Abstract
Molecular characterization of tumor cells is a key step in the diagnosis and optimal treatment of lung cancer. However, analysis of tumor samples, often corresponding to small biopsies, can be difficult and does not accurately reflect tumor heterogeneity. Recent studies have shown that isolation of circulating tumor cells (CTCs) is feasible in non-small cell lung cancer patients, even at early disease stages. The amount of CTCs corresponds to the metastatic potential of the tumor and to patient prognosis. Moreover, molecular analyses, even at the single-cell level, can be performed on CTCs. This review describes the technologies currently available for detecting and capturing CTCs, the potential for downstream molecular diagnostics, and the clinical applications of CTCs isolated from lung cancer patients as screening, prognostic, and predictive tools. Main limitations of CTCs are also discussed.
Keywords: lung cancer, circulating tumor cells, prognosis, predictive marker, molecular diagnosis
INTRODUCTION
Lung cancer is the main cause of cancer-related mortality worldwide [1]. Molecular screening techniques have allowed great advances in the treatment of non-small cell lung cancer (NSCLC) [2]. However, there is an urgent need to develop new tools to improve lung tumor cell characterization and understand the mechanisms underlying prognosis, treatment resistance, and tumor progression. Moreover, easy and reliable methods are still needed for lung cancer screening. Since a few years, the use and impact of liquid biopsy have raised as a surrogate marker of solid biopsy [3]. Liquid biopsy can be performed from many body fluids, but mainly from peripheral blood [4]. Along with circulating tumor DNA (ctDNA), circulating tumor cells (CTCs) are cells originating from the tumor site that migrate to the blood circulatory system and disseminate throughout the body; they have a putative role in metastasis formation [5, 6]. Their presence in patients with solid tumors at all disease stages is well established [7, 8], and they have the same cytomorphologic features as the original tumor [9]. CTC detection and isolation in lung cancer patients is technically difficult. However, molecular and functional analyses of lung cancer CTCs have great clinical potential. This review describes the different techniques currently available for detecting and isolating CTCs from lung cancer patients; the clinical impact on cancer screening, patient prognosis, and predicting the treatment response; and current data concerning the molecular analyses of CTCs.
METHODS FOR CTC DETECTION AND ISOLATION
Magnetic bead assays: EpCAM and CD45 depletion
CTCs can be directly detected or enriched using anti-epithelial cell surface molecule (EpCAM) antibody or indirectly enriched by removing other cells, for example using anti-CD45 antibody (specific for leukocytes). The Cellsearch system (Janssen Diagnostics) is designed to isolate CTCs from peripheral whole blood samples using EpCAM selection with magnetic beads. Cells are also stained with DAPI (nuclear staining), various cytokeratins (cytokeratins 8, 18, and 19), and CD45 for fluorescence microscopy analysis: CTCs are oval/round cytokeratin positive and CD45 negative cells with a visible nucleus (DAPI positive). Allard et al. validated this system in 964 patients with metastatic carcinomas, 199 patients with nonmalignant diseases, and 145 healthy controls [10]. In patients with metastatic lung cancer (n=168), the mean number of CTCs was 30 per 7.5 mL blood, and 20% of patients had ≥2 CTCs per 7.5 mL blood. Similar percentages were found in other studies [11–13]. Other systems aimed to improve the EpCAM-dependent assay, for example by using a microfluidic device with bidirectional flow and a sloped box to concentrate CTCs [14, 15] or by performing RT-PCR to measure expression of a panel of cancer genes after CTC enrichment [16]. The Cellsearch platform is currently approved by the US Food and Drug Administration for CTC isolation from blood samples from breast, colorectal and prostate cancer patients.
Depletion of CD45-positive cells with magnetic beads is another method of isolating CTCs. CD45 is a highly specific marker of leukocytes, the main nucleated cell population in the blood. Wu et al. tested this method in a cohort of 47 lung cancer patients (41 newly diagnosed patients and 6 with recurrent lung cancers), 13 tuberculosis patients and 18 healthy controls [17]. After CD45-positive cell depletion by cell sorting, cytokeratins 18 and 19 immunofluorescence assay was performed. Positive detection was defined as the presence of ≥2 CTCs per 7.5 mL blood. The overall detection rate with this cut-off was 85% in lung cancer patients versus 8% in tuberculosis patients and 0% in healthy patients. When stratified by tumor stage in newly diagnosed patients, the detection rate was 67% for stage I–II (mean, 5 CTCs per 7.5 mL), 72% for stage III (mean, 7 CTCs per 7.5 mL), and 50% for stage IV (mean, 7 CTCs per 7.5 mL). For recurrent cancer patients, the detection rate was 83% (mean, 11 CTCs per 7.5 mL). Another study with a similar design also reported high detection rates (73% for stage I–III cancer patients and 100% for stage IV cancer patients) [18].
Folate receptors are overexpressed in various solid tumors, including NSCLC [19]. A specific two-step method for CTC detection and isolation by recognizing surface folate receptor expression has been developed (CytoploRare, GenoSaber Biotech). The first step is CTC enrichment by leucocyte deletion (using CD45-coated immunomagnetic beads) and the second step is ligand-targeted PCR (LT-PCR) in which CTCs are labeled with the tumor-specific folic acid ligand conjugated to a synthesized oligonucleotide. The conjugated ligand is then annealed and extended onto the RT primer, before amplification and quantitative PCR. Using this method, the CTC detection rate is around 75% in lung cancer patients [20–22].
Chip-based assays
CTCs can also be isolated using a microfluidic platform, called the “CTC-chip,” developed by Nagrath et al. In this assay, an anti-EpCAM antibody tethered to microposts captures EpCAM-positive cells as they pass through the device [23]. After sorting, CTCs were characterized by cytokeratin, CD45, and DAPI staining, as described for other assays. In a cohort of 116 cancer patients (55 metastatic lung cancer patients), the detection rate was 99% for NSCLC patients (with 5–1281 CTCs per ml) and 0% in 20 healthy controls. The captured cell purity (cytokeratin-positive cells/CD45-positive cells ratio) was 52% in NSCLC patients.
Many similar devices have been produced by different research groups based on the same principle (isolation of CTCs using mechanical pumps inducing inertial lift and drag forces). After passing through the chip, CTCs can be characterized by immunofluorescence staining and/or magnetic beads or nanoparticles coated with various antibodies (e.g. CD45, cytokeratins, EpCAM). The detection rate is often high (53–100%) in lung cancer samples [24–35]. Some devices include special membranes to improve CTC isolation, release, and viability [36–38], whereas other devices use centrifugal instead of mechanical force [39–42]; all have good detection rates.
Another device, called Apostream (ApoCell), is a microfluidic system for dielectrophoretic separation of CTCs from normal circulating cells. Enriched CTCs are then labeled with anti-folate receptor alpha antibody for immunofluorescence characterization. However, the CTC detection rate for this method is low: 36% for advanced lung adenocarcinoma patients (n=5/14) and 0% for lung squamous cell carcinoma patients (n=0/6) [43].
Size-based assays
Due to their larger size compared with normal leukocytes, CTCs can be isolated by size-based filtration. This concept was first developed and validated in samples from hepatocellular carcinoma patients by Vona et al. in 2000 with the ISET system (Isolation by Size of Epithelial Tumor cells; Rarecells) [44]. ISET isolation enabled morphological and functional analyses of CTCs. Later, ISET isolation of CTCs was tested in a large cohort of 250 lung cancer patients and 59 healthy patients [45]. Based on their morphology, cells were classified as malignant (meeting at least four of the following criteria: anisonucleosis, nuclei >24 μm, irregular nuclei, high nuclear–cytoplasmic ratio, three-dimensional sheets), uncertain malignant cells (meeting 1–2 of the criteria) or benign cells (meeting none of the criteria). The detection rate was 41% for malignant cells and 6% for uncertain malignant cells; and none of the samples from healthy subject contained CTCs. Interestingly, intra-institution agreement in diagnosing malignant circulating cells (evaluation by three different pathologists) was very good (κ=1), but inter-institution agreement was poor (κ<0.40 for three different institutions compared with the referent institution).
The ScreenCell device uses a similar size-based approach for CTC isolation. For two different pathologists evaluating the presence of CTCs in blood samples from a cohort of 74 cancer patients (including 32 lung cancer patients), the individual detection rates were 53% and 57%, , but the rate of detection by both pathologists was only 43% [46] with a moderate κ value (0.54). Smaller series of lung cancer patients reported similar detection rates [47, 48].
A recent work from Yagi et al. described the development of an automated microcavity array (MCA) system for CTCs isolation [49]. This assay is based on the differences in size and deformability between tumor cells and normal blood cells. After isolation with MCA, cells were considered as CTCs if they were DAPI-positive, cytokeratin-positive, and CD45-negative under the fluorescence microscope. On 50 lung cancer patients and 10 healthy volunteers, sensitivity and specificity were 80 and 90%, respectively, with better performances than the Cellsearch system.
Other locally developed devices for size-based CTCs isolation include microcavity array [50, 51], fluid-assisted separation technology (FAST) [52], home-made membranes [53], and integrated microfluidic chip with membrane filter [54], all with good detection rates (69–100%).
Other methods of CTC detection
Other assays have been tested for CTC detection. As cancer stem cells (CSCs) are the key factors for cancer spreading and metastases induction, Skirecki et al. hypothesized that CTCs had similar features to CSCs [55]. They performed flow cytometry analysis with CD133 (a CSC marker) and EpCAM double staining in both lung tumor tissues (n=7) and peripheral blood samples (n=41) from lung cancer patients. However, the CD133+/EpCAM+ cell detection rate was only 36% in peripheral blood compared with 86% in tumor tissues. Other research groups developed immunofluorescence assays for cell monolayers from blood samples after red blood cell lysis using various antibodies (e.g. against cytokeratins, CD45), with only limited preliminary results [56, 57].
In vivo assays for isolating CTCs directly from the peripheral veins of patient have also been described. Most use a wire coupled to an anti-EpCAM antibody directly inserted into the vein via a catheter, and have a detection rate of 58–100% [58, 59].
