Abstract
Tumor-initiating cells (TICs) have been implicated in pancreatic tumor initiation, progression, and metastasis. Among different markers that define this cell population within the tumor, the CD133+ cancer stem cell (CSC) population has reliably been described in these processes. CD133 expression has also been shown to functionally promote metastasis through NF-κB activation in this population but, the mechanism is unclear. In the current study, overexpression of CD133 increased expression and secretion of IL-1β (IL1B), which activates an autocrine signaling loop that upregulates NF-κB signaling, epithelial-mesenchymal transition (EMT), and cellular invasion. This signaling pathway also induces CXCR4 expression, which in turn is instrumental in imparting an invasive phenotype to these cells. In addition to the autocrine signaling of the CD133 secreted IL-1β, the tumor-associated macrophages (TAMs) also produced IL-1β, that further activated this pathway in TICs. The functional significance of the TIC marker CD133 has remained elusive for a very long time; the current study takes us one step closer to understanding how the downstream signaling pathways in these cells regulate the functional properties of tumor-initiating cells.
Keywords: pancreatic cancer, interleukin-1, cancer stem cells, CD133
INTRODUCTION
Extensive metastasis is one of the major reasons for poor prognosis of pancreatic cancer, which has one of lowest rates of 5-year survival compared with other cancers (1,2). This has been attributed to the presence of tumor initiating cells or TIC (3–6). Several markers have been identified for pancreatic TICs. Our results have consistently shown that among these markers CD133+ cells are responsible for formation of aggressive tumors that undergo metastasis (7–9).
We have previously shown that increased metastasis by pancreatic TIC is mediated via activation of the NF-κB pathway (8,10). NF-κB one of the major pathways that respond to an inflammatory stimulus. It is well established that chronic inflammation is associated with tumor development in several different cancer types (11–13). NF-κB activation has also been shown to be actively involved in pancreatic cancer development (10,14). In the pancreas, chronic inflammation in the form of pancreatitis is known to increase risk of pancreatic cancer development in approximately 20% of patients (15,16). Literature shows that IL-1 signaling within acute pancreatitis is essential for full propagation of disease and absence of functional IL-1 signaling results in attenuation of inflammation, enzyme release, edema, and necrosis (17,18).
Interleukins are an important messenger molecule capable of modulating cellular behaviors and capable of initiating inflammatory responses. These molecules are actively secreted from cells in response to appropriate stimuli, such as tissue damage or infection. Among these, IL-1β, specifically, is synthesized in response to a stimulus and induced by NF-κB activation. NF-κB is also activated by IL-1 receptor stimulation, creating a feed-forward loop between IL-1 and NF-κB signaling (19).
Outside of the cell, secreted IL-1β binds to the IL-1 receptor on the surface of responding cells. This triggers signaling downstream of the receptor and the induction of a cascade of pro-inflammatory gene expression. Further, expression of matrix metalloproteinases, cytokines/chemokines, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). On stromal cells, endothelial cells, and leukocytes, IL-1 stimulation induces the upregulation of high-affinity adhesion molecules, such as integrins and adhesion molecules, promoting infiltration into the tumor from the circulation (20,21).
IL-1 signaling and chronic inflammation produce a tumor-supportive environment for the initiation or progression of the neoplastic process. In humans, polymorphisms of interleukin-1 have been shown to modulate patient prognosis and survival in breast (22), lung (23), prostate (24), and pancreatic cancers (25), as well as, an increased risk for the development of cancer (26). High expression levels of IL-1 in human cancers also correlate with poor patient prognosis (27).
IL-1 in the tumor microenvironment, either from tumor cells, stromal cells, or immune cells, has been shown to aid in tumorigenesis, increase tumor invasiveness, and control the immunosuppressive microenvironment in several different cancer types (28–32). Currently, the role of IL-1 stimulation and signaling in pancreatic cancer stem cell populations is unknown.
In this current study, we evaluated the role of IL-1 signaling in the CD133 subset of pancreatic cancer cells as well as the source of IL-1 secretion in pancreatic cancer. We show that IL-1 signaling inhibition has a greater effect on cells with higher CD133 populations through several mechanisms. Inhibition of IL-1 signaling attenuates NF-κB activity, mesenchymal marker expression, and invasiveness. We further show that this IL1 signaling loop in the pancreatic tumor initiating cells can be activated in an autocrine manner (where the CD133+ cells secrete the cytokine) as well as in a paracrine fashion by the macrophages in the tumor microenvironment.
MATERIALS AND METHODS
Cell culture
MIA PaCa-2 (ATCC) cell line was cultured in DMEM (Hyclone) containing 10% Fetal Bovine Serum. S2-013 (a gift from Dr. Masato Yamamoto, University of Minnesota) cells were maintained in RPMI 1640 (Hyclone) containing 10% Fetal Bovine Serum. Primary cell line derived from tumor of (LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre) spontaneous murine model (KPC001) as described in Banerjee, et al(7) and grown in DMEM supplemented with 10 % fetal bovine serum and 1% penicillin/streptomycin.
Boyden chamber invasion assay
Boyden chamber invasion inserts (Corning Biocoat) were rehydrated for 2 hours in serum free medium at 37°C. Cells were plated in the insert, on top of the Matrigel coated membrane in serum free medium. The well contained the 10% FBS containing medium, serving as the attractant. After 24 hours, inserts were washed with PBS, the top of the membrane was scrubbed with cotton swab to remove any remaining non-invaded cells, fixed in methanol, and stained with crystal violet. Membranes were analyzed by microscopy (Magnification 20X).
IL-1 stimulation and inhibition
Recombinant IL-1beta ( Sigma Aldrich) stimulation was performed at concentrations described. ON-TARGETplus human IL1R1 siRNA-SMARTpool (GE Dharmacon) was transfected using HiPerfect transfection reagent according to manufacturer’s guidelines. IL-1Ra [Sigma Aldrich (SRP3084)] was reconstituted according to manufacturer’s guidelines, and used at 0.2 μg/mL concentration. Anti-hIL1-β-IgG neutralizing antibody was obtained from InvivoGen for blocking IL-1β mediated signaling.
