Abstract
Endometrial cancer arises from the endometrium. It has a slow progression and a reported survival rate of 75%. The identification of soluble biomarkers in the uterine aspirate may be very useful for its early diagnosis. Uterine aspirates from 10 patients with endometrial cancer and 6 non-endometrial cancer controls were analyzed by two-dimensional gel electrophoresis coupled with mass spectrometry and western blotting for data verification. A total of 25 proteins with fold change in %V ≥2 or ≤0.5 in intensity were observed to change significantly (P<0.05). From the discovery phase, four proteins (costars family protein ABRACL, phosphoglycerate mutase 2, fibrinogen beta chain, annexin A3) were found to be present in the uterine aspirate of endometrial cancers and not in healthy aspirates. Western blotting verification data demonstrated that costars family protein ABRACL, phosphoglycerate mutase 2 were present only in endometrial cancer uterine aspirate while fibrinogen beta chain, annexin A3 were also present in healthy aspirates. To our knowledge, phosphoglycerate mutase 2 has not been previously associated with endometrial cancer. In this study we demonstrate that uterine aspirates are a promising biological fluid in which to identify endometrial cancer biomarkers. In our opinion proteins like costars family protein ABRACL and phosphoglycerate mutase 2 have a great potential to reach the clinical phase after a validation phase.
Keywords: endometrial neoplasms, uterine aspirate, two dimensional electrophoresis, mass spectrometry, biomarkers
INTRODUCTION
Endometrial cancer (EC) is the most common gynecological malignant neoplasm [1, 2].
In Italy it represents the fourth malignant tumor in women (5% of all tumors) after breast, colon and lung cancers, and the third if we consider women between 50 and 69 years of age (7%). Its incidence shows a slight upward time trend (+ 0.7% / year), while mortality has decreased in the last 10 years with the 5-year survival rate rised from 73% in 1990 to 77.5% in 2007 [3]. Nowadays, the majority of cases are diagnosed at early stages, while only 30% of EC patients are diagnosed at an advanced stage of the disease [4].
Abnormal uterine bleeding (AUB) is generally the first sign of the disease but AUB occurs in almost all women over the age of 45. Other symptoms include pain, bleeding, infection, and uterine perforation. Diagnosis relies on the results of the endometrial biopsy.
Early detection improves the chances that the endometrial cancer will be treated successfully.
Several risk factors are already known and include: diabetes mellitus, breast cancer, dietary, obesity, high levels of estrogen, and increasing age [5, 6].
Studies have established the involvement of defects in DNA mismatch repair genes, microsatellite instability, and mutations in the PTEN and K-ras and/or B-catenin genes and p53 suppressor gene with mutation of Her-2/neu in endometrial cancer physiopathology [7].
Proteins play a key role in several cellular processes and are often associated with diseases, including cancer. The search for EC biomarkers has been mostly based on tissue analyses. Li and colleagues identified CypA, E-FABP, CAPS as potential EC-associated proteins by using 2-DE coupled with mass spectrometry [8]. Multiple members of peroxiredoxin [9], members of the annexin family [9], prohibitin 2 and bone marrow stromal antigen 2 [10] were identified by proteomics technique as potential biomarkers or potential therapeutic targets in endometrial cancer. However, the detection of these proteins has not led to their use as biomarkers in clinical practice.
Biological fluids are rich with proteins and the different abundance of these proteins can indicate the presence of tumors and are thus valuable markers for the development of noninvasive diagnostic tests [11]. A little number of proteomic studies have been performed on uterine aspirates without a focus on EC biomarkers [12, 13].
Martinez-Garcia et al. conducted the first study focused on EC uterine aspirate using LC-PRM for biomarker identification [14].
An ideal biomarker research should be based on three sequential steps: discovery, verification and validation [15]. Obviously, the discovery phase produces a long list of biomarkers using a limited number of samples, that will need to be verified before entering the validation phase.
Two-dimensional gel electrophoresis (2-DE) is a powerful method for quantitative comparative proteomic studies, able for simultaneous resolution of thousands of proteins including isoform or protein PTM. 2-DE was used for the identification of biomarkers for several types of cancers [16, 17] but also to give more detailed information about these proteins as the presence of alternative splicing and/or isoforms.
The aim of the present study is the identification and verification of soluble biomarkers in the uterine aspirates of ECs, which could be then verified in a further validation phase.
