Abstract
BMI-1, also known as a stem cell factor, is frequently upregulated in several malignancies. Elevated expression of BMI-1 correlates with poor prognosis and is therefore considered a viable therapeutic target in a number of malignancies including ovarian cancer. Realizing the immense pathological significance of BMI-1, small molecule inhibitors against BMI1 are recently being developed. In the current study, we functionally characterize PTC-028, an orally bioavailable compound that decreases BMI-1 levels by post-translational modification We report that PTC-028 treatment selectively inhibits cancer cells in clonal growth and viability assays whereas normal cells remain unaffected. Mechanistically, hyper-phosphorylation mediated depletion of cellular BMI-1 by PTC-028 coupled with a concurrent temporal decrease in ATP and a compromised mitochondrial redox balance potentiates caspase-dependent apoptosis. In vivo, orally administered PTC-028, as a single agent exhibits significant antitumor activity comparable to the standard cisplatin/paclitaxel therapy in an orthotopic mouse model of ovarian cancer. Thus, PTC-028 has the potential to be used as an effective therapeutic agent in patients with epithelial ovarian cancer, where treatment options are limited.
Keywords: BMI-1, PTC-028, apoptosis
Introduction
BMI1/BMI-1 proto-oncogene, polycomb ring finger, a member of the Polycomb Repressor Complex 1 (PRC1) that mediates gene silencing by regulating chromatin structure is frequently upregulated in several types of cancer where its expression correlates with poor prognosis [1-5]. We previously demonstrated that BMI-1 is overexpressed in epithelial ovarian cancer patient samples [3] and targeting BMI-1 sensitizes a variety of cancer cells to chemotherapeutics [3, 5-7]. BMI-1 thus has been implicated in the propagation of several cancers with a role in self-renewal of cancer-initiating cells of glioblastoma and colorectal cancer [1, 2]. Realizing this potential, Kreso et al. first described PTC-209, a small molecule that decreased translation of BMI-1 mRNA [1], and inhibited self-renewal of cancer-initiating cells causing irreversible impairment in primary colorectal tumor growth when administered intra-tumoraly. We subsequently reported that in ovarian cancer cells, PTC-209 potentiated autophagy mediated necroptosis [8] and others reported Cyclin G2 mediated induction of autophagy in leukemia cells [9]. Here, we investigated the biological and therapeutic activity of PTC-028, a novel compound with superior pharmaceutical properties that depletes BMI-1 at the protein level.
We report that PTC-028 significantly impacts clonal growth and viability of ovarian cancer cells by specifically decreasing BMI-1 through hyper-phosphorylation mediated degradation while normal cells, with minimal expression of BMI-1 are unaffected. Compared to PTC-209 (200 nM), PTC-028 (100 nM) depletes steady-state BMI1 protein levels faster and induces depletion of ATP to potentiate caspase-dependent apoptosis through generation of mitochondrial reactive oxygen species (ROS). Importantly, orally administered PTC-028 exhibits significant, single agent antitumor activity in the orthotopic mouse model of ovarian cancer similar to that of the standard-of-care cisplatin/paclitaxel administered intra-peritonealy. Therefore, PTC-028 could potentially be used as an effective therapeutic tool in several malignancies that are characterized by overexpression of BMI-1 including ovarian cancer.
Materials and Methods
Cell culture and chemicals
SV40 transformed primary normal ovarian epithelial cell line (OSE tsT/hTERT, henceforth OSE) [10] was a kind gift from Dr. V. Shridhar, Mayo Clinic, Rochester, MN, USA. SV40 transformed primary normal fallopian tube epithelial cells (henceforth FTE187 and FTE188) [11] were kindly provided by Dr. Jinsong Liu, MD Anderson Cancer Center, Houston, TX, USA. CP20 cell line was a kind gift from Dr. Anil K. Sood, MD Anderson Cancer Center, Houston, TX, and was authenticated through STR profiling facility at MD Anderson Cancer Center. OV90 and OVCAR4 cell lines were purchased from ATCC and NCI respectively. OSE cells were routinely cultured in 1:1 MCDB 105 and Medium 199 (Corning, Corning, NY, USA) + 15% FBS (Gibco, Grand Island, NY); FTE187 and FTE188 were cultured in 1:1 MCDB 105 and Medium 199 + 15% FBS + 0.01ug/ml EGF; CP20, OV90 and OVCAR4 were routinely cultured in RPMI + 10% FBS. All the cells were cultured with 1× penicillin-streptomycin (Gibco, Grand Island, NY) in a 5% CO2 humidified atmosphere and tested for mycoplasma contamination prior to any experiment. PTC-028 was obtained from PTC Therapeutics (South Plainfield, NJ, USA). PTC-209 (SML1143) was obtained from Sigma–Aldrich (St Louis, MO, USA). FLAG-empty vector (FLAG-EV) or FLAG-BMI-1 was kind gift from Dr. Damu Tang, McMaster University, Hamilton, ON, Canada. Gene silencing was performed using Hiperfect (Qiagen, Valencia, CA, USA) and 10 picomoles siRNA (scrambled control D-001206-13-20, Dharmacon; BMI1 siRNA SASI-HS01-00175765 from Sigma (St. Louis, MO, USA) in OPTIMEM (Invitrogen, Grand Island, NY, USA)
Cell Lysis, Cell fractionation, SDS-PAGE, and Western blotting
Total Cell Lysate was prepared in RIPA (purchased from Boston Bioproducts, Ashland, MA, USA). Measurement of protein concentration, independent of the extraction method, was performed using BCA assay kit from Pierce, Grand Island, NY, USA. SDS-PAGE and immunoblotting was performed using standard protocol. The cell lysates were separated on 10% or 15% glycine SDS-PAGE gel and transferred to PVDF membrane. Membranes were blocked in 5% non-fat dry milk in TBS with 0.1% TWEEN-20 (TBST) for 1 h at room temperature followed by incubation with indicated primary antibodies in TBST with 5% BSA. Antibodies were purchased from following vendors. BMI-1 was from Invitrogen (37-5400), Bethyl Laboratories Montgomery, TX, USA (IHC-00606) and Proteintech, Rosemont, IL, USA (66161); uH2A (8240), H2A (2578), RING1A (2820), LC3B (2775), β-Actin (4970) , PARP (9542), Cleaved Caspase-3 (9664), Cleaved Caspase-7 (8438), Cleaved Caspase-9 (7237), NFkB/p65 (4764) from Cell Signaling Technology (Danvers, MA, USA); RIPK1 (374639) from Santa Cruz Biotechnology (Dallas, TX, USA); XIAP (MAB822) from R&D Systems (Minneapolis, MN, USA) and secondary antibodies conjugated with horseradish peroxidase IgG Rabbit (A6154) and Mouse (A4416) from Sigma–Aldrich . Primary antibodies were used in dilutions recommended by the manufacturer. Secondary antibodies were used at a concentration of 1:10,000.