CTCs and epithelial–mesenchymal transition
EpCAM is used as a marker for CTC isolation in several assays, for example in the Cellsearch device [10–13], based on the rational that all CTCs express epithelial markers. Thus, CTCs with a mesenchymal phenotype are not detected using this method. Epithelial–mesenchymal transition (EMT) is the process of conversion from an epithelial to a mesenchymal phenotype, resulting in expression of mesenchymal markers (e.g. vimentin) and loss of epithelial markers (e.g. E-cadherin and EpCAM). EMT is associated with cell migration and metastasis, and is a feature of CSCs [60, 61]. EMT markers are heterogeneously expressed in CTCs, and are mainly detectable in CTCs isolated by ISET compared with Cellsearch [62]. Most CTCs isolated by ISET frequently express both epithelial and mesenchymal makers, but some express only epithelial or mesenchyme markers [63, 64]. Bozzetti et al. showed that only 29% of CTCs isolated by ISET from 55 advanced squamous cell lung carcinoma patients were EpCAM positive, whereas 43% were vimentin positive [65]. De Wit et al. characterized EpCAM-negative cells discarded by the Cellsearch method by filtration and additional cytokeratins staining [66]. When discarded CTCs were included, the detection rate increased from 41% to 74%. Using this method, CTCs were detected in 33% of patients in whom no CTC was detected by Cellsearch. A recent study suggested that CTCs with EMT features were more frequent in EGFR-mutated NSCLC, compared to ALK-rearranged NSCLC and Kras-mutated NSCLC [67].
A direct comparison of CTC detection by ISET and Cellsearch has been performed in lung cancer patients. Krebs et al. showed a higher detection rate for ISET in a cohort of 40 patients with advanced NSCLC: 80%, for ISET versus 23% for Cellsearch [68]. Interestingly, most CTCs isolated with ISET did not express EpCAM. Clusters of CTCs (i.e. circulating tumor microemboli (CTM)) are associated with a greater propensity for metastasis compared with single CTC [69, 70]. CTM were observed in 43% patients when ISET was used for CTC isolation versus 0% with Cellsearch. Farace et al. tested concordance between the two methods in 60 lung cancer patients (20 with advanced NSCLC) [71]. Concordant results were obtained for only 20% of patients: 15% of patients had ≥3 CTCs per 7.5 mL with Cellsearch versus 60% with ISET. A median of 0 CTCs per 7.5 mL was obtained with for Cellsearch and 5 CTCs per 7.5 mL with ISET.
CLINICAL IMPLICATION OF CTCS
Prognostic impact of CTCs
Most studies on CTCs in lung cancer have shown an association between the presence of CTCs and poor prognosis, whatever detection method was used. The presence of CTCs seems to be associated with the TNM stage of the disease [11–13, 72–76]. CTC detection is consistently associated with lower progression-free survival (PFS) and overall survival (OS) [72, 74, 77–81].
For early-stage NSCLC treated by surgery, CTCs have been detected in the peripheral blood [82–89] and in pulmonary venous blood [90–96]. The CTC detection rate seemed to be higher in samples from the pulmonary vein compared with peripheral blood taken during surgery in the same patient [97]. However, whatever the sample site, the presence of CTCs is associated with poor outcome with shorter disease-free survival [82–89].
Huang et al. published a meta-analysis on the prognostic impact of CTCs in lung cancer patients that included 20 studies and 1576 patients [98]. CTCs were positively associated with tumor stage (odds ratio OR 1.95 (95% CI 1.08–3.54)) and nodal stage (OR 2.06 (95% CI 1.18–3.62)), and with poorer OS (relative risk RR 2.19 (95% CI 1.53–3.12)) and PFS (RR 2.14 (95% CI 1.36–3.38)). Another recent meta-analysis (8 studies and 453 patients) showed equivalent results [99].
Predictive impact of CTCs
Changes in the CTC count before and during treatment correlate with treatment response and outcome, respectively. A decrease in CTC numbers during cytotoxic chemotherapy is associated with treatment response and longer PFS, whereas stable or increased CTC numbers during treatment is associated with disease progression and shorter PFS [72, 75, 78, 80, 100]. A meta-analysis by Wu et al. confirmed the predictive impact of CTC numbers [99]: the presence of CTCs at baseline was negatively associated with the disease control rate (RR 2.56; 95% CI 1.36–4.82) but not with the response rate (RR 1.07; 95% CI 0.75–1.53). However, persistence of CTCs after the 3rd cycle of chemotherapy correlated negatively with both the disease control rate (RR 9.39; 95% CI 2.81–31.39) and response rate (RR 1.85; 95% CI 1.04–3.30). Tarumi et al. showed in stage III NSCLC patients (n=9) after induction chemoradiotherapy that the absence of CTCs was associated with a complete pathological response (p=0.012) [101]. Changes in the CTC detection rate during chest radiotherapy also correlated with tumor response and PFS [102–104].
The same association was found for targeted therapies. Patients treated with pertuzumab (HER2 antibody) or erlotinib (EGFR tyrosine kinase inhibitor (TKI)) included in a phase II trial underwent CTC monitoring during treatment (n=41) [105]. A decrease in CTCs during treatment was associated with a tumor response, as evaluated by CT (p=0.019) or PET (p=0.014). For patients with EGFR-mutated NSCLC treated with the EGFR TKI, the CTC count can be followed during treatment (as done for ctDNA) levels) to monitor the treatment response [106, 107].
The use of specific protein expression on CTCs as predictive biomarkers is an interesting possibility. For cytotoxic chemotherapy in NSCLC patients, low excision repair cross-complementation group 1 (ERCC1) expression is associated with platinum sensitivity [108, 109]. Das et al. measured ERCC1 expression by immunohistochemical (IHC) analysis of CTCs from 17 patients with advanced NSCLC treated with platinum-based chemotherapy and found similar results: low ERCC1 expression on CTCs correlated with longer PFS [110]. Similarly, thymidylate synthase expression (associated with pemetrexed sensitivity in tumor samples) on CTCs has the same predictive effect [111]. Concerning immune checkpoints inhibitor (ICI) treatment, PDL1 expression (evaluated by IHC analysis) on tumor samples seems to correlate with the tumor response. Nicolazzo et al. performed serial IHC monitoring of PDL1 expression on CTCs from 24 NSCLC patients treated with nivolumab [112]. The persistence of PDL1-positive CTCs at 6 months was associated with a lack of clinical response to this treatment. Adams et al. showed also the feasibility of tracking the increase of PDL1-positive CTCs during radiotherapy in 100% of patients (n=41), whereas only 24% of primary biopsies had sufficient tissue for PD-L1 testing [113]. Therefore, the expression of predictive biomarkers on CTCs seems to be feasible, and could be a good surrogate to the use of IHC on small lung biopsies.
CTCs as a diagnostic/screening tool
As described in several studies, CTCs are frequently detected in NSCLC patients, even at early disease stages, whereas the detection rate is null in healthy individuals. A small meta-analysis of five studies including control groups (total of 460 lung cancer patients and 239 controls with benign disease) showed that CTCs represent an efficient diagnostic tool, with a sensitivity of 75%, a specificity of 92%, and an area under the ROC curve (AUC) of 0.93 [114]. The presence of CTCs could be used for better characterization of lung nodules. Carlsson et al. studied 80 patients with stage I NSCLC and 25 patients with benign disease who underwent PET [115]. Detection of CTMs (by immunofluorescence assay) in addition to clinical and PET data was better for diagnosing lung cancer compared with clinical and PET only: AUC of 0.87 versus 0.77, respectively.
CTCs can be detected in patients with small lung tumors, and the question of using them as a lung cancer screening tool was partially answered by Ilie et al. [116]. These researchers investigated CTCs by ISET at baseline in 245 subjects without cancer: 168 patients with chronic obstructive pulmonary disease (COPD), 42 control smokers, and 35 nonsmokers. COPD patients underwent low-dose CT once a year for 5 years; they were heavy smokers (median 56 packet-years), and 49% had moderate to severe COPD. CTCs were detected in five COPD patients (3%) but no control patients. All five COPD patients developed NSCLC during the 5-year follow-up, as detected by CT (median time to node detection 3.2 years). None of the control patients developed a lung node during follow-up. Of the five NSCLC tumors, most were adenocarcinomas (n=4) and one was a squamous cell carcinoma. All were treated by surgery, which confirmed that all were stage Ia NSCLCs. However, these exciting results need to be prospectively confirmed in a larger population and the impact on reducing cancer-specific mortality remains a key determinant in validating this tool as an effective screening marker. It also surprising that none of the control patient developed a lung nodule, whereas around 25% of new nodules were found on CT-scan each year in smokers in a large screening clinical trial [117]. Moreover, the ISET technique still has some inter-user variation issues that limit its development for a use in routine so far.
Clinical implication of CTCs in small cell lung carcinoma patients
SCLC is an aggressive lung cancer with high metastatic potential. The interest in monitoring CTC numbers in patients with this disease is therefore justified. As for NSCLC patients, CTCs can be detected and isolated in SCLC patients. Bevilacqua et al. isolated CTCs from four patients with extended SCLC for the first time using the Cellsearch system [118]. These results were confirmed in a cohort of 50 SCLC patients and 85 healthy controls [119]. The CTC detection rate was 86% using the Cellsearch system. The CTC count was a prognostic indicator: patients with >300 CTCs had worse survival than those with <2 CTCs (134 days versus 443 days, p<0.005). The same group confirmed this sensitivity level and the prognostic impact of CTC numbers in another cohort of SCLC patients (n=97) [120]. A multivariate analysis showed that the presence of CTCs at baseline was negatively associated with PFS (HR 2.01; 95% CI 1.17–3.46) and OS (HR 2.45; 95% CI 1.39–4.30), as was their presence after one cycle of chemotherapy (PFS: HR 4.20; 95% CI 1.44–12.25; OS: HR 5.49; 95% CI 1.78–16.91). The change in CTC numbers during chemotherapy was also associated with OS (HR 4.10; 95% CI 1.10–15.10). Similarly, Messaritakis et al. showed, using the Cellsearch system, in 108 SCLC patients, that chemotherapy reduced both the incidence of detection and the absolute number of CTCs, and that the incidence of detection and the number of CTCs were increased at the time of progression. Multivariate analyses confirmed the prognostic impact on OS of CTCs [121]. Several other studies reported similar results [122–129]. A meta-analysis (seven studies, 440 patients) on the prognostic impact of CTCs in SCLC patients found a HR for OS of 1.90 (95% CI 1.19–3.04) and for PFS of 2.60 (95% CI 1.90–3.54) [130].
An interesting approach to using CTCs in SCLC patients is the possibility of testing the chemosensitivity of isolated CTCs as for a reflect of tumor behavior in the patient. Hamilton et al. established two CTC lines from patients with extended SCLC [127] and tested these in vitro for chemosensitivity to topotecan and epirubicin [131]. Hodgkinson et al. implanted CTC explants isolated with the Cellsearch system into immunocompromised mice [132]. These explants had the same sensitivities to platinum and etoposide chemotherapy as the corresponding donor tumor. In comparison, CTCs explants in NSCLC are much more difficult to realize [133]. These possibilities of in vivo culture and CTCs explants in SCLC, associated with the high rate of CTCs isolated in SCLC patients, strengthen the interest of CTCs in this setting.