RESULTS
2-DE and MS
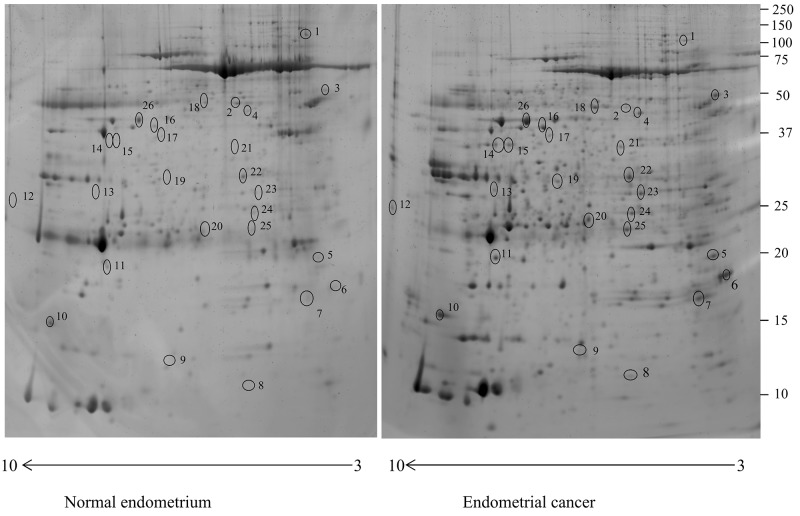
In the present study, the uterine aspirate of six non-EC controls and ten women suffering from EC were resolved using 2-DE. More than a thousand protein spots were detected in each gel. For 2-DE analysis, 40 gels were used and the effectiveness of average-matching between each gel and the corresponding “Master gel” was 80% of the total number of spots. The analysis revealed 28 protein spots with different abundance in the uterine aspirate of the ECs, which corresponded to 25 proteins. Only protein spots significantly different between EC and control (P<0.05) and with a fold change in %V ≥2 or ≤0.5 were considered (Figure 1).
Figure 1. Two-dimensional electrophoresis map of the normal endometrium uterine aspirate and endometrial cancer uterine aspirate proteome.
Immobilized pH gradient pH 3-10 non-linear strips were used for the first dimension and 12% polyacrylamide gels were used for the second dimension.
These proteins were identified by MALDI-TOF/TOF and LTQ-Orbitrap XL, and database searched against the human section of the UniProt database (version 20150401; 90,411 sequences) (Table 1).
Table 1. List of proteins with significantly different abundance in the uterine aspirate of the endometrial cancer compared to the aspirate of healthy controls, as identified by MALDI-TOF/TOF or LTQ-Orbitrap XL mass spectrometry, and classified by their corresponding molecular function. Only the most relevant molecular and cellular functions are reported.
| Accession no | Spot no | Protein description | Gene symbol | Protein score | Molecular function | Fold changea | P-value |
|---|---|---|---|---|---|---|---|
| Q9P1F3 | 8 | Costars family protein ABRACL | ABRACL | 52.25 | Not known | 490 | 0.0273 |
| P02675 | 21 | Fibrinogen beta chain | FGB | 246.69 | Binding | 400 | 0.0050 |
| P15259 | 20 | Phosphoglycerate mutase 2 | PGAM2 | 130.55 | Catalytic activity | 230 | 0.0431 |
| P06733 | 15 | Alpha-enolase | ENO1 | 339.27 | Catalytic activity | 220 | 0.0431 |
| P12429 | 23 | Annexin A3 | ANXA3 | 480.59 | Binding | 180 | 0.0273 |
| P20073-2 | 13 | isoform 2 of Annexin A7 | ANXA7 | 366.98 | Binding | 150 | 0.0412 |
| P06733 | 14 | Alpha-enolase | ENO1 | 50.65 | Catalytic activity | 120 | 0.0431 |
| A6NIW5 | 7 | Peroxiredoxin 2, isoform CRA_a | PRDX2 | 138.93 | Antioxidant activity | 60 | 0.0431 |
| A0A087WT99 | 19 | Ester hydrolase C11orf54 | C11orf54 | 76.43 | Catalytic activity | 60 | 0.0431 |
| P04406 | 12 | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 291.10 | Catalytic activity | 50 | 0.0422 |
| P28072 | 5 | Proteasome subunit beta type-6 | PSMB6 | 82.33 | Catalytic activity/binding | 20 | 0.0251 |
| P48637 | 4 | Glutathione synthetase | GSS | 506.38 | Catalytic activity | 15 | 0.0277 |
| J3KPD9 | 10 | Nucleoside diphosphate kinase B | NME2 | 330.20 | Catalytic activity | 15 | 0.0277 |
| Q7L266-2 | 9 | Isoform 2 Isoaspartyl peptidase/L-asparaginase | ASRGL1 | 126.66 | Catalytic activity | 11.5 | 0.0431 |
| P00558-2 | 25 | Isoform 2 of Phosphoglycerate kinase 1 | PGK1 | 132.98 | Catalytic activity | 10 | 0.0180 |
| P31146 | 18 | Coronin-1A | CORO1A | 70.29 | Binding | 8.5 | 0.0431 |
| P16930-2 | 17 | isoform 2 of Fumarylacetoacetase | FAH | 50.32 | Catalytic activity | 7 | 0.0277 |
| F5H3C5 | 11 | Superoxide dismutase | SOD2 | 108.73 | Catalytic activity | 5 | 0.0218 |
| Q13938-3 | 6 | isoform 2 of Calcyphosin | CAPS | 235.58 | Binding | 5 | 0.0431 |
| Q06323-3 | 24 | isoform-3 Proteasome activator complex subunit 1 | PSME1 | 73.07 | Catalytic activity | 3.3 | 0.0180 |
| P30101 | 3 | Protein disulfide-isomerase A3 | PDIA3 | 253.77 | Catalytic activity | 3.3 | 0.0277 |
| O75874 | 16 | Isocitrate dehydrogenase [NADP] cytoplasmic | IDH1 | 698.72 | Catalytic activity | 3.2 | 0.0180 |
| P06733 | 26 | Alpha-enolase | ENO1 | 373.86 | Catalytic activity | 2.9 | 0.0284 |
| P07195 | 22 | L-lactate dehydrogenase B chain | LDHB | 239.73 | Catalytic activity | 2 | 0.0241 |
| Q96KP4 | 2 | Cytosolic non-specific dipeptidase | CNDP2 | 186.34 | Catalytic activity | 0.3 | 0.0116 |
| A0A0C4DGB6 | 1 | Serum albumin | ALB | 183.91 | Binding | 0.1 | 0.0249 |
aFold change was defined as the ratio of the mean %V according to the formula %V=Vsingle spot/Vtotal spot of cases vs. controls.