Determination of apoptosis, cell viability and clonal growth
Apoptosis was determined by using the ApoTox-Glo™ triplex assay kit (G6321) from Promega (Madison, WI, USA). Briefly, PTC-028 or PTC-209 treated and untreated cells were incubated simultaneously to measure two protease activities; one is a marker of cell viability, and the other is a marker of cytotoxic cell death. The live- and dead-cell proteases produce different products, AFC and R110, which have different excitation and emission spectra, allowing them to be detected concurrently. The second part of the assay utilizes a luminogenic Caspase-3/Caspase-7 substrate (the tetrapeptide sequence DEVD), in a reagent optimized for caspase activity. Luminescence was proportional to the amount of caspase activity present. For clonal growth assay, cells were seeded as single cells (200cells/well) in six-well plates for 24 h, treated with or without PTC-028 and cells were cultured for additional 7 days before staining with crystal violet (0.75% crystal violet, 50% ethanol, 0.25% NaCl, 1.57% formaldehyde) and counted.
Measurement of ATP and mitochondrial ROS
ATP was measured using CellTiter-Glo® 2.0 Assay (G7571) from Promega (Madison, WI, USA) as per the manufacturer's instruction. Briefly, cells were treated with PTC-028 or PTC-209 and the luminescence was recorded at various time intervals upto 48h using CLARIOstar (BMG Labtech, Ortenberg, Germany). Luminescence intensity determined (ATP) and normalized with respective viable cells by ApoTox-Glo™ triplex assay kit (G6321) in each group; then expressed relative to respective control. Mitochondrial ROS was measured in cells by MitoSOX (Invitrogen) staining (2.5 μM for 10 minutes at 37°C). Data were acquired with a FACS Calibur (BD Biosciences) and analyzed with FlowJo analytical software.
Preclinical Model of Ovarian Cancer
Female athymic nude mice (NCr-nu; 6 to 8 wks old) were purchased from the Harlan Laboratory (Indianapolis, IN). All mice were housed and maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the U.S. Department of Agriculture, U.S. Department of Health and Human Services, and NIH. All studies were approved and supervised by the University of Oklahoma Animal Facility under the guidance of the IACUC #13-101-SSH. For the generation of orthotopic ovarian tumor models, OV90 cells (1 million in 100ul PBS) was orthotopically implanted in both the ovaries of each mice. Mice were randomized and divided into 3 groups (N= 7-10 mice/group): (a) vehicle control (0.5% HPMC,1% Tween 80), (b) Bmi-1 inhibitor PTC-028, and (c) cisplatin+paclitaxel. Treatment was initiated 1 week after implantation and continued for another 3-4 weeks. After the final treatment and assessing tumor growth/ regression in these animals, mice were sacrificed by CO2 inhalation with tumors and tissue harvested for further analysis.
Immunohistochemistry
Tumor grafts or mouse tissues were embedded in paraffin and sectioned (4 μm). These sections were deparaffinized in xylene, rehydrated in graded alcohol, subjected to heat-induced antigen retrieval with Target Retrieval Solution, and blocked with Protein Block. Immunohistochemistry was performed according to standard protocols. Antigen retrieval was achieved by heating sections in 95°C citrate buffer for 10 minutes. Sections were incubated with specific antibodies overnight at 4°C. For BMI-1 (1:300) and Ki67 (1:100) staining, the dark brown signal was revealed after incubation with the ABC kit (Vector), followed by a diaminobenzidine (DAB) and hydrogen peroxide reaction using the DAB detection kit (Vector). Counterstaining was performed by incubating the slides in Hematoxylin for 5 min. Images were taken using Nikon Eclipse Ni microscope. To detect the apoptosis in tissue, TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) positivity was assessed with a TUNEL apoptosis detection kit (DeadEnd Fluorometric TUNEL system, Promega) according to the supplier's instructions. Immunohistochemistry analysis of mouse tissues was performed in a blinded fashion.
Data analysis and Statistics
All the experiments unless otherwise stated were repeated independently 3 times. Data are expressed as means ± standard deviation (SD). Analysis of Variance (ANOVA) was performed to compare the mean among three or more groups and Student's t test was was performed to compare the mean between two groups. Statistical significance was set at P < 0.05, using GraphPad Prism 6 software.