MOLECULAR ANALYSES OF CTCS
For several years, molecular characterization has enabled the specific targeting of key oncogenic alterations (i.e. oncogenic addiction) in patients with advanced NSCLC with specific TKIs. [134–137]. However, the small size of tumor samples from NSCLC patients can make molecular screening a challenge in daily practice. Moreover, the need for a second round of molecular screening at the time of progression to identify resistance means that new biopsies are necessary during follow-up. The identification of somatic mutations in ctDNA isolated from blood samples (liquid biopsy) is a validated method of identifying EGFR mutations. However, detection of genetic rearrangements (such as ALK) in ctDNA is technically difficult. Molecular analysis of oncogenic addiction in CTCs therefore has huge potential for fully characterizing NCSLCs at baseline but also during the treatment of NSCLC patients with oncogenic addiction.
EGFR mutations
In 2008, Maheswaran et al. reported the feasibility and sensitivity of detecting EGFR mutations in CTCs [138]. These researchers analyzed CTCs isolated by CTC-chip from 27 NSCLC patients. EGFR mutations were screened in ctDNA and CTCs using the Scorpion amplification refractory mutation system (SARMS) and in tumor samples using SARMS or standard sequencing. Of 20 patients with EGFR-mutated NSCLCs, the EGFR mutation was identified in 95% of the corresponding CTCs (n=19/20). Other studies confirmed these results using different molecular techniques, such as RT-PCR and melting curve analysis (sensitivity 100%, n=8) [139], next-generation sequencing (sensitivity 84%, n=37) [140], and SARMS (sensitivity 50%, n=4) [141]. Moreover, EGFR mutation can be easily detected in single CTCs [139, 142, 143].
In addition to detecting sensitizing EGFR mutations at diagnosis, longitudinal molecular screening of CTCs can also detect the emergence of resistance mutations, such as the exon 20 T790M EGFR mutation. Maheswaran et al. detected the T790M EGFR mutation at baseline in CTCs from 9 out of 14 patients who had disease progression after treatment with the EGFR TKI (64%) [138]. Serial molecular screening of CTCs was feasible, with the appearance and increasing prevalence of the T790M EGFR allele during gefitinib treatment (n=4). In seven advanced NSCLC patients who developed EGFR TKI resistance, Yeo et al. performed molecular screening of both tumor (re-biopsy) and CTC samples by direct sequencing [143]. The T790M EGFR mutation was detected in CTCs in four patients (57%) and in tumor samples from five patients (71% (one patient did not have detectable CTCs). The concordance rate for detection of the T790M EGFR mutation between CTC and tumor sample was good (κ=0.70). A larger study of 40 EGFR-mutated advanced NSCLC patients compared the incidence of the T790M EGFR mutation in tumor samples (n=40), CTCs (isolated by CTC-chip) (n=28), and ctDNA (n=32) [144]. All patients had disease progression after EGFR TKI treatment. The T790M EGFR mutation rate was 63% in tumor biopsies, 50% in CTCs, and 50% in ctDNA. Agreement between the different techniques was low to moderate: CTCs versus biopsy, κ=0.49; ctDNA versus biopsy, κ=0.29; and CTCs/ctDNA versus biopsy, κ=0.35. Notably, molecular screening of CTCs and ctDNA identified the T790M EGFR mutation in 35% of patients (n=14) with negative or indeterminate tumor sample findings. This discordance could be explained by technical issues, but also by tumor heterogeneity because CTCs and ctDNA represent all tumor clones whereas tumor biopsy represents a single site, as previously described [145, 146]. A meta-analysis in 2016 of EGFR mutation detection in CTCs (eight studies, 170 patients) found good sensitivity (91%) and specificity (99%), and an AUC of 0.99 [147].
However, despite this excellent performance, the role of EGFR screening in CTCs is still to be defined. The role of ctDNA is currently growing, notably for detecting the T790M EGFR mutation to monitor disease progression with EGFR TKI treatment. ctDNA analysis has fewer technical steps compared with CTC analysis, with similar performance [145, 146].
ALK and ROS1 rearrangements
Detection of ALK/ROS1 rearrangements is currently based on IHC or fluorescence in situ hybridization (FISH) in NSCLC tumor samples. Several studies have shown that these tests are feasible in CTCs from NSCLC patients. Ilie et al. performed ALK FISH and IHC analyses of CTCs (isolated by ISET) from 87 NSCLC patients [148]. There was perfect concordance for ALK status between CTC and tumor samples: ALK rearrangement was detected in CTCs and tumor samples in five patients (6%), whereas both CTC and tumor samples were negative in the remaining 82 patients. Several larger series of ALK-positive patients have confirmed the excellent performance of ALK FISH in CTCs [149–151]. Moreover, as shown with other anti-tumor treatments, the pattern of change in CTC count seems to correlate with the tumor response to ALK TKI [149–152]. Some assays have been developed for the simultaneous isolation and molecular analysis of CTCs by FISH. As an example, Pailler et al. developed a semi-automated microscopy method to identify filtration-enriched CTCs (ISET) by combining phenotypic and cytomorphological analysis with filter-adapted FISH, which enabled detection of ALK-rearranged CTCs in 82% of patients with ALK-rearranged NSCLC [153].
As with EGFR TKI treatment, it is also feasible to perform serial molecular screening of CTCs during treatment of ALK-rearranged NSCLC patients. Zhang et al. described a NSCLC patient with an ALK translocation identified in both the tumor sample and CTCs (by FISH) and treated with crizotinib [154]. At the time of disease progression, an ALK resistance mutation was detected in both a new tumor biopsy (L1196M) by whole exome sequencing and in CTCs isolated at the same time using Sanger sequencing. The patient then received treatment with a second-generation ALK TKI (ceritinib).
similar to ALK rearrangement, ROS1 rearrangement can also be detected in CTCs by filter-adapted FISH [155].
Other molecular abnormalities
Kras mutation is the most frequent molecular abnormality in NSCLC patients, occurring in around 25% of tumors [2]. This mutation can be detected in both ctDNA and CTCs. However, several studies have highlighted that Kras mutation detection in CTCs is less sensitive compared with ctDNA (e.g. using high-sensitive assays such as digital droplet PCR (ddPCR)) [156, 157]. Similar results for other mutations, e.g. Braf [158], question the place of CTC analysis in point mutation screening.
Extensive molecular screening of CTCs
Compared with screening for one molecular abnormality, large-scale screening of multiple abnormalities is more challenging but represents a promising tool for developing new targeted therapies. The rapid development of new assays for extensive gene profiling, most commonly in single CTCs, provides further applications of CTCs in NSCLC patients. Park et al. performed multigene (four-gene) expression and mutation profiling of single CTCs from 35 stage IV lung adenocarcinoma patients, and reported high sensitivity [159]. Larger panels of cancer-associated genes can also be used. For example, Yoo et al. reported the feasibility of large molecular screening with a panel of 381 cancer-related genes in single CTCs, confirmed by ddPCR. In a cohort of 13 NSCLC patients, the detection rate of point mutations was 62% (n=8) in CTCs and 85% (n=11) in tumor samples, mainly in EGFR, TP53, and FGFR genes. Interestingly, some mutations detected in CTCs were not observed in the corresponding tumor samples, suggesting that CTCs can have a distinct genetic profile. Copy-number alterations (CNAs) can also be evaluated in CTCs [160–162]. Carter et al. evaluated CNAs in CTCs from two SCLC cohorts (training set, n=13 patients; validation set, n=18 patients) [162]. These researchers were able to classify 83% of tumors as chemosensitive or chemorefractory based on the CNAs profile. Moreover, the CNA-based classification had a prognostic impact on PFS. Extensive exome analysis by whole exome sequencing is also possible in single CTCs. The inherent limitation of low-quantity of DNA extracted from single CTCs can be partly resolved by whole exome amplification, which enables sufficient DNA to be produced to perform further deep genome analyses [163]. However, DNA quality affected by the CTC isolation process, cell fixation, DNA amplification, and the low starting DNA quantity remain potential issues [163]. Last, whole transcriptome analysis (WTA) enables global gene expression in CTCs to be evaluated and key regulators of lung cancer cells to be identified. For example, WTA analysis of EpCAM-positive CTCs from 42 NSCLC patients showed that the most highly expressed genes in CTCs are linked to cell movement, cell adhesion, and cell–cell communication, such as Notch1, and have high prognostic value [164].
LIMITATIONS AND PERSPECTIVES
Despite the exciting results on CTCs in lung cancer, the applicability of a clinical use of CTCs in the management of NSCLC is still subject to discussion. Beyond the wide diversity of available assays, each one with potential limitation (Table 1), the main problem is still to have a consistent definition of CTCs. One major question is whether CTCs really represent the tumor, or if they are only particular cancer cells, with EMT and/or CSC features. As detailed above with the multiple techniques developed for CTCs isolation, there is currently no universal definition of CTCs. Depending on the device, CTCs will be EpCAM-expressing cells, CD45-negative cells, and/or large circulating cells. This question is a major limitation for comparison of results in the literature, integration in clinical trials in oncology and for generalization of CTCs use in the daily practice. Consortia such as Cancer-ID are aiming to standardize these aspects. Moreover, it is important to note that, until now, CTCs use has never been shown to prolong patient survival yet. A prospective randomized trial using CTCs to manage advanced NSCLC treatment is still awaited, but should use validated assay for CTCs isolation, strict time-points for CTCs analyses and meaningful thresholds, as these parameters can largely influence the results of the trial, as shown in other malignancies [165, 166]. Current recruiting phase I-III clinical trials using CTCs in lung cancer are presented in Table 2.
