Abstract
Introduction
Bipolar disorder is characterized by recurring episodes of depression and mania. Defining differences in brain function during these states is an important goal of bipolar disorder research. However, few imaging studies have directly compared brain activity between bipolar mood states. Herein, we compare functional magnetic resonance imaging (fMRI) responses during a flashing checkerboard stimulus between bipolar participants across mood states (euthymia, depression, and mania) in order to identify functional differences between these states.
Methods
40 participants with bipolar I disorder and 33 healthy controls underwent fMRI during the presentation of the stimulus. A total of 23 euthymic-state, 16 manic-state, 15 depressed-state, and 32 healthy control imaging sessions were analyzed in order to compare functional activation during the stimulus between mood states and with healthy controls.
Results
A reduced response was identified in the visual cortex in both the depressed and manic groups compared to euthymic and healthy participants. Functional differences between bipolar mood states were also observed in the cerebellum, thalamus, striatum, and hippocampus.
Conclusions
Functional differences between mood states occurred in several brain regions involved in visual and other sensory processing. These differences suggest that altered visual processing may be a feature of mood states in bipolar disorder.
Limitations
The key limitations of this study are modest mood-state group size and the limited temporal resolution of fMRI which prevents the segregation of primary visual activity from regulatory feedback mechanisms.
Keywords: Bipolar Disorder, fMRI, mood state, mania, depression, visual cortex
1. Introduction
Bipolar I disorder is a psychiatric illness characterized by intermittent episodes of depressed and manic mood states as well as periods of euthymia when mood is normal (American Psychiatric et al., 2013; Mitchell and Malhi, 2004). However, the vast majority of imaging studies have only examined participants during the euthymic state and only a handful of studies have looked at participants during bipolar depression (Altshuler et al., 2008; Cerullo et al., 2014; Cerullo et al., 2012; Chen et al., 2006; Diler et al., 2013; Li et al., 2015; Liu et al., 2012; Pomarol-Clotet et al., 2015), mania (Cerullo et al., 2012; Chen et al., 2006; Chen et al., 2010; Strakowski et al., 2011), or have compared these mood states directly with each other (Cerullo et al., 2012; Chen et al., 2006; Chen et al., 2010; Li et al., 2015; Liu et al., 2012; Pomarol-Clotet et al., 2015). Because bipolar disorder is accompanied by deficits in executive function (Strakowski et al., 2004; Strakowski et al., 2005a), emotional processing (Chen et al., 2006; Lawrence et al., 2004; Mitchell and Malhi, 2004), and emotional regulation (Chen et al., 2011; Phillips et al., 2008), most imaging studies have been devoted to identifying neural correlates of these deficits using a variety of behavioral tasks that interrogate executive function and limbic processes. Consequently, neuroimaging studies of bipolar disorder have primarily implicated brain regions in the prefrontal cortex, limbic system, and basal ganglia (Chen et al., 2011; Keener and Phillips, 2007; Strakowski et al., 2012; Strakowski et al., 2005b). Thus, there is a paucity of knowledge about brain function and dysfunction in bipolar disorder across mood states and in broad domains of brain activity.
An emerging body of imaging research into bipolar disorder suggests impairment in sensory processing networks (Javitt, 2009; Veer et al., 2010). These findings suggest that psychiatric disorders may result from disruptions early in sensory processing rather than from deficits in higher-order processing networks. Deficits in early sensory processing including reduced visual evoked potentials (VEP) (Elvsashagen et al., 2012; Liu et al., 2014; Nazhvani et al., 2013; Yeap et al., 2009) and auditory-evoked potentials such as the P50 (Morla-Sanchez et al., 2008) have been identified using electroencephalography (EEG). These findings suggest that there may be functional differences between people with bipolar disorder and healthy controls within the visual cortex and other visual processing areas. Few imaging studies have attempted to assess functional responses to visual stimuli in bipolar disorder. However, one MR spectroscopy study used a flashing pinwheel stimulus to assess metabolic differences in bipolar disorder and found abnormal rates of change in ATP and phosphocreatine levels in the occipital cortex (Yuksel et al., 2015). These findings suggest abnormal bioenergetics may underlie functional differences in brain activity and, taken together with the EEG findings suggest that disrupted brain function in bipolar disorder may occur on a more fundamental level than previously thought, impairing both sensory processing and perception.
For assessing sensory processing and perception, the flashing checkerboard paradigm offers a number of advantages. First, this paradigm evokes robust responses (Drobyshevski et al., 2006), thus increasing sensitivity to detect even subtle differences in brain function. Second, because no complex motor or cognitive tasks are required, responses are likely to exhibit less interference from non-visual brain circuits. Similarly, because the flashing checkerboard should not evoke emotive content, it therefore avoids emotion-related confounds. Finally, because the flashing checkerboard places little demand on subjects, the paradigm is well-suited for imaging more symptomatic participants, particularly those experiencing mania.
The purpose of this study was to determine whether the activity of visual processing networks is altered in people with bipolar disorder versus healthy controls. We hypothesized that people with bipolar disorder would exhibit reduced functional activation in vision processing circuits, which would indicate deficits in early sensory processing in bipolar disorder. We also explored whether any such differences are state or trait characteristics of bipolar disorder by contrasting functional responses to the flashing checkerboard paradigm between participants in different mood states (euthymia, depression, and mania).
2. Methods
2.1 Participants
A total of 40 people with bipolar disorder and 33 healthy controls with no history of psychiatric illness balanced for age and gender participated in this fMRI study conducted between July 2012 and April 2015. We previously published data from this sample in Johnson et al. (Johnson et al., 2015a) and Johnson et al. (Johnson et al., 2015b). All participants provided written informed consent according to the study protocol approved by the University of Iowa Institutional Review Board. Potential participants were excluded for a history of brain damage, neurological problems, seizure disorder, heart problems, lung disease, alcohol or drug dependence, or contraindications for MRI including pregnancy. Healthy controls were also excluded if they had a history of a DSM-IV-TR Axis I or Axis II psychiatric disorder. The diagnosis for the participants in the bipolar disorder group was confirmed by psychiatric evaluation (JGF) using DSM-IV-TR diagnostic criteria. Participants with bipolar I disorder underwent imaging during one or more distinct disease states: euthymia (E), depression (D), and/or mania (M). Subsamples of these participants have previously been described (Fiedorowicz et al., 2015; Johnson et al., 2015a). The mood state was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) and the Young Mania Rating Scale (YMRS) (Young et al., 1978). For the purposes of this study, we defined mania as YMRS ≥ 20, depression as MADRS > 20, and euthymia as YMRS ≤ 12 and MADRS < 10 at the time of the fMRI session.