NF-κB activity assays
NF-κB activity was determined by both p50 binding ELISA (Thermo Scientific) and Dual-Luciferase reporter assay (Qiagen). Binding ELISA was performed according to the manufacturer’s protocol using whole cell lysates and values were normalized to μg protein as determined by protein estimation (Pierce). Dual-Luciferase reporter assay system (Promega) results were determined by Synergy2 luminometer (Biotek).
Generation of stable cell lines MIA-CD133hi, MIA-CXCR4hi, and shCD133-S2VP10
MIA PaCa-2 (ATCC) and stable MIA-derivatives were maintained in DMEM (Hyclone) containing 10% fetal bovine serum. MIA PaCa-2 cells were stably transfected with empty vector and CD133 or CXCR4 expression vectors (pReceiver-M02 expression vector) and selected by G418 treatment (600 ug/mL) and maintained in 150 ug/mL G418. Selected clones were characterized for expression levels by mRNA expression and surface expression by flow cytometry analysis (CD133 Ab Miltenyi Biotec, CXCR4 Ab BD. S2-VP10 cells were cultured in RPMI 1640 (Hyclone) containing 10% fetal bovine serum. Cells were infected by lentivirus and expression of non-silencing (NS) shRNA or CD133 shRNA was determined by GFP expression. Stable clones were selected and maintained in Geneticin (Invitrogen) and Puromycin (Clontech) for MIA PaCa-2 and S2-VP10 derivatives, respectively.
Plasmids and vectors
Human cDNA empty vector plasmid (EX-NEG-M02) and CD133 expression plasmid (EX-Z0396-M02) were attained from GeneCopoeia. Lentiviral shRNA pGIPZ vectors; NS (RHS4348) and αCD133 (V2LHS_71816) were acquired from Thermo Scientific. NF-kB activity was inhibited after transfecting cells with pBabe-puro-IKBalphamut suprerepressor (a gift from William Hahn [ Addgene plasmid#15291]. In this plasmid, the IκBα is mutated at S32A, which keeps NF-κB pathway constitutively repressed (33).
Immunofluorescence staining
Slides were deparaffinized in xylene and hydrated through graded ethanol solutions. Slides were steamed with a Reveal Decloaker (Biocare Medical, Concord, CA), blocked in 1% BSA/PBS. Antibodies were diluted as per manufacturer’s instructions in 1% BSA/PBS and stained overnight (F4/80 Ab Abcam). Slides were washed 3x in PBS. Secondary antibodies (Alexafluor) were diluted in 1% BSA/PBS 1:1000 and slides were stained for 1 hour at room temperature. Slides were washed 3x in PBS and mounted using Prolong Gold anti-fade with DAPI (Molecular Probes). Slides were dried overnight and imaged by confocal microscopy.
Murine macrophage isolation and stimulation
Murine macrophages were elicited and harvested as described by Zhang et al(34). Briefly, C57B/6 mice were injected intraperitoneally with 3% brewer thioglycollate to induce an inflammatory response. Elicited peritoneal macrophages were harvested by injecting 10 mL cold PBS into the peritoneal cavity with a 20G needle and aspirating the fluid. As a positive control, macrophages were stimulated with LPS (50 ng/mL).
Statictical Analysis
Values are expressed as the mean ± standard error. All experiments were performed at least three times. The significance of the difference between two samples was determined with an unpaired Student’s t-test. P-values of less than 0.05 were considered statistically significant
RESULTS
IL-1β expression and secretion increases with CD133 expression
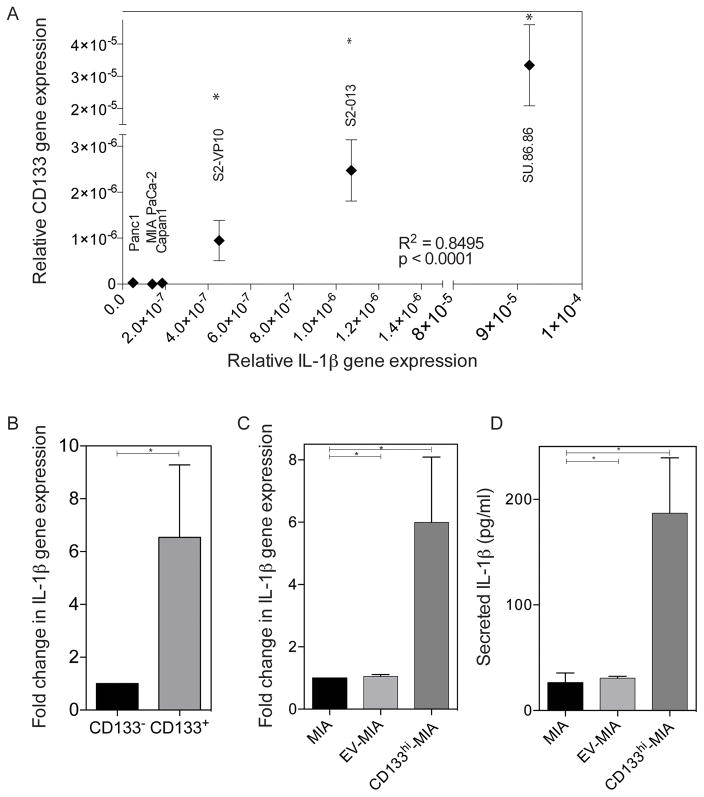
IL-1 signaling in cancer has been associated with poor prognosis and survival. Its signaling activates many pathways important in cancer survival and progression, such as the activation of NF-κB. Our previous work demonstrated that the expression of CD133 in pancreatic cancer activates NF-κB signaling and induces epithelial-mesenchymal transition, increasing the invasiveness of cells (8). This led us to inquire how NF-κB is activated upon the expression of the surface marker, CD133. To discern the role of IL-1 autocrine signaling, we evaluated IL-1β gene expression in several of the established pancreatic cancer cells lines. We have previously shown that these cell lines vary in CD133 expression, which correlates with their invasiveness (35). The IL-1β gene expression also compares with the aggressiveness of the cells, with Panc-1 and MIA PaCa-2 cell lines with low IL-1β gene expression and more aggressive, invasive cell lines, such as the SUIT-2 derived S2-VP10 and S2-013 cell lines with higher IL-1β gene expression (Figure 1A). The IL-1β gene expression positively correlates with CD133 expression. When separating cells derived from the KPC (LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre) tumor based on CD133 surface expression, IL-1β gene expression was 6.541 fold higher in the CD133+ population, as compared to the CD133− population (Figure 1B).