Results
Depletion of BMI-1 by PTC-028 inhibits ovarian cancer cell viability and clonal growth
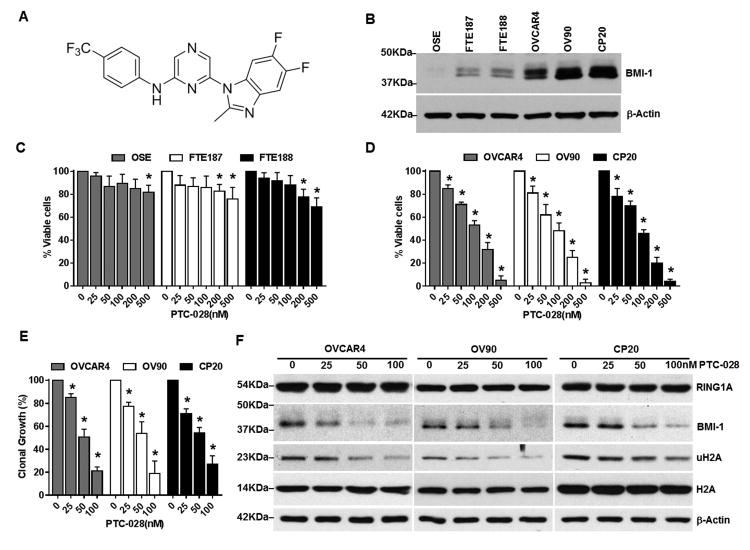
The chemical structure of PTC-028 is shown in Fig. 1A and its synthesis is described in Fig. S1. BMI-1 levels were determined in the immortalized non-malignant ovarian surface epithelial cells (OSE) and fallopian tube epithelial cells (FTE 187 and 188), as well as in the malignant cisplatin resistant CP20, OVCAR4 and OV90 epithelial ovarian cancer cells using immunoblotting. Compared to the normal OSE and FTE, BMI-1 levels were higher in the ovarian cancer cells (Fig. 1B). Using the MTS assay we next evaluated the effect of PTC-028 on cell viability. In normal OSE and FTE cells, upto 500 nM treatment with PTC-028 for 48 h had minimal effect (∼18-30% decrease) (Fig. 1C). However, OVCAR4, OV90 and CP20 cells demonstrated significant dose dependent decrease in cell viability with a half-maximum inhibitory concentration (IC50) of ∼100 nM and ∼95% decrease at 500 nM (Fig. 1D). The lack of effect in normal cells that express minimal BMI-1 supports specificity of PTC-028 and was further confirmed later. Because targeting BMI-1 in various systems inhibits self-renewal and clonal growth, we next evaluated the effect of PTC-028 in clonal growth assays. A significant dose-dependent decrease in clonal growth was observed in all the ovarian cancer cell lines tested (Fig. 1E). These results confirm that PTC-028 effectively inhibits ovarian cancer cell viability and clonal growth. Using immunoblotting we then determined that treatment with PTC-028 significantly reduced levels of BMI-1 and its functional readout, ubiquitinated histone 2A (uH2A) in ovarian cancer cells (Fig. 1F). However RING 1A, a polycomb repressor complex 1 (PRC1) partner of BMI-1 or total H2A levels remained unchanged (Fig. 1F) indicating specificity of PTC-028 towards BMI-1. These results together confirm that compared to non-malignant cells, PTC-028 selectively inhibits cancer cells that express relatively more BMI-1.
Figure 1. PTC-028 depletes BMI-1 and decreases ovarian cancer cell viability and clonal growth.
(A) The chemical structure of PTC-028. (B) Expression of BMI-1 in normal and malignant ovarian cells. (C) Immortalized ovarian surface epithelium (OSE) or the fallopian tube epithelium (FTE) cells and (D) ovarian cancer cells were treated with PTC-028 at the indicated concentrations for 48h and cellular viability was assessed using the MTS assay. Vehicle treated control cells were set to 100%. Data are the mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective control by a two-way ANOVA. (E) Ovarian cancer cells were treated with PTC-028 at the indicated concentrations for up to 7 days for CP20 and 10 days for OV90 and OVCAR4 respectively and clonal growth determined by counting crystal violet stained colonies. Vehicle treated control cells were set to 100%. Data are mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective control by a two-way ANOVA. (F) Ovarian cancer cells were treated with PTC-028 at the indicated concentrations for 48h. Expression of RING1A, BMI-1, uH2A and total H2A was determined by immunoblotting.
PTC-028 induces hyper-phosphorylation and decreases BMI-1 function
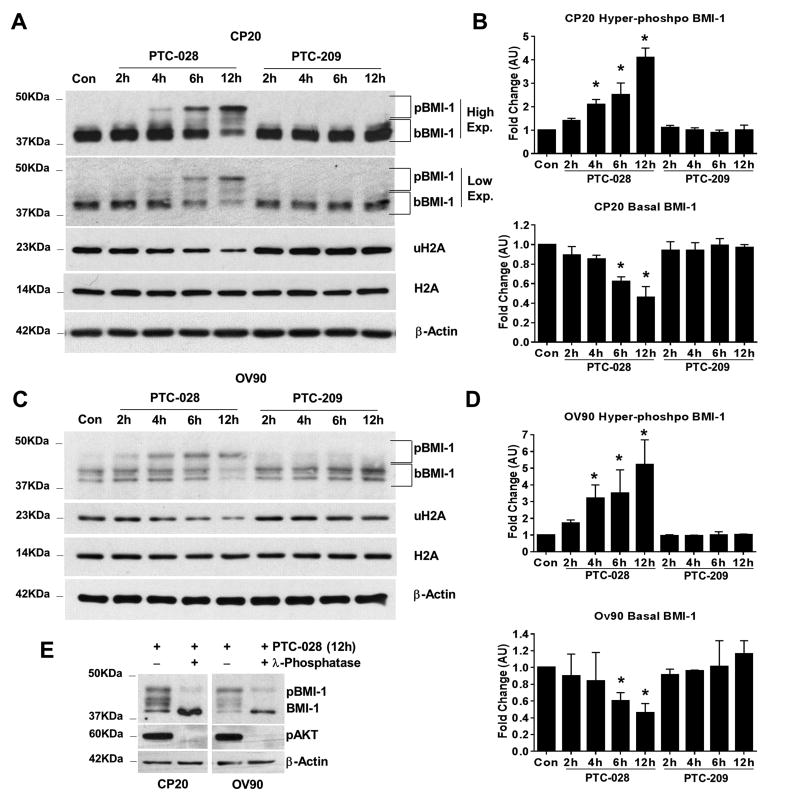
To determine temporal differences in depletion of BMI-1 by PTC-209 and PTC-028, we performed immunoblotting after treating with different concentrations of the compounds for 0-12 h. A time-dependent increase in the phosphorylated BMI-1 species and subsequent reduction in the biochemical functional readout, uH2A was observed up to 12 h with PTC-028 (100 nM) in both CP20 (Fig. 2A, 2B) and OV90 cells (Fig. 2C, 2D) while total H2A levels remained unchanged. Accordingly, basal BMI-1 levels were reduced in CP20 and OV90 cells by ∼53% and ∼54% respectively at 12 h with PTC-028 (Fig 2B and Fig. 2D), suggesting depletion of BMI-1 following its hyper-phosphorylation. At 200 nM, PTC-209 however did not affect BMI-1 levels up to 12 h in either cell line. That PTC-028 mediated depletion of BMI-1 was due to hyper-phosphorylation was confirmed by treating CP20 and OV90 cells with PTC-028 for 12 h. Subsequently, the cellular lysates were treated with or without lambda phosphatase (λ-phosphatase) and subjected to immunoblotting. Disappearance of the lower mobility hyper-phospho bands and accumulation of basal BMI-1 in the phosphatase treated samples (Fig. 2E) confirmed induction of phosphorylation by PTC-028. Significant reduction of phosphorylated AKT in the PTC-028 and λ-phosphatase, dual treated samples confirmed efficiency of phosphatase action (Fig. 2E). These results clearly indicate a more potent depletion of BMI1 by PTC-028 at a lower concentration than PTC-209 by 12 h.