A total of 91 imaging sessions were acquired of which five sessions were excluded from this analysis based upon insufficient participant responses to the stimulus, leaving a total of 86 sessions which were used for cross-sectional comparisons between groups. Participant demographics for these 86 sessions are summarized in Table 2, which included 23 euthymic group sessions (13 male; 10 female; mean age = 40 ± 13 years), 15 depressed group sessions (8 male; 7 female; mean age = 45 ± 12 years), 16 manic group sessions (9 male; 7 female; mean age = 39 ± 14 years), and 32 healthy control sessions (19 male; 13 female; mean age = 42 ± 13 years). Of the 40 participants with bipolar I disorder, nine were imaged in two mood states (6 E,D; 2 D, M; 1 E, M) and three were imaged in all three mood states. The ordering of these repeated sessions is shown in Supplemental Table 1. A mixed-effect regression model was used to account for these repeated sessions. Participants with bipolar disorder and healthy controls did not differ with regard to age, sex, race, ethnicity, or handedness. However, healthy controls had significantly more years of education (Table 1).
Table 2.
Functional Differences Between Mood State Groups
| Cluster | Size | Peak Coordinates |
Peak | Mean | mean % signal change |
||||
|---|---|---|---|---|---|---|---|---|---|
| Number | (voxels) | x | y | z | t-stat | t-stat | Group A |
Group B |
Regions |
| Bipolar (A) vs. Healthy (B) | |||||||||
| 1 | 6188 | −32 | 99 | 5 | −4.70 | −2.93 | 1.49 | 2.07 | Visual Areas |
|
| |||||||||
| Depressed (A) vs. Healthy (B) | |||||||||
| 1 | 4132 | −18 | 103 | 5 | −4.70 | −2.64 | 1.44 | 2.27 | Visual Areas |
| 2 | 3469 | −14 | 8 | −7 | −4.34 | −2.77 | 0.07 | 0.25 | R Thalamus |
| 3 | 2741 | 13 | 18 | 8 | −3.67 | −2.76 | 0.05 | 0.23 | L Thalamus |
| 4 | 2355 | −6 | 66 | −33 | −4.52 | −3.05 | 0.003 | 0.16 | Cerebellar Vermis |
|
| |||||||||
| Manic (A) vs. Healthy (B) | |||||||||
| 1 | 15641 | 23 | 27 | −9 | −4.20 | −2.49 | −0.18 | 0.10 | L Thalamus, Hippocampus, and Striatum |
| 2 | 13865 | −46 | 42 | 40 | −3.40 | −2.47 | −0.15 | 0.14 | R supramarginal gyrus, R Thalamus, Hippocampus, and striatum |
| 3 | 7610 | 17 | 56 | −38 | −4.01 | −2.78 | −0.14 | 0.09 | Cerebellar Vermis |
| 4 | 6544 | −19 | 101 | −4 | −5.34 | −3.07 | 1.35 | 2.17 | Visual Areas |
|
| |||||||||
| Depressed (A) vs. Euthymic (B) | |||||||||
| 1 | 2579 | 16 | 50 | 8 | −4.28 | −2.59 | −0.07 | 0.02 | L corpus callosum and L posterior forceps |
| 2 | 2486 | −3 | 17 | 5 | −3.44 | −2.55 | 0.07 | 0.33 | R Thalamus |
|
| |||||||||
| Manic (A) vs. Euthymic (B) | |||||||||
| 1 | 2646 | 14 | 62 | −35 | −6.52 | −2.41 | −0.12 | 0.10 | Cerebellar vermis and L cerebellum |
| 2 | 2564 | 33 | 52 | 9 | −8.05 | −2.67 | −0.18 | 0.01 | L corpus callosum and L posterior forceps |
| 3 | 2511 | −3 | 18 | 1 | −8.81 | −2.53 | −0.11 | 0.32 | R Thalamus |
L – Left, R - Right
Table 1.