Figure 1. IL-1β expression and secretion increases with CD133 expression.
(a) Positive correlation of CD133 and IL-1β gene expression in several established pancreatic cancer cell lines p<0.0001 (linear regression). (b) IL-1β gene expression in the CD133− and CD133+ sorted populations of KPC tumor cells * p<0.05 (unpaired t-test). (c) Increased IL-1β gene expression upon overexpression of CD133 in MIA PaCa-2 cells *p<0.05 as determined by analysis of variance (ANOVA). (d) Increased IL-1β secretion upon overexpression of CD133 in MIA PaCa-2 cells *p<0.05 as determined by analysis of variance (ANOVA). Each bar is representative of three or more independent experiments; error bars are represented in s.e.m.
Our group has shown that CD133 surface expression in pancreatic cancer has a functional role in NF-κB activation and metastasis. Additionally, upon over expression of CD133 in MIA PaCa-2 there is also a 6.00 fold (± 2.10) upregulation of IL-1β gene expression (Figure 1C). Overexpression of CD133 increased IL-1β secretion from 26.23 pg/mL (± 9.28) and 30.72 pg/mL (± 1.59) in MIA and empty vector (EV), respectively, to 186.8 pg/(± 52.6) upon the overexpression of CD133 (Figure 1D). To study if IL1 receptors were overexpressed in the CD133hi cells, we estimated the expression of IL1R in MIA PaCa-2, EV-MIA and CD133hi-MIA. Our results showed that CD133hi-MIA had much higher expression of IL1R compared to the others (Supplementary Figure 1).
IL-1β stimulation increases NF-κB activation, EMT, and invasion
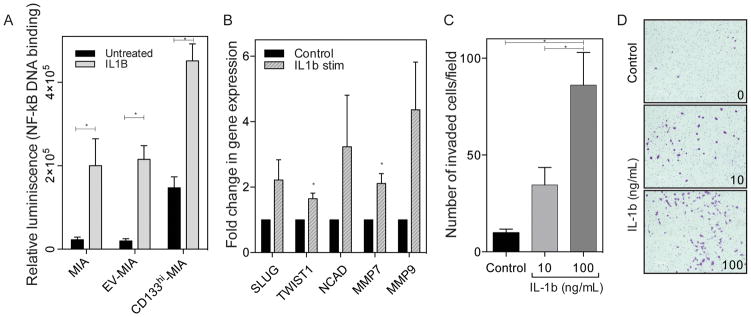
As we have previously demonstrated that CD133 expression activates NF-κB signaling, we next evaluated if IL-1β stimulation leads to the activation of NF-κB in pancreatic cancer. Exogenous stimulation with IL-1β drastically increased NF-κB activation in control cells, with a lesser, but still significant increase in cells overexpressing CD133, which are already secreting high levels of IL-1β prior to exogenous stimulation (Figure 2A). This increased NF-κB activity upon IL-1β stimulation led to an increase in epithelial-mesenchymal transition (EMT) related gene expression (Figure 2B). Then to verify that IL-1β is indeed responsible for the increased invasiveness we have previously seen upon the overexpression of CD133, exogenous IL-1β treatment was used in low and high doses. An increase in Boyden chamber invasion was demonstrated in a dose dependent manner (Figure 2C) increasing 3.5 fold (10 ng/mL) to 8.7 fold (100 ng/mL), as compared to unstimulated control, with representative images of invaded cells (Figure 2D).
Figure 2. IL-1β stimulation increases NF-κB, EMT, and invasion.
(a) Exogenous IL-1β stimulation increases NF-κB activity, as seen by p50 DNA binding in MIA PaCa-2, empty vector, and CD133 overexpressing cells. (b) Exogenous IL-1β stimulation increases EMT gene expression in MIA PaCa-2 cell line. * p<0.05 (unpaired t-tests). (c) Exogenous IL-1β stimulation increases in vitro Boyden chamber invasion in MIA PaCa-2 *p<0.05 as determined by analysis of variance (ANOVA) and (d) representative images of invaded cells. Each bar is representative of three or more independent experiments; error bars are represented in s.e.m.
Inhibition of IL-1 signaling, in the presence of CD133, decreases invasion
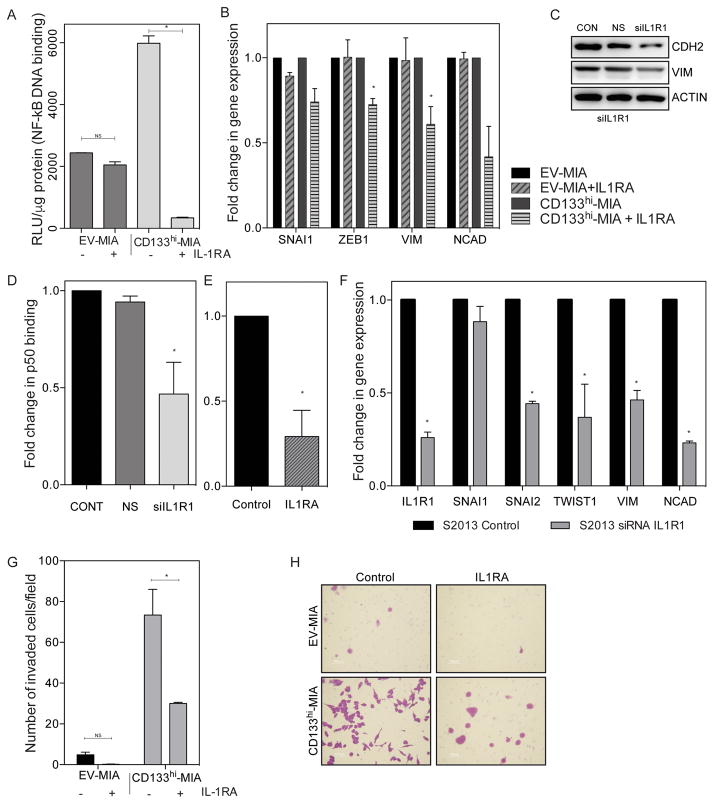
Next, to verify that IL-1 signaling is responsible for invasiveness in CD133 expressing cells, IL1 antagonist (IL-1RA) was used to block the signaling from the IL-1 receptor. Inhibition of IL-1 signaling decreased NF-κB p50 binding activity (Figure 3A). In CD133hi-MIA cells, p50 binding to the consensus sequence was 5748 RLU/μg protein (± 238.2), but with treatment of IL1RA this was less than 10% of control to 314.7 RLU/μg protein (± 21.75). In empty vector control cells, the p50 binding in untreated and IL1RA treated cells was not significantly different (2440 RLU/μg protein (± 4.881) and 2048 (± 101.4), respectively).
