Abstract
Targeted therapy options are currently lacking for the heterogeneous population of patients whose melanomas lack BRAF or NRAS mutations (~35% of cases). We undertook a chemical biology screen to identify potential novel drug targets for this understudied group of tumors. Screening a panel of 8 BRAF/NRAS-WT melanoma cell lines against 240 targeted drugs identified ceritinib and trametinib as potential hits with single agent activity. Ceritinib enhanced the efficacy of trametinib across the majority of the BRAF/NRAS-WT cell lines, and the combination showed increased cytotoxicity in both 3D spheroid culture and long-term colony formation experiments. Co-administration of ceritinib and trametinib led to robust inhibition of tumor growth in an in vivo xenograft BRAF/NRAS-WT melanoma model; this was not due to ALK inhibition by ceritinib. Mechanistic studies showed the ceritinib-trametinib combination to increase suppression of MAPK and TORC1 signaling. Similar results were seen when BRAF/NRAS-WT melanoma cells were treated with a combination of trametinib and the TORC1/2 inhibitor INK128. We next used mass spectrometry-based chemical proteomics and identified known and new ceritinib targets, such as IGF1R and ACK1, respectively. Validation studies suggested that ceritinib could suppress mTORC1 signaling in the presence of trametinib through inhibition of IGF1R and/or ACK1 in a cell line-dependent manner. Together our studies demonstrated that combining a specific inhibitor (trametinib) with a more broadly targeted agent (ceritinib) has efficacy against tumors with heterogeneous mutational profiles.
Keywords: melanoma, ceritinib, BRAF, NRAS, chemical biology, combination therapy
Introduction
Major breakthroughs have been made in the development of therapeutic strategies to treat advanced melanoma. At this time, the choice of frontline therapy is determined both by the BRAF mutational status of the tumor and by the kinetics of disease progression (1, 2). For the ~45–50% of patients whose melanomas harbor activating position 600 mutations in the serine-threonine kinase BRAF, the combination of a BRAF inhibitor (e.g., vemurafenib or dabrafenib) with a MEK inhibitor (e.g., cobimetinib or trametinib) leads to high response rates, with progression-free survival rates of close to a year and survival durations approaching 2 years (3). The BRAF-MEK inhibitor combination has the advantage of working relatively rapidly with mild off-target effects (3, 4). This combination is thought to be ineffective, however, and may even be deleterious in individuals whose melanomas are wild-type (WT) for BRAF (5). The current frontline treatment for patients with BRAF-WT melanoma is immunotherapy, with the combination of ipilimumab plus nivolumab being associated with response rates similar to that achieved with targeted therapy in BRAF-mutant melanoma (6, 7). Although highly effective, the immune-related adverse effects associated with this combination are frequently severe with nearly 40% of those treated having to discontinue therapy. Other therapeutic options, and in particular second-line therapies, are urgently needed for patients with BRAF-WT melanoma.
Melanoma is one of the most genetically diverse tumors and carries a high mutational burden (8). Approximately 15–20% of melanoma patients have tumors that are NRAS-mutant. Another recently identified group of melanomas are those with mutation/inactivation of the tumor suppressor NF1 (9, 10). These mutations, which can also co-occur with BRAF and NRAS, are found in ~13% of melanomas (9–11).
The goal of precision medicine is to identify genetic predictors of response that will help guide therapy selection. Although there are numerous instances in which strong predictions can be made (e.g., EML4-ALK fusions and sensitivity to ALK inhibitors, TSC2 mutations and sensitivity to rapalogs), tumor genetic profiles are complex and sensitivity predictions are often confounded by the presence of bypass pathways (e.g., EGFR signaling in BRAF-mutant colorectal carcinoma) (12–14). With this in mind, we have undertaken the first comprehensive screen of curated (FDA-approved or in clinical development) anti-cancer drugs to identify potential therapeutic strategies for BRAF/NRAS-WT melanoma.
Materials and Methods
Cell Lines
M257 and M285 were a gift from Antoni Ribas (UCLA Medical Center). 1205Lu, WM1366, WM209, WM1963, WM3438, WM3681, and WM3918 were acquired from the Wistar Institute. A375 and SK-MEL-23 were acquired from the ATCC. The identities of all cell lines were confirmed by STR fingerprinting performed by Bio-synthesis Inc. (Lewisville, TX). All cell lines were grown in RPMI + 5% FBS. Mycoplasma testing was performed using Plasmotest every three months (Invivogen, San Diego, CA). Fresh cells were thawed every 3 months.
Western blotting
Western blotting was performed as previously described in (15). See Supplemental Materials for antibody details.
Cell proliferation assay
Cells proliferation was measured as previously described (15).
Compounds
Compounds used included trametinib (Chemitek #CT-GSK212), ceritinib (Selleck #S7083), INK128 (Cayman #11811) (16) and AIM-100 (Bio-Techne #4946) (17).
RNAi knockdown
RNAi was performed using Dharmacon Smartpools: IGF1R (Dharmacon #L-003012-00-0005), ALK (#L-003103-00-0005), and ACK (#L-003102-01-0005).
Three-dimensional Spheroid Assay
Spheroids were grown as previously described (15). Spheroids were treated for 24h prior to 2X washes with PBS and staining with calcein-AM and propidium iodide (20ng/mL). Images were acquired with an AMG Evos FL system.
Colony formation assay
Colony formation assays were performed as previously described (18).
Drug screening
Cells were seeded in 384-well plates at 1000 cells/well. All compounds were diluted to 0.5 or 2.5μM, and all cell lines were treated in duplicate. A total of 240 compounds from an in-house library were tested. Compounds were aliquoted by a Biotek Precision Pipetting robot. Cell viability was measured by Cell-Titer-Glo (Promega G7572) at 72h post treatment.
Xenograft studies
WM209 were virally transduced with eGFP-NanoLuc plasmid (19) and tumor xenografts generated following injection of 2.5×106 cells in Matrigel into each flank of NSG mice. Mice were randomized at day 10 day (n=5), and treatment was started. Each mouse was dosed daily with vehicle, 25mg/kg ceritinib, 1mg/kg trametininb, or the combination of 25mg/kg ceritinib and 1mg/kg trametinib. Drugs were formulated in 0.5% methycellulose + 0.5% Tween-80. Luciferase expression was visualized on an IVIS-100. The work was performed under the approval of the IACUC of the University of South Florida (IS00000324).
Drug Affinity Chromatography
Drug affinity chromatography experiments were conducted as described previously (20). Briefly, the immobilizable analog c-ceritinib (21) and ampicillin were tethered to NHS-activated Sepharose for Fast Flow resin (GE Healthcare) and blocked with ethanolamine overnight. SK-MEL-23 cells were lysed and total cell lysate containing 5mg of protein was added to the affinity matrix for 2hr. Competition experiments were conducted by incubating total cell lysates with 20μM ceritinib or 20μM GSK1838705A for 30 min prior to affinity chromatography. Following affinity chromatography, SDS-PAGE and in-gel digestion with trypsin was performed.
