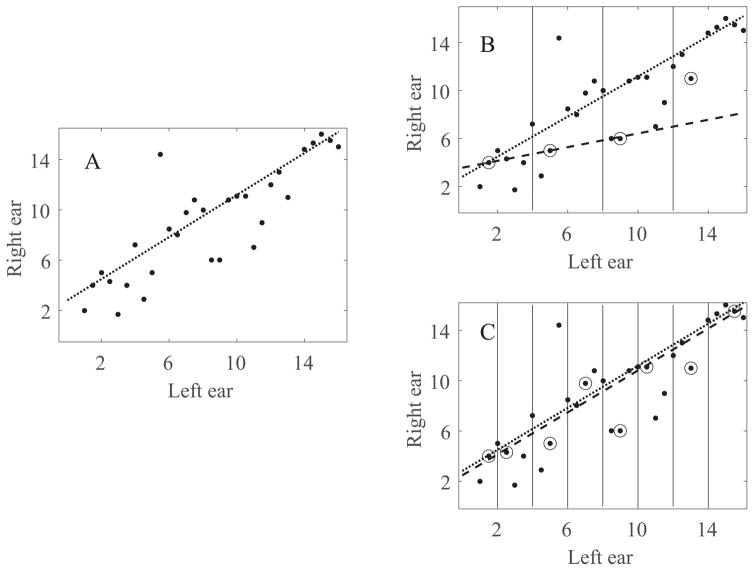
In order for this task to be clinically feasible, it needs to be fast; which means determining the fewest number of pitch matches that are needed. Reduced data sets were generated based on 4 to 16 pseudo-randomly selected data points, using the procedure shown in Figure 1. Starting from the original data set (Figure 1, panel A) reduced data sets were created by dividing the reference ear array into four to sixteen equal sections (Figure 1, panel B uses four sections, C uses eight sections). One data point was randomly sampled from each section. Next, a linear fit was calculated based on each reduced data set using a least trimmed squares regression (dashed line in Figure 1, panels B and C). Finally, the bilaterally paired stimulation sites were determined based on the slope and intercept of the linear fit for the reduced data sets and compared to those based on the slope and intercept of the linear fit from the full data set (dotted lines in Figure 1). If, for example, electrode 2 on the left ear were matched with electrode 4 on the right ear when using the full set, but it was matched with electrode 5 when using the reduced set, this would indicate a one electrode error with the reduced set. This process of random sampling with replacement, calculating a linear fit, selecting the bilaterally paired stimulation sites, and comparing those to the paired sites based on the full data set was repeated 599 times for each participant and each number of samples (4–16). The 20% trimmed mean of the difference between the pitch matched pairs for the full and reduced data sets was then calculated for each participant and each number of samples.
Figure 1.
An example of the procedure used to calculate and analyze reduced data sets. This particular example is derived from I07s data. (A) The full data set of all pitch match samples collected. (B) The reduced data set divided into four equal sections, with one data point randomly sampled from each section. (C) The reduced data set divided into eight equal sections, with one data point randomly sampled from each section. The dotted line indicates the linear fit of the full data set and the dashed line indicates the linear fit of the reduced data set.
The determination of how many pitch matches would be necessary was based on calculating the point at which additional pitch matches provided minimal improvement in reducing the differences between pitch matches based on the reduced data set and those based on the full data set. For the purposes of this study, minimal improvement was defined as a reduction in error (i.e., the difference between electrodes paired with the full and reduced data set) of less than 0.375 mm when compared to pitch matches derived using all electrodes. This is smaller than the inter-electrode spacing for current cochlear implant arrays.
Results
Robust statistical techniques were adopted to minimize the potential effects of outliers and non-normality (see the Appendex in the supplemental digital content in Aronoff, Stelmach et al., 2016). These included bootstrap analyses, which avoid assumptions of normality by using distributions based on the original data rather than an assumed normal distribution. These also included 20% trimmed means. With medians, the upper and lower approximately 50% of the data are treated as ordinal values and the mean of the remaining interval data is calculated. With the 20% trimmed means used here, the upper and lower 20% of the data are treated as ordinal values and the mean of the remaining interval data is calculated.
To determine if there was a significant difference between pitch matching with the different reduced datasets, a bootstrap analysis of variance (ANOVA) using 20% trimmed means was conducted. There was a main effect of the number of samples (Fcrit = 15.9, Ft = 18.1, where Ft > Fcrit indicates significant results for α= 0.05).