Table 1. Main assays developed for CTCs detection and isolation.
| CTCs detection/isolation assay | Sensitivity | Specificity | Limitations | Ref |
|---|---|---|---|---|
| EpCAM-based assay (Cellsearch) | 20-100% | 80-95% | no EMT CTCs detection | [10–16] |
| CD45- based assay | 73-100% | 82% | limited number of studies | [17–18] |
| Chip-based assay (CTC-chip…) | 36-99% | 100% | purity sometimes low | [23–43] |
| Size-based assay (ISET, ScreenCell, MCA…) | 41-80% | 90-100% | poor inter-institution correlation rate (ISET) | [44–54] |
Table 2. Recruiting phase I-III clinical trials using CTCs in lung cancer.
| Type of trial | Study title | ClinicalTrials.gov Identifier |
|---|---|---|
| Phase II | Liquid Biopsy as a Tool to Evaluate Resistance to First and Third (AZD9291) (EGFR) (TKIs) in (EGFR) Mutant NSCLC | NCT02771314 |
| Phase I-II | High-activity Natural Killer Immunotherapy for Small Metastases of Non-small Cell Lung Cancer | NCT03007875 |
| Phase I-II | Combination of Cryosurgery and NK Immunotherapy for Advanced Non-Small Cell Lung Cancer | NCT02843815 |
| Phase I-II | Combination of Cetuximab and NK Immunotherapy for Recurrent Non-small Cell Lung Cancer | NCT02845856 |
| Phase I-II | Safety and Efficiency of γδ T Cell Against Lung Cancer | NCT03183232 |
| Phase II-III | Bioinformation Therapy for Lung Cancer | NCT03239171 |
Another potential issue for the future development of CTCs in lung cancer is the putative advantage of CTCs among ctDNA. ctDNA is now widely used in lung cancer as a diagnostic, prognostic and predictive biomarker. Current techniques allow very low detection rate for somatic mutation in ctDNA, lower than 0.01% with ddPCR [3]. Large molecular screening by NGS is feasible on ctDNA, with good performances [167]. Based on these data, the detection of somatic mutations in lung cancer through analysis of CTCs does not seem to give a definitive advantage over ctDNA. Besides, it adds a supplemental technical step (CTCs isolation), that raises potential issue by itself, as discussed above, with additional costs. However, CTCs could have an interest in specific situations in addition with ctDNA. Interestingly, in the study of Sundaresan et al. on the T790M mutation detection (n=37), the mutation could be detected in CTCs and not in corresponding ctDNA (n=5), or in ctDNA and not in corresponding CTCs (n=1). Moreover, the use of both CTCs and ctDNA increased the T790M detection rate to 100%, and allowed the detection of the mutation in 35% patients s in whom the concurrent biopsy was indeterminate or T790M negative. CTCs offer also several advantages compared to ctDNA analysis (Figure 1). Notably, the possibility of transcriptome analyses (gene expression screening…) and biomarkers expression assessment (PDL1 for example) are real advantages of CTCs that cannot be performed with ctDNA. At last, the use of CTCs for functional analyses (chemosensitivity…) with tumor explants in vivo, especially in SCLC, is probably the most exciting perspective for CTCs use in the future.
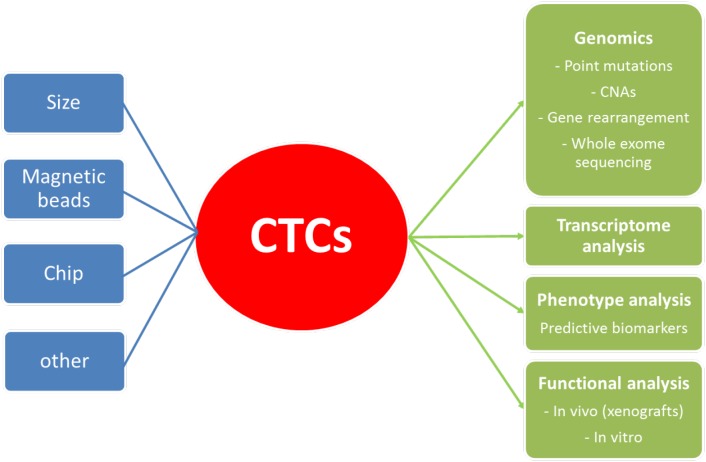
Figure 1. Methods of detection and molecular applications of CTCs.
CNAs: copy number alterations.
In conclusion, the use of CTCs in lung cancer opens a wide range of applications that need to be validated for a clinical use, especially in comparison with other liquid biopsies (ctDNA). Moreover, the diversity of assays for CTCs detection and isolation, with several limitations for each technique, limits currently the application of CTCs in clinical research. A work of standardization concerning the definition of CTCs, the way to isolate them and their application in clinical trials is therefore needed.
Footnotes
Author contributions
EGL, BH, JFE and TC conceived the project, EGL and LW wrote the manuscript, all authors reviewed the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, Ouafik L, Besse B, Rouquette I, Westeel V, Escande F, Monnet I, Lemoine A, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–26. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 3.Diaz LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–86. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolfo C, Castiglia M, Hong D, Alessandro R, Mertens I, Baggerman G, Zwaenepoel K, Gil-Bazo I, Passiglia F, Carreca AP, Taverna S, Vento R, Santini D, et al. Liquid biopsies in lung cancer: the new ambrosia of researchers. Biochim Biophys Acta. 2014;1846:539–46. doi: 10.1016/j.bbcan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massagué J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashworth T. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J. 1869;14:146–9. [Google Scholar]
- 8.Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–8. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 9.Marrinucci D, Bethel K, Luttgen M, Bruce RH, Nieva J, Kuhn P. Circulating tumor cells from well-differentiated lung adenocarcinoma retain cytomorphologic features of primary tumor type. Arch Pathol Lab Med. 2009;133:1468–71. doi: 10.1043/1543-2165-133.9.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka F, Yoneda K, Kondo N, Hashimoto M, Takuwa T, Matsumoto S, Okumura Y, Rahman S, Tsubota N, Tsujimura T, Kuribayashi K, Fukuoka K, Nakano T, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res. 2009;15:6980–6. doi: 10.1158/1078-0432.CCR-09-1095. [DOI] [PubMed] [Google Scholar]
- 12.Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, Ranson M, Dive C, Blackhall FH. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29:1556–63. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 13.Earhart CM, Hughes CE, Gaster RS, Ooi CC, Wilson RJ, Zhou LY, Humke EW, Xu L, Wong DJ, Willingham SB, Schwartz EJ, Weissman IL, Jeffrey SS, et al. Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab Chip. 2014;14:78–88. doi: 10.1039/c3lc50580d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji JL, Jiang YZ, Tang QQ, He XD, Shen ZJ, Zhang BY. Detection of circulating tumor cells using a novel immunomagnetic bead method in lung cancer patients. J Clin Lab Anal. 2016;30:656–62. doi: 10.1002/jcla.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B, Wang B, Zhang D, Guo H, Zhang L, Zhou W. Clinical test on circulating tumor cells in peripheral blood of lung cancer patients, based on novel immunomagnetic beads. Artif Cells Nanomed Biotechnol. 2016;44:892–7. doi: 10.3109/21691401.2014.998827. [DOI] [PubMed] [Google Scholar]
- 16.Devriese LA, Bosma AJ, van de Heuvel MM, Heemsbergen W, Voest EE, Schellens JH. Circulating tumor cell detection in advanced non-small cell lung cancer patients by multi-marker QPCR analysis. Lung Cancer. 2012;75:242–7. doi: 10.1016/j.lungcan.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Wu C, Hao H, Li L, Zhou X, Guo Z, Zhang L, Zhang X, Zhong W, Guo H, Bremner RM, Lin P. Preliminary investigation of the clinical significance of detecting circulating tumor cells enriched from lung cancer patients. J Thorac Oncol. 2009;4:30–6. doi: 10.1097/JTO.0b013e3181914125. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Ge F, Cui W, Wang F, Yang Z, Guo Y, Li L, Bremner RM, Lin PP. Lung cancer circulating tumor cells isolated by the EpCAM-independent enrichment strategy correlate with Cytokeratin 19-derived CYFRA21-1 and pathological staging. Clin Chim Acta. 2013;419:57–61. doi: 10.1016/j.cca.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Nunez MI, Behrens C, Woods DM, Lin H, Suraokar M, Kadara H, Hofstetter W, Kalhor N, Lee JJ, Franklin W, Stewart DJ, Wistuba II. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J Thorac Oncol. 2012;7:833–40. doi: 10.1097/JTO.0b013e31824de09c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Chen Z, Dong J, Wei P, Hu R, Zhou C, Sun N, Luo M, Yang W, Yao R, Gao Y, Li J, Yang G, et al. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol. 2013;6:697–702. doi: 10.1593/tlo.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Zhou F, Li X, Yang G, Zhang L, Ren S, Zhao C, Deng Q, Li W, Gao G, Li A, Zhou C. Folate receptor-positive circulating tumor cell detected by LT-PCR-based method as a diagnostic biomarker for non-small-cell lung cancer. J Thorac Oncol. 2015;10:1163–71. doi: 10.1097/JTO.037205R1037205R10606. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Wu C, Qiao L, Yu W, Guo Q, Zhao M, Yang G, Zhao H, Lou J. Clinical significance of folate receptor-positive circulating tumor cells detected by ligand-targeted polymerase chain reaction in lung cancer. J Cancer. 2017;8:104–10. doi: 10.7150/jca.16856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang T, Jia CP, Jun-Yang, Sun WJ, Wang WT, Zhang HL, Cong H, Jing FX, Mao HJ, Jin QH, Zhang Z, Chen YJ, Li G, et al. Highly sensitive enumeration of circulating tumor cells in lung cancer patients using a size-based filtration microfluidic chip. Biosens Bioelectron. 2014;51:213–8. doi: 10.1016/j.bios.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 25.Lim E, Tay A, Von Der Thusen J, Freidin MB, Anikin V, Nicholson AG. Clinical results of microfluidic antibody-independent peripheral blood circulating tumor cell capture for the diagnosis of lung cancer. J Thorac Cardiovasc Surg. 2014;147:1936–8. doi: 10.1016/j.jtcvs.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 26.Sollier E, Go DE, Che J, Gossett DR, O’Byrne S, Weaver WM, Kummer N, Rettig M, Goldman J, Nickols N, McCloskey S, Kulkarni RP, Di Carlo D. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip. 2014;14:63–77. doi: 10.1039/c3lc50689d. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe M, Serizawa M, Sawada T, Takeda K, Takahashi T, Yamamoto N, Koizumi F, Koh Y. A novel flow cytometry-based cell capture platform for the detection, capture and molecular characterization of rare tumor cells in blood. J Transl Med. 2014;12:143. doi: 10.1186/1479-5876-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoo BL, Warkiani ME, Tan DS, Bhagat AA, Irwin D, Lau DP, Lim AS, Lim KH, Krisna SS, Lim WT, Yap YS, Lee SC, Soo RA, et al. Clinical validation of an ultra high-throughput spiral microfluidics for the detection and enrichment of viable circulating tumor cells. PLoS One. 2014;9:e99409. doi: 10.1371/journal.pone.0099409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murlidhar V, Zeinali M, Grabauskiene S, Ghannad-Rezaie M, Wicha MS, Simeone DM, Ramnath N, Reddy RM, Nagrath S. A radial flow microfluidic device for ultra-high-throughput affinity-based isolation of circulating tumor cells. Small. 