Mass Spectrometry and Bioinformatic Analysis
A nanoflow ultra-high performance liquid chromatograph (RSLC, Dionex, Sunnyvale, CA) coupled to an electrospray bench top Orbitrap mass spectrometer (Q-Exactive plus, Thermo, San Jose, CA) was used for tandem mass spectrometry (MS) peptide-sequencing experiments. Sixteen tandem mass spectra were collected in a data-dependent manner following each survey scan. Both MS and MS/MS scans were performed in the Orbitrap to obtain accurate mass measurement using 60sec exclusion for previously sampled peptide peaks. Data was searched by Mascot (v2.4.1) using the Swiss-Prot human database (downloaded 9/2015). Following protein ID, the data was filtered (95% minimum peptide threshold, 95% protein threshold; 0.3% peptide FDR, 0.0% protein FDR) using Scaffold 4.6.1 (Proteome Software). A maximum of two missed cleavages was allowed. A minimum of two unique spectrum counts was required for protein identification.
In vitro kinase assays
In vitro profiling of a panel of kinases selected from the chemical proteomic experiments was performed at Reaction Biology Corporation (Malvern, PA) using the “HotSpot” assay platform (22).
Statistics
For all experiments in which P values are shown, the unpaired student T-test was used. A P value of ≤0.05 was considered statistically significant.
Results
Screening for small molecule inhibitors with activity against BRAF/NRAS wild-type melanoma
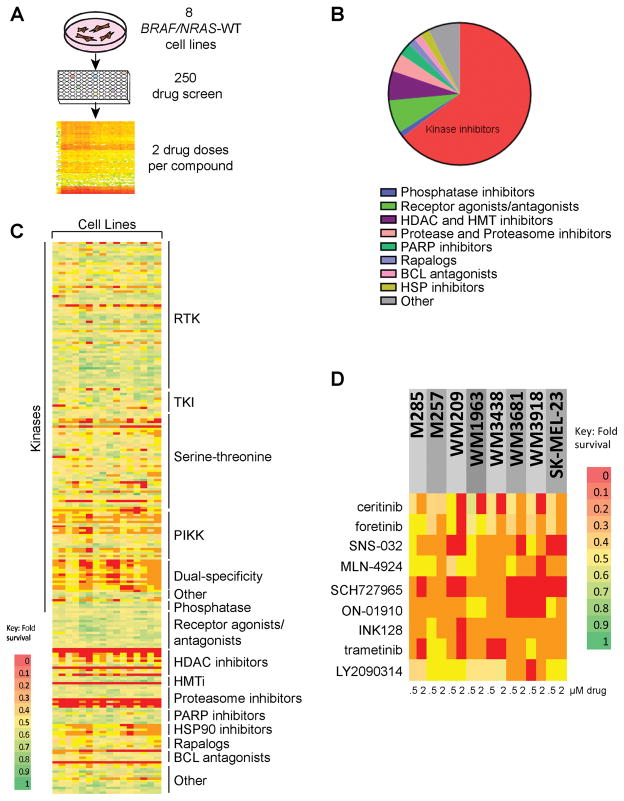
We began by screening a panel of 8 BRAF/NRAS-WT melanoma cell lines against a customized library of 240 drugs. The screen involved treating the cells with two concentrations of each drug, with cell viability measured after 72h (Figure 1A) (raw data and drug list in Supplemental Table 1). The library covered all major target classes, such as kinases, receptor tyrosine kinases, phosphatases, receptor agonists, proteases/proteasome, PARP1, epigenetic enzymes, Hedgehog, HSP90 and Notch, and was chosen to reflect the current landscape of targeted agents approved for use or have been considered for clinical development (Figure 1B). Our analysis identified several classes of compounds with activity across all of the cell lines. These included the proteasome inhibitor bortezomib, pan-histone deacetylase inhibitors, HSP90 inhibitors and histone methyltransferase inhibitors. These compounds were not selected for further analysis based upon prior poor performance in melanoma clinical trials. The selected compounds included the ALK inhibitor ceritinib, the MET/VEGFR2 inhibitor foretinib, the cyclin dependent kinase (CDK) inhibitor SNS-032, the NEDD8 activating enzyme (NAE) inhibitor MLN-4924, the CDK1/2/9 inhibitor SCH727965, the polo-like kinase (PLK1) inhibitor ON-01910, the mTORC1/2 inhibitor INK128, the MEK inhibitor trametinib and the glycogen synthase kinase (GSK3)-β inhibitor LY2090314 that all showed activity against >50% of the cell lines, suggesting some selectivity (Figures 1C–D).
Figure 1. Identification of drugs with single agent activity against BRAF/NRAS wild-type (WT) melanoma.
A: Overview of the workflow of the drug screen. Eight BRAF/NRAS-WT melanoma cell lines were treated with two concentrations of each drug (0.5 and 2.5μM) for 72h before cell viability was assessed using the Cell-Titer-Glo assay. B: Overview of the drugs in the panel by type. C: Response of individual cell lines to each drug in the panel. (RTK: receptor tyrosine kinase. TKI: tyrosine kinase inhibitor. PIKK: Phosphatidylinositol 3-kinase related kinase. Dual-Specificity: dual specificity phosphatases. HDACi: histone deacetylase inhibitors. HMTi: histone methyl transferase inhibitors) Scale indicates the percentage growth inhibition at 0.5 and 2.5μM of drug relative to vehicle. D: Detailed view of the responses of the drugs selected for follow-up in the cell line panel. Data shows the inhibition of growth per cell line at 0.5 and 2.5μM of drug relative to vehicle.