Demographics of Imaging Sample
| HC | BD | E | D | M | BD v. HC | E v. D | E v. M | D v. M | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # Scans | 32 | 54 | 23 | 15 | 16 | ||||||
|
| |||||||||||
| Age | M | 42.1 | 41.1 | 39.6 | 45.3 | 39.4 | t(df) | −0.34(84) | −1.31(36) | 0.03(37) | 1.23(29) |
| SD | 12.5 | 12.5 | 13.5 | 13.4 | 13.5 | p | 0.74 | 0.20 | 0.98 | 0.23 | |
|
| |||||||||||
| Sex | M | 19 | 30 | 13 | 8 | 9 | X2(df) | 0.12(1) | 0.04(1) | 0.00(1) | 0.03(1) |
| F | 13 | 24 | 10 | 7 | 7 | p | 0.73 | 0.85 | 0.99 | 0.99 | |
|
| |||||||||||
| Race | AI | 0 | 4 | 2 | 1 | 1 | X2(df) | 4.14(3) | 1.64(3) | 1.49(3) | 0.00(1) |
| As | 0 | 1 | 1 | 0 | 0 | p | 0.25 | 0.65 | 0.69 | 0.96 | |
| AA | 0 | 1 | 1 | 0 | 0 | ||||||
| W | 32 | 44 | 16 | 13 | 12 | ||||||
|
| |||||||||||
| Ethnicity | H | 0 | 1 | 0 | 0 | 1 | X2(df) | 0.72(1) | - | 1.72(1) | 1.13(1) |
| N | 32 | 44 | 20 | 13 | 11 | p | 0.40 | 0.19 | 0.29 | ||
|
| |||||||||||
| Education | M | 16.6 | 13.8 | 14.1 | 13.8 | 13.3 | t(df) | −5.94(84) | 0.46(36) | 1.24(37) | 0.56(29) |
| SD | 2.1 | 2.1 | 1.7 | 1.7 | 1.7 | p | 0.00* | 0.65 | 0.22 | 0.58 | |
|
| |||||||||||
| Handedness | R | 28 | 44 | 18 | 13 | 10 | X2(df) | 1.87(2) | 0.50(2) | 0.53(2) | 1.36(2) |
| L | 4 | 7 | 3 | 1 | 3 | p | 0.39 | 0.78 | 0.77 | 0.51 | |
| A | 0 | 3 | 1 | 1 | 1 | ||||||
|
| |||||||||||
| MADRS | M | 0.41 | 11.80 | 4.65 | 29.47 | 6.25 | |||||
| SD | 0.67 | 11.78 | 2.09 | 5.78 | 4.06 | ||||||
|
| |||||||||||
| YMRS | M | 0.06 | 9.74 | 2.12 | 3.43 | 26.69 | |||||
| SD | 0.35 | 11.71 | 2.76 | 3.39 | 3.94 | ||||||
|
| |||||||||||
| Medication Class | Li | 0 | 24 | 8 | 6 | 7 | X2(df) | 19.73(1) | 0.16(1) | 0.00(1) | 0.14(1) |
| p | 0.00* | 0.69 | 0.98 | 0.71 | |||||||
| AC | 0 | 17 | 4 | 7 | 5 | X2(df) | 12.56(1) | 1.89(1) | 0.38(1) | 0.56(1) | |
| p | 0.00* | 0.17 | 0.54 | 0.46 | |||||||
| AD | 0 | 23 | 8 | 9 | 3 | X2(df) | 18.61(1) | 0.54(1) | 2.59(1) | 5.00(1) | |
| p | 0.00* | 0.46 | 0.11 | 0.03* | |||||||
| AP | 0 | 24 | 4 | 8 | 9 | X2(df) | 19.73(1) | 3.02(1) | 4.39(1) | 0.14(1) | |
| p | 0.00* | 0.08 | 0.04* | 0.71 | |||||||
| SH | 0 | 26 | 9 | 10 | 6 | X2(df) | 22.08(1) | 0.62(1) | 0.54(1) | 2.14(1) | |
| p | 0.00* | 0.43 | 0.46 | 0.14 | |||||||
AI: American Indian or Alaskan Native, As: Asian, AA: African-American, W: White ; HC: healthy controls, BD: bipolar disorder group, E: euthymic group, D: depressed group, M: manic group; H: Hispanic, N: not Hispanic; R: Right, L: Left, A: Ambidextrous; MADRS: Montgomery Asberg Depression Rating Scale, YMRS: Young Mania Rating Scale; Li: Lithium, AC: Anti-convulsants, AD: Anti-depressants, AP: Anti-psychotics, SH: Sedative-hypnotics
p<0.05
2.2 Imaging Protocol
Participants were imaged using a Siemens TIM Trio 3T MRI system (Siemens Healthcare; Erlangen, Germany) and a vendor-provided 12-channel head receive coil. First, high-resolution T1- and T2-weighted anatomical images were acquired in order to calculate transformations to register individual participant brain images to a common atlas space. T1-weighted imaging parameters were: coronal 3D MP-RAGE; field-of-view = 256×256×256 mm3; sampling matrix = 256×256×256; resolution = 1.0 mm3; TR = 2530 ms; TE = 2.8 ms; TI = 909 ms; flip angle = 10°; BW = 180 Hz/px; and R=2 GRAPPA. T2-weighted imaging parameters were: sagittal 3D SPACE; field-of-view = 260×228×176 mm3; sampling matrix = 256×230×176; resolution = 1.0 mm3; TR = 4000 ms; TE = 406 ms; BW = 592 Hz/px; turbo factor = 121; slice turbo factor = 2; and R = 2 GRAPPA. Second, BOLD fMRI was performed using a T2*-weighted single-shot gradient-echo echo planar imaging with parameters: voxel size = 3.4×3.4×4.0 mm3; field-of-view = 220×220 mm2; sampling matrix = 64×64; # slices = 30; slice thickness/gap = 4.0/1.0 mm; TR = 2000 ms; TE = 30 ms; BW = 2004 Hz/px; fat saturation; and 140 measurements.
The flashing checkerboard stimulus utilized a block design with three flashing checkerboard blocks and four rest blocks (Supplemental Figure 1). The paradigm began with a rest block and then alternated between the flashing checkerboard and rest conditions. Each was 40 s in duration. During the rest condition, the screen was left black, while during the flashing checkerboard condition, a black and white checkerboard pattern was alternated with its inverse at a rate of 4 Hz. A red square (target) was briefly presented in the center of the screen every 4 s throughout the flashing checkerboard blocks, and participants were asked to press a button in response in order to ensure that the participants were awake. Two runs of the functional paradigm were acquired for each participant for each of the 91 imaging sessions.
2.3 Image Processing
Between-participant registration of the percent activation maps was carried out by first using each participant’s T1- and T2-weighted anatomical images to calculate a deformable transformation to a common brain atlas (Halle et al., 2013) using BRAINS Auto Workup (Pierson et al., 2011) and Advanced Normalization Tools (Avants et al., 2011) After aligning the percent activation map to the T1-weighted anatomical image using AFNI, the deformable transformation was then applied to align all participants’ percent activation maps in the common atlas space, thereby allowing voxel-wise group analyses.
Functional MRI data was processed using AFNI (AFNI_2011_12_21_1014 compiled in September, 2015) (Cox, 1996) in order to evaluate the percent activation map in response to the visual stimulus for each participant. Pre-processing included skull-stripping, de-spiking, spatial Gaussian smoothing (5.0 mm full-width-at-half-maximum), and co-registration with the high-resolution T1-weighted anatomical images (including interpolation of the calculated percent activation maps to 1 mm3 voxels).