2014;10:4895–904. doi: 10.1002/smll.201400719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Y, Zhang Y, Sun S, Wang Z, Wang M, Yu B, Czajkowsky DM, Liu B, Li Y, Wei W, Shi Q. An integrated microfluidic chip system for single-cell secretion profiling of rare circulating tumor cells. Sci Rep. 2014;4:7499. doi: 10.1038/srep07499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Ye M, Cheng L, Li R, Zhu W, Shi Z, Fan C, He J, Liu J, Liu Z. Simultaneous isolation and detection of circulating tumor cells with a microfluidic silicon-nanowire-array integrated with magnetic upconversion nanoprobes. Biomaterials. 2015;54:55–62. doi: 10.1016/j.biomaterials.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Magbanua MJ, Pugia M, Lee JS, Jabon M, Wang V, Gubens M, Marfurt K, Pence J, Sidhu H, Uzgiris A, Rugo HS, Park JW. A novel strategy for detection and enumeration of circulating rare cell populations in metastatic cancer patients using automated microfluidic filtration and multiplex immunoassay. PLoS One. 2015;10:e0141166. doi: 10.1371/journal.pone.0141166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CL, Huang W, Jalal SI, Chan BD, Mahmood A, Shahda S, O’Neil BH, Matei DE, Savran CA. Circulating tumor cell detection using a parallel flow micro-aperture chip system. Lab Chip. 2015;15:1677–88. doi: 10.1039/C5LC00100E. [DOI] [PubMed] [Google Scholar]
- 34.Gao W, Yuan H, Jing F, Wu S, Zhou H, Mao H, Jin Q, Zhao J, Cong H, Jia C. Analysis of circulating tumor cells from lung cancer patients with multiple biomarkers using high-performance size-based microfluidic chip. Oncotarget. 2016;8:12917–28. doi: 10.18632/oncotarget.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Che J, Yu V, Dhar M, Renier C, Matsumoto M, Heirich K, Garon EB, Goldman J, Rao J, Sledge GW, Pegram MD, Sheth S, Jeffrey SS, et al. Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology. Oncotarget. 2016;7:12748–60. doi: 10.18632/oncotarget.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Shiratsuchi H, Lin J, Chen G, Reddy RM, Azizi E, Fouladdel S, Chang AC, Lin L, Jiang H, Waghray M, Luker G, Simeone DM, et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget. 2014;5:12383–97. doi: 10.18632/oncotarget.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan X, Jia C, Yang J, Li G, Mao H, Jin Q, Zhao J. A microfluidic chip integrated with a high-density PDMS-based microfiltration membrane for rapid isolation and detection of circulating tumor cells. Biosens Bioelectron. 2015;71:380–6. doi: 10.1016/j.bios.2015.04.080. [DOI] [PubMed] [Google Scholar]
- 38.Reátegui E, Aceto N, Lim EJ, Sullivan JP, Jensen AE, Zeinali M, Martel JM, Aranyosi AJ, Li W, Castleberry S, Bardia A, Sequist LV, Haber DA, et al. Tunable nanostructured coating for the capture and selective release of viable circulating tumor cells. Adv Mater. 2015;27:1593–9. doi: 10.1002/adma.201404677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan DS, Lim WT, Han J, Bhagat AA, Lim CT. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci Rep. 2013 doi: 10.1038/srep01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee A, Park J, Lim M, Sunkara V, Kim SY, Kim GH, Kim MH, Cho YK. All-in-one centrifugal microfluidic device for size-selective circulating tumor cell isolation with high purity. Anal Chem. 2014;86:11349–56. doi: 10.1021/ac5035049. [DOI] [PubMed] [Google Scholar]
- 41.Lee SW, Hyun KA, Kim SI, Kang JY, Jung HI. Enrichment of circulating tumor cells using a centrifugal affinity plate system. J Chromatogr A. 2014;1373:25–30. doi: 10.1016/j.chroma.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Park JM, Kim MS, Moon HS, Yoo CE, Park D, Kim YJ, Han KY, Lee JY, Oh JH, Kim SS, Park WY, Lee WY, Huh N. Fully automated circulating tumor cell isolation platform with large-volume capacity based on lab-on-a-disc. Anal Chem. 2014;86:3735–42. doi: 10.1021/ac403456t. [DOI] [PubMed] [Google Scholar]
- 43.O’Shannessy DJ, Davis DW, Anderes K, Somers EB. Isolation of circulating tumor cells from multiple epithelial cancers with ApoStream(®) for detecting (or monitoring) the expression of folate receptor alpha. Biomark Insights. 2016;11:7–18. doi: 10.4137/BMI.S35075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, Lacour B, Bréchot C, Paterlini-Bréchot P. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofman V, Long E, Ilie M, Bonnetaud C, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, Mourad N, Butori C, Selva E, Marquette CH, Poudenx M, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology. 2012;23:30–8. doi: 10.1111/j.1365-2303.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- 46.Freidin MB, Tay A, Freydina DV, Chudasama D, Nicholson AG, Rice A, Anikin V, Lim E. An assessment of diagnostic performance of a filter-based antibody-independent peripheral blood circulating tumour cell capture paired with cytomorphologic criteria for the diagnosis of cancer. Lung Cancer. 2014;85:182–5. doi: 10.1016/j.lungcan.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Mascalchi M, Falchini M, Maddau C, Salvianti F, Nistri M, Bertelli E, Sali L, Zuccherelli S, Vella A, Matucci M, Voltolini L, Pegna AL, Luconi M, et al. Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J Cancer Res Clin Oncol. 2016;142:195–200. doi: 10.1007/s00432-015-2021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chudasama D, Barr J, Beeson J, Beddow E, McGonigle N, Rice A, Nicholson A, Anikin V. Detection of circulating tumour cells and survival of patients with non-small cell lung cancer. Anticancer Res. 2017;37:169–73. doi: 10.21873/anticanres.11302. [DOI] [PubMed] [Google Scholar]
- 49.Yagi S, Koh Y, Akamatsu H, Kanai K, Hayata A, Tokudome N, Akamatsu K, Endo K, Nakamura S, Higuchi M, Kanbara H, Nakanishi M, Ueda H, et al. Development of an automated size-based filtration system for isolation of circulating tumor cells in lung cancer patients. PLoS One. 2017;12:e0179744. doi: 10.1371/journal.pone.0179744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosokawa M, Kenmotsu H, Koh Y, Yoshino T, Yoshikawa T, Naito T, Takahashi T, Murakami H, Nakamura Y, Tsuya A, Shukuya T, Ono A, Akamatsu H, et al. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS One. 2013;8:e67466. doi: 10.1371/journal.pone.0067466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosokawa M, Yoshikawa T, Negishi R, Yoshino T, Koh Y, Kenmotsu H, Naito T, Takahashi T, Yamamoto N, Kikuhara Y, Kanbara H, Tanaka T, Yamaguchi K, et al. Microcavity array system for size-based enrichment of circulating tumor cells from the blood of patients with small-cell lung cancer. Anal Chem. 2013;85:5692–8. doi: 10.1021/ac400167x. [DOI] [PubMed] [Google Scholar]
- 52.Kim TH, Lim M, Park J, Oh JM, Kim H, Jeong H, Lee SJ, Park HC, Jung S, Kim BC, Lee K, Kim MH, Park DY, et al. FAST: size-selective, clog-free isolation of rare cancer cells from whole blood at a liquid-liquid interface. Anal Chem. 2017;89:1155–62. doi: 10.1021/acs.analchem.6b03534. [DOI] [PubMed] [Google Scholar]
- 53.Fiorelli A, Accardo M, Carelli E, Angioletti D, Santini M, Di Domenico M. Circulating tumor cells in diagnosing lung cancer: clinical and morphologic analysis. Ann Thorac Surg. 2015;99:1899–905. doi: 10.1016/j.athoracsur.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Lu W, Tang C, Liu Y, Sun J, Mu X, Zhang L, Dai B, Li X, Zhuo H, Jiang X. Label-free isolation and mRNA detection of circulating tumor cells from patients with metastatic lung cancer for disease diagnosis and monitoring therapeutic efficacy. Anal Chem. 2015;87:11893–900. doi: 10.1021/acs.analchem.5b03484. [DOI] [PubMed] [Google Scholar]
- 55.Skirecki T, Hoser G, Kawiak J, Dziedzic D, Domagała-Kulawik J. Flow cytometric analysis of CD133- and EpCAM-positive cells in the peripheral blood of patients with lung cancer. Arch Immunol Ther Exp (Warsz) 2014;62:67–75. doi: 10.1007/s00005-013-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nieva J, Wendel M, Luttgen MS, Marrinucci D, Bazhenova L, Kolatkar A, Santala R, Whittenberger B, Burke J, Torrey M, Bethel K, Kuhn P. High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis. Phys Biol. 2012;9:016004. doi: 10.1088/1478-3975/9/1/016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wendel M, Bazhenova L, Boshuizen R, Kolatkar A, Honnatti M, Cho EH, Marrinucci D, Sandhu A, Perricone A, Thistlethwaite P, Bethel K, Nieva J, Heuvel M, Kuhn P. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Phys Biol. 2012;9:016005. doi: 10.1088/1478-3967/9/1/016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saucedo-Zeni N, Mewes S, Niestroj R, Gasiorowski L, Murawa D, Nowaczyk P, Tomasi T, Weber E, Dworacki G, Morgenthaler NG, Jansen H, Propping C, Sterzynska K, et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol. 2012;41:1241–50. doi: 10.3892/ijo.2012.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorges TM, Penkalla N, Schalk T, Joosse SA, Riethdorf S, Tucholski J, Lücke K, Wikman H, Jackson S, Brychta N, von Ahsen O, Schumann C, Krahn T, et al. Enumeration and molecular characterization of tumor cells in lung cancer patients using a novel in vivo device for capturing circulating tumor cells. Clin Cancer Res. 2016;22:2197–206. doi: 10.1158/1078-0432.CCR-15-1416. [DOI] [PubMed] [Google Scholar]