Figure 2. Temporal depletion of BMI-1 by PTC-028 and PTC-209.
(A) CP20 cells were treated with PTC-028 at 100 nM or with PTC-209 at 200 nm for the indicated times. Expression of BMI-1, uH2A, total H2A and beta actin was determined by immunoblotting. (B) Top panel represents NIH Image J quantitation of the hyper-phosphorylated (pBMI-1) bands (high exposure) as indicated in A. Bottom panel shows similar representation of the basal BMI-1 (bBMI-1) (low exposure) as indicated in A. Data represent mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective control by a one-way ANOVA. (C) OV90 cells were treated with PTC-028 at 100 nM or with PTC-209 at 200 nm for the indicated times. Expression of BMI-1, uH2A, total H2A and beta actin was determined by immunoblotting. (D) Top panel represents NIH Image J quantitation of the hyper-phosphorylated bands as indicated in C. Bottom panel shows similar representation of the basal BMI-1 as indicated in C. Data represent mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective control by a one-way ANOVA. (E) CP20 or OV90 cells were treated with PTC-028 at 100 nm for 12 h with or without treatment with λ-phosphatase. The expression of BMI-1, pAKT (S473) and beta actin was determined by immunoblot.
Depletion of BMI-1 by PTC-028 induces apoptosis
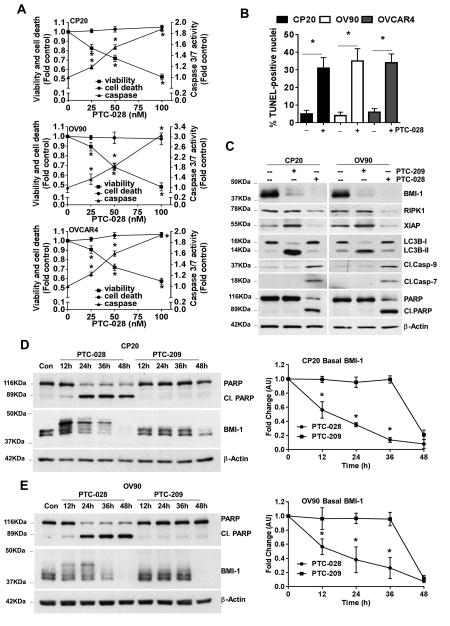
To determine the mechanism by which PTC-028 inhibits cancer cell growth, CP20, OV90 and OVCAR4 cells were treated with increasing concentrations of PTC-028 for 48 h followed by the ApoTox-Glo Triplex assay that simultaneously determines cell viability, cell death and Caspase 3/7 activity by utilizing two different protease markers and a luminogenic caspase substrate respectively [8]. A dose-dependent decrease in cell viability and simultaneous increase in Caspase-3/7 activity was observed, without any effect on cytotoxic cell death (Fig. 3A). We previously confirmed that a cytotoxic cell death signal is generated in this assay only by a disruption of the cellular membrane (Triton-X) but not by an apoptotic stimuli such as cisplatin [8]. Recent evidences suggest a fallopian tube origin of ovarian cancer [12, 13] rendering the FTE188 cells a suitable model for normal ovarian cells. In FTE188 cells that express minimal BMI1 (Fig. 1B), PTC-028 had no effect (Fig. 1C), however, forced expression of BMI1 (Fig. S2A) sensitized FTE188 cells to PTC-028 demonstrating specificity towards BMI1 (Fig. S2B). Furthermore, silencing BMI-1 in CP20 or OV90 cells rendered them insensitive to PTC-028 confirming specificity (Fig S2C). To determine if decreased cellular viability was due to apoptosis, TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) positivity was evaluated in the ovarian cancer cells that were treated with PTC-028 (100nM) for 48 h. Compared to the untreated control significant TUNEL positivity was observed in the PTC-028 treated CP20 (∼32%), OV90 (∼34%) and OVCAR4 (∼35%) cells respectively (Fig. 3B). Having demonstrated that OVCAR4 cells responded similarly to PTC-028 with respect to cellular viability and apoptosis, henceforth further experiments were performed using CP20 and OV90 cell lines. In contrast to PTC-028, PTC-209 potentiates autophagy leading to RIPK-mediated necroptosis in ovarian cancer cells [8]. To assess potential differences in signaling we treated ovarian cancer cells with PTC-209 (200 nM) or PTC-028 (100 nM) for 48 h followed by immunoblotting. Appreciable depletion of BMI-1 was observed by both the compounds (Fig. 3C). Consistent with our prior report [8], PTC-209 induced the expression of LC3B-II and X-linked inhibitor of apoptosis (XIAP) while RIPK1 levels remained unchanged (Fig. 3C). PTC-028 however decreased the expression of XIAP and RIPK1 while LC3B levels remained unchanged compared to that of the control (Fig. 3C). Additionally, significant cleavage of Caspase 7, Caspase 9 and poly ADP-ribose polymerase (PARP) was observed in PTC-028 but not in PTC-209 treated ovarian cancer cells (Fig. 3C). These results clearly suggest that depletion of BMI-1 by PTC-028 induces caspase-mediated apoptosis.
Figure 3. PTC-028 induces caspase-dependent apoptosis.
(A) CP20, OV90 and OVCAR4 cells were treated with increasing concentrations of PTC-028 for 48h and cell viability, cytotoxic cell death and caspase 3/7 activity was evaluated using the ApoTox-Glo Triplex assay. Data are mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective vehicle treated control by a two-way ANOVA. (B) CP20, OV90 and OVCAR4 cells were treated with 100 nM PTC-028 for 48h, cells were subjected to the TUNEL assay and analyzed by fluorescence microscopy. The number of TUNEL positive nuclei were counted from ∼300 cells per treatment group. Data represent the mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing with respective control by Student's t-test. (C) CP20 or OV90 cells were treated with 100 nM PTC-028 or 200 nM PTC-209 for 48h. Expression of BMI-1, RIPK1, XIAP, LC3B, cleaved caspase 7, cleaved caspase 9, PARP and beta actin was determined by immunoblotting. (D) CP20 cells were treated with PTC-028 at 100 nM or with PTC-209 at 200 nm for the indicated times. Expression of BMI-1, PARP and β-actin was determined by immunoblotting (left panel). The quantitation of basal, residual BMI-1 by NIH Image J is shown in the right panel. (E) OV90 cells were treated with PTC-028 at 100 nM or with PTC-209 at 200 nm for the indicated times. Expression of BMI-1, PARP and β-actin was determined by immunoblotting (left panel). NIH Image J quantitation of the basal, residual BMI-1 is shown in the right panel. Data represent mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing between each group at respective time point by a two-way ANOVA.
