Abstract
The contamination of aflatoxins in maize or maize-related products synthesized by Aspergillus flavus causes severe economical loss and threat to human health. Use of eco-friendly phytochemicals has shown potential to inhibit secondary metabolites in Aspergillus species. Thus, A. flavus cultured in corn flour (CF) and corn flour with quercetin (CFQ) was used for protein extraction for proteome analysis using nLC-Q-TOF mass spectrometer. Proteome analysis revealed the expressions of 705 and 843 proteins in CFQ and CF, respectively. Gene Ontology Slim Categories (GOSC) of CF exhibited major transcriptional factors; involved in acetylation and deacetylation of histone proteins, carbohydrate metabolism, and hydrolase activity, whereas GOSC analysis of CFQ showed membrane transport activity, including both influx and efflux proteins. cAMP/PKA signaling pathway was observed in CFQ, whereas MAPK pathway in CF. To quantify biosynthesis of aflatoxin B1 (AFB1) in CF and CFQ, HPLC analysis at 7, 12, 24 and 48 h was carried out which showed decrease in AFB1 (1%) at 7–24 h in CFQ. However, remarkable decrease in AFB1 biosynthesis (51%) at 48 h time point was observed. Thus, the present study provided an insight into the mechanism of quercetin-mediated inhibition of aflatoxin biosynthesis in A. flavus and raises the possibility to use quercetin as an anti-aflatoxigenic agent.
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1067-0) contains supplementary material, which is available to authorized users.
Keywords: Aspergillus flavus, Corn flour, nLC-Q-TOF, Quantitative HPLC analysis, Quercetin
Introduction
Aspergillus flavus is a saprophytic, soil-borne mold frequently encountered in agricultural crops during pre-harvest and post-harvest stage by producing most potent mycotoxin, aflatoxin (AFB1 and AFB2) (Giray et al. 2007; Reddy et al. 2011). Approximately 4.5 billion people living in developing countries are exposed to unchecked amount of aflatoxin which results in acute aflatoxicosis (Mwalwayo and Thole 2016; Obrian et al. 2007). The world health organization advice that low doses with dietary exposure to aflatoxin is a major risk as they can lead to hepatocellular carcinoma (Magnussen and Parsi 2013). Also, A. flavus is one of the major causes of aspergillosis in humans (Thakur et al. 2015). To restrict exposure of aflatoxin, a limit of 20 ppb by most countries, and 8 ppb for AFB1 by European commission has been set up (van Egmond and Jonker 2005; Van Egmond et al. 2007). Due to restrictions, significant economic loss in the agricultural industry has been reported (Guchi 2015).
Maize (Zea mays L.) is a prime food source after rice and wheat (Ranum et al. 2014). Aflatoxin-contaminated maize, if consumed by humans and animals, leads aflatoxin biotransformation to AFB1-exo-8,9-epoxide, which possesses a high carcinogenic activity (Bbosa et al. 2013). A recent research revealed that aflatoxin in corn flour was about 80% which was 10% higher than that of limit set by EU regulation for AFB1 (Krishnan et al. 2015). Recently, RNA-seq studies on maize–A. flavus interaction showed enrichment in aflatoxin biosynthesis pathway genes (Musungu et al. 2016). A considerable amount of research related to maize–pathogen interaction has been carried out (Domijan et al. 2005). Corn products are consumed by human and animals, often susceptible to aflatoxin contagion in comparison to pre- or post-harvested maize crops. Recently, co-culture of A. flavus on maize in different conditions (injured maize kernels, sterilized maize kernels and in vivo conditions) was carried out genotypically and phenotypically, which showed expression of 819 unique genes in A. flavus in different condition at 48 h time point (Reverberi et al. 2013). Until now, limited study has been reported on protein profiling of A. flavus during the interaction with corn. Protein profile of A. flavus has advantage over transcripts as it undergoes post-transcriptional regulation, possibly alter gene products or biosynthetic pathways (Bai et al. 2015).
A sustainable and economical source is required to detoxify aflatoxin from the food chain. Natural phytochemicals (phenolic, thiols, carotenoids, flavonoids, anthocyanin and tocopherol) extracted from different parts of plants such as fruits, vegetables and herbs have showed a wide range of biological effects, including antioxidant, antimicrobial and anti-inflammatory actions. This scenario, therefore, calls for alternate approaches which are economically feasible and eco-friendly to control the contamination and increase the yield (Kumar et al. 2006; Salim et al. 2008).