The time series images from both runs for each participant were fit voxel-wise using a general linear model that included a hemodynamic response function model based on the stimulus block design and nuisance regressors for second-order baseline fluctuations as well as six degrees of motion using AFNI. The voxel-wise general linear model was used to determine the beta coefficient between the hemodynamic response function and BOLD signal. A percent activation map was then generated by normalizing this beta coefficient with the zeroth-order baseline coefficient.
2.4 Statistical Analysis
Demographic information and behavioral performance were analyzed using a t-test for continuous variables and a chi-square test for categorical variables implemented in MATLAB (R2015a). Groups were treated as independent in these analyses.
Group contrasts of percent signal change were performed using a linear mixed model implemented using the fitlme function in MATLAB. In order to test whether it was meaningful to compare mood-state group differences, we first performed an omnibus statistical test on all participants using a full regression model that included a variable for each of the three mood-state groups as well as covariates for age and sex and a reduced model that only included the age and sex covariates. For each voxel, we compared these two models using a likelihood ratio test with three degrees of freedom and only voxels where these two models were significantly different (p < 0.05) were included and non-significant voxels were excluded from between-mood-state-group contrasts.
Contrasts between groups were performed using separate regression models for each comparison. These models included a single variable for group as well as age and sex covariates. Additionally, a random effect for participant (intercept only) allowed the analysis to account for some participants being imaged in multiple mood states. At each voxel, a two-tailed t-statistic and p-value were calculated for each fixed variable. A cluster-based correction method was utilized by calculating an appropriate threshold volume for α= 0.05 with the 3dClustSim function in AFNI. Group contrast images were then thresholded at a cluster (2.309 cm3) level significance of p < 0.05. These clusters were then registered to the Montreal Neurological Imaging (MNI) atlas (Evans et al., 1993) using an affine transform implemented in AFNI.
3. Results
3.1 Bipolar Disorder vs. Healthy Controls
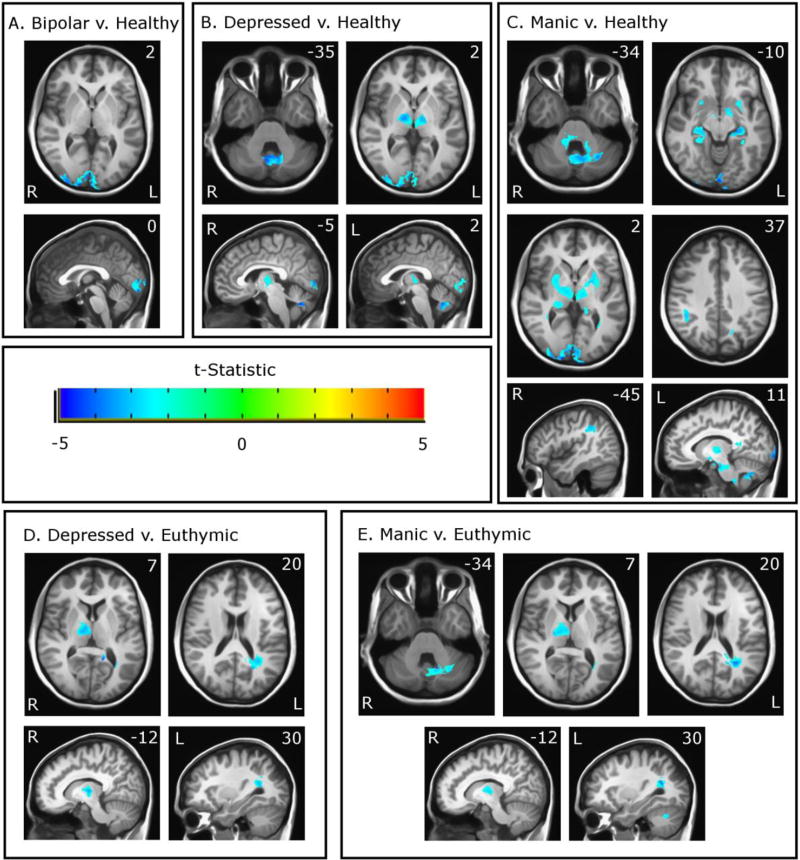
A whole-brain voxel-wise analysis approach was used in order to identify differences in functional activation during the flashing checkerboard paradigm between participants with bipolar disorder and healthy controls (Figure 1 and Table 2). Grouped together, the participants with bipolar disorder exhibited a decreased functional response compared to controls in bilateral visual areas including the cuneal cortex and the occipital pole (Figure 1a).
Figure 1.
Functional differences between the bipolar group and healthy controls (A), between the depressed group and healthy controls (B), between the manic group and healthy controls (C), between the depressed group and the euthymic group (D), and between the manic group and the euthymic group (E) overlaid on MNI space. Colors reflect t-statistic value as shown in color bar. Images are thresholded at p < 0.05 based on cluster-based family-wise error correction. Areas where Group 1 exhibit reduced activation compared to Group 2 are shown in blue.
3.2 Mood State Contrasts
We then compared functional activity during the flashing checkerboard paradigm between mood-state groups (euthymic, depressed, manic) and between mood-state groups and healthy controls. We found no significant differences in functional activity in the contrast between the euthymic group and healthy controls nor did we find significant differences in the contrast between the depressed and manic groups. However, mood-state sensitive differences in functional activity were present between both the manic and depressed group versus healthy controls and between both the manic and depressed groups versus the euthymic group. These differences were present in a set of brain regions including visual areas, thalamus, striatum, hippocampus, cerebellum, and supramarginal gyrus (Figure 1b–e, Table 2). These differences are described below:
Visual Areas
Both the depressed and manic groups exhibited decreased activity in visual areas compared with healthy controls. There were no differences in visual areas between these groups versus the euthymic group.
Thalamus
Functional activity was significantly lower in both the depressed and manic groups in bilateral thalami when compared with healthy controls. When compared with the euthymic group, functional activation in the thalamus was reduced in only the right hemisphere in both the depressed and manic groups.