We performed a ROC analysis to determine the sensitivity and specificity of each biomarker. The best-performing individual protein controls (sensitivity 100%, specificity 100%) (Supplementary Table 2) were: PDIA3, isoform 2 of CAPS, isoform CRA_a PRDX2, ABRACL, NME2, Isoform 2 ASRGL1, GAPDH, isoform 2 ANXA7, ENO1, CORO1A, C11orf54, PGAM2, FGB, ANXA3, PGK1. Of these, ABRACL, PGAM2, FGB, ANXA3 were present only in EC. Based on this data we decided to validate ABRACL, PGAM2, FGB, ANXA3 by western blotting.
Functional analysis of the EC aspirate proteome
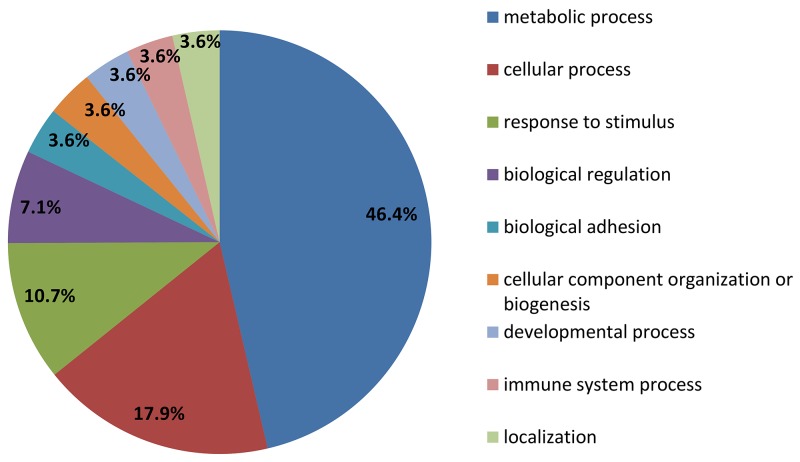
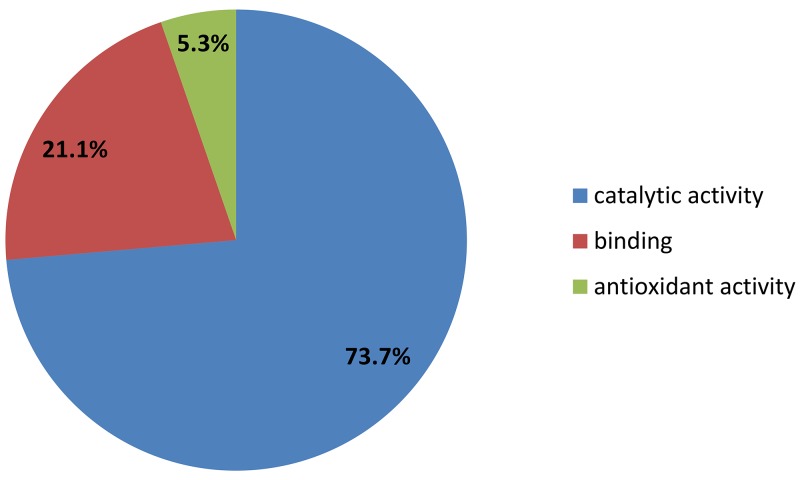
The 25 identified proteins were divided into groups, based on the PANTHER classification system, according to their biological processes and molecular functions. In terms of biological processes, proteins were grouped into four main categories: metabolic process, cellular process, response to stimulus and biological regulation (Figure 2). For molecular function the proteins were grouped into three categories: catalytic activity, binding, antioxidant activity (Figure 3). Fifteen of these proteins (PGAM2, IDH1, C11orf54, GSS, NME2, GAPDH, PSME1, PDIA3, FAH, PGK1, LDHB, ASRGL1, SOD2, ENO1, CNDP2) were found to be associated with the catalytic activity, according to the PANTHER prediction (Table 1). These data show the involvement of different types of enzymes in endometrial cancer disease.