To further appreciate the temporal link between depletion of cellular steady-state BMI1 levels and apoptosis, we treated CP20 and OV90 cells with 100 nM PTC-028 or 200 nM PTC-209 for 0-48 h. A gradual depletion of cellular BMI1 levels was induced by PTC-028, however maximal depletion by PTC-209 was observed between 36-48 h (Fig. 3D and 3E). At 48 h however, both the compounds significantly inhibited BMI1 to similar levels. Interestingly, while there was no PARP cleavage in PTC-209 treated cells, earliest PARP cleavage could be observed as early as 12 h after treatment with PTC-028 (Fig 3D and 3E) suggesting activation of apoptosis.
To establish that PTC-028 mediated decrease in cell viability was due to apoptosis, we treated the cells with the pan caspase inhibitor z-VAD-fmk (10μM) for 3h with or without PTC-028 (100 nM) for 48h and analyzed cell viability using the MTS assay. Compared to PTC-028 only, dual treatment with z-VAD-fmk significantly increased cell viability; by ∼ 30% in CP20, ∼41% in OV90 cells respectively (Fig. S3A). To further confirm involvement of the caspase cascade in PTC-028-mediated apoptosis, z-VAD-fmk pre-treated cells with or without PTC-028 were subjected to immunoblotting. Compared to PTC-028 only, dual treatment with z-VAD-fmk partially restored RIPK1, XIAP and NF-κB/p65 levels (Fig. S3B). Prior reports indicate that caspase-mediated depletion of RIPK1 potentiates apoptosis if levels of anti-apoptotic NF-κB or XIAP are low; furthermore, such apoptosis can be blocked by treatment with z-VAD-fmk [14]. Together, these results establish that PTC-028 mediated apoptotic cell death is specific towards high BMI-1 expressing cells and is caspase-dependent.
PTC-028 mediated apoptosis is dependent on mitochondrial ROS
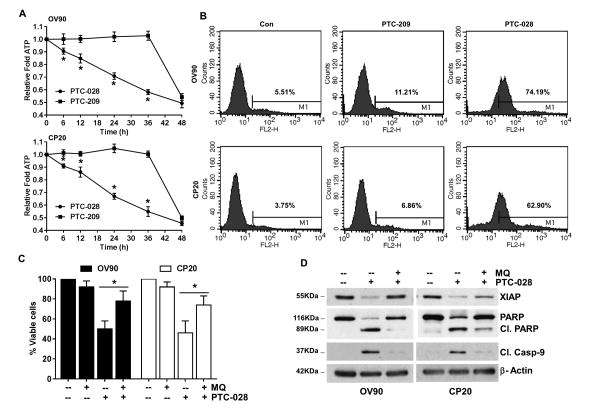
While caspase activation is a manifestation of apoptotic cell death, reduced ATP levels and compromised redox balance have been attributed as causes [15]. Therefore, we determined ATP levels in PTC-028 and PTC-209 treated cells (Fig. 4A). Similar to BMI1 depletion (Fig. 3D and 3E), a gradual depletion in cellular ATP levels was observed in PTC-028 treated cells (Fig 4A). However in PTC-209 treated cells ATP levels remained steady up to 36 h after which a significant drop was observed at 48 h (Fig. 4A). Next using MitoSOX Red, we determined mitochondrial superoxide formation in PTC-028 or PTC-209 treated cells at 48h. A robust increase in mean fluorescence intensity of oxidized MitoSOX was evidenced after PTC-028 but not PTC-209 treatment (Fig. 4B). To evaluate if mitochondrial ROS was required for the induction of apoptotic cell death, both OV90 and CP20 cells were pre-treated with mitoquinone (MitoQ), a mitochondria-targeted antioxidant [16] followed by PTC-028 for 48 h and cell viability determined by the MTS assay. Pre-treatment with MitoQ at 10 μM rescued PTC-028 mediated decrease in cell viability by ∼28% in OV90 and by ∼29% in CP20 cells respectively (Fig. 4C). To determine the status of the apoptotic markers, MitoQ pre-treated cells with or without PTC-028 were subjected to immunoblotting. Compared to PTC-028 only, dual treatment with MitoQ, restored XIAP and PARP levels to near control while cleaved caspase 9 was absent from the dual MitoQ and PTC-028 treated cells (Fig. 4D). Together these results confirm that PTC-028 induces mitochondria-mediated apoptosis in ovarian cancer cells.
Figure 4. PTC-028 mediated apoptosis is dependent on cellular ATP depletion and mitochondrial ROS.
(A) CP20 or OV90 cells were treated with PTC-028 at 100 nM or with PTC-209 at 200 nm for the indicated times and intracellular ATP levels determined and normalized with respective number of viable cells in each group. Data represent mean ± S.D. of three independent experiments performed in triplicate. *P<0.05 when comparing between each group at respective time point by a two-way ANOVA. (B) OV90 or CP20 cells were treated with 200 nm PTC-209 or 100 nM PTC-028 for 48 h followed by MitoSOX staining and analyzed by a FACS Calibur flow cytometer. A representative histogram depicting mean florescence intensity from three independent replicates is shown. (C) OV90 or CP20 cells were pre-treated with or without Mitoquinone (MQ) 10 μM for 3h followed by PTC-028 at 100 nM for 48 h. Cell viability was assessed by the MTS assay. Vehicle treated control cells were set to 100%. Data represent mean ± S.D. of three independent experiments performed in triplicate and *P<0.05 by a two-way ANOVA. (D) OV90 or CP20 cells were pre-treated with or without Mitoquinone at 10 μM for 3h followed by PTC-028 at 100 nM for 48 h. Expression of XIAP, PARP, cleaved caspase 9 and beta actin was determined by immunoblotting.