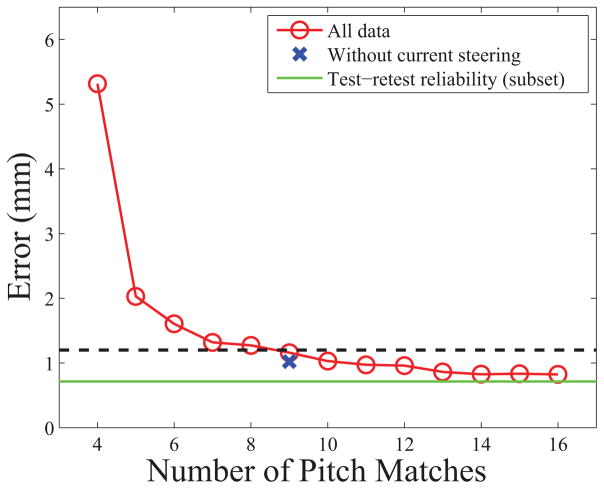
Figure 2 summarizes the results, showing the comparison of pitch matches with 4–16 samples. The error (i.e., the difference between electrodes paired based on the full and reduced data set) continued to be reduced with additional pitch match samples, reaching our criteria that additional pitch matches resulted in a change in error of less than 0.375 mm when nine samples were acquired.
Figure 2.
Benefit of obtaining additional pitch matches. The circles represent the least trimmed mean of all participants for the number of samples. The x-axis shows the number of pitch matches. The Y-axis shows the average error compared to the full pitch matching dataset. The dashed line represents a 0.375 mm difference in the magnitude of error compared to 16 samples. The “x” shows what the result would be for nine samples without the use of current steering. The solid line represents the test-retest difference for a subset of users. (Color available online).
Another analysis was completed looking at whether the number of needed pitch matched electrodes would differ across manufacturers, where the number of electrodes and the availability of current steering differs. In the current experiment virtual channels were used since current steering is used in AB devices clinically. To determine how the results would be affected without current steering, the analysis was restricted to physical reference electrodes (no virtual channels) and the responses were rounded to the nearest electrode. Note that this meant that the number of reference locations was cut in half and the spacing between references was doubled. The “x” in figure 2 shows the error for nine non-virtual electrode samples compared to the full non-virtual electrode data. The results suggest that, even without current steering and with increased spacing between stimulation locations, the error with nine pitch matches is similar.
To evaluate test-retest reliability an analysis was completed looking at a subset of the participants (I02, I03, I05, and I06) who had a second pitch matching data set acquired at a different date. The electrodes that would be paired based on the two data sets were compared for each subject. Results showed, on average, a magnitude of 0.715 mm difference between the electrodes paired in the two data sets, less than the difference between the full data set and the 16 sample reduced data set.
The difficult problem with adding a new task (such as pitch matching) in the clinic is that CI fittings are already time intensive. As part of the current dataset, timed trials were completed for over one hundred pitch matching trials, which included data from six of the participants in this study. The 20% trimmed mean for the amount of time required to conduct a pitch matching trial was 46 seconds, equaling approximately seven minutes of testing for nine pitch matches. Cutting the time to the minimal possible time increases the feasibility of this task in the clinic.
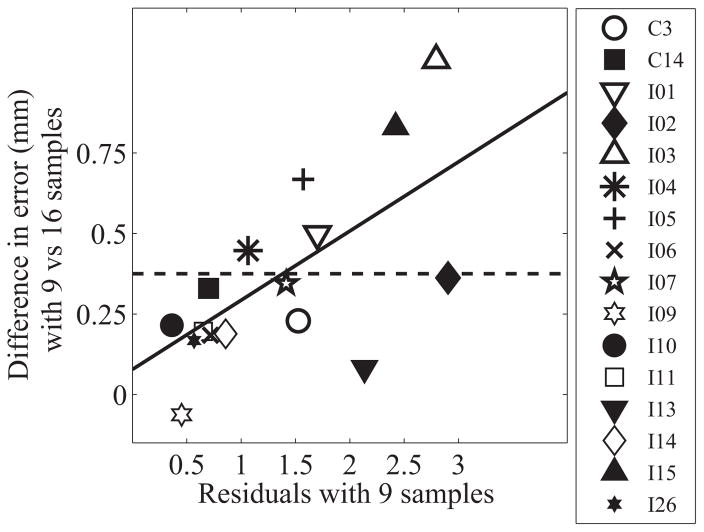
For some participants with highly variable pitch matches, more pitch matched electrodes will need to be determined to accurately capture pitch matching across the array. In practice, clinicians will not have access to the full data set when determining if nine pitch matches are sufficient. Thus, a method is required to use the nine sample pitch match data to predict whether a patient requires additional pitch matches or not. It was anticipated that those participants whose data were not closely clustered around a linear fit, indicating increased idiosyncrasy of each individual pitch match judgment, would also require more than nine pitch matches (i.e., noisier pitch-matching data would be harder to accurately measure with a small number of samples). To determine if that were the case, the magnitude of the residuals for a linear fit of the nine sample dataset were compared to the increased error when using nine reference electrodes instead of all sixteen electrodes. For each participant the residuals for each bootstrap distribution for the nine sample dataset was calculated and the 20% trimmed mean of the residuals was calculated. A least trimmed squares regression analysis comparing the average residuals with nine samples and the total distance from the original fit was calculated, which indicated a slope of 0.21 mm and an intercept of 0.08 mm (see Figure 3). This means that, if the residual is below 1.38 mm (where the 0.375 mm criteria intersects with the linear fit in Figure 3) then no further pitch matching is needed; however, if it is greater than 1.38 mm, finding a pitch match for each electrode is suggested.