BRAF/NRAS wild-type melanomas show frequent aberrations in the MAPK pathway and show sensitivity to MEK inhibition
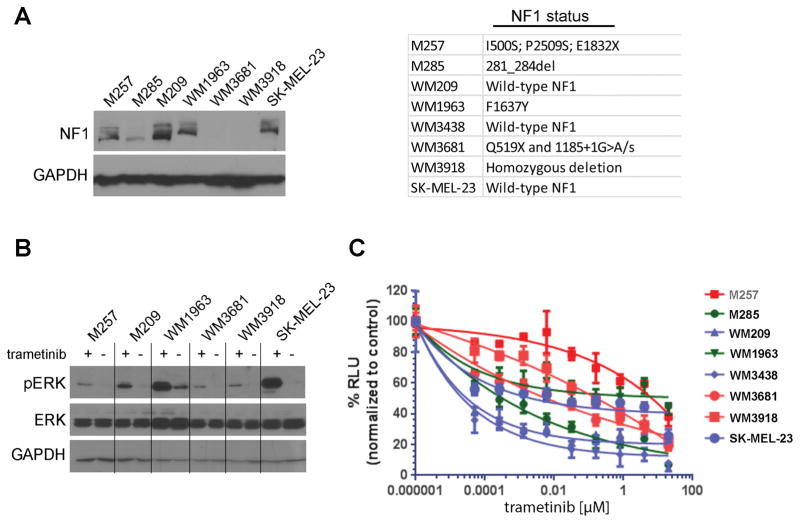
Cutaneous melanoma is uniquely addicted to signals through the mitogen-activated protein kinase (MAPK) pathway. Despite this, and the fact that melanomas frequently harbor mutations in MAPK pathway drivers such as BRAF or RAS, the sensitivity of BRAF/NRAS-wild-type melanoma cell lines to MAPK inhibitors has been little studied. One gene that was frequently mutated in the BRAF/NRAS-WT melanoma cell lines was the tumor suppressor NF1. Western blot analysis revealed NF1 expression to be either reduced or absent in 3/7 cell lines tested including M285, WM3681 and WM3918 (Figure 2A), with other cell lines harboring one or more non-synonymous NF1 mutations. Only the WM209, WM3438, and SK-Mel-23 melanoma cell lines had no identifiable mutations in NF1. All of the BRAF/NRAS-WT cell lines tested showed constitutive phosphorylation of phospho-ERK (Figure 2B) and treatment with the MEK inhibitor trametinib (10nM, 24h) was associated with phospho-ERK inhibition. Treatment of the BRAF/NRAS-WT melanoma cell lines with increasing concentrations of trametinib led to decreased cell growth (Figure 2C). Across the panel, the growth inhibitory effect of trametinib was highly variable and although there was some trend towards increased trametinib sensitivity in NF1 intact cell lines, this was not true in all cases (Figure 2C).
Figure 2. BRAF/NRAS-WT melanoma cell lines have constitutive ERK activity and show a range of MEK inhibitor sensitivities.
A: Western Blot showing the expression of NF1 in BRAF/NRAS WT melanoma cells. Panel: NF1 mutations in the BRAF/NRAS-WT cell lines. B: Trametinib inhibits pERK in the majority of BRAF/NRAS-WT melanoma cells. Cells were treated with trametinib (10nM, 24h) before being subject to Western blot for pERK. C: Trametinib inhibits the growth of BRAF/NRAS-WT cell lines. Cells were treated with increasing concentrations of trametinib for 72h before being analyzed by Cell Titer Glo assay. Data shows the mean of three experiments ± SEM. Blue indicates cell lines with wild-type NF1, Green indicates cell lines with heterozygous NF1 mutations and Red indicates cell lines with no NF1 expression/NF1 mutation.
Ceritinib enhances the activity of the MEK inhibitor trametinib in BRAF/NRAS-WT melanoma
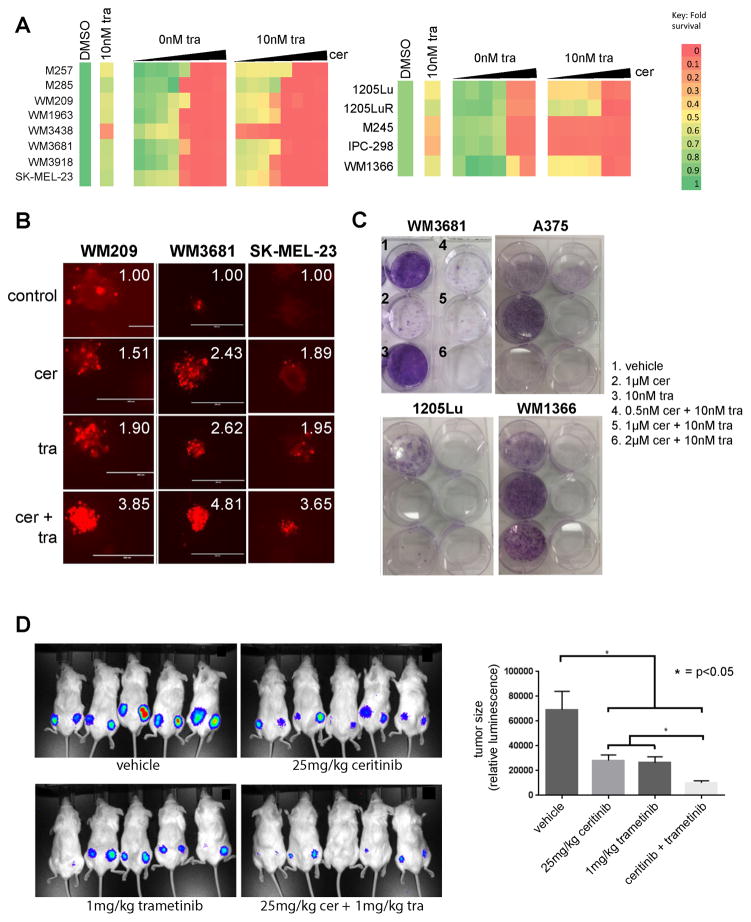
Drug combinations that limit the adaptive signaling are typically associated with more durable responses than the single agents. In the majority of the cell lines tested, concentrations of ceritinib that are clinically achievable (2.5μM) reduced cell growth to <50% when combined with trametinib (Figure 3A). The combination also had activity in melanoma cell lines with BRAF-V600E mutations (1205Lu and 1205LuR), and 3 NRAS-mutant (M245, IPC-298, WM1366) melanoma cell lines (Figure 3A). Under more physiologically relevant collagen-implanted spheroid culture conditions, the combination of ceritinib and trametinib enhanced cell death in a representative panel of 3 BRAF/NRAS-WT melanoma cell lines (WM209, WM3681, SK-MEL-23) compared to either agent alone (Figure 3B). The effects of the trametinib-ceritinib combination were also durable, suppressing the outgrowth of resistant clones in long-term colony formation assays in BRAF/NRAS-WT (WM3681), BRAF-mutant (1205Lu, A375) and NRAS-mutant (WM1366) melanoma cell lines (Figure 3C).
Figure 3. Ceritinib enhances the efficacy of trametinib.
A: Heatmap showing the inhibition of growth to a selection of drugs identified from the screen in Figure 1 ± 10nM trametinib. Cells were treated with each drug alone and in the presence of trametinib for 72h before being analyzed by MTT assay. B: WM209, SK-MEL-23 and WM3681 spheroids were implanted into a gel of collagen before being treated with vehicle, ceritinib (2 μM), trametinib (10 nM) or ceritinib + trametinib (2 μM/10nM). Spheroids were stained with propidium iodide to indicate fold-increase in dead cells. Panels show fold-increase in dead cells. C: The combination of trametinib and ceritinib prevents outgrowth of WM3681, A375, 1205Lu and WM1366 cells in long-term colony formation assays. Cells were seeded out and treated with vehicle, ceritinib, trametinib or ceritinib plus trametinib for 2 weeks before being stained with crystal violet. D: Luciferase-expressing WM209 cells were grown as xenografts in NSG mice before being dosed daily with vehicle, ceritinib (25mg/kg), trametinib (1mg/kg) or the ceritinib-trametinib combination (25mg/kg and 1mg/kg) daily by oral gavage. Left panel shows luminescence following 24 days of treatment. Right panel shows quantification of mean fluorescence following treatment with the drugs (day 24).