Figure 3. Inhibition of IL-1 signaling, in the presence of CD133, decreases invasion.
(a) Blocking IL-1 signaling with IL-1R antagonist decreases NF-κB in cells with high CD133 as compared to MIA PaCa-2 * p<0.05 (unpaired t-tests). (b) Gene expression of EMT transcription factors and mesenchymal markers upon silencing of IL1R1 in both EV-MIA and CD133hi-MIA * p<0.05 (unpaired t-tests). (c) Mesenchymal marker expression in CD133hi-MIA upon silencing of IL1R1. NF-κB activity upon (d) IL1R1 silencing *p<0.05 as determined by analysis of variance (ANOVA) and (e) IL-1R antagonist * p<0.05 (unpaired t-test). (f) Silencing of IL1R decreases EMT gene expression * p<0.05 (unpaired t-tests). (g) IL1R antagonist decreases cellular invasiveness * p<0.05 (unpaired t-tests), (h) including representative membrane photos. Each bar is representative of three or more independent experiments; error bars are represented in s.e.m.
Inhibition of IL-1 signaling also led to decreased gene expression of several EMT related markers: SNAI1, ZEB1, VIM, CDH2 (Figure 3B) in cells overexpressing CD133, with little effect on control, empty vector cells. IL-1RA treatment of EV-MIA changed SNAI1 0.893 fold (± 0.021), ZEB1 1.003 fold (± 0.102), VIM 0.985 fold (± 0.132), and CDH2 0.994 fold (± 0.038). Whereas in CD133hi-MIA, these genes were significantly decreased: SNAI1 0.741 fold (± 0.078), ZEB1 0.725 fold (± 0.035), VIM 0.609 fold (± 0.104), and CDH2 0.419 fold (± 0.178). Subsequently, this led to a reduction in the protein expression of n-cadherin and vimentin (Figure 3C).
In S2-013 cells, inhibition of IL-1 signaling by silencing of IL1R1 by siRNA as well as treatment of IL1R antagonist led to decreased NF-kB activity (Figures 3D and E). IL1R1 silencing decreased SNAI1 (0.884 fold ± 0.082), SNAI2 (0.442 fold ± 0.013), TWIST1 (0.370 fold ± 0.178), VIM (0.462 fold ± 0.052), and CDH2 (0.232 fold ± 0.010), as compared to control (Figure 3F), with a similar effect upon IL1R antagonist treatment. Additionally, upon treatment with exogenous IL-1Ra, Boyden chamber invasion in CD133hi-MIA cells was decreased significantly (Figure 3G). In the representative invasion chamber images (Figure 3H), not only is the number of invaded cells decreased, but also a loss in fibroblast-like morphology can be seen with IL-1RA treatment in cells overexpressing CD133.
These data suggest that IL-1 stimulation and downstream signaling in cells expressing high levels of CD133 has a greater role in EMT induction and invasion, than in cells expressing lower levels of CD133.
IL-1 increases invasiveness through upregulating CXCR4 receptor expression
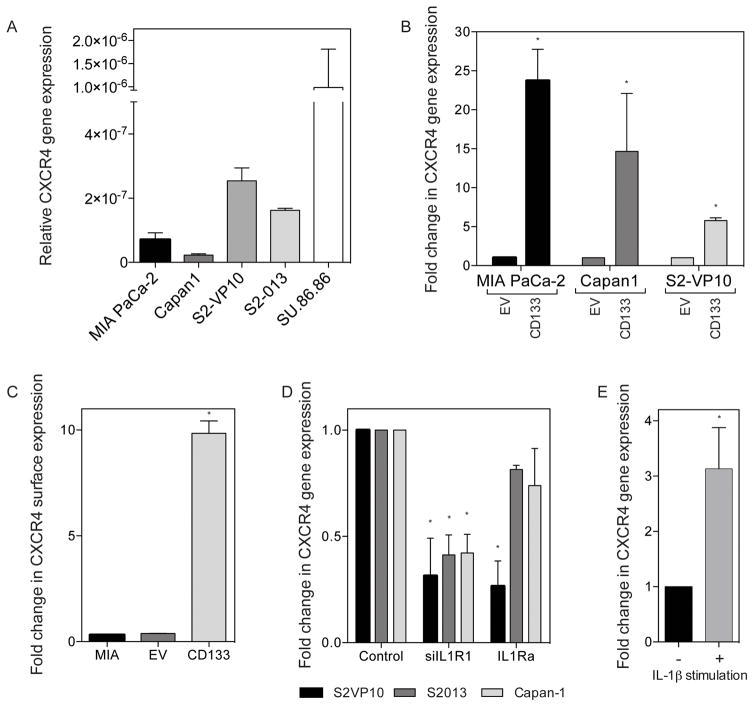
The CD133+CXCR4+ subset has been identified as an extremely aggressive and invasive population among pancreatic TIC. We first examined the expression of CXCR4 in various pancreatic cancer cell lines and saw a similar correlation; cell lines with higher CD133 expression also show an increased CXCR4 expression (Figure 4A). Additionally, overexpression of CD133 in MIA PaCa-2, Capan1, and S2-VP10 cell lines, resulted in a significant increase in CXCR4 gene expression and surface expression (Figure 4B and C, respectively). Inhibition of IL-1 signaling by silencing IL1R1 or treatment with the IL-1 antagonist, CXCR4 gene expression is decreased in S2-VP10, S2-013 and Capan-1 cell lines (Figure 4D). Conversely, stimulation of IL-1 signaling by exogenous IL-1β treatment, led to a 3.13 fold (± 0.74) increase in CXCR4 gene expression (Figure 4E). Together, these data indicate that CD133 expression upregulates CXCR4 expression through IL-1β stimulated IL-1R signaling. We further suppressed IL1 signaling using a blocking antibody for IL1-β and studied the expression of CXCR4. Consistent with above, the gene expression of CXCR4 was diminished in this condition indicating that IL1 stimulation and signaling was required for CXCR4 activation (Supplementary Figure 2A). We next transfected S2VP10 cells a super-repressor plasmid for NF-κB in which the NF-kB activity is constitutively repressed, and checked the CXCR4 gene expression in this. CXCR4 expression was significantly downregulated when NF-kB was repressed (Supplementary Figure 2B). To study if IL1-b signaling was indeed instrumental in NF-kB transcriptional activity, we blocked IL1-b signaling using the Anti-IL1-b antibody and studied the NF-kB transcriptional activity using a Dual Luciferase reporter system. Our results showed that when IL1-b signaling was blocked by the antibody there was significantly reduced NF-kB activity as well (Supllementary Figure 2C.). This confirmed that IL1-β stimulation activated NF-κB activity leading to an upregulation of CXCR4 expression in pancreatic cancer TIC.