Cerebellar Vermis
Both the depressed and manic groups exhibited decreased functional activity within the cerebellar vermis when compared with healthy controls. The depressed group did not differ from the euthymic group in the cerebellum, but the manic group showed decreased functional activity in the cerebellar vermis in a cluster that also included adjacent areas of the left posterior lobe.
Striatum and Hippocampus
The manic group had decreased functional activity in bilateral striatum and hippocampus when compared with healthy controls. There were no significant differences in these regions in other contrasts.
Right Supramarginal gyrus
Decreased functional activity was present in the manic group compared with the healthy control group in the right supramarginal gyrus.
Left Posterior Forceps
A cluster of significantly reduced functional activity was present in the manic and depressed groups compared with the euthymic group in a white matter region located immediately posterior to the left lateral ventricle.
3.3 Behavioral Performance
Participants were instructed to press a button in response to a red square (target) presented periodically in the center of the checkerboard. We quantified responses in terms of hits (responding when target present), misses (no response to target), latency (time from target presentation to response), and false positives (responses when the target was absent). Groups did not differ in terms of hits, misses, or latency (Supplemental Tables 2, 3), however the number of false positives was significantly greater in the bipolar disorder group compared to the healthy control group, suggesting a tendency towards disinhibition or hyperactivity in the participants with bipolar disorder. The increase in false positive responses was observed in all of the bipolar subgroups, and thus occurred regardless of mood state.
4. Discussion
This study is unique in investigating functional differences in the processing of visual stimuli between mood states in bipolar disorder. Briefly, our results showed a reduction in stimulus-related activation in visual cortex in bipolar disorder compared with healthy controls. However, mood-state dependent differences in functional activity were observed in a greater number of other brain regions including the cerebellum, thalamus, and striatum while functional activity in these areas during the euthymic state was indistinguishable from healthy controls.
The flashing checkerboard paradigm produced robust activation in visual areas including the cuneal cortex and occipital pole. While differences in metabolic markers (Yuksel et al., 2015) and evoked response potentials have been identified in bipolar disorder (Elvsashagen et al., 2012; Liu et al., 2014; Nazhvani et al., 2013; Yeap et al., 2009) in response to visual stimulation, these prior studies have not explored a relationship between functional abnormalities in visual areas and mood state in bipolar disorder. We identified group differences in these regions not only between bipolar disorder and healthy control groups, but also between both the depressed and manic states and the euthymic state. Our findings suggest that abnormal visual processing, evidenced by reduced functional activity, may be a previously unknown feature of mood states in bipolar disorder.
It remains unclear whether the reduced functional responses in visual cortices represent disruptions that are primary to these regions or whether they reflect differences in regulatory feedback to the visual cortex from other brain regions. There is some evidence for volume reductions in the occipital cortex in Bipolar disorder (Abé et al., 2016), which might explain the reduction in functional activity observed in this study, however preliminary volume analyses of our participant sample did not reveal any significant volume differences (unpublished data). Furthermore, it is unlikely that volume changes would be present between participants in different mood-states. An increased role for occipital connectivity has been observed in seasonal affective disorder (Borchardt et al., 2015) and decreased GABA and increased glutamate in visual areas has been observed in major depression (Sanacora et al., 2004), suggesting that increased baseline connectivity and diminished regulatory feedback in occipital regions may be associated with mood symptoms. Similarly, prior EEG findings suggest a role for attention mechanisms, as the P1 component of the visual evoked potential, (Luck et al., 1994), which has been shown to be reduced in bipolar disorder (Elvsashagen et al., 2012; Liu et al., 2014; Nazhvani et al., 2013; Yeap et al., 2009). Taken together, such studies support the notion that the bipolar disorder-related differences in activation observed in this study occur due to impaired feedback mechanisms. It may therefore be important that these differences were present in both the depressed and manic states, but not in the euthymic state, as this suggests that periodic failures in the regulation of sensory information may underlie transitions between euthymia and active mood-states.
Similarly, previous studies have identified cortical thinning in the right supramarginal gyrus (Hatton et al., 2013) as well as reduced functional activity in response to visual attention, verbal fluency, and emotion processing tasks (Hatton et al., 2013; Sepede et al., 2015). In light of these findings, we might expect that reduced activity in the right supramarginal gyrus during the flashing checkerboard stimulus is related to deficits in visual attention.
Numerous previous studies have identified thalamic and striatal abnormalities in bipolar disorder including increased tissue volume (Aylward et al., 1994; Strakowski et al., 2005b; Strakowski et al., 1999), increased functional signal (Blumberg et al., 2003; Blumberg et al., 2000; O'Connell et al., 1995; Strakowski et al., 2005b), and increased metabolism (Ketter et al., 2001). Furthermore, reward tasks have found increased reward-related activity within the striatum in bipolar disorder (Caseras et al., 2013; Mason et al., 2014; Pacifico and Davis, 2016).
Our findings showed reduced task-related activity in the thalamus and striatum in the depressed and manic groups compared with healthy controls and with the euthymic group. Closer examination of the mean percent signal change in these areas indicates that this occurs due to diminished activation of these regions in the depressed group and a slight deactivation of these areas in the manic group in response to the flashing checkerboard stimulus. Given that prior studies suggest elevated baseline levels of activity within these regions, it is possible that mood-state related reductions in activity within these regions are due to a ceiling effect on BOLD signal due to these regions being constitutively active. We also found a similar reduction in activity within the hippocampus. These three regions are known to play a role in attention and salience (Goldfarb et al., 2016; Kermadi and Boussaoud, 1995; Portas et al., 1998; Rees, 2009), and so altered functional activity within these regions may also be consistent with the presence of altered visual attention in bipolar disorder. Alternatively, these regions are also involved in regulating mood (Cardinal et al., 2002; Haber and Calzavara, 2009; Hare et al., 2005; Phelps, 2004; Vertes, 2006) and so elevated baseline activity and diminished task-related activity may instead be a reflection of altered mood regulation during depressed and manic mood states.