Figure 3.
The linear fit between the average residuals based on nine pitch matching samples and the decrease in error when 16 samples are used compared to nine. Each point represents an individual participant. The solid diagonal line shows the linear fit. The horizontal dashed line represents a 0.375 mm difference in the magnitude of error compared to 16 samples.
Discussion
The results from this study suggest that an accurate measure of pitch matching between the ears can be obtained with nine pitch matches, with the added benefit beyond nine pitch matches typically being a reduction in error of less than 0.375 mm. In comparison, Kan, Stoelb et al. (2013) found that interaural mismatches needed to be 3 mm or less to allow binaural fusion and lateralization. This suggests that determining pitch matches for nine electrodes instead of measuring pitch matches for all electrodes should add little error to the measurement and still result in preserved binaural fusion and lateralization.
Although this experiment included only AB participants, this approach can be used with devices from all three major cochlear implant companies. While current steering was used in this study, the number of samples necessary to accurately estimate pitch matching across the arrays corresponded to a spacing of approximately 1.8 mm, greater than the spacing between electrodes for Advanced Bionics and Cochlear devices. Although this is smaller than the distance between Med-El electrodes, it is possible to implement current steering with Med-El arrays to obtain that precision. Additionally, even when current steering was not used, the effect on the magnitude of error with nine samples was minimal. This suggests that this clinical pitch matching task does not depend on the specific characteristics of the Advanced Bionics implants used in this study.
Clinical maps are typically created by assigning a given frequency region to the same numbered electrode in both ears (i.e. electrode 1 on the right and electrode 1 on the left array both having the same frequency allocation). Pitch matching data can be used to create pitch matched maps by adjusting the frequency allocations based on the pitch matching data. For example, I05 had a pitch match between electrode 3 on the right and electrode 5 on the left; for that participant, their pitch matched map would provide the same frequency allocation for electrode 3 on the right processor and electrode 5 on the left processor. Although the pitch matching procedures used here utilized specialized research hardware, the method can be adapted to clinical use with current clinical hardware. However, alterations of clinical software would be needed. Pitch matching could be added to clinical software similar to how bilateral loudness balancing has been added in recent years. Based on the participant’s pitch matches, the software could automatically update the frequency allocations of the maps based on the pitch matches obtained. The frequency allocations would be shifted by adjusting the filters so that electrodes that produce the same pitch across ears also receive the same frequency allocation.
Conclusion
In conclusion, it was found that nine pitch matches are sufficient for accurately determining pitch matches across the array. This requires approximately seven minutes of testing, indicating that accurate pitch matching can be accomplished in a clinically feasible timeframe.
Acknowledgments
We thank our participants for the time and effort they put in to this experiment. We thank Advanced Bionics for providing equipment for this study. We also thank Drs. Karen Kirk and Ron Chambers for their commentary and feedback. This work was supported by the National Organization for Hearing Research, NIH/NIDCD, R03-DC-013380, T32DC009975, R01-DC12152, R01-DC001526, R01-DC004993, R03-DC010064.
Abbreviations
- CI
Cochlear Implants
- NH
Normal Hearing
- ITD
Interaural Time Difference
- BEDCS
Bionic Ear Data Collection System
- ANOVA
Analysis of Variance
Footnotes
Declaration of Interest
National Organization for Hearing Research, NIH/NIDCD, R03-DC013380, T32DC009975, R01-DC12152, R01-DC001526, R01-DC004993, R03-DC010064. The data were presented previously as a poster presented at Association for Research in Otolaryngology (2/22/16), San Diego, CA & AudiologyNOW The American Academy of Audiology Conference (4/15/16), Phoenix, AZ.