Figure 4. IL-1 increases invasiveness through upregulating CXCR4 receptor expression.
(a) CXCR4 gene expression in several established PDAC cell lines. Increased CXCR4 upon CD133 expression of (b) gene expression *p<0.05 as determined by analysis of variance (ANOVA) and (c) surface expression by flow cytometry *p<0.05 as determined by analysis of variance (ANOVA). (d) Decreased CXCR4 gene expression upon blocking IL1R signaling through IL1R1 siRNA and IL1Ra *p<0.05 as determined by analysis of variance (ANOVA). (e) Increased CXCR4 expression in MIA PaCa-2 with IL-1β stimulation * p<0.05 (unpaired t-test). Each bar is representative of three or more independent experiments; error bars are represented in s.e.m.
IL-1 signaling mediated CXCR4 expression increases invasiveness
Simultaneous CD133 and CXCR4 expression in pancreatic cancer has been shown as a metastatic subset of pancreatic cancer cells (6). In addition, this study showed a regulatory link between CD133 and CXCR4 expression. We therefore next wanted to examine the functional consequence of CD133 expression mediated upregulation of CXCR4.
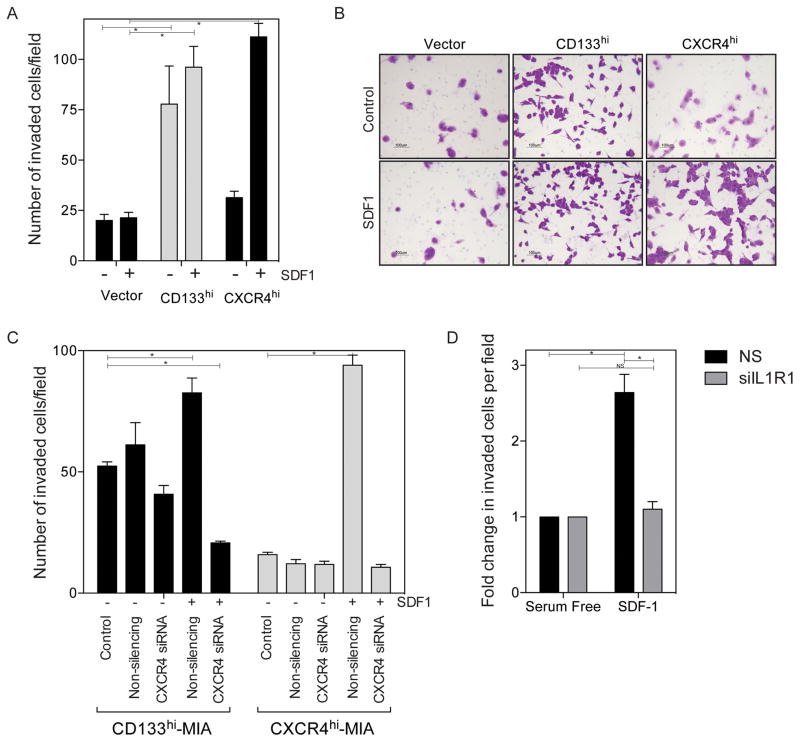
First we examined the importance of CXCR4 expression in cellular invasiveness. We have previously shown that CD133+ cells and cells overexpressing CD133 have increased in vitro invasion(7,8). We wanted to further examine this in the context of the CXCR4 ligand, SDF1 (CXCL12), in in vitro invasion. Overexpression of CD133 increased invasion, with a slight increase in invasion when SDF1 is used as the attractant. However, upon overexpression of CXCR4 (without CD133 expression) in the absence of SDF1, invasiveness is similar to vector controls and there is only an increase in invasiveness when SDF1 is used as an attractant (Figure 5A) and shown in representative Boyden chamber images (Figure 5B). This effect is inhibited in both cells overexpressing CD133 or CXCR4 when CXCR4 is silenced by siRNA (Figure 5C). Further, upon silencing of IL1R1 in cell lines with endogenously high CD133 and CXCR4, such as S2-013, we also see a decrease in the extent of invasion (Figure 5D). These data indicate that CXCR4 mediated invasion requires the expression of both CD133 as well as IL-1 stimulation and downstream signaling.
Figure 5. IL-1 signaling mediated CXCR4 expression increases invasiveness.
(a) Increased invasion with SDF1 stimulation in CD133 and CXCR4 over expressing cell lines *p<0.05 as determined by analysis of variance (ANOVA). (b) and representative images of invaded cells. (c) Silencing of CXCR4 in CD133 overexpressing cells and CXCR4 overexpressing cells with SDF1 gradient *p<0.05 as determined by analysis of variance (ANOVA). (d) Silencing IL1R1 in S2-013 cell line decreased invasion in the presence of SDF1 gradient *p<0.05 as determined by analysis of variance (ANOVA). Each bar is representative of three or more independent experiments; error bars are represented in s.e.m.