Functionally, the cerebellum has been traditionally thought of as being primarily involved in motor function and timing, and functional deficits in postural control (Bolbecker et al., 2011b), finger tapping (Bolbecker et al., 2011a), and eye blink conditioning (Bolbecker et al., 2009) have been observed in bipolar disorder. However, a role for the cerebellum in emotion processing is now also being appreciated (Konarski et al., 2005; Schmahmann and Caplan, 2006; Schmahmann and Sherman, 1998). Previous studies by our group have shown elevated cerebellar T1ρ in bipolar disorder (Johnson et al., 2015a, b) and others have shown reduced volume in the cerebellar vermis that is associated with the number of previous manic episodes (DelBello et al., 1999; Monkul et al., 2008; Strakowski et al., 2002; Strakowski et al., 2005b). Other cerebellar abnormalities have also been observed in bipolar disorder including altered neurochemistry in the offspring of people with bipolar disorder (Singh et al., 2011) and altered sex-linked morphology (Womer et al., 2009). Our findings here showed mood-state related reductions in functional activity within the cerebellar vermis that were most pronounced in the manic group but were also present in the depressed group compared with healthy controls. Taken together, these findings suggest that the cerebellum is affected by bipolar disorder and may be a key region involved in regulating mood state, particularly manic episodes.
4.1 Limitations
While our findings suggest that alterations in sensory processing networks are present in bipolar disorder and that these differences are mood-state dependent, there are several limitations to this approach. First, the size of the bipolar disorder mood-state groups was modest.
One limitation of the flashing checkerboard paradigm used in this study is the predictable frequency of the target stimuli, which may require less attention than an unpredictably timed target. We were nonetheless able to achieve the desired robust functional activation of visual areas in all participants suggests that this did not occur. Furthermore, response latencies did not differ between groups, suggesting that all groups were performing the task in a similar fashion.
Nearly all participants in the bipolar group were being treated by multiple classes of medications; however our study had too few participants to disentangle their effects. However, the break-down of medication use by class (lithium, anti-convulsants, anti-depressants, anti-psychotics, and sedative/hypnotics) was similar across mood state groups (Table 1) except increased utilization of anti-depressants in participants in the depressed mood state as compared to the manic mood state and increased utilization of anti-psychotics during the manic mood state as compared to euthymia. Targeted enrollment of participants based on use of a specific medication could help mitigate this limitation, but at the cost of generalizability to individuals receiving other treatments. Likewise, a dedicated within-subjects longitudinal design may help better control for medication effects (particularly chronic effects). However, addressing this through a longitudinal design remains challenging, as only a portion of bipolar disorder participants transition between mood states in a given period of time and with these transitions, changes in medication are common.
Finally, these studies tested only a single sensory modality. It would be of interest to discern whether the observations herein are restricted to vision or whether they extend to other sensory modalities. Auditory stimuli might be ideal, as they can also be easily performed in an MRI machine and EEG studies of bipolar disorder have shown deficits in early auditory potentials such as the P50 (Morla-Sanchez et al., 2008).
4.2 Conclusion
We identified reduced functional activity in several brain areas in bipolar disorder in response to a flashing checkerboard stimulus. These reductions were sensitive to mood state, occurring in the depressed and manic states, but not during euthymia. These regions included visual areas, thalamus, striatum, hippocampus, cerebellum, and supramarginal gyrus. Taken together, these differences suggest that visual attention may be altered in bipolar mood states. Further investigation in other sensory modalities and in other illnesses such as major depression, schizophrenia, or ADHD may clarify the role of sensory processing and attention underlying physiology of bipolar mood states and may reveal distinguishing or shared features of these separate illnesses.
Supplementary Material
Supplemental Table 1: Order of Repeated Imaging Sessions by Mood State
Supplemental Table 2: Behavioral Performance by clinical state group
Supplemental Table 3: Behavioral Performance of HC vs. BD
Acknowledgments
The authors would like to thank Janie Myers, Ashley Schumacher, Robin Follmer, Lois Warren, Autumn Craig, and Marla Kleingartner for their help in recruiting and assessing study participants.
Funding:
This study was supported by the generous donation provided by a Roger Koch. Archive and storage of imaging data was supported by the University of Iowa Institute for Clinical and Translational Science (U54TR001013).
J.A.W. was supported by the Department of Veterans Affairs (Merit Award), the NIMH (5R01MH085724), NHLBI (R01HL113863) and a NARSAD Independent Investigator Award.
JGF was supported by the NIMH (K23MH083695) and NHLBI (P01HL014388).
C.P.J. was supported in part by a NARSAD Young Investigator Award.
V.A.M. was supported in part by a NARSAD Independent Investigator Award.
Footnotes
Author Contributions:
Joseph J. Shaffer: Completed statistical analysis and was primary author of the manuscript.
Casey Johnson: Contributed to experimental design, data collection, data analysis, manuscript preparation and review.
Jess Fiedorowicz: Responsible for clinical assessments and managed participant recruitment, data collection, and data management. Contributed to experimental design, interpretation, and manuscript revision.
Gary Christensen: Contributed to study design, interpretation, and manuscript revision.
John Wemmie: Contributed to conception, experimental design, interpretation, writing and editing of manuscript.
Vincent Magnotta: Contributed to conception, experimental design, interpretation, writing and editing of manuscript.
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Iowa Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants provided informed written consent.
Conflicts of Interest: The authors do not have any conflicts of interest to report.