We next determined the efficacy of the trametinib-ceritinib combination in a BRAF/NRAS-WT melanoma cell mouse xenograft model. Here, luciferase-tagged WM209 cells were injected subcutaneously into the flanks of NSG mice, and allowed to grow to 1mm3 in size before being treated with vehicle, ceritinib (25mg/kg), trametinib (1mg/kg) or the combination of ceritinib (25mg/kg) and trametinib (1mg/kg) for 24 days. At experiment termination, it was noted that the growth of the tumors treated with the combination was significantly reduced compared to those receiving trametinib or ceritinib monotherapy (Figure 3D).
Ceritinib-trametinib inhibits MAPK and TORC1 signaling
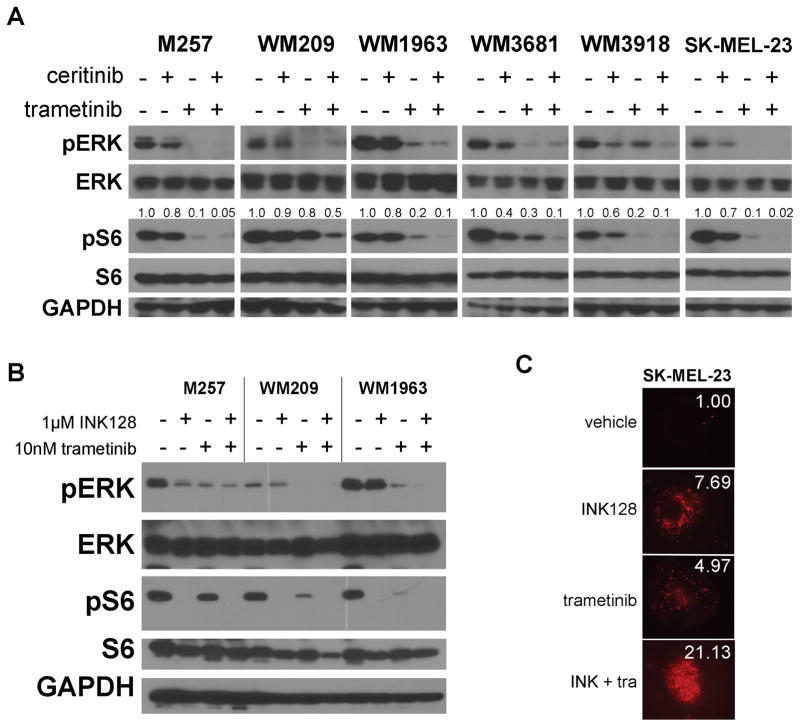
To determine the mechanism of action of trametinib-ceritinib we began by performing kinome arrays on BRAF/NRAS-WT melanoma cells (Supplemental Figure 1). The combination had marked effects upon signaling through the MAPK, AKT/mTOR, RSK1/2 and JNK/c-JUN pathways. As RSK1/2 are downstream targets of both PI3K/AKT and MEK/ERK we decided to focus on the MAPK, AKT/mTOR and JNK/c-JUN pathways (Supplemental Figure 2). Analysis of c-JUN phosphorylation under drug treatment showed variable results across the cell line panel, with some cell lines showing the expected inhibition of c-JUN phosphorylation and loss of c-JUN expression (WM209, SK-MEL-23, WM1936) while some (WM3681, WM3918) showed the opposite effect (Supplemental Figure 3). We next focused upon MAPK and TORC1, as dual inhibition of these pathways are well correlated with BRAF inhibitor responses in BRAF-mutant melanoma (23). It was observed in the majority of the cell lines that maximal inhibition of pS6 occurred following treatment with both trametinib and ceritinib (Figure 4A). Ceritinib had little effect upon pS6 levels as a single agent, indicating the importance of ceritinib target kinases in the escape from trametinib. Ceritinib had some minor inhibitory activity against pERK in some cell lines, including WM3918 and SK-MEL-23 (Figure 4A). The effects of ceritinib-trametinib on signaling were highly durable, with no recovery of pERK or pS6 signaling seen up to 72h of treatment (Supplemental Figure 4). No alterations in the MAPK signaling regulator Sprouty-2 were noted (Supplemental Figure 5). Further studies showed that the combination of trametinib and the mTOR inhibitor INK128 inhibited both pS6 and MEK signaling (Figure 4B) and that co-treatment of SK-MEL-23 cells with trametinib and INK128 led to enhanced cytotoxic effects in 3D collagen-implanted spheroid assays, in a manner that was equivalent to the ceritinib-trametinib combination (Figure 4C and Figure 3B).
Figure 4. The combination of trametinib and ceritinib leads to enhanced suppression of TORC1 signaling.
A: BRAF/NRAS-WT cell lines were treated with vehicle, ceritinib, trametinib or ceritinib-trametinib for 24h before extraction of protein and Western blot for pERK or pS6. Numbers above pS6 indicate the densitometry measurements expressed as a proportion of total S6. B: The combination of trametinib and the TORC inhibitor INK128 causes the dual suppression of MAPK and S6. BRAF/NRAS WT cell lines were treated with vehicle, INK128 (1μM), trametinib (10nM) or INK128-trametinib (1μM/10nM) for 24h before the extraction of protein and Western blot for pERK and pS6. C: The ceritinib-trametinib combination shows equivalent effects to trametinib-INK128 in a 3D collagen-implanted spheroid assay. SK-MEL-23 spheroids were implanted into a collagen gel before being treated with vehicle, INK128 (1μM), trametinib (10nM) or INK128-trametinib (1μM/10nM). Spheroids were stained with propidium iodide to indicate dead cells. Panels show fold-increase in dead cells relative to controls.