Studies conducted by Zhou et al. (2015) on the inhibition of AFB1 production in A. flavus by quercetin showed the promising results, viz. aflatoxin inhibition at 800 µg/mL. Quercetin showed activity via inhibiting the production of reactive oxygen species; cytotoxicity and lipid peroxidation in aflatoxin-mediated hepatic damage cells (HepG2) in mice (Choi et al. 2010).
Thus, to gain insight into quercetin-mediated inhibition of aflatoxin biosynthesis in A. flavus, we have carried out quantitative proteome analysis of Aspergillus flavus cultured in corn flour media (CF) and corn flour media with quercetin using nano liquid chromatography-quadrupole time-of-flight mass spectrometry (nLC-Q-TOF). Our study demonstrated that trans-membrane transporter proteins were highly expressed in response to CFQ in comparison to CF. In addition, cAMP/PKA signaling pathway was observed in CFQ in comparison to CF. Also, AFB1 at different time points (7, 12, 24 and 48 h) using quantitative high-performance liquid chromatography (HPLC) was determined. Overall, we also demonstrated the inhibition of aflatoxin biosynthesis in the presence of quercetin in A. flavus.
Materials and methods
Aspergillus flavus culture conditions and quercetin treatment
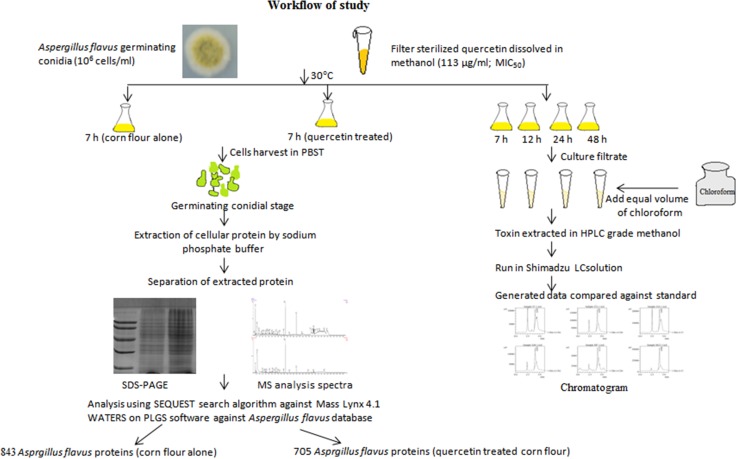
Aspergillus flavus conidia (MTCC9367) were cultured in corn flour powder (20 g/L), peptone (5 g/L) in distilled water for 7 days at 30 °C (Patel et al. 2014). Spores were harvested in phosphate buffer saline (PBS) with 0.05% Tween 20 (PBST), centrifuged, followed by a viability count (numbers of CFU/mL). Working conidial culture of 106 cells/mL was used (Tiwari et al. 2016). A. flavus morphogenesis was assessed at different time points; 2 h through 8 h until germinating conidial stage (conidia with germ tube) was achieved. Morphotypes of conidia or germinating conidia were examined using by Magnus MPS-USB microscope at 40× (Olympus, India). To achieve maximum cellular homogeneity, media, inoculum size, and growth conditions were optimized. Germinating conidial stage was obtained at 7 h, thus considered for large-scale culture. Results from previous studies showed quercetin exhibit MIC50 value of 113 μg/mL, which has been used in our study (Tiwari et al. 2017). Working solution of quercetin (HiMedia, India) at MIC50 (113 μg/mL) was prepared in HPLC grade methanol (Sigma Aldrich, India). For CF and CFQ, 106 cells/mL were used for inoculation (pH 5.7), incubated at 30 °C for 7 h. A. flavus conidia with germ tube were collected for protein extraction and Q-TOF analysis. For HPLC analysis, culture filtrate was extracted at different time points (7, 12, 24 and 48 h) and stored at 4 °C for further analysis. Figure 1 depicts workflow of our study.
Fig. 1.