- 60.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 62.Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, Clack G, Ranson M, Blackhall F, Dive C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178:989–96. doi: 10.1016/j.ajpath.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria JC, Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer. 2011;105:1338–41. doi: 10.1038/bjc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu S, Liu S, Liu Z, Huang J, Pu X, Li J, Yang D, Deng H, Yang N, Xu J. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10:e0123976. doi: 10.1371/journal.pone.0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bozzetti C, Quaini F, Squadrilli A, Tiseo M, Frati C, Lagrasta C, Azzoni C, Bottarelli L, Galetti M, Alama A, Belletti S, Gatti R, Passaro A, et al. Isolation and characterization of circulating tumor cells in squamous cell carcinoma of the lung using a non-EpCAM-based capture method. PLoS One. 2015;10:e0142891. doi: 10.1371/journal.pone.0142891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Wit S, van Dalum G, Lenferink AT, Tibbe AG, Hiltermann TJ, Groen HJ, van Rijn CJ, Terstappen LW. The detection of EpCAM+ and EpCAM– circulating tumor cells. Sci Rep. 2015 doi: 10.1038/srep12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindsay CR, Faugeroux V, Michiels S, Pailler E, Facchinetti F, Ou D, Bluthgen MV, Pannet C, Ngo-Camus M, Bescher G, Caramella C, Billiot F, Remon J, et al. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann Oncol. 2017;28:1523–31. doi: 10.1093/annonc/mdx156. [DOI] [PubMed] [Google Scholar]
- 68.Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, Ranson M, Blackhall FH, Dive C. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7:306–15. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 69.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 70.Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122:3203–8. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- 71.Farace F, Massard C, Vimond N, Drusch F, Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L, Planchard D, Le Moulec S, André F, Fizazi K, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105:847–53. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu L, Liao G, He P, Zhu H, Liu P, Qu Y, Song X, Xu Q, Gao Q, Zhang Y, Chen W, Yin Y. Detection of circulating cancer cells in lung cancer patients with a panel of marker genes. Biochem Biophys Res Commun. 2008;372:756–60. doi: 10.1016/j.bbrc.2008.05.101. [DOI] [PubMed] [Google Scholar]
- 73.Ding M, Li X, Qiu T. Combination of multiple gene markers to detect circulating tumor cells in the peripheral blood of patients with non-small cell lung cancer using real-time PCR. Genet Mol Res. 2015;14:13033–40. doi: 10.4238/2015.October.21.24. [DOI] [PubMed] [Google Scholar]
- 74.Yie S, Lou B, Ye S, He X, Cao M, Xie K, Ye N, Lin R, Wu S, Xiao H, Gao E. Clinical significance of detecting survivin-expressing circulating cancer cells in patients with non-small cell lung cancer. Lung Cancer. 2009;63:284–90. doi: 10.1016/j.lungcan.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 75.Chen YY, Xu GB. Effect of circulating tumor cells combined with negative enrichment and CD45-FISH identification in diagnosis, therapy monitoring and prognosis of primary lung cancer. Med Oncol. 2014;31:240. doi: 10.1007/s12032-014-0240-0. [DOI] [PubMed] [Google Scholar]
- 76.Wan JW, Gao MZ, Hu RJ, Huang HY, Wei YY, Han ZJ, Yan ZH. A preliminary study on the relationship between circulating tumor cells count and clinical features in patients with non-small cell lung cancer. Ann Transl Med. 2015;3:352. doi: 10.3978/j.issn.2305-5839.2015.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sher YP, Shih JY, Yang PC, Roffler SR, Chu YW, Wu CW, Yu CL, Peck K. Prognosis of non-small cell lung cancer patients by detecting circulating cancer cells in the peripheral blood with multiple marker genes. Clin Cancer Res. 2005;11:173–9. [PubMed] [Google Scholar]
- 78.Yin J, Wang Y, Yin H, Chen W, Jin G, Ma H, Dai J, Chen J, Jiang Y, Wang H, Liu Z, Hu Z, Shen H. Circulating tumor cells enriched by the depletion of leukocytes with bi-antibodies in non-small cell lung cancer: potential clinical application. PLoS One. 2015;10:e0137076. doi: 10.1371/journal.pone.0137076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nel I, Jehn U, Gauler T, Hoffmann AC. Individual profiling of circulating tumor cell composition in patients with non-small cell lung cancer receiving platinum based treatment. Transl Lung Cancer Res. 2014;3:100–6. doi: 10.3978/j.issn.2218-6751.2014.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muinelo-Romay L, Vieito M, Abalo A, Nocelo MA, Barón F, Anido U, Brozos E, Vázquez F, Aguín S, Abal M, López RL. Evaluation of circulating tumor cells and related events as prognostic factors and surrogate biomarkers in advanced NSCLC patients receiving first-line systemic treatment. Cancers (Basel) 2014;6:153–65. doi: 10.3390/cancers6010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Z, Xiao Y, Zhao J, Chen M, Xu Y, Zhong W, Xing J, Wang M. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology. 2016;21:519–25. doi: 10.1111/resp.12696. [DOI] [PubMed] [Google Scholar]
- 82.Yamashita JI, Kurusu Y, Fujino N, Saisyoji T, Ogawa M. Detection of circulating tumor cells in patients with non-small cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: a potential hazard for intraoperative hematogenous tumor cell dissemination. J Thorac Cardiovasc Surg. 2000;119:899–905. doi: 10.1016/S0022-5223(00)70084-5. [DOI] [PubMed] [Google Scholar]
- 83.Yamashita J, Matsuo A, Kurusu Y, Saishoji T, Hayashi N, Ogawa M. Preoperative evidence of circulating tumor cells by means of reverse transcriptase-polymerase chain reaction for carcinoembryonic antigen messenger RNA is an independent predictor of survival in non-small cell lung cancer: a prospective study. J Thorac Cardiovasc Surg. 2002;124:299–305. doi: 10.1067/mtc.2002.124370. [DOI] [PubMed] [Google Scholar]
- 84.Ge MJ, Shi D, Wu QC, Wang M, Li LB. Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in peroperative period. J Cancer Res Clin Oncol. 2006;132:248–56. doi: 10.1007/s00432-005-0059-3. [DOI] [PubMed] [Google Scholar]
- 85.Sawabata N, Okumura M, Utsumi T, Inoue M, Shiono H, Minami M, Nishida T, Sawa Y. Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen Thorac Cardiovasc Surg. 2007;55:189–92. doi: 10.1007/s11748-007-0101-2. [DOI] [PubMed] [Google Scholar]
- 86.Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, Mourad N, Butori C, Selva E, Poudenx M, Sibon S, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17:827–35. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 87.Hofman V, Ilie MI, Long E, Selva E, Bonnetaud C, Molina T, Vénissac N, Mouroux J, Vielh P, Hofman P. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011;129:1651–60. doi: 10.1002/ijc.25819. [DOI] [PubMed] [Google Scholar]
- 88.Yoon SO, Kim YT, Jung KC, Jeon YK, Kim BH, Kim CW. TTF-1 mRNA-positive circulating tumor cells in the peripheral blood predict poor prognosis in surgically resected non-small cell lung cancer patients. Lung Cancer. 2011;71:209–16. doi: 10.1016/j.lungcan.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 89.Bayarri-Lara C, Ortega FG, Cueto Ladrón de Guevara A, Puche JL, Ruiz Zafra J, de Miguel-Pérez D, Ramos AS, Giraldo-Ospina CF, Navajas Gómez JA, Delgado-Rodriguez M, Lorente JA, Serrano MJ. Circulating tumor cells identify early recurrence in patients with non-small cell lung cancer undergoing radical resection. PLoS One. 2016;11:e0148659. doi: 10.1371/journal.pone.0148659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong Q, Huang J, Zhou Y, Li L, Bao G, Feng J, Sha H. Hematogenous dissemination of lung cancer cells during surgery: quantitative detection by flow cytometry and prognostic significance. Lung Cancer. 2002;37:293–301. doi: 10.1016/s0169-5002(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 91.Sienel W, Seen-Hibler R, Mutschler W, Pantel K, Passlick B. Tumour cells in the tumour draining vein of patients with non-small cell lung cancer: detection rate and clinical significance. Eur J Cardiothorac Surg. 2003;23:451–6. doi: 10.1016/s1010-7940(02)00865-5. [DOI] [PubMed] [Google Scholar]
- 92.Okumura Y, Tanaka F, Yoneda K, Hashimoto M, Takuwa T, Kondo N, Hasegawa S. Circulating tumor cells in pulmonary venous blood of primary lung cancer patients. Ann Thorac Surg. 2009;87:1669–75. doi: 10.1016/j.athoracsur.2009.03.073. [DOI] [PubMed] [Google Scholar]
- 93.Funaki S, Sawabata N, Nakagiri T, Shintani Y, Inoue M, Kadota Y, Minami M, Okumura M. Novel approach for detection of isolated tumor cells in pulmonary vein using negative selection method: morphological classification and clinical implications. Eur J Cardiothorac Surg. 2011;40:322–7. doi: 10.1016/j.ejcts.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 94.Hashimoto M, Tanaka F, Yoneda K, Takuwa T, Matsumoto S, Okumura Y, Kondo N, Tsubota N, Tsujimura T, Tabata C, Nakano T, Hasegawa S. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg. 2014;18:775–83. doi: 10.1093/icvts/ivu048. [DOI] [PubMed] [Google Scholar]
- 95.Crosbie PA, Shah R, Krysiak P, Zhou C, Morris K, Tugwood J, Booton R, Blackhall F, Dive C. Circulating tumor cells detected in the tumor-draining pulmonary vein are associated with disease recurrence after surgical resection of NSCLC. J Thorac Oncol. 2016;11:1793–7. doi: 10.1016/j.jtho.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reddy RM, Murlidhar V, Zhao L, Grabauskiene S, Zhang Z, Ramnath N, Lin J, Chang AC, Carrott P, Lynch W, Orringer MB, Beer DG, Nagrath S. Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early-stage lung cancer. J Thorac Cardiovasc Surg. 2016;151:852–7. doi: 10.1016/j.jtcvs.2015.09.126. [DOI] [PubMed] [Google Scholar]
- 97.Sawabata N, Funaki S, Hyakutake T, Shintani Y, Fujiwara A, Okumura M. Perioperative circulating tumor cells in surgical patients with non-small cell lung cancer: does surgical manipulation dislodge cancer cells thus allowing them to pass into the peripheral blood? Surg Today. 2016;46:1402–9. doi: 10.1007/s00595-016-1318-4. [DOI] [PubMed] [Google Scholar]