Ceritinib inhibits multiple targets in BRAF/NRAS –WT melanoma
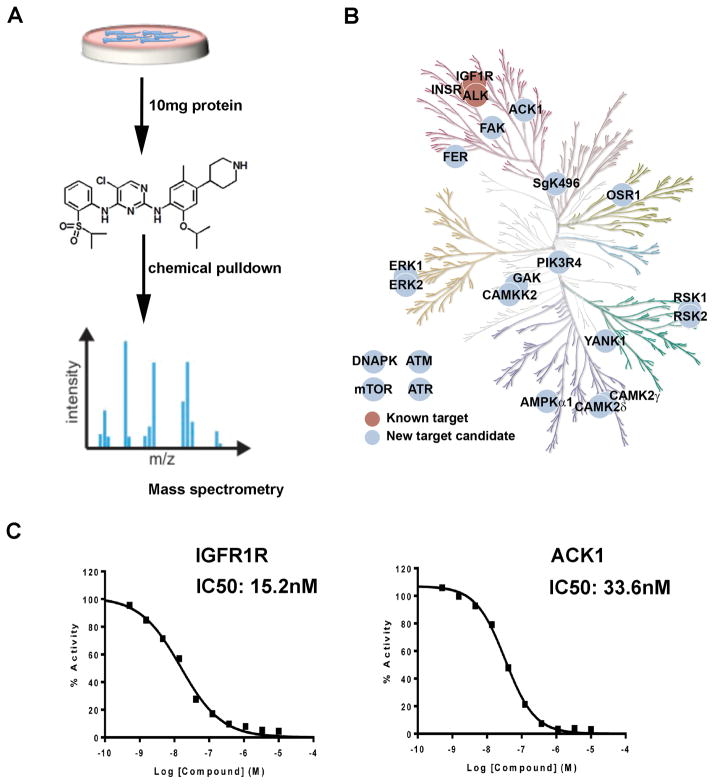
Ceritinib was developed as an inhibitor of the ALK fusion protein. As ALK fusions are rare in melanoma we performed a chemical proteomic screen to identify potential interactors/binding partners of ceritinib. In these studies an immobilized ceritinib analogue was used for drug affinity chromatography with total cell lysates from SK-MEL-23 melanoma cells and the resulting drug pull downs were analyzed by LC-MS/MS (Figure 5A: Structure shown in Supplemental Figure 6 (21)). Using ampicillin beads and ceritinib competition as independent controls, these studies identified the known ceritinib targets ALK, IGF1R and InsR, as well as several new ceritinib target candidates, such as ACK1, FER, FAK, and CAMKK2 (Figure 5B). In vitro kinase assays were then performed to validate the chemical proteomics studies (Table 1). Dose response analysis showed ceritinib to potently inhibit IGF1R and ACK1 (IC50s 15.2nM and 33.6nM, respectively) (Figure 5C).
Figure 5. Chemical proteomics identifies IGF1R and ACK1 as potential targets of ceritinib in BRAF/NRAS-WT melanoma.
A: Workflow of the chemical proteomic experiment. B: Kinome tree of potential kinase targets of ceritinib in SK-MEL-23 melanoma cells. C: Certinib inhibits IGF1R and ACK1 in in vitro kinase assays.
Table 1.
Inhibitory potency of ceritinib and staurosporine against the kinases identified in the chemical proteomic screen.
| Kinase | Ceritinib | Staurosporine |
|---|---|---|
| ACK1 | 33.7nM | 255nM |
| AMPK | 2360nM | 0.00813nM |
| CAMKK2 | 75.3nM | 455nM |
| FAK | 16.3nM | 173nM |
| FER | 11.7nM | 0.303nM |
| IGF1R | 15.2nM | 52.7nM |
| IR | 72.6nM | 32.1nM |
| OSR1 | 881nM | 58.4nM |
| RSK1 | 966nM | 0.09nM |
| RSK2 | 418nM | 0.11nM |
Ceritinib limits TORC1 signaling via inhibition of ACK1 and IGF1R
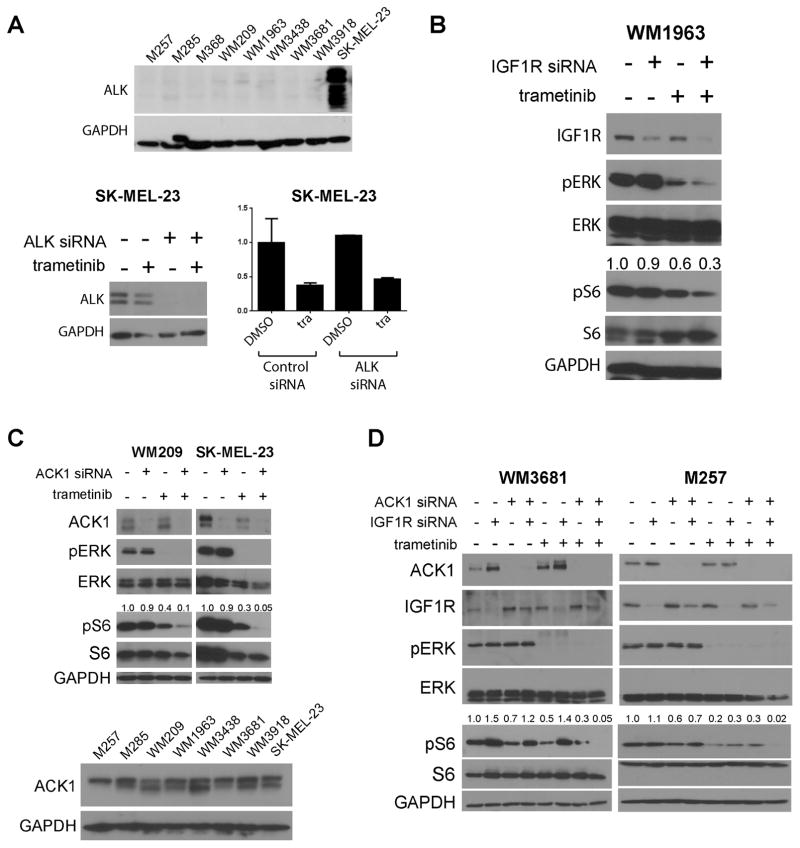
Among all of the cell lines, only SK-MEL-23 expressed any ALK, with siRNA studies showing ALK knockdown to have little impact upon cell survival (Figure 6A). We next turned our attention to other potential targets of ceritinib. Seven of the cell lines (aside from M285) expressed IGF1R (Supplemental Figure 7), with IGF1R being the only receptor tyrosine kinase to be inhibited following the treatment of WM3681 and SK-MEL-23 BRAF/NRAS-WT melanoma cells with ceritinib (Supplemental Figure 8). The WM1963 and WM209 cells showed a requirement for IGF1R signaling with its siRNA knockdown being associated with reduced viability (Supplemental Figure 9). A role for IGF1R in the maintenance of pS6 signaling in WM1963 cells was suggested by the observation that knockdown of IGF1R in combination with trametinib led to a suppression of both pS6 and pERK signaling (Figure 6B). None of the other cell lines showed any decrease in pS6 following knockdown of IGF1R (alone and in combination with trametinib). Systematic siRNA knockdown of ceritinib targets showed that only ACK1 appeared to be a regulator of TORC1 signaling following MEK inhibition. In particular, no effects of ceritinib upon pFAK were noted, nor were dramatic effects seen upon siRNA knockdown of FER (Supplemental Figures 10–11). All of the BRAF/NRAS-WT melanoma cell lines expressed similar levels of ACK1 expression (Figure 6C). siRNA knockdown of ACK1 in combination with trametinib led to the near-complete suppression of pS6 in both the WM209 and SK-MEL-23 cell lines (Figure 6C). The ACK1 inhibitor AIM-100 was noted to suppress long-term colony formation growth in WM209 and SK-MEL-23 cells when combined with trametinib (Supplemental Figure 12). Combined knockdown of ACK1 and IGF1R in combination with trametinib blocked pS6 signaling in WM3681 and M257 cells (Figure 6D). The observation that ACK1 and IGF1R inhibition had distinct, cell line specific effects upon pS6, indicated the value of combining a broad specificity drug (ceritinib) with a highly specific drug (trametinib).