Tumor mediated macrophage secretion of IL-1β stimulates tumor cell IL-1 signaling
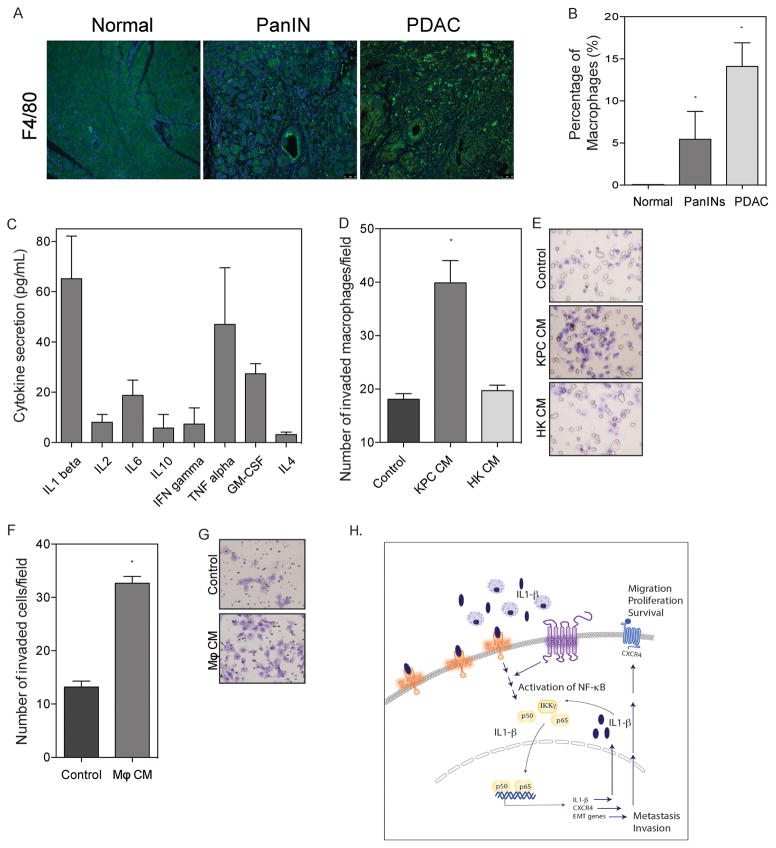
As IL-1β is an important inflammatory mediator of the immune regulation, we next examined tumor associated macrophages (TAM) as a potential source of IL-1β tumor stimulation. We first assessed macrophage infiltration during tumor initiation and progression using the LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre model of spontaneous pancreatic cancer. F4/80 staining was used for a marker for macrophages in normal pancreas, PanIN, and PDAC sections (Figure 6A). Normal pancreas did not show macrophage infiltration, whereas PanIN stages displayed 5.5% macrophages, which increased to 14.1% of cells within 6-month old mice with fully developed pancreatic tumors (Figure 6B). These tumor associated macrophages showed high levels of IL-1β secretion as compared to other cytokines (Figure 6C).
Figure 6. Tumor mediated macrophage secretion of IL-1β stimulated tumor cell IL-1 signaling.
(a) Immunofluorescence images of KPC pancreatic cancer progression stained with F4/80. (b) Quantification of macrophage infiltration in KPC tumor images *p<0.05 as determined by analysis of variance (ANOVA). (c) Cytokine profile from KPC tumor. (d) Increased macrophage invasion using conditioned medium from KPC cell line and heat killed conditioned medium *p<0.05 as determined by analysis of variance (ANOVA). with (e) representative images of invaded macrophages. (f) Increased invasion of KPC cells using conditioned medium from macrophages * p<0.05 (unpaired t-test). with (g) representative images of invaded KPC cells. Each bar is representative of three or more independent experiments; error bars are represented in s.e.m. (h) Schematic diagram demonstrating that CD133hi cells have increased IL1R1 levels and thus have increased IL1-b (secreted by infiltrating macrophages) mediated paracrine signaling. This results in increased NF-kB activity in the CD133 hi cells leading to a feed-forward loop of IL1 signaling in a autocrine manner as well as increased expression of CXCR4 and EMT genes.
We further looked at the functional effect these populations have on invasiveness in both tumor cells and macrophages. Using conditioned medium from KPC cells as an attractant, invasion of macrophages increased 2.2 fold as compared to FBS control. Macrophage invasion did not increase when using heat killed KPC conditioned medium as an attractant (Figure 6D and E). Further, KPC tumor cell invasiveness increased 2.5 fold using macrophage conditioned medium as an attractant (Figure 6F and G).
Taken together, these data indicate that infiltrating macrophages could be a paracrine source of IL-1β for the stimulation of pancreatic cancer cells and this interaction increases tumor cell invasiveness.
DISCUSSION
The connection between chronic inflammation and cancer has been previously well established. Inflammatory signaling has been shown to mediate several stages of tumor progression, from initiation to metastasis. A dominant mediator of inflammation, interleukin-1, has been implicated in pancreatic cancer as an inducer of invasion and metastasis (31). This study demonstrates the importance of IL-1 signaling in EMT and invasion in cells with high CD133 expression.
It has not yet been shown that the expression of CD133 in pancreatic cancer leads to an upregulation of IL-1β expression or secretion, which we observed in several pancreatic cancer cell lines (Figure 1C and D). CD133 expression was previously described to increase the invasiveness of cells and we therefore wanted to determine if the increased IL-1β secretion upon overexpression of CD133 increased invasion. Exogenous IL-1β stimulation in MIA PaCa-2 cell lines showed a dose dependent increase in invasion (Figure 1C).
We have previously established that pancreatic cancer stem cells expressing CD133, have high levels of NF-κB signaling as compared to the CD133 negative population within the tumor (7). This was also observed upon the overexpression of CD133 in pancreatic cancer cells with very low endogenous CD133 levels (8). We next determined that IL-1β stimulation did increase NF-κB activity, specifically in control cells secreting little IL-1β as compared to a lesser increase in CD133 overexpressing cells with a higher secretion of IL-1β (Figure 2A).