References
- Abé C, Ekman CJ, Sellgren C, Petrovic P, Ingvar M, Landén M. Cortical thickness, volume and surface area in patients with bipolar disorder types I and II. J Psychiatry Neurosci. 2016:240–250. doi: 10.1503/jpn.150093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, Cohen MS. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric, A., American Psychiatric, A., Force, D.S.M.T. Diagnostic and statistical manual of mental disorders : DSM-5 2013 [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, Peyser CE, Pearlson GD. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry. 1994;151:687–693. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH, Peterson BS. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, Silbersweig DA. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- Bolbecker AR, Hong SL, Kent JS, Forsyth JK, Klaunig MJ, Lazar EK, O'Donnell BF, Hetrick WP. Paced finger-tapping abnormalities in bipolar disorder indicate timing dysfunction. Bipolar Disord. 2011a;13:99–110. doi: 10.1111/j.1399-5618.2011.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbecker AR, Hong SL, Kent JS, Klaunig MJ, O'Donnell BF, Hetrick WP. Postural control in bipolar disorder: increased sway area and decreased dynamical complexity. PLoS One. 2011b;6:e19824. doi: 10.1371/journal.pone.0019824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbecker AR, Mehta C, Johannesen JK, Edwards CR, O'Donnell BF, Shekhar A, Nurnberger JI, Steinmetz JE, Hetrick WP. Eye blink conditioning anomalies in bipolar disorder suggest cerebellar dysfunction. Bipolar Disord. 2009;11:19–32. doi: 10.1111/j.1399-5618.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- Borchardt V, Krause AL, Starck T, Nissila J, Timonen M, Kiviniemi V, Walter M. Graph theory reveals hyper-functionality in visual cortices of Seasonal Affective Disorder patients. World J Biol Psychiatry. 2015;16:123–134. doi: 10.3109/15622975.2014.966144. [DOI] [PubMed] [Google Scholar]
- Cardinal R, Parkinson J, Hall J, Everitt B. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo MA, Eliassen JC, Smith CT, Fleck DE, Nelson EB, Strawn JR, Lamy M, DelBello MP, Adler CM, Strakowski SM. Bipolar I disorder and major depressive disorder show similar brain activation during depression. Bipolar Disord. 2014;16:703–712. doi: 10.1111/bdi.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo MA, Fleck DE, Eliassen JC, Smith MS, DelBello MP, Adler CM, Strakowski SM. A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disord. 2012;14:175–184. doi: 10.1111/j.1399-5618.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Lennox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, Suckling J, Bullmore E. Explicit and implicit facial affect recognition in manic and depressed States of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Ooi C, Jacob R, Lupson V, Bullmore ET, Lennox BR. A longitudinal fMRI study of the manic and euthymic states of bipolar disorder. Bipolar Disord. 2010;12:344–347. doi: 10.1111/j.1399-5618.2010.00801.x. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacology. 1999;21:63–68. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Diler RS, de Almeida JR, Ladouceur C, Birmaher B, Axelson D, Phillips M. Neural activity to intense positive versus negative stimuli can help differentiate bipolar disorder from unipolar major depressive disorder in depressed adolescents: a pilot fMRI study. Psychiatry Res. 2013;214:277–284. doi: 10.1016/j.pscychresns.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevski A, Baumann S, Scheider W. A Rapid fMRI Task Battery for Maping of Visual, Motor, Cognitive, and Emotional Function. Neuroimage. 2006;31:732–744. doi: 10.1016/j.neuroimage.2005.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvsashagen T, Moberget T, Boen E, Boye B, Englin NO, Pedersen PO, Andreassen OA, Dietrichs E, Malt UF, Andersson S. Evidence for impaired neocortical synaptic plasticity in bipolar II disorder. Biol Psychiatry. 2012;71:68–74. doi: 10.1016/j.biopsych.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Evans A, Collins D, Mills S, Brown E, Kelly R, Peters T. 3D statistical neuroanatomical models from 305 MRI volumes. Nuclear Science Symposium and Medical Imaging Conference, 1993; 1993 IEEE Conference Record; 1993. pp. 1813–1817. [Google Scholar]
- Fiedorowicz JG, Prossin AR, Johnson CP, Christensen GE, Magnotta VA, Wemmie JA. Peripheral inflammation during abnormal mood states in bipolar I disorder. J Affect Disord. 2015;187:172–178. doi: 10.1016/j.jad.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb EV, Chun MM, Phelps EA. Memory-Guided Attention: Independent Contributions of the Hippocampus and Striatum. Neuron. 2016;89:317–324. doi: 10.1016/j.neuron.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S, Calzavara R. The cortico-basal ganglia integrative network: The role of the thalamus. Brain Research Bulletin. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle M, Talos I-F, Jakab M, Makris N, Meier D, Wald L, Fischl B, Kikinis R. Multi-modality MRI-based Atlas of the Brain. SPL; Boston, MA: 2013. [Google Scholar]
- Hare T, Tottenham N, Davidson M, Glover G, Casey B. Contributions of amygdala and striatal activity in emotion regulation. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hatton SN, Lagopoulos J, Hermens DF, Scott E, Hickie IB, Bennett MR. Cortical thinning in young psychosis and bipolar patients correlate with common neurocognitive deficits. Int J Bipolar Disord. 2013;1 doi: 10.1186/2194-7511-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophr Bull. 2009;35:1059–1064. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Follmer RL, Oguz I, Warren LA, Christensen GE, Fiedorowicz JG, Magnotta VA, Wemmie JA. Brain abnormalities in bipolar disorder detected by quantitative T1ρ mapping. Mol Psychiatry. 2015a;20:201–206. doi: 10.1038/mp.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Follmer RL, Oguz I, Warren LA, Christensen GE, Fiedorowicz JG, Magnotta VA, Wemmie JA. Quantitative T1ρ mapping links the cerebellum and lithium use in bipolar disorder. Mol Psychiatry. 2015b;20:149. doi: 10.1038/mp.2015.10. [DOI] [PubMed] [Google Scholar]
- Keener MT, Phillips ML. Neuroimaging in bipolar disorder: a critical review of current findings. Curr Psychiatry Rep. 2007;9:512–520. doi: 10.1007/s11920-007-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermadi I, Boussaoud D. Role of the primate striatum in attention and sensorimotor processes: comparison with premotor cortex. Neuroreport. 1995;6:1177–1181. doi: 10.1097/00001756-199505300-00026. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, Willis MW, Danielson A, Frye MA, Herscovitch P, Post RM. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–186. [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Li M, Huang C, Deng W, Ma X, Han Y, Wang Q, Li Z, Guo W, Li Y, Jiang L, Lei W, Hu X, Gong Q, Merikangas K, Palaniyappan L, Li T. Contrasting and convergent patterns of amygdala connectivity in mania and depression: A resting-state study. Journal of Affective Disorders. 2015;173:53–58. doi: 10.1016/j.jad.2014.10.044. [DOI] [PubMed] [Google Scholar]