Therapeutic activity of PTC-028 in vivo
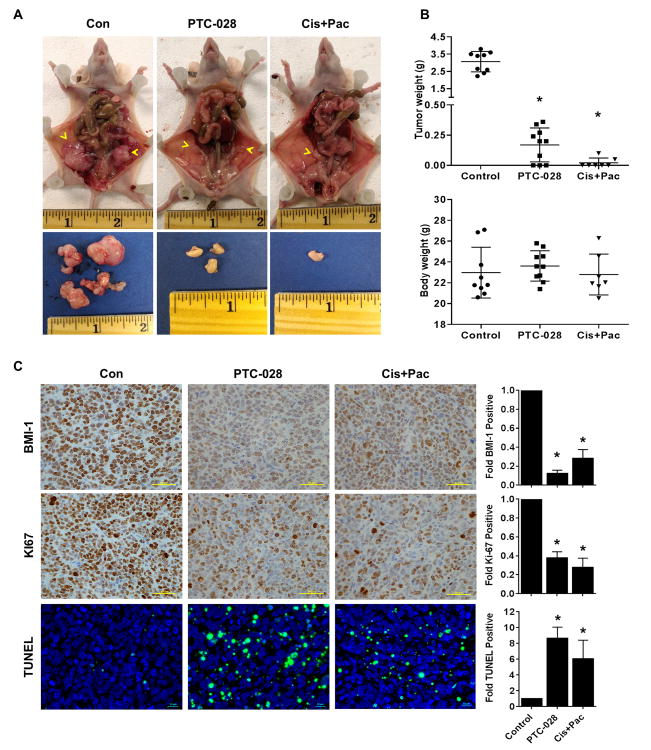
To translate our in vitro results in vivo, we determined plasma concentration of PTC-028 after administration of single oral doses to the CD-1 mice. Total plasma AUC0-24h were 10.9 and 26.1 μg·h/mL at doses of 10 and 20 mg/kg, which suggests dose proportional pharmacokinetics. The Cmax for PTC-028 at 10 and 20 mg/kg was 0.79 and 1.49 ug/mL, respectively. The Cmax was reached at both dose levels 1h post dose after which plasma concentrations slowly reduced (Fig. S4). Next, we implanted OV90 cells in the bilateral ovarian bursa of athymic female nude mice. A week after implantation, mice were randomized in to three different groups. PTC-028 was administered orally at a dose of 15 mg/kg twice weekly to one group. The second group received intraperitoneal injections of cisplatin at a dose of 3 mg/kg/weekly and paclitaxel at 15 mg/kg/weekly following the established standard-of-care for treatment of epithelial ovarian cancer [17, 18], while the third group received vehicle control orally and intraperitoneally. Three weeks after treatment the mice were euthanized and tumor tissues collected, weighed and processed for further analysis by immunohistochemistry (IHC). Compared to the control (average tumor weight, ∼3g), single agent PTC-028 and cisplatin+paclitaxel caused ∼94% (0.169 g) and ∼99% (0.025 g) reduction in tumor weight respectively (Fig. 5A, 5B). No obvious toxicity was noted in the animals during therapy experiments as assessed by mean body weight (Fig. 5B). The tumor tissues were further analyzed by IHC and compared to the control, the expression of BMI-1 was reduced by 8 fold in the PTC-028 group and by 3.5 fold in the cisplatin/paclitaxel treated group. Similarly the proliferation marker Ki67 decreased by 2.6 fold and 3.6 fold in the PTC-028 and the cisplatin/paclitaxel treated group respectively (Fig. 5C). Consequently a significant increase in TUNEL positivity was observed in the PTC-028 (8.7 fold) and cisplatin/paclitaxel (6.1 fold) treated groups compared to the control (Fig. 5C). These results confirm the specificity of PTC-028 towards BMI-1 in vivo and indicate that anti-BMI-1 strategies are comparable in their efficacy to standard therapy in the orthotopic OV90 model.
Figure 5. Therapeutic efficacy of PTC-028 in the orthotopic OV90 model.
(A) OV90 cells were implanted in the bursa of bilateral ovaries in athymic female nude mice. One week later mice were randomized into 3 groups of 7-10 each and treatment initiated. PTC-028 was administered orally at 15 mg/kg twice weekly. Cisplatin at 3 mg/kg/weekly and paclitaxel at 15 mg/kg/weekly were administered by intraperitoneal injections and vehicle control given orally and intraperitoneally. Three weeks after treatment, the mice were euthanized and tumor tissues collected, weighed and processed. Representative images of the tumors within the ovary and after resection from each group are shown. (B) Top panel depicts average and individual tumor weight ± SD from each group. *P<0.05 compared to the control group by a one-way ANOVA. Bottom panel depicts average body weight of the mice after treatment from each group. (C) Immunohistochemical staining of tumor xenografts for BMI-1 (upper panel), Ki67 (middle panel) and TUNEL positivity (lower panel). After quantification fold changes with respect to the control are shown graphically on the right, scale bar represent 50 μm for BMI-1, Ki67 and 10 μm for TUNEL. *P<0.05 compared to the control group by a one-way ANOVA.
Discussion
Accumulating evidences have established BMI-1 as an important therapeutic target in several different malignancies including ovarian cancer [1, 3-5, 19]. Consequently PTC-209, a small-molecule, that inhibited BMI-1 reporter expression [1] potentiated autophagy leading to RIPK-mediated necroptotic cell death [8] in ovarian cancer and impaired primary colorectal tumor growth. Here we report for the first time that PTC-028, a second generation inhibitor that depletes BMI-1 at the protein level, activates caspase-dependent apoptosis and significantly reduces ovarian tumor growth similar to the standard-of-care cisplatin/paclitaxel.
The specificity of PTC-028 towards BMI-1 was demonstrated by the fact that normal OSE and FTE cells that express low levels of BMI-1 were non-responsive while ovarian cancer cells that express higher BMI-1 showed a dose-dependent decrease in cell viability upon treatment with PTC-028. Furthermore, forced expression of BMI-1, sensitized FTE188 cells to PTC-028, thus confirming specificity towards BMI-1.