To verify that IL-1 signaling was indeed responsible for the increased invasiveness upon overexpression of CD133, several different ways of IL-1 signaling inhibition were utilized. IL-1 receptor antagonist (IL-1Ra) and IL1R1 siRNA silencing were used to block IL-1R signaling in cells overexpressing CD133. This led to a decrease in invasion, NF-κB activation, and EMT gene expression (Figure 3), demonstrating that IL-1 signaling was important for the activation of NF-κB and downstream events in the presence of CD133 surface expression.
In cells with high populations of CD133 positive cells, IL-1 signaling is also of importance. Inhibition of IL-1 signaling had similar effects as seen with inhibition in cells overexpressing CD133 (Figure 3). In addition, gene expression of IL-1β significantly correlates with CD133 expression in several human pancreatic cancer cell lines (Figure 1A). Exhibiting an important signaling axis for invasion in pancreatic cancer.
IL-1 signaling in pancreatic cancer has not yet been described in the context of cancer stem cells or CD133 function in invasion. This study demonstrates the upregulation of gene expression and secretion of IL-1β upon the expression of CD133, and the importance of IL-1 signaling in CD133 positive cells in the context of EMT induction and invasion.
Clinically, blockade of interleukin signaling and specifically IL-1β signaling is standard care in autoimmune disease patients or patients with lymphomas. One specific treatment is the endogenous IL-1 receptor antagonist (IL-1Ra), anakinra (KineretTM), which was FDA approved for use in rheumatoid arthritis in 2001 (36,37). Recently, Zhuang et al described the use of anakinra in an orthotopic, xenograft model of pancreatic cancer in combination with gemcitabine treatment(38). Anakinra treatment at a concentration of 1.5 mg/kg i.p. did show some efficacy, but not complete regression of the tumors. This indicates the importance of IL-1 signaling for tumor growth, however, tumor progression in vivo must still be elucidated. Their data, in combination with this study indicates that IL-1 stimulation is essential for tumor cell invasion.
Another pathway altered by IL-1 stimulation is the activation of COX2 by IL-1β stimulation, which was shown to confer chemoresistance in pancreatic cancer. This study indicates the IL-1 signaling has a greater effect on cells expressing CD133 and perhaps this previously described mechanism may contribute to the higher chemoresistance characteristics in pancreatic cancer stem cells (39).
Our results in this study indicate that IL-1 signaling may be an important mediator of epithelial-mesenchymal transition induction and invasiveness in pancreatic cancer stem cells and could be a valuable signaling pathway to target for treatment of pancreatic cancer.
Tumor Initiating Cells (TICs) are typically present in hypoxic niches of the tumor. Our previous studies have demonstrated that the microenvironment actually plays a role in enriching for the CD133+ cells(40). Our current study showed that macrophage infiltration into the pancreas increases with tumor progression (Figure 6A). These pancreatic cancer associated macrophages secrete a large amount of IL-1β (Figure 6C) in addition to several other cytokines, providing a paracrine source of IL-1 stimulation for pancreatic tumor cells in the tumor microenvironment.
Since the TICs typically have a high expression of IL1 receptors compared to the non-TICs (Supplementary Figure 1A), the paracrine IL1-β activates the NF-κB pathway in these cells to a greater extent, thereby resulting in increased invasion and metastasis (Figure 6H). Once NF-kB is activated within the cells, a feed-forward loop is triggered in which the autocrine IL1-β mediated signaling is activated.
This study demonstrates the important role of tumor and macrophage derived IL-1β stimulation in pancreatic cancer. IL-1 signaling is increased in cells with CD133 expression, leading to increased NF-kB activity, EMT induction, and invasion. Further, increased invasiveness via IL-1β stimulation is mediated by the upregulation of CXCR4 expression. In conclusion, we show the importance of IL-1 in the activation of NF-κB signaling and invasion in pancreatic cancer.
Supplementary Material
Implications.
This study demonstrates the important role of tumor- and macrophage-derived IL-1β stimulation in pancreatic cancer. IL-1 signaling is increased in cells with CD133 expression, leading to increased NF-kB activity, EMT induction, and invasion. Increased invasiveness via IL-1β stimulation is mediated by the upregulation of CXCR4 expression. The study highlights the importance of IL1-mediated signaling in tumor-initiating cells.
Acknowledgments
The authors would like to acknowledge the Flow Cytometry Core and the Analytical Imaging Core for help with experiments in the study.
FINANCIAL SUPPORT
This study was funded by NIH grants R01-CA170946 and CA124723 (to AKS); NIH grant R01-CA184274 (to SB).
Footnotes
COMPETING FINANCIAL INTEREST STATEMENTS
University of Minnesota has a patent for Minnelide (WO/2010/129918/Triptolide Prodrugs), which has been licensed to Minneamrita Therapeutics, LLC. AKS is the co-founder and the Chief Scientific Officer of this company. Dr. Banerjee is a compensated consultant with Minneamrita Therapeutics LLC and this relationship is managed by University of Miami.