Figure 2. PANTHER classification of proteins upregulated in leiomyoma according to their biological process and molecular function.
Figure 3. PANTHER classification of proteins upregulated in leiomyoma according to their molecular function.
Western blotting analysis
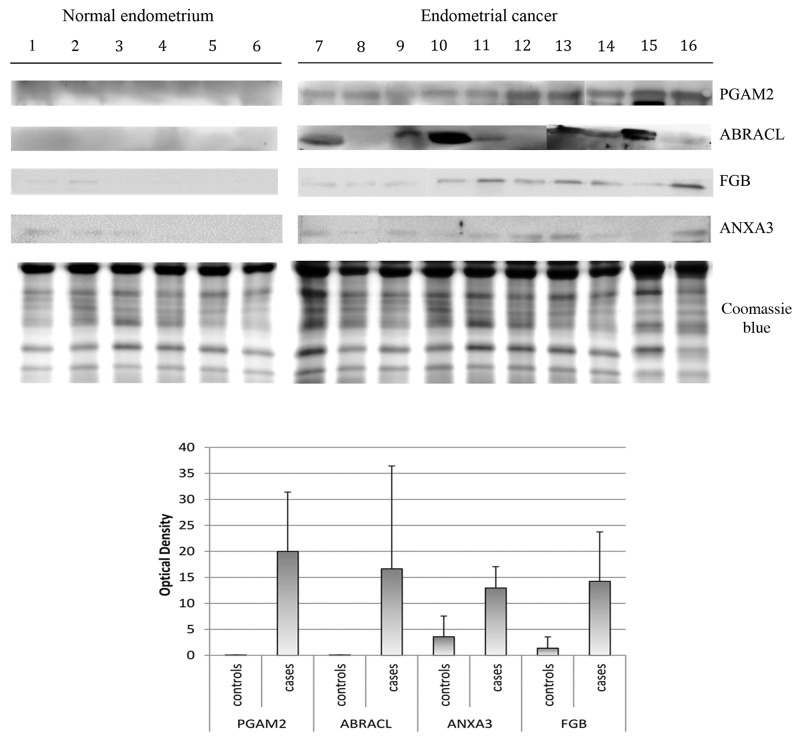
In this study, western blot (WB) analysis from ten endometrial cancer vs six normal endometrium was used to validate the abundance of the four proteins which discriminate unambiguously between cases and controls (ABRACL, PGAM2, FGB, ANXA3). As seen from Figure 4, only ABRACL and PGAM2 are able to unambiguously discriminate the controls, from the EC patients.
Figure 4. Western blot analysis of PGAM2, ABRACL, ANXA3, FBG normal endometrium uterine aspirate and endometrial cancer uterine aspirate.
The intensities of the immunostained bands were normalized with the protein intensities measured by Coomassie blue from the same blot. The bar graph shows the relative expression (band density) of proteins in the normal endometrium uterine aspirate and endometrial cancer uterine aspirate. The results are shown as a histogram (mean) with error bars representing the standard deviation. All differences were observed to be significant (Wilcoxon signed-rank test for matched samples, P<0.05).
The western blot data verified the presence of PGAM2 in all EC patients, behaving as a marker for G1, G2, G3, G2-G3 and serous papillary adenocarcinoma EC phase.
For ABRACL, western blotting verified its presence in eight EC patients G1, G2, G3, serous papillary adenocarcinoma phase, while we did not observe the presence of ABRACL in one G2 and one G2-G3 phase.
In case of FGB, we found the protein in all the EC samples but this protein was also present in the samples of two healthy subjects (hyperplastic endometrium and normal endometrium). For ANXA3 we found this protein in nine EC samples. This protein was absent in G3 samples and was found in three healthy patients (hyperplastic endometrium and two normal endometrium). FGB and ANXA3 were not able to discriminate the normal endometrium from the EC, although their expression level is significantly increased in EC.
DISCUSSION
To date, several studies have been carried out for the identification of EC biomarkers, but no protein has yet reached the stage of clinical application. This may be related to the lack of studies on biofluids for the identification of soluble biomarkers and overall to the difficulty in performing such kind of studies on large cohorts of samples for the validation phase. The studies conducted until now focused on tissues and on plasma/serum [18, 19]. However, the research for biomarkers in serum/plasma is very difficult due to the large dynamic range of protein concentration and the low concentration of the potential biomarkers [20, 21].
To our knowledge, our study is the first using 2-DE with SYPRO Ruby and data verification by western blotting in the EC uterine aspirate. We identified 25 potential biomarkers, but only four of them were present only in ECs. Further data validation by western blotting showed that ABRACL and PGAM2 fully discriminate the healthy from EC samples. Our verification data showed the presence of PGMA2 in all EC patients. This protein has the potential to be identified as a biomarker for all EC phases and in serous papillary adenocarcinoma. While ABRACL verification data showed the presence of this protein in eight EC patients, only two samples (G2 and G2-G3 phase) did not show the presence of this protein, and this may be associated with the low abundance of these protein in the samples. However, the protein may be a potential biomarker for EC in G1, G2, G3 and serous papillary adenocarcinoma.