Experimental design to obtain protein data and HPLC analysis of A. flavus cultured on corn flour with and without quercetin (113 μg/ml) at 30 °C, followed by protein profiling from PLGS software using UniProt database
Protein isolation and sample preparation
CF and CFQ at germinating conidial stage was obtained (7 h), followed by separation by filtration through sterilized muslin cloth, lyophilized (Allied frost FD-3) in liquid nitrogen and subjected to sodium phosphate extraction buffer (Gautam et al. 2008). Precipitation was performed with 5% TCA and collected in rehydration buffer after washing with cold acetone (Tiwari et al. 2016). Protein concentration was estimated by Bradford’s method (Bradford 1976).
SDS-PAGE analysis and nLC-Q-TOF mass spectrometer
As described by LaemmLi et al. (1970) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of proteins obtained from two samples of independent experiment. Protein pellet was reconstituted in sample buffer followed by the addition of β-mercaptoethanol and bromophenol blue 0.02% (w/v). SDS-PAGE (12%) was carried out that showed similar banding pattern from biological replicates. Thus, one of the two biological replicates was used for nLC-Q-TOF analysis. Protein sample was prepared in ammonium bicarbonate buffer (50 mM), precipitated in acetone overnight at − 20 °C. 10 mM DTT was added in precipitated protein followed by incubation at 56 °C followed by overnight trypsin digestion at 37 °C. C18 nano-LC column was used for injection of supernatant for the separation of peptides. nLC-Q-TOF for mass spectrometric analysis was performed for digested samples at Sandor Life sciences Pvt. Ltd., Hyderabad, India (sandorlifesciences.co.in) (Tiwari et al. 2016).
MS and MS/MS analysis and protein identification
Synapt G2 (Waters, India Inc.) was used for the analysis of peptide fractions obtained from nLC column. Ionization of separated peptides was performed using electrospray ionization. The mass spectra were obtained in data dependent mode to acquire MS spectra (m/z 300–1150), MS resolution (60,000) and MS/MS resolution (15,000). MS/MS fragmentation was performed by collisional dissociation cell mode of higher energy with normalized collision energy. Peptides having unassigned charge stage or charge less than 3 were excluded. Quadruple time-of-flight analyzer was used to acquire fragmented spectra followed by acquisition of mass spectra. Parameters were controlled by Protein Lynx Global Services (PLGS) software. Data analysis was done by Mass Lynx 4.1 WATERS based on SEQUEST search algorithm with > 95.0% identity. MS/MS spectra of individual peptides were matched on PLGS software, WATERS (Du et al. 2014). The data, obtained from Aspergillus flavus and Aspergillus species Uniprot database, were generated after removing the outliers. Consistent data showed 843 and 705 cellular proteins in CF and CFQ, respectively, considered for further analysis. Based on identified proteins and their matching peptides, the proteins from CF and CFQ samples were compared (Pieragostino et al. 2012).
Isolation and HPLC analysis of culture filtrate for AFB1 detection
Production of AFB1 was assessed in CF and CFQ in different time points, viz. 7, 12, 24 and 48 h. Conidia at 106 were inoculated aseptically in each flask and kept at 30 °C at 250 × g. Culture supernatant was extracted with three volumes of chloroform (1:1 v/v) at room temperature (25 °C). Organic phase was separated using separating funnel. An equal amount of chloroform was added to different flasks and agitated for 30 min (Ren et al. 2016). Chloroform containing aflatoxin was filtered through Whatman no. 1 filter paper and evaporated to dryness at 37 °C in air-circulated oven. The residues then re-dissolved in 500 µL of HPLC grade methanol (Merck, India) and filtered through a 0.22-µm syringe filter. AFB1 standard was obtained from Sigma, USA, dissolved in HPLC grade methanol (Merck, India) with a concentration of 1 mg/mL, and stored at 4 °C in dark until use. After filtration using 0.22-µm microporous membrane, samples were subjected to Shimadzu LC solution HPLC system. The mobile phase was acetone:methanol:water (1:1:2 v/v) at a flow rate of 1 mL/min. UV detection was at 365 nm. For each injection, a volume of 10 µL of AFB1 standard and unknown samples was loaded. Qualitative HPLC analysis was performed at Institute of Bioengineering and Biological sciences, Varanasi, India. The amount to analyte was calculated by external standard quantitation method using the following formula (Farthing et al. 1992):
Keeping all the values in the above formula, the concentrations of unknown samples were calculated and tabulated for analysis.