References
- 1.Society AC. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Kim HS, Yoo SY, Kim KT, Park JT, Kim HJ, Kim JC. Expression of the stem cell markers CD133 and nestin in pancreatic ductal adenocarcinoma and clinical relevance. International journal of clinical and experimental pathology. 2012;5(8):754–61. [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, et al. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. British journal of cancer. 2008;98(8):1389–97. doi: 10.1038/sj.bjc.6604307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balic A, Dorado J, Alonso-Gomez M, Heeschen C. Stem cells as the root of pancreatic ductal adenocarcinoma. Experimental cell research. 2012;318(6):691–704. doi: 10.1016/j.yexcr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell stem cell. 2007;1(3):313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Nomura A, Sangwan V, Chugh R, Dudeja V, Vickers SM, et al. Minnelide reduces CD133+ tumors initiating “stem-like” cells in a syngenic murine model of pancreatic ductal adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-13-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nomura A, Banerjee S, Chugh R, Dudeja V, Yamamoto M, Vickers SM, et al. CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget. 2015;6(10):8313–22. doi: 10.18632/oncotarget.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomura A, McGinn O, Dudeja V, Sangwan V, Saluja AK, Banerjee S. Minnelide effectively eliminates CD133(+) side population in pancreatic cancer. Mol Cancer. 2015;14:200. doi: 10.1186/s12943-015-0470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer cell. 2012;21(1):105–20. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Hausmann S, Kong B, Michalski C, Erkan M, Friess H. The role of inflammation in pancreatic cancer. Adv Exp Med Biol. 2014;816:129–51. doi: 10.1007/978-3-0348-0837-8_6. [DOI] [PubMed] [Google Scholar]
- 13.Marusawa H, Jenkins BJ. Inflammation and gastrointestinal cancer: an overview. Cancer Lett. 2014;345(2):153–6. doi: 10.1016/j.canlet.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5(1):119–27. [PubMed] [Google Scholar]
- 15.Whitcomb DC, Pogue-Geile K. Pancreatitis as a risk for pancreatic cancer. Gastroenterol Clin North Am. 2002;31(2):663–78. doi: 10.1016/s0889-8553(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 16.Pinho AV, Chantrill L, Rooman I. Chronic pancreatitis: a path to pancreatic cancer. Cancer Lett. 2014;345(2):203–9. doi: 10.1016/j.canlet.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Norman JG, Fink G, Franz M, Guffey J, Carter G, Davison B, et al. Active interleukin-1 receptor required for maximal progression of acute pancreatitis. Ann Surg. 1996;223(2):163–9. doi: 10.1097/00000658-199602000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman J, Franz M, Messina J, Riker A, Fabri PJ, Rosemurgy AS, et al. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995;117(6):648–55. doi: 10.1016/s0039-6060(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 19.Hiscott J, Marois J, Garoufalis J, D’Addario M, Roulston A, Kwan I, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13(10):6231–40. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bochner BS, Luscinskas FW, Gimbrone MA, Jr, Newman W, Sterbinsky SA, Derse-Anthony CP, et al. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173(6):1553–7. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nourshargh S, Larkin SW, Das A, Williams TJ. Interleukin-1-induced leukocyte extravasation across rat mesenteric microvessels is mediated by platelet-activating factor. Blood. 1995;85(9):2553–8. [PubMed] [Google Scholar]
- 22.Hefler LA, Grimm C, Lantzsch T, Lampe D, Leodolter S, Koelbl H, et al. Interleukin-1 and interleukin-6 gene polymorphisms and the risk of breast cancer in caucasian women. Clin Cancer Res. 2005;11(16):5718–21. doi: 10.1158/1078-0432.CCR-05-0001. [DOI] [PubMed] [Google Scholar]
- 23.Hu Z, Shao M, Chen Y, Zhou J, Qian J, Xu L, et al. Allele 2 of the interleukin-1 receptor antagonist gene (IL1RN*2) is associated with a decreased risk of primary lung cancer. Cancer Lett. 2006;236(2):269–75. doi: 10.1016/j.canlet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Lindmark F, Zheng SL, Wiklund F, Balter KA, Sun J, Chang B, et al. Interleukin-1 receptor antagonist haplotype associated with prostate cancer risk. Br J Cancer. 2005;93(4):493–7. doi: 10.1038/sj.bjc.6602729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber MD, Powell JJ, Lynch SF, Fearon KC, Ross JA. A polymorphism of the interleukin-1 beta gene influences survival in pancreatic cancer. Br J Cancer. 2000;83(11):1443–7. doi: 10.1054/bjoc.2000.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sehouli J, Mustea A, Konsgen D, Katsares I, Lichtenegger W. Polymorphism of IL-1 receptor antagonist gene: role in cancer. Anticancer Res. 2002;22(6A):3421–4. [PubMed] [Google Scholar]
- 27.Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Furuse J, Inagaki M, et al. Serum levels of IL-6 and IL-1beta can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 2013;108(10):2063–9. doi: 10.1038/bjc.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25(3):387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 29.Apte RN, Voronov E. Is interleukin-1 a good or bad ‘guy’ in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222:222–41. doi: 10.1111/j.1600-065X.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 30.Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, et al. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res. 2006;12(4):1088–96. doi: 10.1158/1078-0432.CCR-05-1603. [DOI] [PubMed] [Google Scholar]
- 31.Melisi D, Niu J, Chang Z, Xia Q, Peng B, Ishiyama S, et al. Secreted interleukin-1alpha induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-kappaB. Mol Cancer Res. 2009;7(5):624–33. doi: 10.1158/1541-7786.MCR-08-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voronov E, Apte RN. IL-1 in Colon Inflammation, Colon Carcinogenesis and Invasiveness of Colon Cancer. Cancer Microenviron. 2015;8(3):187–200. doi: 10.1007/s12307-015-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129(6):1065–79. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14(Unit 14):1. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee S, Nomura A, Sangwan V, Chugh R, Dudeja V, Vickers SM, et al. CD133+ tumor initiating cells in a syngenic murine model of pancreatic cancer respond to Minnelide. Clin Cancer Res. 2014;20(9):2388–99. doi: 10.1158/1078-0432.ccr-13-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(3):614–24. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- 37.Cohen SB, Moreland LW, Cush JJ, Greenwald MW, Block S, Shergy WJ, et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63(9):1062–8. doi: 10.1136/ard.2003.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhuang Z, Ju HQ, Aguilar M, Gocho T, Li H, Iida T, et al. IL1 Receptor Antagonist Inhibits Pancreatic Cancer Growth by Abrogating NF-kappaB Activation. Clin Cancer Res. 2016;22(6):1432–44. doi: 10.1158/1078-0432.CCR-14-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angst E, Reber HA, Hines OJ, Eibl G. Mononuclear cell-derived interleukin-1 beta confers chemoresistance in pancreatic cancer cells by upregulation of cyclooxygenase-2. Surgery. 2008;144(1):57–65. doi: 10.1016/j.surg.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomura A, Dauer P, Gupta V, McGinn O, Arora N, Majumdar K, et al. Microenvironment mediated alterations to metabolic pathways confer increased chemo-resistance in CD133+ tumor initiating cells. Oncotarget. 2016 doi: 10.18632/oncotarget.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.