Maxwell GL at colleagues conducted a detailed study on the proteomic profile of EC and endometrium samples using label-free GeLC-MS/MS [9]. We compared our data with their data. The comparison confirmed that the expression of FGB, ENO1, ANXA3, PRDX2, GAPDH, PSMB6, GSS, ASRGL1, PGK1, CORO1A, PSME1, PDIA3, IDH1, LDHB was upregulated. In Martinez-Garcia et al., ENO1 was confirmed as an up-regulated protein in endometrial cancer [14].
DeSouza L et al. studied the proteomic profile of EC and endometrium samples using labeled tags iTRAQ and cICAT: NME2, SOD2, CAPS were upregulated while ALB was down-regulated [22].
Our proteomic study revealed the dysregulation of fifteen metabolic enzymes showing a clear disruption of cancer metabolism.
ABRACL is a 82 amino acid protein that increase actin dynamics and cell motility [23].
PGAM2 is a glycolytic enzyme that catalyzes the reversible conversion of 3-phosphoglycerate (3-PG) to 2-phosphoglycerate, which is highly expressed in muscle tissues [24]. This enzyme increase NADPH homeostasis in response to the oxidative stress that impacts cell proliferation and tumor growth [25]. Further studies with larger groups of patients and controls are needed for the validation of ABRACL and PGAM2 as biomarkers.
Both proteins are involved in cell motility and proliferation, and in our opinion further studies are needed to verify the association between the expression of these proteins and tumor metastatization. In addition, PGAM2 being a glycolytic enzyme can be very useful in monitoring glycolysis as a fundamental metabolic pathway in cancer development.
In this study we demonstrate that uterine aspirates are a valuable biological fluid for the identification of cancer biomarkers.
The use of diagnostic biomarkers for EC can fasten the diagnostic process and reduce the sanitary costs.
Uterine aspirate may be an alternative to serum/plasma due to its high specificity [14].
In conclusion, our study expands the knowledge on the search for biomarkers in EC uterine aspirates. This study confirms the potential of 2-DE for biomarker discovery and western blotting for data verification. Proteins like ABRACL and PGAM2 have a great potential to reach the clinical phase after a validation phase.
MATERIALS AND METHODS
Patients, sample collection and treatment
A total of 16 patients (10 women suffering from EC and 6 non-EC controls) were recruited at the Institute for Maternal and Child Health – IRCCS “Burlo Garofolo” (Trieste, Italy) during 2014 and 2015. All procedures complied with the Declaration of Helsinki and were approved by the Technical and Scientific Committee of the Institute for Maternal and Child Health – IRCCS “Burlo Garofolo” (approval no. RC 19/08). All patients signed an informed consent. The clinical and pathological characteristics of the patients are described in Supplementary Table 1. Samples from control patients were obtained during the proliferative phase endometrium and the median age was 43 years. Samples from patients with endometrial cancer were obtained during postmenopause and the median age was 72.5 years.
Uterine aspirates were collected by aspiration with a Cornier Pipelle in the office of the clinician or in the operating room prior to surgery, and transferred to 10 ml microtubes and 3 ml of NaCl 0.9% was added with a protease inhibitor mixture (2 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 1 mM EDTA and 10 mM NaF). The supernatant was centrifuged at 5,000 x g for 30min at 4°C followed by centrifugation at 15,000 x g for 30min at 4°C to remove cell debris. Approximately 3 ml of supernatant was desalted and concentrated using an Ultrafree-4 Centrifugal Filter Unit (EMD Millipore, Billerica, MA, USA) with a molecular weight cut-off of 3 kDa at 4,000 x g at 25°C until the remaining volume reached 50 μl. The protein content of the supernatant was determined by Bradford assay.
2-DE and image analysis
2-DE was performed like previously described [26]. For 2-DE analysis, 400 μg of proteins from each uterine aspirate sample was denatured in 300 μl of dissolution buffer [7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 40 mM Tris, 65 mM dithiothreitol (DTT) and 0.24% Bio-Lyte 3/10 (Bio-Rad Laboratories, Inc., Hercules, CA, USA)] and a trace of bromophenol blue. ReadyStrip™ pH 3-10 Non-linear (NL) 18-cm immobilized pH gradient (IPG) strips (Bio-Rad Laboratories, Inc.) were rehydrated in a dissolution buffer at 50 V for 12h at 20°C, and isoelectric focusing (IEF) was performed in a PROTEAN IEF Cell (Bio-Rad Laboratories, Inc.). After the IEF, serial incubations were performed: first, the IPG strips were equilibrated for 20min in an equilibration buffer [6 M urea, 2% SDS, 50 mM Tris-HCl (pH 8.8), 30% glycerol and 1% DTT] and then in another equilibration buffer containing 4% iodoacetamide instead of DTT. For the second dimension, the equilibrated IPG strips were transferred to a 12% polyacrylamide gel. After electrophoresis, gels were fixed in 40% methanol and 10% acetic acid for 1h, and then stained for 16h with SYPRO Ruby. These gels, after SYPRO Ruby destaining, were stained for 48h with colloidal Coomassie Brilliant Blue. Double experimental replicates were performed per sample. Molecular weights were determined by comparison with Precision Plus Protein™ Prestained Standards (Bio-Rad Laboratories, Inc.), covering a range from 10 to 250 kDa. 2-DE gels were scanned with a Molecular Imager PharosFX System and analyzed using the Proteomweaver 4.0 software (both from Bio-Rad Laboratories, Inc.).