Figure 6. Silencing of IGF1R or ACK1 enhances suppression of pS6 when combined with trametinib.
A: (Upper panel) Expression of ALK in the eight BRAF/NRAS-WT melanoma cell lines. Western Blot shows expression of ALK and GAPDH. (Lower panel) Silencing of ALK in SK-MEL-23 cells does not affect viability +/− trametinib in cell survival assays. Cells were treated with siRNA for 24h and then with ALK siRNA or non-silencing control alone and in the presence of trametinib (10nM) for 72h. Cell numbers were measured by MTT assay. B: Silencing of IGF1R in WM1963 leads to enhanced pS6 suppression when combined with trametinib. Cells were treated with siRNA for 24h and then with vehicle or drug for 24h. Numbers are the densitometry values corresponding to pS6/tS6. C: (Upper) Silencing of ACK1 enhances the suppression of pS6 in WM209 and SK-MEL-23 cells when combined with trametinib. Cells were treated with siRNA for 24h and then with vehicle or drug for 24h before Western blot analysis for pERK and pS6. (Lower) Western Blot shows the expression of ACK1 across the cell line panel. D: Combined silencing of ACK1 and IGF1R suppresses pS6 signaling in combination with trametinib (10nM). WM3681 and M257 Cells were treated with siRNA as above and then with trametinib for 24h before Western blot analysis for pERK and pS6.
Discussion
Although targeted therapies have proven effective for BRAF-mutant melanoma and immune therapies are showing great promise across all genotypes (both BRAF-mutant and WT), new treatment strategies are still urgently needed. As a subgroup, BRAF/NRAS-WT melanomas have been little studied and attempts to date have not uncovered consistent, therapeutically tractable, oncogenic drivers (8, 24). This analysis has been complicated by the extremely high mutational load of melanomas; a likely result of the role of UV-radiation exposure in the etiology of the disease (25). It therefore seems likely that the transformation of BRAF/NRAS-WT melanomas may depend upon the complex interplay of multiple genetic hits that work together to drive the pathways required for maintenance of the oncogenic state. At this time, immunotherapy approaches such as nivolumab and the ipilimumab-nivolumab combination are routinely used to treat BRAF-WT melanoma (6, 7). For those who fail checkpoint inhibitor therapy, the only second-line therapy options currently available are chemotherapy or a clinical trial. Against this backdrop we sought to define novel targeted therapy strategies for BRAF/NRAS-WT melanoma. As our goal was to define drug combinations with potential for rapid clinical translation, we focused upon drugs and targeted inhibitors that were either 1) FDA-approved or 2) currently in clinical development.
Our drug screen identified multiple hits. The compounds identified were diverse and included pan-RTK inhibitors (ceritinib, foretinib), inhibitors of CDK2 (SNS-032), CDK1/2/9 (SCH727965), GSK3-β (LY2090314), PLK1 (ON-01910), NEDD8 activation enzyme (NAE) (MLN-4924), mTOR (INK128) and MEK (trametinib). Many of these classes of drugs, including CDK2, CDK1/2/9, GSK3β, NAE, mTOR and MEK inhibitors, have been previously shown to have efficacy against melanoma cell lines in in vitro and in vivo studies (26–31). We next determined the sensitivity of our BRAF/NRAS-WT melanoma cell line panel to the MEK inhibitor trametinib. Our focus upon MEK inhibition was based upon previous work demonstrating the reliance of nearly all melanomas upon the MAPK pathway (32). Although BRAF/NRAS-WT melanomas lack more commonly known MAPK drivers such as mutant BRAF and NRAS, they do harbor other mutations that lead to MAPK pathway activation. In particular, ~13% of all BRAF/NRAS-WT melanomas harbor inactivating mutations in the RAS-GAP NF1, with a further 15–36% of melanoma specimens showing loss of or reduced NF1 expression (10, 11). Loss of NF1 function typically leads to increased MAPK signaling that is secondary to loss of negative regulation of Ras signaling (11). In BRAF/NRAS-WT melanomas, NF1 mutations frequently co-occur with lesions in other “Ras-opathy” genes including SOS1, RASA2 and PTPN1 (11) - which often lead to increased MAPK signaling. In line with published work, our BRAF/NRAS-WT cell line panel showed recurrent mutations in and loss of expression of the tumor suppressor NF1. All of the cell lines in the BRAF/NRAS-WT panel exhibited high levels of pERK activation, with some cell lines showing good sensitivity to trametinib monotherapy (30). In agreement with previously published work, NF1 status was not highly predictive of MEK inhibitor sensitivity (11, 30).
Single agent trametinib has been evaluated in BRAF-WT melanoma patients with some partial responses noted (33, 34). In light of this, and the general observation that kinase inhibitor monotherapy typically leads to signaling adaptation and resistance (5, 35), we reasoned that trametinib would make a good backbone therapy for BRAF/NRAS-WT melanoma. We then asked which of the candidates from our screen would constitute a suitable combination partner and identified ceritinib on the basis of its positive interactions with trametinib and its clinical availability (36).
Ceritinib is a multi-RTK inhibitor that is FDA-approved for the treatment of EML4-ALK fusion-positive non-small cell lung cancer (NSCLC). To our knowledge, ceritinib has never been evaluated in melanoma, either preclinically or clinically. The combination of ceritinib and trametinib was associated with increased cytotoxicity in long term colony formation experiments and in 3D collagen-implanted spheroid assays that more closely mimic the in vivo tumor microenvironment (15). These responses were not limited to BRAF/NRAS-WT melanoma and were also observed in the BRAF and NRAS-mutant melanoma cell lines we tested. Previous work has shown the progression of BRAF-mutant melanoma to be dependent upon signals through both the MAPK and PI3K/AKT/mTOR/S6 signaling pathways. A role for both of these pathways in melanoma initiation has been demonstrated by the observation that loss of PTEN is critical for both the escape of BRAF mutant melanocytes from oncogene-induced senescence as well the initiation of BRAF mutant melanoma in GEM models (37, 38). Studies from our own group and others have shown adaptive AKT/mTOR signaling is a frequent event in the escape of BRAF mutant melanoma cells from BRAF and MEK inhibitor therapy (39–41). These findings are not restricted to BRAF-mutant melanoma, with synergy between inhibitors of MEK and TORC1/2 inhibitors also reported for NRAS-mutant melanoma (42). Mechanistically it appears that MAPK and PI3K/AKT/mTOR converge on key cell survival hubs, with their combined inhibition leading to increased apoptosis (43).