References
- Aronoff JM, Freed DJ, Fisher LM, Pal I, Soli SD. Cochlear implant patients’ localization using interaural level differences exceeds that of untrained normal hearing listeners. J Acoust Soc Am. 2012;131:EL382–EL387. doi: 10.1121/1.3699017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff JM, Shayman C, Prasad A, Suneel D, Stelmach J. Unilateral spectral and temporal compression reduces binaural fusion for normal hearing listeners with cochlear implant simulations. Hear Res. 2015;320:24–29. doi: 10.1016/j.heares.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff JM, Stelmach J, Padilla M, Landsberger DM. Interleaved Processors Improve Cochlear Implant Patients’ Spectral Resolution. Ear Hear. 2016;37:e85–90. doi: 10.1097/AUD.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschendorff A, Kubalek R, Turowski B, Zanella F, Hochmuth A, et al. Quality control after cochlear implant surgery by means of rotational tomography. Otol Neurotol. 2005;26:34–37. doi: 10.1097/00129492-200501000-00007. [DOI] [PubMed] [Google Scholar]
- Dunn CC, Noble W, Tyler RS, Kordus M, Gantz BJ, et al. Bilateral and unilateral cochlear implant users compared on speech perception in noise. Ear Hear. 2010;31:296–298. doi: 10.1097/AUD.0b013e3181c12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CC, Tyler RS, Oakley S, Gantz BJ, Noble W. Comparison of speech recognition and localization performance in bilateral and unilateral cochlear implant users matched on duration of deafness and age at implantation. Ear Hear. 2008;29:352–359. doi: 10.1097/AUD.0b013e318167b870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad J, Linthicum FH, Jr, Otto SR, Galey FR, House WF. Cochlear implants: histopathologic findings related to performance in 16 human temporal bones. Ann Otol Rhinol Laryngol. 1991;100:807–811. doi: 10.1177/000348949110001004. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MB, Kan A, Goupell MJ. Bilateral Loudness Balancing and Distorted Spatial Perception in Recipients of Bilateral Cochlear Implants. Ear Hear. 2015;36:e225–236. doi: 10.1097/AUD.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupell MJ, Stoelb C, Kan A, Litovsky R. Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening. J Acoust Soc Am. 2013;133:2272–2287. doi: 10.1121/1.4792936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Dietz M. Comparison of Interaural Electrode Pairing Methods for Bilateral Cochlear Implants. Trends Hear. 2015;19:1–22. doi: 10.1177/2331216515617143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan A, Litovsky R, Goupell MJ. Effects of Interaural Pitch Matching and Auditory Image Centering on Binaural Sensitivity in Cochlear Implant Users. Ear Hear. 2015;36(3):e62–68. doi: 10.1097/AUD.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan A, Stoelb C, Litovsky RY, Goupell MJ. Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users. J Acoust Soc Am. 2013;134:2923–2936. doi: 10.1121/1.4820889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber S, Seeber BU. Sound localization in noise by normal-hearing listeners and cochlear implant users. Ear Hear. 2012;33:445–457. doi: 10.1097/AUD.0b013e318257607b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Fu QJ. Effects of spectral shifting on speech perception in noise. Hear Res. 2010;270:81–88. doi: 10.1016/j.heares.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Goupell MJ, Godar S, Grieco-Calub T, Jones GL, et al. Studies on bilateral cochlear implants at the University of Wisconsin’s Binaural Hearing and Speech Laboratory. J Am Acad Audiol. 2012;23:476–494. doi: 10.3766/jaaa.23.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J. Spatial hearing and speech intelligibility in bilateral cochlear implant users. Ear Hear. 2009;30:419–431. doi: 10.1097/AUD.0b013e3181a165be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak L. BEDCS Bionic Ear Data Collection System. Version 1.16 user manual. 2003. [Google Scholar]
- Loizou PC, Litovsky R, Yu G, Peters R, Lake J, et al. Speech recognition by bilateral cochlear implant users in a cocktail-party setting. J Acoust Soc Am. 2009;125:372–383. doi: 10.1121/1.3036175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CJ, Eddington DK, Colburn HS, Rabinowitz WM. Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user. J Acoust Soc Am. 2003;114:1565–1574. doi: 10.1121/1.1603765. [DOI] [PubMed] [Google Scholar]
- Marsh MA, Xu J, Blamey PJ, Whitford LA, Xu SA, et al. Radiologic evaluation of multichannel intracochlear implant insertion depth. Am J Otol. 1993;14:386–391. [PubMed] [Google Scholar]
- Nogueira W, Buechner A. Conveying low frequency information through analog electrical stimulation in cochlear implants. 20th European Signal Processing Conference; Bucharest, Romania. 2012. pp. 509–513. [Google Scholar]
- Poon BB, Eddington DK, Noel V, Colburn HS. Sensitivity to interaural time difference with bilateral cochlear implants: Development over time and effect of interaural electrode spacing. J Acoust Soc Am. 2009;126:806–815. doi: 10.1121/1.3158821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts TA, Grantham DW, Ashmead DH, Haynes DS, Labadie RF. Speech recognition for unilateral and bilateral cochlear implant modes in the presence of uncorrelated noise sources. Ear Hear. 2006;27:763–773. doi: 10.1097/01.aud.0000240814.27151.b9. [DOI] [PubMed] [Google Scholar]
- Rom DM. A sequentially rejective test procedure based on a modified Bonferroni inequality. Biometrika. 1990;77:663–666. [Google Scholar]