Quantification of spot levels
2-DE image analysis was performed using the Proteomweaver 4.0 software. The analysis was performed by matching all gels from each uterine aspirate sample with a reference gel for the same condition, having the best resolution and the greatest number of spots, named “Master gel”.
Differences were considered to be significant when the ratio of the mean percentage of relative volume (%V), determined as %V = Vsingle spot/Vtotal spot, was ≥2.0 for up-regulated or ≤ 0.5 for down-regulated proteins, and the non-parametric Wilcoxon signed-rank test for matched samples resulted as being significant (P<0.05). Fold change was calculated as the ratio between the mean %V of the EC uterine aspirate and that of the normal uterine aspirate.
Western blotting
Protein extracts (30 μg) used for 2-DE were separated by 12% or 15% SDS-PAGE and then transferred to a nitrocellulose membrane. The residual binding sites on the membrane were blocked by treatment with 5% dry milk in TBS-tween 20. After milk saturation the membrane was incubated overnight at 4°C with 1:200 diluted primary rabbit polyclonal antibody against ABRACL, 1:200 diluted primary rabbit polyclonal antibody against FGB, 1:1000 diluted primary rabbit polyclonal antibody against ANXA3, 1:300 diluted primary rabbit polyclonal antibody against PGAM2. The membrane was washed three times in TBST for 10min, and then incubated for 90min at 4°C with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G antibody (Sigma-Aldrich; Merck KGaA) at 1:3.000 dilution. Protein expression was visualized by chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate; Thermo Fisher Scientific, Inc.), and the intensity of the signals was quantified by VersaDoc Imaging System (Bio-Rad Laboratories, Inc.). The intensities of the immunostained bands were normalized with the protein intensities measured by Coomassie blue (Sigma-Aldrich; Merck KGaA) from the same blot.
Trypsin digestion and MS analysis
Spots from 2-DE were digested with sequencing grade-modified trypsin (Promega, Madison, WI, USA) and analyzed by mass spectrometry, as described by Ura et al. [27].
Spots extracted from 2-DE gels were washed with 50 mM NH4HCO3 and acetonitrile (ACN; Sigma-Aldrich) and dried under vacuum in a SpeedVac system. To each spot, three microliters of 12.5 ng/μl sequencing grade modified trypsin (Promega, Madison, WI, USA) in 50 mM NH4HCO3 were added, samples were allowed the rehydrate at 4°C and then covered with 50 mM NH4HCO3 and digested overnight at 37°C. Peptides were extracted with three changes of 50% ACN/0.1% formic acid (FA; Fluka), dried under vacuum and dissolved in 10 μl of 0.1% trifluoroacetic acid (TFA; Riedel-de Haën).
One microliter of matrix solution (α-cyano-4hydroxycinnamic acid 5 mg/ml in 70% acetonitrile/0.1% TFA) was mixed with 1 μl of each sample, and 0.8 μl of the final sample/matrix mixture was spotted onto a stainless steel MALDI target plate. Tandem mass spectrometry (MS/MS) analysis was performed on a MALDI-TOF/TOF 4800 mass spectrometer (AB Sciex, Framingham, MA, USA) in a data dependent mode: a full MS scan was acquired, followed by MS/MS spectra of the 10 most intense signals.
Samples that could not be identified by MALDI-TOF/TOF analysis were further analyzed by LC-MS/MS on an LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Rockford, IL, USA), coupled with a nano-HPLC Ultimate 3000 (Dionex – Thermo Fisher Scientific). The analysis was performed in a data dependent mode, and a full scan at 60,000 resolution on the Orbitrap was followed by MS/MS fragmentation scans on the 10 most intense ions acquired with CID fragmentation in the linear trap.
Data were converted into MGF (Mascot generic format) files to be elaborated with Proteome Discoverer 1.4 (Thermo Fisher Scientific), while raw data files from the LTQ-Orbitrap XL mass spectrometer were directly analyzed with the software. Proteome Discoverer was interfaced to a Mascot search engine, version 2.2.4 (Matrix Science, London, UK).
Enzyme specificity was set to trypsin with 1 missed cleavage. The database used for protein identification was UniProt Human (version 20150401; 90,411 sequences).
The tolerances were 50 ppm (parent) and 0.3 Da (fragment ions) for the MALDI-TOF/TOF data, and were set to 10 ppm for parent mass and to 0.6 Da for fragment ions for the files from LTQ-Orbitrap XL. Carbamidomethylation of cysteine residues was set to ‘fixed modification’ and methionine oxidation to ‘variable modification’.