Interestingly although at 48 h relative depletion of cellular BMI-1 by either PTC-028 (100 nM) or PTC-209 (200 nM) was comparable there was a distinct difference at earlier time points. A gradual depletion of cellular BMI-1 that reached approximately ∼50% by 12 h was observed in PTC-028 treated cells. However in PTC-209 treated cells a severe decline in BMI-1 levels was observed only between 36-48 h of treatment. Similar to depletion of BMI-1, cellular ATP levels also gradually decreased over time with PTC-028 but drastically dropped between 36-48 h in PTC-209 treated cells. Furthermore at 48 h significant induction of mitochondrial ROS was observed with PTC-028 but not PTC-209 treatment. These results lead us to conclude that a gradual decrease in cellular BMI-1 levels is linked to the decrease in ATP and increase in mitochondrial ROS generation. Prior studies from our and other groups have demonstrated that BMI-1 supports mitochondrial complex activity, absence of which leads to decreased ATP production and enhanced ROS generation [8, 20]. We therefore speculate that a gradual drop in ATP coupled with a compromised redox balance potentiates apoptosis [15] in PTC-028 treated cells. However a swift, drastic drop in ATP coupled with induction of XIAP and lack of ROS prevents apoptosis in PTC-209 treated cells. Indeed prior studies have demonstrated that intracellular ATP levels determine cell death fate [21, 22]. A transient and moderate depletion of ATP in cells induces apoptosis while potent and prolonged depletion causes necrosis [23-27] because apoptosis is an energy requiring process while necrosis is not [21].
PTC-028 mediated apoptotic cell death was exemplified by the enhanced caspase activity, TUNEL positivity and cleavage of PARP. Furthermore, decreased cell viability could be rescued by the pan-caspase inhibitor z-VAD-fmk. Additionally, RIPK1 and XIAP levels were significantly decreased by PTC-028 but not by PTC-209. Complex roles of RIPK1 in regulating NF-κB mediated inflammatory signaling, FADD/Caspase-8 mediated apoptotic signaling or RIPK1/RIPK3 mediated necroptotic signaling are beginning to be appreciated [14, 28]. It is noteworthy that the RIPK1-/- mice display extensive apoptosis in their lymphoid and adipose tissue and die within 1-3 days of birth [14, 29]. Also RIPK1 is an important component of major cellular death pathways such as those regulated by Fas/FADD, TRAIL-R and TNFR signaling [14, 30-32]. In all these pathways, Caspase 8 mediated cleavage of RIPK1 potentiates apoptosis if levels of anti-apoptotic NF-κB or XIAP are low that can be blocked by treatment with z-VAD-fmk (Fig. 6) [14]. Our results are consistent with these observations in that z-VAD-fmk restores levels of RIPK1, XIAP and NF-κB/p65 in PTC-028 treated cells.
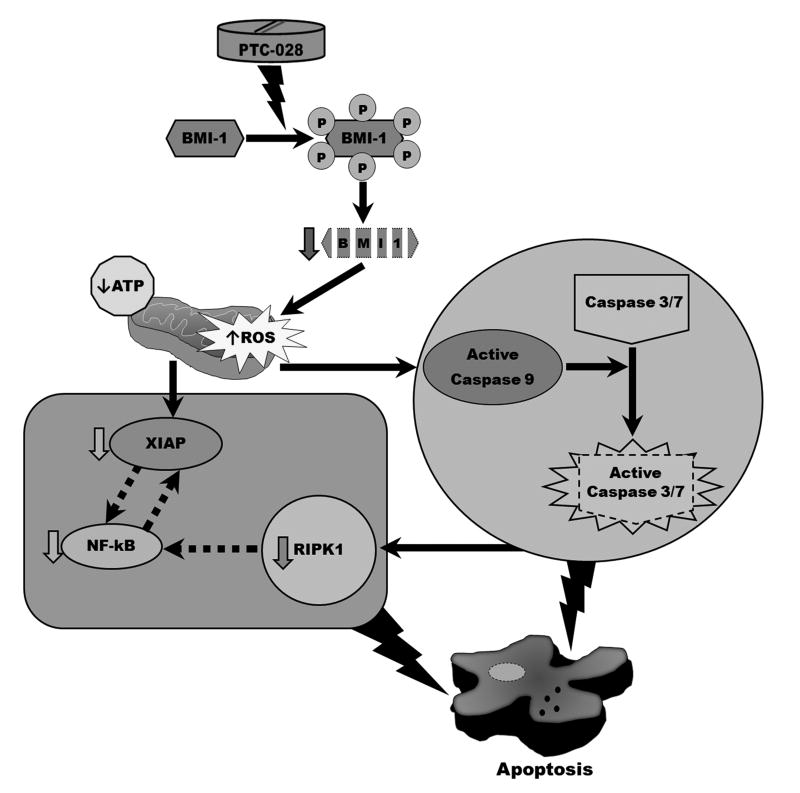
Figure 6. Scheme of action of PTC-028.
PTC-028 induces hyper-phosphorylation and subsequent depletion of BMI-1 that induces reduction of ATP and induction of mitochondrial ROS along with inhibition in expression of RIPK1 and XIAP. Low XIAP and increased ROS then activate caspase 9 that is followed by caspase-3/7. RIPK1 can be targeted by caspases leading to downregulation of NFkB that reciprocally regulates XIAP. Together these signaling ultimately lead to apoptosis. Dashed arrows represent published reports as elaborated in the discussion section.
Furthermore, in contrast to PTC-209 that was administered sub-cutaneously at 60 mg/kg/day for 10 days to inhibit sub-cutaneous xenograft tumors [1], PTC-028, administered orally at a dose of 15 mg/kg twice weekly showed impressive single agent activity similar to that of cisplatin/paclitaxel administered by intra-peritoneal delivery in the orthotopic OV90 ovarian cancer model without any obvious toxicity.
In summary, PTC-028 induces hyper-phosphorylation and gradual depletion of BMI-1 that results in depletion of ATP and induction of mitochondrial ROS with decreased expression of both RIPK1 [33] and XIAP [34] leading to activation of the caspase cascade. Additionally, caspase targeting of RIPK1 can lead to downregulation of NF-kB which has a reciprocal feedback regulation with XIAP [35, 36]. Together these signaling ultimately lead to apoptotic cell death (Fig. 6). Therefore PTC-028 could potentially be used as an effective therapeutic for management of patients with epithelial ovarian cancer, for whom treatment options are limited.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health (NIH) CA 157481 to R. Bhattacharya, Foundation for Women's cancer (FWC), St. Louis Ovarian Cancer Awareness Research Grant to A. Dey. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank an Institutional Development Award (IDeA) grant (P20 GM103639) from the National Institute of General Medical Sciences of the National Institutes of Health supporting the use of the Histology and Immunohistochemistry Core. We thank the Laboratory for Molecular Biology and Cytometry Research at OUHSC for the use of the Flow Cytometry and Imaging facility.