- 98.Huang J, Wang K, Xu J, Huang J, Zhang T. Prognostic significance of circulating tumor cells in non-small-cell lung cancer patients: a meta-analysis. PLoS One. 2013;8:e78070. doi: 10.1371/journal.pone.0078070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu ZX, Liu Z, Jiang HL, Pan HM, Han WD. Circulating tumor cells predict survival benefit from chemotherapy in patients with lung cancer. Oncotarget. 2016;7:67586–96. doi: 10.18632/oncotarget.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hirose T, Murata Y, Oki Y, Sugiyama T, Kusumoto S, Ishida H, Shirai T, Nakashima M, Yamaoka T, Okuda K, Ohnishi T, Ohmori T. Relationship of circulating tumor cells to the effectiveness of cytotoxic chemotherapy in patients with metastatic non-small-cell lung cancer. Oncol Res. 2012;20:131–7. doi: 10.3727/096504012X13473664562583. [DOI] [PubMed] [Google Scholar]
- 101.TTarumi S, Gotoh M, Kasai Y, Matsuura N, Okuda M, Go T, Ishikawa S, Yokomise H. Innovative method using circulating tumor cells for prediction of the effects of induction therapy on locally advanced non-small cell lung cancer. J Cardiothorac Surg. 2013;8:175. doi: 10.1186/1749-8090-8-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ge M, Shi D, Wu Q, Wang M, Li L. Fluctuation of circulating tumor cells in patients with lung cancer by real-time fluorescent quantitative-PCR approach before and after radiotherapy. J Cancer Res Ther. 2005;1:221–6. doi: 10.4103/0973-1482.19591. [DOI] [PubMed] [Google Scholar]
- 103.Martin OA, Anderson RL, Russell PA, Cox RA, Ivashkevich A, Swierczak A, Doherty JP, Jacobs DH, Smith J, Siva S, Daly PE, Ball DL, Martin RF, et al. Mobilization of viable tumor cells into the circulation during radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88:395–403. doi: 10.1016/j.ijrobp.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 104.Dorsey JF, Kao GD, MacArthur KM, Ju M, Steinmetz D, Wileyto EP, Simone CB, Hahn SM. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: pilot study results. Cancer. 2015;121:139–49. doi: 10.1002/cncr.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Punnoose EA, Atwal S, Liu W, Raja R, Fine BM, Hughes BG, Hicks RJ, Hampton GM, Amler LC, Pirzkall A, Lackner MR. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res. 2012;18:2391–401. doi: 10.1158/1078-0432.CCR-11-3148. [DOI] [PubMed] [Google Scholar]
- 106.Morabito A, Sandomenico C, Costanzo R, Montanino A, Caraco C, De Lutio E, Bevilacqua S, Pasquale R, Caronna A, Botti G, Normanno N, Rocco G. Positron emission tomography and circulating tumor cells to monitor a dramatic response to gefitinib. J Thorac Oncol. 2012;7:e27–28. doi: 10.1097/JTO.0b013e3182653da2. [DOI] [PubMed] [Google Scholar]
- 107.Yanagita M, Redig AJ, Paweletz CP, Dahlberg SE, O’Connell A, Feeney N, Taibi M, Boucher D, Oxnard GR, Johnson BE, Costa DB, Jackman DM, Jänne PA. A prospective evaluation of circulating tumor cells and cell-free DNA in EGFR-mutant non-small cell lung cancer patients treated with erlotinib on a phase II trial. Clin Cancer Res. 2016;22:6010–20. doi: 10.1158/1078-0432.CCR-16-0909. [DOI] [PubMed] [Google Scholar]
- 108.Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, Sabatier L, Pignon JP, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 109.Reynolds C, Obasaju C, Schell MJ, Li X, Zheng Z, Boulware D, Caton JR, Demarco LC, O’Rourke MA, Shaw Wright G, Boehm KA, Asmar L, Bromund J, et al. Randomized phase III trial of gemcitabine-based chemotherapy with in situ RRM1 and ERCC1 protein levels for response prediction in non-small-cell lung cancer. J Clin Oncol. 2009;27:5808–15. doi: 10.1200/JCO.2009.21.9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Das M, Riess JW, Frankel P, Schwartz E, Bennis R, Hsieh HB, Liu X, Ly JC, Zhou L, Nieva JJ, Wakelee HA, Bruce RH. ERCC1 expression in circulating tumor cells (CTCs) using a novel detection platform correlates with progression-free survival (PFS) in patients with metastatic non-small-cell lung cancer (NSCLC) receiving platinum chemotherapy. Lung Cancer. 2012;77:421–6. doi: 10.1016/j.lungcan.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 111.Christoph DC, Hoffmann AC, Gauler TC, Asuncion BR, Loewendick H, Peglow A, Hassan B, Tran C, Wynes MW, Schuler M, Eberhard WE, Hirsch FR. Detection of circulating lung cancer cells with strong thymidylate synthase reactivity in the peripheral blood of a patient with pulmonary adenocarcinoma treated with pemetrexed. J Thorac Oncol. 2012;7:766–7. doi: 10.1097/JTO.0b013e3182460fa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nicolazzo C, Raimondi C, Mancini M, Caponnetto S, Gradilone A, Gandini O, Mastromartino M, Del Bene G, Prete A, Longo F, Cortesi E, Gazzaniga P. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep. 2016;6:31726. doi: 10.1038/srep31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adams DL, Adams DK, He J, Kalhor N, Zhang M, Xu T, Gao H, Reuben JM, Qiao Y, Komaki R, Liao Z, Edelman MJ, Tang CM, et al. Sequential tracking of PD-L1 expression and RAD50 induction in circulating tumor and stromal cells of lung cancer patients undergoing radiotherapy. Clin Cancer Res. 2017;23:5948–58. doi: 10.1158/1078-0432.CCR-17-0802. [DOI] [PubMed] [Google Scholar]
- 114.Huang H, Shi Y, Huang J, Wang X, Zhang R, Chen H. Circulating tumor cells as a potential biomarker in diagnosis of lung cancer: a systematic review and meta-analysis. Clin Respir J. 2016 doi: 10.1111/crj.12573. [DOI] [PubMed] [Google Scholar]
- 115.Carlsson A, Nair VS, Luttgen MS, Keu KV, Horng G, Vasanawala M, Kolatkar A, Jamali M, Iagaru AH, Kuschner W, Loo BW, Shrager JB, Bethel K, et al. Circulating tumor microemboli diagnostics for patients with non-small-cell lung cancer. J Thorac Oncol. 2014;9:1111–9. doi: 10.1097/JTO.037205R1037205R10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud JM, Padovani B, Mouroux J, Marquette CH, Hofman P. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9:e111597. doi: 10.1371/journal.pone.0111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD, National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bevilacqua S, Gallo M, Franco R, Rossi A, De Luca A, Rocco G, Botti G, Gridelli C, Normanno N. A “live” biopsy in a small-cell lung cancer patient by detection of circulating tumor cells. Lung Cancer. 2009;65:123–5. doi: 10.1016/j.lungcan.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 119.Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, Board R, Amir E, Hughes S, Krebs M, Hughes A, Ranson M, Lorigan P, Dive C, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol. 2009;175:808–16. doi: 10.2353/ajpath.2009.090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, Ward T, Blackhall FH, Dive C. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–32. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 121.Messaritakis I, Politaki E, Kotsakis A, Dermitzaki EK, Koinis F, Lagoudaki E, Koutsopoulos A, Kallergi G, Souglakos J, Georgoulias V. Phenotypic characterization of circulating tumor cells in the peripheral blood of patients with small cell lung cancer. PLoS One. 2017;12:e0181211. doi: 10.1371/journal.pone.0181211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Naito T, Tanaka F, Ono A, Yoneda K, Takahashi T, Murakami H, Nakamura Y, Tsuya A, Kenmotsu H, Shukuya T, Kaira K, Koh Y, Endo M, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol. 2012;7:512–9. doi: 10.1097/JTO.0b013e31823f125d. [DOI] [PubMed] [Google Scholar]
- 123.Hiltermann TJ, Pore MM, van den Berg A, Timens W, Boezen HM, Liesker JJ, Schouwink JH, Wijnands WJ, Kerner GS, Kruyt FA, Tissing H, Tibbe AG, Terstappen LW, Groen HJ. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol. 2012;23:2937–42. doi: 10.1093/annonc/mds138. [DOI] [PubMed] [Google Scholar]
- 124.Fu L, Liu F, Fu H, Liu L, Yuan S, Gao Y, Fu Z, Yu J. Circulating tumor cells correlate with recurrence in stage III small-cell lung cancer after systemic chemoradiotherapy and prophylactic cranial irradiation. Jpn J Clin Oncol. 2014;44:948–55. doi: 10.1093/jjco/hyu109. [DOI] [PubMed] [Google Scholar]
- 125.Normanno N, Rossi A, Morabito A, Signoriello S, Bevilacqua S, Di Maio M, Costanzo R, De Luca A, Montanino A, Gridelli C, Rocco G, Perrone F, Gallo C. Prognostic value of circulating tumor cells’ reduction in patients with extensive small-cell lung cancer. Lung Cancer. 2014;85:314–9. doi: 10.1016/j.lungcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 126.Igawa S, Gohda K, Fukui T, Ryuge S, Otani S, Masago A, Sato J, Murakami K, Maki S, Katono K, Takakura A, Sasaki J, Satoh Y, et al. Circulating tumor cells as a prognostic factor in patients with small cell lung cancer. Oncol Lett. 2014;7:1469–73. doi: 10.3892/ol.2014.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang CH, Wick JA, Sittampalam GS, Nirmalanandhan VS, Ganti AK, Neupane PC, Williamson SK, Godwin AK, Schmitt S, Smart NJ, Spencer S, Van Veldhuizen PJ. A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker in patients with extensive small-cell lung cancer. Front Oncol. 2014;4:271. doi: 10.3389/fonc.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng Y, Liu XQ, Fan Y, Liu YP, Liu Y, Liu Y, Ma LX, Liu XH, Li H, Bao HZ, Liu JJ, Zhang S, Wu CJ. Circulating tumor cell counts/change for outcome prediction in patients with extensive-stage small-cell lung cancer. Future Oncol. 2016;12:789–99. doi: 10.2217/fon.15.346. [DOI] [PubMed] [Google Scholar]
- 129.Messaritakis I, Politaki E, Plataki M, Karavassilis V, Kentepozidis N, Koinis F, Samantas E, Georgoulias V, Kotsakis A. Heterogeneity of circulating tumor cells (CTCs) in patients with recurrent small cell lung cancer (SCLC) treated with pazopanib. Lung Cancer. 2017;104:16–23. doi: 10.1016/j.lungcan.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 130.Zhang J, Wang HT, Li BG. Prognostic significance of circulating tumor cells in small--cell lung cancer patients: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:8429–33. doi: 10.7314/apjcp.2014.15.19.8429. [DOI] [PubMed] [Google Scholar]