A false discovery rate (FDR) was calculated by Proteome Discoverer based on the parallel search against a randomized database. Proteins were considered as positive hits if at least two independent peptides were identified with medium (95%) or high (99%) confidence.
Functional analysis
The different abundant proteins identified were analyzed by PANTHER 11.0 (Protein Analysis through Evolutionary Relationships; http://www.pantherdb.org). Proteins were then classified according to their involvement in biological processes and molecular function. As most of the proteins take part in multiple processes, only the most relevant were reported.
Statistical analysis
Statistical analyses were carried out with the non-parametric Wilcoxon signed-rank test for matched samples for both 2-DE and western blot data. P<0.05 was considered to indicate a statistically significant difference. All analyses were conducted with Stata/IC 14.1 for Windows (StataCorp LP, College Station, TX, USA).
SUPPLEMENTARY MATERIALS TABLES
Abbreviations
- MALDI-TOF/TOF
Matrix-assisted laser desorption/ionization
- LC-PRM
Targeted Proteomics by Parallel-Reaction Monitoring
- LTQ Orbitrap XL
Hybrid Ion Trap-Orbitrap Mass Spectrometer
- NaCl
Sodium chloride
- EDTA
Ethylenediaminetetraacetic acid
- NaF
Sodium fluoride
- SDS
Sodium Dodecyl Sulphate
- Tris-HCL
2-Amino-2-(hydroxymethyl)-1,3-propanediol hydrochloride
- IPG
Immobilized pH gradient
- SDS-PAGE
Sodium Dodecyl Sulphate - Poly-Acrylamide Gel Electrophoresis
- NH4HCO3
Ammonium bicarbonate
- LC-MS/MS
Liquid chromatography–mass spectrometry
Footnotes
Author contributions
Conceived and designed the experiments: Giuseppe Ricci, Federica Scrimin; Performed the experiments: Blendi Ura, Giorgio Arrigoni, Cinzia Franchin; Analyzed the data: Lorenzo Monasta, Blendi Ura, Oriano Radillo, Isabel Peterlunger; Contributed reagents/materials/analysis tools: Giorgio Arrigoni, Cinzia Franchin; Wrote the paper: Blendi Ura, Lorenzo Monasta, Federica Scrimin, Giuseppe Ricci.
CONFLICTS OF INTEREST
The authors have no financial nor personal conflicts of interest to disclose.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database Syst Rev. 2012:CD003916. doi: 10.1002/14651858.CD003916.pub3. [DOI] [PubMed] [Google Scholar]
- 3. AIOM-AIRTUM (2013) I numeri del cancro in italia 2013. http://www.registri-tumori.it, http://www.registri-tumori.it/PDF/AIOM 2013/I_numeri_del_cancro_2013.pdf.
- 4.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 5.Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, Ghaem-Maghami S. Endometrial cancer. BMJ. 2011;343:d3954. doi: 10.1136/bmj.d3954. [DOI] [PubMed] [Google Scholar]
- 6.Lucenteforte E, Talamini R, Montella M, Dal Maso L, Tavani A, Deandrea S, Pelucchi C, Greggi S, Zucchetto A, Barbone F, Parpinel M, Franceschi S, La Vecchia C, et al. Macronutrients, fatty acids and cholesterol intake and endometrial cancer. Ann Oncol. 2008;19:168–72. doi: 10.1093/annonc/mdm446. [DOI] [PubMed] [Google Scholar]
- 7.Talhouk A, McAlpine JN. New classification of endometrial cancers: the development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016;3:14. doi: 10.1186/s40661-016-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Zhao X, Bai S, Wang Z, Chen L, Wei Y, Huang C. Proteomics identification of cyclophilin a as a potential prognostic factor and therapeutic target in endometrial carcinoma. Mol Cell Proteomics. 2008;7:1810–23. doi: 10.1074/mcp.M700544-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxwell GL, Hood BL, Day R, Chandran U, Kirchner D, Kolli VS, Bateman NW, Allard J, Miller C, Sun M, Flint MS, Zahn C, Oliver J, et al. Proteomic analysis of stage I endometrial cancer tissue: identification of proteins associated with oxidative processes and inflammation. Gynecol Oncol. 2011;121:586–94. doi: 10.1016/j.ygyno.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama T, Enomoto T, Serada S, Morimoto A, Matsuzaki S, Ueda Y, Yoshino K, Fujita M, Kyo S, Iwahori K, Fujimoto M, Kimura T, Naka T. Plasma membrane proteomics identifies bone marrow stromal antigen 2 as a potential therapeutic target in endometrial cancer. Int J Cancer. 2013;132:472–84. doi: 10.1002/ijc.27679. [DOI] [PubMed] [Google Scholar]
- 11.Monge M, Colas E, Doll A, Gil-Moreno A, Castellvi J, Diaz B, Gonzalez M, Lopez-Lopez R, Xercavins J, Carreras R, Alameda F, Canals F, Gabrielli F, et al. Proteomic approach to ETV5 during endometrial carcinoma invasion reveals a link to oxidative stress. Carcinogenesis. 2009;30:1288–97. doi: 10.1093/carcin/bgp119. [DOI] [PubMed] [Google Scholar]