We observed that the combination of trametinib and ceritinib increased the suppression of pS6. Ceritinib had little impact upon pS6 levels as a single agent, indicating its role in inhibiting the signaling adaptations that occurred secondary to MEK inhibition. The critical nature of dual MAPK and TORC1 signaling inhibition was demonstrated by the ability of the trametinib and the mTOR inhibitor INK128 combination to phenocopy the trametinib-ceritinib combination in 3D spheroid cytotoxicity assays.
The results that we describe here have parallels with studies in both BRAF mutant melanoma and PIK3CA-mutant breast cancer, in which complete inhibition of TORC1 (indicated by suppression of pS6) is a biomarker of sensitivity to inhibitors of BRAF and PI3K, respectively (23, 44). In BRAF-mutant melanoma, complete suppression of TORC1 was required for apoptosis induction, with PUMA being identified as the key mediator of cell death (23). The importance of TORC1 inhibition as a biomarker of response to many different targeted therapies is reflective of the central role of pS6 as a signal activity readout from multiple upstream inputs. It is therefore likely that maximal pS6 inhibition reflects the near total shut down of adaptive signaling. Although the level of suppression effected in some cell lines by combined ceritinib-trametinib was sometimes only slightly more than single agent trametinib, this is not say it is not significant. Even low levels of signaling are sufficient to allow survival following drug treatment. This is most clearly demonstrated in melanoma patients treated with BRAF inhibitors, in which >90% pERK inhibition is required for efficacy to be observed (45).
It is worth noting that the ceritinib-trametinib combination affected multiple signaling pathways in addition to MAPK and TORC1, including JNK and c-JUN. The JNK signaling pathway is known to be a critical mechanism of adaptation of BRAF-mutant melanoma cells following BRAF inhibitor treatment, where it drives RTK signaling that limits the effects of MEK/ERK inhibition (46). Although likely to be important in some of the BRAF/NRAS-WT cell lines evaluated here, the responses were variable across the cell line panel, indicating the complexity and cell line specificity of the drug combination.
Ceritinib is known primarily as an inhibitor of ALK. An examination of the BRAF/NRAS-WT melanoma cell lines demonstrated only one of the 8 to express any ALK protein by Western Blot. In the one cell line that did express ALK (SK-MEL-23), its knockdown did not affect cell viability. It therefore seems unlikely that the effects of ceritinib are mediated through ALK inhibition. We next used chemical proteomics to define other potential targets of ceritinib. One of the top hits for ceritinib was IGF1R, a previously validated target of the drug in NSCLC (47). The importance of IGF1R as a ceritinib target in some melanoma cell lines was confirmed through RTK arrays, with no other RTK found to be significantly inhibited. The majority of melanomas express IGF1R, with its signaling being implicated in proliferation, cell invasion and apoptosis resistance. In the monotherapy setting, IGF1R inhibition has proven relatively weak; a likely consequence of the high signaling redundancy in melanoma cells (48). In other contexts, IGF1R signaling may be important, with some studies implicating this RTK in BRAF inhibitor resistance (49). IGF1R signaling has also shown to be an important adaptive signaling mechanism in other tumor systems, such as following the knockdown of KRAS in colon cancer (50).
Although the majority of the BRAF/NRAS WT melanoma cell lines express IGF1R, its siRNA knockdown had minimal effects upon cell growth, both alone and in combination with trametinib. Despite this, one of the cell lines - WM1963 - did show sensitivity to IGF1R knockdown, and more importantly, showed a decrease in pS6 when IGF1R knockdown was combined with trametinib. As melanoma cells are known to be genetically complex, we reasoned that multiple ceritinib targets were involved in the regulation of adaptive TORC1 signaling. One candidate was the non-receptor tyrosine kinase ACK1/TNK2 (activated CDC42 associated kinase), whose knockdown suppressed pS6 signaling when combined with MEK inhibition in a further two BRAF/NRAS-WT cell lines. ACK1 is best characterized as an intermediary non-receptor tyrosine kinase that links upstream receptor activation to downstream signaling pathways. Extensive work has already shown ACK1 to be an EGFR interactor, where EGF stimulation leads to the association of ACK1 and EGFR, resulting in EGFR degradation (51). ACK1 also interacts with many other RTKs, with its kinase activity being increased following treatment with GAS6, IGF-I and heregulin (52).
Together our data illustrate the utility of combining drugs that are highly specific (such as MEK inhibitors) with agents that are broadly targeted (such as ceritinib). The utility of this approach is demonstrated in genetically complex cancers where the effects of potent inhibition of a critical growth pathway in combination with the simultaneous inhibition of multiple signaling adaptations can prove highly effective. This strategy can be particularly advantageous in instances where melanoma cell lines have multiple, simultaneous mechanisms of escape (e.g. ACK1, IGF1R) that converge upon single signaling nodes, such as S6. Our study also illustrates the strength of using unbiased drug screens combined with chemical proteomics to identify novel off-target effects of FDA-approved drugs that can be efficacious in cancers that lack effective targeted therapy options.
Supplementary Material
Acknowledgments
Funding: This work was supported by R01 CA161107-01 and P50 CA168536 (to K.S.M. Smalley), R01 CA181746 (to U. Rix), F99 CA212456 (to B.M. Kuenzi), and the Cancer Center Support Grant P30-CA076292.
The authors would like to thank Dr. Franz Schaub for technical assistance.