Financial Support: This study was supported by the National Institutes of Health (NIH) CA 157481 to R. Bhattacharya; Foundation for Women's cancer (FWC), St. Louis Ovarian Cancer Awareness Research Grant to A. Dey.
References
- 1.Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, Cao L, Baiazitov R, Du W, Sydorenko N, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20(1):29–36. doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- 2.Abdouh M, Facchino S, Chatoo W, Balasingam V, Ferreira J, Bernier G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J Neurosci. 2009;29(28):8884–8896. doi: 10.1523/JNEUROSCI.0968-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya R, Nicoloso M, Arvizo R, Wang E, Cortez A, Rossi S, Calin GA, Mukherjee P. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res. 2009;69(23):9090–9095. doi: 10.1158/0008-5472.CAN-09-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya R, Mustafi SB, Street M, Dey A, Dwivedi SK. Bmi-1: At the crossroads of physiological and pathological biology. Genes Dis. 2015;2(3):225–239. doi: 10.1016/j.gendis.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang E, Bhattacharyya S, Szabolcs A, Rodriguez-Aguayo C, Jennings NB, Lopez-Berestein G, Mukherjee P, Sood AK, Bhattacharya R. Enhancing chemotherapy response with Bmi-1 silencing in ovarian cancer. PLoS One. 2011;6(3):e17918. doi: 10.1371/journal.pone.0017918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca EA, Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68(22):9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- 7.Song LB, Zeng MS, Liao WT, Zhang L, Mo HY, Liu WL, Shao JY, Wu QL, Li MZ, Xia YF, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66(12):6225–6232. doi: 10.1158/0008-5472.CAN-06-0094. [DOI] [PubMed] [Google Scholar]
- 8.Dey A, Mustafi SB, Saha S, Kumar Dhar Dwivedi S, Mukherjee P, Bhattacharya R. Inhibition of BMI1 induces autophagy-mediated necroptosis. Autophagy. 2016;12(4):659–670. doi: 10.1080/15548627.2016.1147670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mourgues L, Imbert V, Nebout M, Colosetti P, Neffati Z, Lagadec P, Verhoeyen E, Peng C, Duprez E, Legros L, et al. The BMI1 polycomb protein represses cyclin G2-induced autophagy to support proliferation in chronic myeloid leukemia cells. Leukemia. 2015;29(10):1993–2002. doi: 10.1038/leu.2015.112. [DOI] [PubMed] [Google Scholar]
- 10.Chien J, Narita K, Rattan R, Giri S, Shridhar R, Staub J, Beleford D, Lai J, Roberts LR, Molina J, et al. A role for candidate tumor-suppressor gene TCEAL7 in the regulation of c-Myc activity, cyclin D1 levels and cellular transformation. Oncogene. 2008;27(58):7223–7234. doi: 10.1038/onc.2008.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan W, Mercado-Uribe I, Zhang J, Rosen D, Zhang S, Wei J, Liu J. Mucinous adenocarcinoma developed from human fallopian tube epithelial cells through defined genetic modifications. Cell cycle. 2012;11(11):2107–2113. doi: 10.4161/cc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26(32):5284–5293. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levanon K, Ng V, Piao HY, Zhang Y, Chang MC, Roh MH, Kindelberger DW, Hirsch MS, Crum CP, Marto JA, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29(8):1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ. 2007;14(3):400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 15.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22(1):58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko GR, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124(9):3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winterhoff B, Freyer L, Hammond E, Giri S, Mondal S, Roy D, Teoman A, Mullany SA, Hoffmann R, von Bismarck A, et al. PG545 enhances anti-cancer activity of chemotherapy in ovarian models and increases surrogate biomarkers such as VEGF in preclinical and clinical plasma samples. Eur J Cancer. 2015;51(7):879–892. doi: 10.1016/j.ejca.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueno NT, Bartholomeusz C, Herrmann JL, Estrov Z, Shao R, Andreeff M, Price J, Paul RW, Anklesaria P, Yu D, et al. E1A-mediated paclitaxel sensitization in HER-2/neu-overexpressing ovarian cancer SKOV3.ip1 through apoptosis involving the caspase-3 pathway. Clin Cancer Res. 2000;6(1):250–259. [PubMed] [Google Scholar]
- 19.Bommi PV, Dimri M, Sahasrabuddhe AA, Khandekar J, Dimri GP. The polycomb group protein BMI1 is a transcriptional target of HDAC inhibitors. Cell cycle. 2010;9(13):2663–2673. doi: 10.4161/cc.9.13.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459(7245):387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsujimoto Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death and Differentiation. 1997;4(6):429–434. doi: 10.1038/sj.cdd.4400262. [DOI] [PubMed] [Google Scholar]
- 22.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20(1):1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 23.Hartley A, Stone JM, Heron C, Cooper JM, Schapira AH. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson's disease. J Neurochem. 1994;63(5):1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- 24.Richter C, Schweizer M, Cossarizza A, Franceschi C. Control of apoptosis by the cellular ATP level. FEBS Lett. 1996;378(2):107–110. doi: 10.1016/0014-5793(95)01431-4. [DOI] [PubMed] [Google Scholar]
- 25.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57(10):1835–1840. [PubMed] [Google Scholar]
- 26.Lieberthal W, Menza SA, Levine JS. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol. 1998;274(2 Pt 2):F315–327. doi: 10.1152/ajprenal.1998.274.2.F315. [DOI] [PubMed] [Google Scholar]
- 27.Ohgoh M, Shimizu H, Ogura H, Nishizawa Y. Astroglial trophic support and neuronal cell death: influence of cellular energy level on type of cell death induced by mitochondrial toxin in cultured rat cortical neurons. J Neurochem. 2000;75(3):925–933. doi: 10.1046/j.1471-4159.2000.0750925.x. [DOI] [PubMed] [Google Scholar]
- 28.Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14(11):727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 29.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 30.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81(4):513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 31.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15(22):6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4(4):387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13(19):2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999;18(19):5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188(1):211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winsauer G, Resch U, Hofer-Warbinek R, Schichl YM, de Martin R. XIAP regulates bi-phasic NF-kappaB induction involving physical interaction and ubiquitination of MEKK2. Cell Signal. 2008;20(11):2107–2112. doi: 10.1016/j.cellsig.2008.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.