- 12.Casado-Vela J, Rodriguez-Suarez E, Iloro I, Ametzazurra A, Alkorta N, Garcia-Velasco JA, Matorras R, Prieto B, Gonzalez S, Nagore D, Simon L, Elortza F. Comprehensive proteomic analysis of human endometrial fluid aspirate. J Proteome Res. 2009;8:4622–32. doi: 10.1021/pr9004426. [DOI] [PubMed] [Google Scholar]
- 13.Hannan NJ, Stephens AN, Rainczuk A, Hincks C, Rombauts LJ, Salamonsen LA. 2D-DiGE analysis of the human endometrial secretome reveals differences between receptive and nonreceptive states in fertile and infertile women. J Proteome Res. 2010;9:6256–64. doi: 10.1021/pr1004828. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Garcia E, Lesur A, Devis L, Campos A, Cabrera S, van Oostrum J, Matias-Guiu X, Gil-Moreno A, Reventos J, Colas E, Domon B. Development of a sequential workflow based on LC-PRM for the verification of endometrial cancer protein biomarkers in uterine aspirate samples. Oncotarget. 2016;7:53102–53115. doi: 10.18632/oncotarget.10632. https://doi.org/10.18632/oncotarget.10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker CE, Borchers CH. Mass spectrometry based biomarker discovery, verification, and validation—quality assurance and control of protein biomarker assays. Mol Oncol. 2014;8:840–58. doi: 10.1016/j.molonc.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Zang Z, Xu X, Zhang Z, Zhong L, Zan W, Zhao Y, Sun L. Differential proteomics identification of HSP90 as potential serum biomarker in hepatocellular carcinoma by two-dimensional electrophoresis and mass spectrometry. Int J Mol Sci. 2010;11:1423–33. doi: 10.3390/ijms11041423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gromova I, Gromov P, Honma N, Kumar S, Rimm D, Talman ML, Wielenga VT, Moreira JM. High level PHGDH expression in breast is predominantly associated with keratin 5-positive cell lineage independently of malignancy. Mol Oncol. 2015;9:1636–54. doi: 10.1016/j.molonc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian W, Zhu Y, Wang Y, Teng F, Zhang H, Liu G, Ma X, Sun D, Rohan T, Xue F. Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol Oncol. 2013;129:505–12. doi: 10.1016/j.ygyno.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Yurkovetsky Z, Ta’asan S, Skates S, Rand A, Lomakin A, Linkov F, Marrangoni A, Velikokhatnaya L, Winans M, Gorelik E, Maxwell GL, Lu K, Lokshin A. Development of multimarker panel for early detection of endometrial cancer. High diagnostic power of prolactin. Gynecol Oncol. 2007;107:58–65. doi: 10.1016/j.ygyno.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–67. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 21.Xue H, Lu B, Lai M. The cancer secretome: a reservoir of biomarkers. J Transl Med. 2008;6:52. doi: 10.1186/1479-5876-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeSouza LV, Grigull J, Ghanny S, Dubé V, Romaschin AD, Colgan TJ, Siu KW. Endometrial carcinoma biomarker discovery and verification using differentially tagged clinical samples with multidimensional liquid chromatography and tandem mass spectrometry. Mol Cell Proteomics. 2007;6:1170–82. doi: 10.1074/mcp.M600378-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Pang TL, Chen FC, Weng YL, Liao HC, Yi YH, Ho CL, Lin CH, Chen MY. Costars, a dictyostelium protein similar to the C-terminal domain of STARS, regulates the actin cytoskeleton and motility. J Cell Sci. 2010;123:3745–55. doi: 10.1242/jcs.064709. [DOI] [PubMed] [Google Scholar]
- 24.Fothergill-Gilmore LA, Watson HC. The phosphoglycerate mutases. Adv Enzymol Relat Areas Mol Biol. 1989;62:227–313. doi: 10.1002/9780470123089.ch6. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Li F, Lv L, Li T, Zhou X, Deng CX, Guan KL, Lei QY, Xiong Y. Oxidative stress activates SIRT2 to deacetylate and stimulate phosphoglycerate mutase. Cancer Res. 2014;74:3630–42. doi: 10.1158/0008-5472.CAN-13-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ura B, Scrimin F, Arrigoni G, Franchin C, Monasta L, Ricci G. A proteomic approach for the identification of up-regulated proteins involved in the metabolic process of the leiomyoma. Int J Mol Sci. 2016;17:540. doi: 10.3390/ijms17040540. https://doi.org/10.3390/ijms17040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ura B, Scrimin F, Arrigoni G, Athanasakis E, Aloisio M, Monasta L, Ricci G. Abnormal expression of leiomyoma cytoskeletal proteins involved in cell migration. Oncol Rep. 2016;35:3094–100. doi: 10.3892/or.2016.4688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.