- Liu J, Blond BN, van Dyck LI, Spencer L, Wang F, Blumberg HP. Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disord. 2012;14:432–441. doi: 10.1111/j.1399-5618.2012.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Chen Y, Su T, Hsieh J, Chen L. Abnormal early gamma responses to emotional faces differentiate unipolar from bipolar disorder patients. BioMed Research International. 2014;2014:1–9. doi: 10.1155/2014/906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S, Hillyard S, Mouloua M, Woldorff M, Clark V, Hawkins H. Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. J Exp Psychol Hum Percept Perform. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Montaldi D, Bentall RP, El-Deredy W. Decision-making and trait impulsivity in bipolar disorder are associated with reduced prefrontal regulation of striatal reward valuation. 2014 doi: 10.1093/brain/awu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PB, Malhi GS. Bipolar depression: phenomenological overview and clinical characteristics. Bipolar Disord. 2004;6:530–539. doi: 10.1111/j.1399-5618.2004.00137.x. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Hatch JP, Sassi RB, Axelson D, Brambilla P, Nicoletti MA, Keshavan MS, Ryan ND, Birmaher B, Soares JC. MRI study of the cerebellum in young bipolar patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:613–619. doi: 10.1016/j.pnpbp.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S, Asberg M. A New Depression Scale Designed to be Sensitive to Change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morla-Sanchez E, Garcia-Jimenez M, Barabash A, Martinez-Vizcaino V, Mena J, Cabranes-Diaz J, Baca-Baldomero E, Santos J. P50 sensory gating deficit is a common marker of vulnerability to bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2008;117:313–318. doi: 10.1111/j.1600-0447.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- Nazhvani AD, Boostani R, Afrasiabi S, Sadatnezhad K. Classification of ADHD and BMD patients using visual evoked potential. Clin Neurol Neurosurg. 2013;115:2329–2335. doi: 10.1016/j.clineuro.2013.08.009. [DOI] [PubMed] [Google Scholar]
- O'Connell RA, Van Heertum RL, Luck D, Yudd AP, Cueva JE, Billick SB, Cordon DJ, Gersh RJ, Masdeu JC. Single-photon emission computed tomography of the brain in acute mania and schizophrenia. J Neuroimaging. 1995;5:101–104. doi: 10.1111/jon199552101. [DOI] [PubMed] [Google Scholar]
- Pacifico R, Davis RL. Transcriptome sequencing implicates dorsal striatum-specific gene network, immune response and energy metabolism pathways in bipolar disorder. Molecular Psychiatry. 2016 doi: 10.1038/mp.2016.94. [DOI] [PubMed] [Google Scholar]
- Phelps E. Human emotion and memory: interactions of the amygdala and hippocampal complex. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson R, Johnson H, Harris G, Keefe H, Paulsen JS, Andreasen NC, Magnotta VA. Fully automated analysis using BRAINS: Auto Workup. Neuroimage. 2011;54:328–336. doi: 10.1016/j.neuroimage.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Alonso-Lana S, Moro N, Sarro S, Bonnin M, Goikolea J, Fernandez-Corcuera P, Amann B, Romaguera A, Vieta E, Blanch J, McKenna P, Salvador R. Brain functional changes across the different phases of bipolar disorder. The British Journal of Psychiatry. 2015;206:136–144. doi: 10.1192/bjp.bp.114.152033. [DOI] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A Specific Role for the Thalamus in Mediating the Interaction of Attention and Arousal in Humans. 1998 doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G. Visual Attention: The Thalamus at the Centre? Current Biology. 2009;19:R213–R214. doi: 10.1016/j.cub.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–290. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Sepede G, De Berardis D, Campanella D, Perrucci MG, Ferretti A, Salerno RM, Di Giannantonio M, Romani GL, Gambi F. Neural correlates of negative emotion processing in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:1–10. doi: 10.1016/j.pnpbp.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Singh MK, Spielman D, Libby A, Adams E, Acquaye T, Howe M, Kelley R, Reiss A, Chang KD. Neurochemical deficits in the cerebellar vermis in child offspring of parents with bipolar disorder. Bipolar Disord. 2011;13:189–197. doi: 10.1111/j.1399-5618.2011.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, DelBello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, DelBello MP. Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord. 2002;4:80–88. doi: 10.1034/j.1399-5618.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005a;162:1697–1705. [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005b;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, Lee JH, Welge JA, DelBello MP, Fleck DE, Adler CM. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ, Aleman A, van Buchem MA, van der Wee NJ, Rombouts SA. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes R. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neurosciencw. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Womer FY, Wang F, Chepenik LG, Kalmar JH, Spencer L, Edmiston E, Pittman BP, Constable RT, Papademetris X, Blumberg HP. Sexually dimorphic features of vermis morphology in bipolar disorder. Bipolar Disord. 2009;11:753–758. doi: 10.1111/j.1399-5618.2009.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Reilly RB, Thakore JH, Foxe JJ. Visual sensory processing deficits in patients with bipolar disorder revealed through high-density electrical mapping. Journal of Psychiatry & Neuroscience. 2009;34:459–464. [PMC free article] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A Rating Scale for Mania: Reliability, Validity, and Sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yuksel C, Du F, Ravichandran C, Goldbach JR, Thida T, Lin P, Dora B, Gelda J, O'Connor L, Sehovic S, Gruber S, Ongur D, Cohen BM. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol Psychiatry. 2015;20:1079–1084. doi: 10.1038/mp.2015.13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Order of Repeated Imaging Sessions by Mood State
Supplemental Table 2: Behavioral Performance by clinical state group
Supplemental Table 3: Behavioral Performance of HC vs. BD