Results
Identification of cellular proteins expressed in CF and CFQ
Cellular proteins from CF and CFQ at 7 h time point using nLC-Q-TOF proteome analysis showed the expressions of 843 and 705 proteins, respectively (Table S1). Molecular weight of identified proteins ranged between 2.4–248 kDa and 10.5–249 kDa, respectively. PLGS score was found to be between 9.17–2231.95 in CF and 1.13–2409.65 in CFQ. The estimated sequence coverage ranged from 7% to 100% and 2% to 61%, respectively. Further, 843 and 705 identified proteins were assigned to Gene Ontology Slim Category (GOSC) from the biological process, molecular functions and cellular components (Figure S1). CF showed majority of proteins involved in hydrolase activity such as 1,4-β-xylosidase XlnD, exopolygalacturonase B, feruloyl esterase B 1, glucan 1,3-β-glucosidase A, followed by nucleic acid binding activity such as exosome complex exonuclease Rrp6, SacI domain and endonuclease exonuclease phosphatase, exonuclease. Cellular functions showed the abundance of cytoplasmic protein followed by membrane proteins. Major biological functions showed proteins involved in transcriptional process such as fungal-specific transcription factor, aflatoxin biosynthesis regulatory protein, BZIP transcription factor, apoptosis antagonizing transcription factor, C2H2 transcription factor AmdX, Swi5, Spt6, and carbohydrate metabolism such as α-xylosidase, xlnD, α-galactosidase B, α-l arabinofuranosidase A, β-galactosidase A. In addition, a set of proteins was associated with protein transport activity transport protein Sec23, Sec10, Sterol-3-β glucosyltransferase, etc. On the other side, protein profile of CFQ showed that a majority of proteins were involved in transferase activity such as 1,3-β-glucanosyltransferase, acetyltransferase GNAT domain, AICARFTIMPCHase bienzyme, and amino acid N-acetyltransferase subunit Mak10, followed by protein binding activity such as 26S proteasome regulatory subunit Rpn2, 39S mitochondrial ribosomal protein L46, and 5-AMP-activated protein kinase. Cellular ontology showed that the majority of proteins were from protein complex. Out of 705 proteins, 86 proteins from integral components of membrane showed expression. As shown in biological functions, majority of proteins were involved in organic substrate transport and cellular response to stress. CFQ also showed expression of quercetin 2,3-dioxygenase family protein that is reported in A. flavus for dioxygenase activity involved in defense.
Comparative analysis of trans-membrane proteins expressed in A. flavus grown on CF and CFQ
Gene ontology analysis revealed that 120 proteins were involved in organic compound transport in CFQ. Among these, 34 proteins were involved in trans-membrane transport activity such as ABC multidrug transporter, ABC bile acid transporter, ABC transporter trans-membrane, integral plasma membrane protein, Na+/H+ antiporter Nha1, adaptin, allantoate permease, ankyrin repeat protein, importin-β-N-terminal domain protein, and E1-E2 ATPase. Proteins involved in amino acid transport were also observed, viz. amino acid permeases, aspartate aminotransferase, intracellular protein transporter UsoA, and OPT oligopeptide transporter protein. Various carbohydrate trans-membrane transporter proteins were also observed such as MFS sugar transport, monocarboxylate permease, sugar, and other transporter, sterol-3-β-glucosyltransferase. Other class of trans-membrane transporter proteins included proteins involved in ion transport activity are calcium-transporting ATPase, cation chloride co-transporter, phosphate transporter, plasma membrane zinc ion transporter, and siderophore ion transporter. These all proteins may provide an insight into the transportation mechanism of CFQ. Results revealed that CF showed less expression of trans-membrane proteins in comparison with CFQ. Current data on proteins was compared with different studies on A. flavus and are presented in Table 1.
Table 1.