- 131.Hamilton G, Rath B, Holzer S, Hochmair M. Second-line therapy for small cell lung cancer: exploring the potential role of circulating tumor cells. Transl Lung Cancer Res. 2016;5:71–7. doi: 10.3978/j.issn.2218-6751.2015.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, Trapani F, Polanski R, Burt DJ, Simpson KL, Morris K, Pepper SD, Nonaka D, Greystoke A, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 133.Morrow CJ, Trapani F, Metcalf RL, Bertolini G, Hodgkinson CL, Khandelwal G, Kelly P, Galvin M, Carter L, Simpson KL, Williamson S, Wirth C, Simms N, et al. Tumourigenic non-small-cell lung cancer mesenchymal circulating tumour cells: a clinical case study. Ann Oncol. 2016;27:1155–60. doi: 10.1093/annonc/mdw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 135.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 136.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 137.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 138.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW, Digumarthy S, Muzikansky A, Irimia D, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Breitenbuecher F, Hoffarth S, Worm K, Cortes-Incio D, Gauler TC, Köhler J, Herold T, Schmid KW, Freitag L, Kasper S, Schuler M. Development of a highly sensitive and specific method for detection of circulating tumor cells harboring somatic mutations in non-small-cell lung cancer patients. PLoS One. 2014;9:e85350. doi: 10.1371/journal.pone.0085350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marchetti A, Del Grammastro M, Felicioni L, Malatesta S, Filice G, Centi I, De Pas T, Santoro A, Chella A, Brandes AA, Venturino P, Cuccurullo F, Crinò L, et al. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS One. 2014;9:e103883. doi: 10.1371/journal.pone.0103883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sawada T, Watanabe M, Fujimura Y, Yagishita S, Shimoyama T, Maeda Y, Kanda S, Yunokawa M, Tamura K, Tamura T, Minami H, Koh Y, Koizumi F. Sensitive cytometry based system for enumeration, capture and analysis of gene mutations of circulating tumor cells. Cancer Sci. 2016;107:307–14. doi: 10.1111/cas.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ran R, Li L, Wang M, Wang S, Zheng Z, Lin PP. Determination of EGFR mutations in single cells microdissected from enriched lung tumor cells in peripheral blood. Anal Bioanal Chem. 2013;405:7377–82. doi: 10.1007/s00216-013-7156-y. [DOI] [PubMed] [Google Scholar]
- 143.Yeo T, Tan SJ, Lim CL, Lau DP, Chua YW, Krisna SS, Iyer G, Tan GS, Lim TK, Tan DS, Lim WT, Lim CT. Microfluidic enrichment for the single cell analysis of circulating tumor cells. Sci Rep. 2016;6:22076. doi: 10.1038/srep22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sundaresan TK, Sequist LV, Heymach JV, Riely GJ, Jänne PA, Koch WH, Sullivan JP, Fox DB, Maher R, Muzikansky A, Webb A, Tran HT, Giri U, et al. Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin Cancer Res. 2016;22:1103–10. doi: 10.1158/1078-0432.CCR-15-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thress KS, Brant R, Carr TH, Dearden S, Jenkins S, Brown H, Hammett T, Cantarini M, Barrett JC. EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 2015;90:509–15. doi: 10.1016/j.lungcan.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 146.Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, Yang JC, Barrett JC, Jänne PA. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:3375–82. doi: 10.1200/JCO.2016.66.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu Y, Xing Z, Zhan P, Liu H, Ye W, Lv T, Song Y. Is it feasible to detect epidermal growth factor receptor mutations in circulating tumor cells in nonsmall cell lung cancer?: a meta-analysis. Medicine (Baltimore) 2016;95:e5115. doi: 10.1097/MD.037205R1037205R15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ilie M, Long E, Butori C, Hofman V, Coelle C, Mauro V, Zahaf K, Marquette CH, Mouroux J, Paterlini-Bréchot P, Hofman P. ALK-gene rearrangement: a comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann Oncol. 2012;23:2907–13. doi: 10.1093/annonc/mds137. [DOI] [PubMed] [Google Scholar]
- 149.Pailler E, Adam J, Barthélémy A, Oulhen M, Auger N, Valent A, Borget I, Planchard D, Taylor M, André F, Soria JC, Vielh P, Besse B, et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J Clin Oncol. 2013;31:2273–81. doi: 10.1200/JCO.2012.44.5932. [DOI] [PubMed] [Google Scholar]
- 150.He W, Xu D, Wang Z, Xiang X, Tang B, Li S, Hou M, Zhang Y, Chen JF, Lin M, Wang L, Hou S, Tseng HR, et al. Detecting ALK-rearrangement of CTC enriched by nanovelcro chip in advanced NSCLC patients. Oncotarget. 2016 doi: 10.18632/oncotarget.8305. [DOI] [Google Scholar]
- 151.Tan CL, Lim TH, Lim TK, Tan DS, Chua YW, Ang MK, Pang B, Lim CT, Takano A, Lim AS, Leong MC, Lim WT. Concordance of anaplastic lymphoma kinase (ALK) gene rearrangements between circulating tumor cells and tumor in non-small cell lung cancer. Oncotarget. 2016;7:23251–62. doi: 10.18632/oncotarget.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aieta M, Facchinetti A, De Faveri S, Manicone M, Tartarone A, Possidente L, Lerose R, Mambella G, Calderone G, Zamarchi R, Rossi E. Monitoring and characterization of circulating tumor cells (CTCs) in a patient with EML4-ALK-positive non-small cell lung cancer (NSCLC) Clin Lung Cancer. 2016;17:e173–7. doi: 10.1016/j.cllc.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 153.Pailler E, Oulhen M, Billiot F, Galland A, Auger N, Faugeroux V, Laplace-Builhé C, Besse B, Loriot Y, Ngo-Camus M, Hemanda M, Lindsay CR, Soria JC, et al. Method for semi-automated microscopy of filtration-enriched circulating tumor cells. BMC Cancer. 2016;16:477. doi: 10.1186/s12885-016-2461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang Z, Shiratsuchi H, Palanisamy N, Nagrath S, Ramnath N. Expanded circulating tumor cells from a patient with ALK-positive lung cancer present with EML4-ALK rearrangement along with resistance mutation and enable drug sensitivity testing: a case study. J Thorac Oncol. 2017;12:397–402. doi: 10.1016/j.jtho.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pailler E, Auger N, Lindsay CR, Vielh P, Islas-Morris-Hernandez A, Borget I, Ngo-Camus M, Planchard D, Soria JC, Besse B, Farace F. High level of chromosomal instability in circulating tumor cells of ROS1-rearranged non-small-cell lung cancer. Ann Oncol. 2015;26:1408–15. doi: 10.1093/annonc/mdv165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Freidin MB, Freydina DV, Leung M, Montero Fernandez A, Nicholson AG, Lim E. Circulating tumor DNA outperforms circulating tumor cells for KRAS mutation detection in thoracic malignancies. Clin Chem. 2015;61:1299–304. doi: 10.1373/clinchem.2015.242453. [DOI] [PubMed] [Google Scholar]
- 157.Guibert N, Pradines A, Farella M, Casanova A, Gouin S, Keller L, Favre G, Mazieres J. Monitoring KRAS mutations in circulating DNA and tumor cells using digital droplet PCR during treatment of KRAS-mutated lung adenocarcinoma. Lung Cancer. 2016;100:1–4. doi: 10.1016/j.lungcan.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 158.Guibert N, Pradines A, Casanova A, Farella M, Keller L, Soria JC, Favre G, Mazières J. Detection and monitoring of the BRAF mutation in circulating tumor cells and circulating tumor DNA in BRAF-mutated lung adenocarcinoma. J Thorac Oncol. 2016;11:e109–112. doi: 10.1016/j.jtho.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 159.Park SM, Wong DJ, Ooi CC, Kurtz DM, Vermesh O, Aalipour A, Suh S, Pian KL, Chabon JJ, Lee SH, Jamali M, Say C, Carter JN, et al. Molecular profiling of single circulating tumor cells from lung cancer patients. Proc Natl Acad Sci U S A. 2016;113:E8379–86. doi: 10.1073/pnas.1608461113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yoo CE, Park JM, Moon HS, Joung JG, Son DS, Jeon HJ, Kim YJ, Han KY, Sun JM, Park K, Park D, Park WY. Vertical magnetic separation of circulating tumor cells for somatic genomic-alteration analysis in lung cancer patients. Sci Rep. 2016;6:37392. doi: 10.1038/srep37392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ni X, Zhuo M, Su Z, Duan J, Gao Y, Wang Z, Zong C, Bai H, Chapman AR, Zhao J, Xu L, An T, Ma Q, et al. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc Natl Acad Sci U S A. 2013;110:21083–8. doi: 10.1073/pnas.1320659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carter L, Rothwell DG, Mesquita B, Smowton C, Leong HS, Fernandez-Gutierrez F, Li Y, Burt DJ, Antonello J, Morrow CJ, Hodgkinson CL, Morris K, Priest L, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med. 2017;23:114–9. doi: 10.1038/nm.4239. [DOI] [PubMed] [Google Scholar]
- 163.Swennenhuis JF, Reumers J, Thys K, Aerssens J, Terstappen LW. Efficiency of whole genome amplification of single circulating tumor cells enriched by CellSearch and sorted by FACS. Genome Med. 2013;5:106. doi: 10.1186/gm510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mariscal J, Alonso-Nocelo M, Muinelo-Romay L, Barbazan J, Vieito M, Abalo A, Gomez-Tato A, Maria de Los Angeles CC, Garcia-Caballero T, Rodriguez C, Brozos E, Baron F, Lopez-Lopez R, Abal M. Molecular profiling of circulating tumour cells identifies notch1 as a principal regulator in advanced non-small cell lung cancer. Sci Rep. 2016;6:37820. doi: 10.1038/srep37820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bidard FC, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J, Caldas C, Gazzaniga P, Manso L, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–14. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 166.Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, Tejwani S, Schott AF, O’Rourke MA, Lew DL, Doyle GV, Gralow JR, Livingston RB, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32:3483–9. doi: 10.1200/JCO.2014.56.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schwaederle M, Chattopadhyay R, Kato S, Fanta PT, Banks KC, Choi IS, Piccioni DE, Ikeda S, Talasaz A, Lanman RB, Bazhenova L, Kurzrock R. Genomic alterations in circulating tumor DNA from diverse cancer patients identified by next-generation sequencing. Cancer Res. 2017;77:5419–27. doi: 10.1158/0008-5472.CAN-17-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]