References
- 1.Smalley KS, Eroglu Z, Sondak VK. Combination Therapies for Melanoma: A New Standard of Care? Am J Clin Dermatol. 2016;17:99–105. doi: 10.1007/s40257-016-0174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grob JJ, Long GV, Schadendorf D, Flaherty K. Disease kinetics for decision-making in advanced melanoma: a call for scenario-driven strategy trials. Lancet Oncol. 2015;16:e522–6. doi: 10.1016/S1470-2045(15)00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Overall survival for dabrafenib and trametinib versus dabrafenib and placebo in V600 BRAF-mutant melanoma: a multi-center, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–51. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 4.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–88. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 8.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A Landscape of Driver Mutations in Melanoma. Cell. 2012;150:251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nissan MH, Pratilas CA, Jones AM, Ramirez R, Won H, Liu C, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Research. 2014;74:2340–50. doi: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maertens O, Johnson B, Hollstein P, Frederick DT, Cooper ZA, Messaien L, et al. Elucidating distinct roles for NF1 in melanomagenesis. Cancer Discov. 2012;3:338–49. doi: 10.1158/2159-8290.CD-12-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, Wu C, et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet. 2015;47:996–1002. doi: 10.1038/ng.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–35. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahajan K, Challa S, Coppola D, Lawrence H, Luo Y, Gevariya H, et al. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. Prostate. 2010;70:1274–85. doi: 10.1002/pros.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haarberg HE, Paraiso KH, Wood E, Rebecca VW, Sondak VK, Koomen JM, et al. Inhibition of Wee1, AKT, and CDK4 underlies the efficacy of the HSP90 inhibitor XL888 in an in vivo model of NRAS-mutant melanoma. Molecular Cancer Therapeutics. 2013;12:901–12. doi: 10.1158/1535-7163.MCT-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaub FX, Reza MS, Flaveny CA, Li W, Musicant AM, Hoxha S, et al. Fluorophore-NanoLuc BRET Reporters Enable Sensitive In Vivo Optical Imaging and Flow Cytometry for Monitoring Tumorigenesis. Cancer Res. 2015;75:5023–33. doi: 10.1158/0008-5472.CAN-14-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumi NJ, Kuenzi BM, Knezevic CE, Remsing Rix LL, Rix U. Chemoproteomics Reveals Novel Protein and Lipid Kinase Targets of Clinical CDK4/6 Inhibitors in Lung Cancer. ACS Chem Biol. 2015;10:2680–6. doi: 10.1021/acschembio.5b00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuenzi BM, Remsing Rix LL, Stewart PA, Fang B, Kinose F, Bryant AT, et al. Polypharmacology-based ceritinib repurposing using integrated functional proteomics. Nature Chemical Biology. 2017 doi: 10.1038/nchembio.2489. Epub 9th October 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1039–45. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corcoran RB, Rothenberg SM, Hata AN, Faber AC, Piris A, Nazarian RM, et al. TORC1 suppression predicts responsiveness to RAF and MEK inhibition in BRAF-mutant melanoma. Science translational medicine. 2013;5:196ra98. doi: 10.1126/scitranslmed.3005753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research N. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015;372:2481–98. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–6. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai BM, Villanueva J, Nguyen TT, Lioni M, Xiao M, Kong J, et al. The anti-melanoma activity of dinaciclib, a cyclin-dependent kinase inhibitor, is dependent on p53 signaling. PLoS ONE. 2013;8:e59588. doi: 10.1371/journal.pone.0059588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks K, Oakes V, Edwards B, Ranall M, Leo P, Pavey S, et al. A potent Chk1 inhibitor is selectively cytotoxic in melanomas with high levels of replicative stress. Oncogene. 2013;32:788–96. doi: 10.1038/onc.2012.72. [DOI] [PubMed] [Google Scholar]
- 28.Smalley KS, Contractor R, Haass NK, Kulp AN, Atilla-Gokcumen GE, Williams DS, et al. An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 2007;67:209–17. doi: 10.1158/0008-5472.CAN-06-1538. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Hawkins OE, Vilgelm AE, Pawlikowski JS, Ecsedy JA, Sosman JA, et al. Combining an Aurora Kinase Inhibitor and a Death Receptor Ligand/Agonist Antibody Triggers Apoptosis in Melanoma Cells and Prevents Tumor Growth in Preclinical Mouse Models. Clin Cancer Res. 2015;21:5338–48. doi: 10.1158/1078-0432.CCR-15-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranzani M, Alifrangis C, Perna D, Dutton-Regester K, Pritchard A, Wong K, et al. BRAF/NRAS wild-type melanoma, NF1 status and sensitivity to trametinib. Pigment Cell Melanoma Res. 2015;28:117–9. doi: 10.1111/pcmr.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong KM, Micel LN, Selby HM, Tan AC, Pitts TM, Bagby SM, et al. Targeting the protein ubiquitination machinery in melanoma by the NEDD8-activating enzyme inhibitor pevonedistat (MLN4924) Invest New Drugs. 2016 doi: 10.1007/s10637-016-0398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedorenko IV, Gibney GT, Sondak VK, Smalley KSM. Beyond BRAF: where next for melanoma therapy? British Journal of Cancer. 2015;112:217–26. doi: 10.1038/bjc.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, DeMarini DJ, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncology. 2012;13:782–9. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. The lancet oncology. 2013;14:249–56. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 35.Emmons MF, Faiao-Flores F, Smalley KS. The role of phenotypic plasticity in the escape of cancer cells from targeted therapy. Biochem Pharmacol. 2016 doi: 10.1016/j.bcp.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vredeveld LC, Possik PA, Smit MA, Meissl K, Michaloglou C, Horlings HM, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Gene Dev. 2012;26:1055–69. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopal YNV, Rizos H, Chen G, Deng WL, Frederick DT, Cooper ZA, et al. Inhibition of mTORC1/2 Overcomes Resistance to MAPK Pathway Inhibitors Mediated by PGC1 alpha and Oxidative Phosphorylation in Melanoma. Cancer Research. 2014;74:7037–47. doi: 10.1158/0008-5472.CAN-14-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Research. 2011;71:2750–60. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xing F, Persaud Y, Pratilas CA, Taylor BS, Janakiraman M, She QB, et al. Concurrent loss of the PTEN and RB1 tumor suppressors attenuates RAF dependence in melanomas harboring (V600E)BRAF. Oncogene. 2011 doi: 10.1038/onc.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Posch C, Moslehi H, Feeney L, Green GA, Ebaee A, Feichtenschlager V, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4015–20. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 Is a Key Effector of the Oncogenic Activation of the AKT and ERK Signaling Pathways that Integrates Their Function in Tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, et al. mTORC1 inhibition is required for sensitivity to PI3K p110alpha inhibitors in PIK3CA-mutant breast cancer. Science translational medicine. 2013;5:196ra99. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsdale R, Jorissen RN, Li FZ, Al-Obaidi S, Ward T, Sheppard KE, et al. The transcription cofactor c-JUN mediates phenotype switching and BRAF inhibitor resistance in melanoma. Sci Signal. 2015;8:ra82. doi: 10.1126/scisignal.aab1111. [DOI] [PubMed] [Google Scholar]
- 47.Lovly CM, McDonald NT, Chen H, Ortiz-Cuaran S, Heukamp LC, Yan Y, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med. 2014;20:1027–34. doi: 10.1038/nm.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeh AH, Bohula EA, Macaulay VM. Human melanoma cells expressing V600E B-RAF are susceptible to IGF1R targeting by small interfering RNAs. Oncogene. 2006;25:6574–81. doi: 10.1038/sj.onc.1209674. [DOI] [PubMed] [Google Scholar]
- 49.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebi H, Corcoran RB, Singh A, Chen Z, Song Y, Lifshits E, et al. Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. Journal of Clinical Investigation. 2011;121:4311–21. doi: 10.1172/JCI57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen F, Lin Q, Gu Y, Childress C, Yang W. Activated Cdc42-associated kinase 1 is a component of EGF receptor signaling complex and regulates EGF receptor degradation. Mol Biol Cell. 2007;18:732–42. doi: 10.1091/mbc.E06-02-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahajan K, Mahajan NP. ACK1/TNK2 tyrosine kinase: molecular signaling and evolving role in cancers. Oncogene. 2015;34:4162–7. doi: 10.1038/onc.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.