Comparative analysis of proteins present/absent in different studies on the basis of different biological functions
| Identified proteins | A. flavus grown on CFQ | A. flavus grown on CF | A. flavus grown on SD broth (Tiwari et al. 2016) | A. flavus–maize interaction, mycelia stage (Reverberi et al. 2013) | A. flavus mycelia stage proteins (Pechanova et al. 2013) |
|---|---|---|---|---|---|
| Trans-membrane transporter proteins | |||||
| ABC bile acid transporter | + | − | − | + | − |
| ABC multidrug transporter | + | + | − | − | − |
| ABC transporter trans-membrane | + | + | − | + | + |
| ABC-2 type transporter | + | − | − | − | |
| Adaptin N-terminal region | + | − | − | + | − |
| Allantoate permease | + | − | − | − | − |
| Amino acid permease | + | − | − | + | + |
| Ankyrin repeat protein | + | + | − | − | + |
| Aspartate aminotransferase | + | + | − | + | − |
| Ferric reductase | + | − | − | − | − |
| ATP-binding cassette transporter | + | − | − | − | − |
| Calcium-transporting ATPas | + | − | − | − | − |
| Cation chloride co-transporter | + | − | − | − | − |
| Importin-β N-terminal domain protein | + | + | − | + | − |
| Importin 13 | + | + | − | − | − |
| Major facilitator super family protein | + | − | − | − | − |
| Meiotically up-regulated protein 113 | + | − | − | − | − |
| MFS sugar transporter | + | − | − | + | − |
| Sugar and other transporters | + | + | − | + | − |
| MIT microtubule interacting and transport domain protein | + | − | − | − | − |
| Monocarboxylate permease | + | − | − | − | − |
| Nitrogen permease regulator of amino acid transport activity 3 | + | − | − | − | − |
| OPT oligopeptide transporter | + | − | − | − | − |
| Phosphate transporter | + | − | − | − | + |
| Plasma membrane zinc ion transporter | + | − | − | + | − |
| Siderophore iron transporter | + | − | − | − | − |
| Sugar and other transporters | + | − | − | + | − |
| VHS domain protein | + | − | − | − | − |
| Vitamin H transporter | + | − | − | − | − |
| V-type proton ATPase proteolipid | + | − | − | − | − |
| Nitrate transporter | − | + | − | + | − |
| Get1 | − | + | + | − | − |
| Mch1 | − | + | − | − | − |
| Oxidative stress response | |||||
| Catalase A | + | + | − | + | − |
| Catalase-peroxidase | + | + | + | − | − |
| PpoA | + | − | − | − | − |
| PpoC | + | − | − | − | − |
| Fatty acid oxygenase | + | − | + | − | − |
| Cat1 | + | + | − | − | + |
| Pgh2/cox2 | + | − | − | − | − |
| RTA1 | + | − | − | − | − |
| Xyl1 | − | + | + | − | − |
| Psi-producing oxygenase A | − | + | + | − | − |
| Nst1 | + | + | + | − | − |
| Erp38 | − | + | − | − | + |
| TigA | + | − | + | − | − |
| Uba4 | + | + | + | − | − |
Comparative analysis of proteins related to oxidative stress
Production of harmful and toxigenic aflatoxin B1 in A. flavus has been postulated to be the result of oxidative stress conditions (Fountain et al. 2016). Hence, oxidative stress and metabolism of secondary metabolite is possibly interlinked. Protein profile of CFQ showed the production of various antioxidant proteins/enzymes such as catalase A, catalase-peroxidase, mycelia catalase Cat1, fatty acid oxygenase PpoA and PpoC, cyclooxygenase Cox2, Nst, RTA1-like protein, Uba4, and Hsp70. These all findings revealed that quercetin-induced A. flavus produces various oxidative stress response proteins. To confirm the expression of oxidative stress-related proteins, it was compared with control which showed the expression of less protein in response to oxidative stress; major proteins were NADPH-dependent d-xylose reductase Xyl1, Nst1, PpoC, Hsp60, Hsp70, etc. However, the differential expression analysis showed up-regulation of PpoC (2.48-fold) and down-regulation of Nst1 (0.74-fold) and Cat1 (0.22-fold) in CFQ v/s CF. Expression of proteins in response to oxidative stress in our study was compared with different reported studies such as A. flavus grown on SD broth (7 h), microarray studies on maize kernels and related products (48 h) and A. flavus mycelia stage (48 h). The analysis has been listed in Table 1.
Signaling pathway
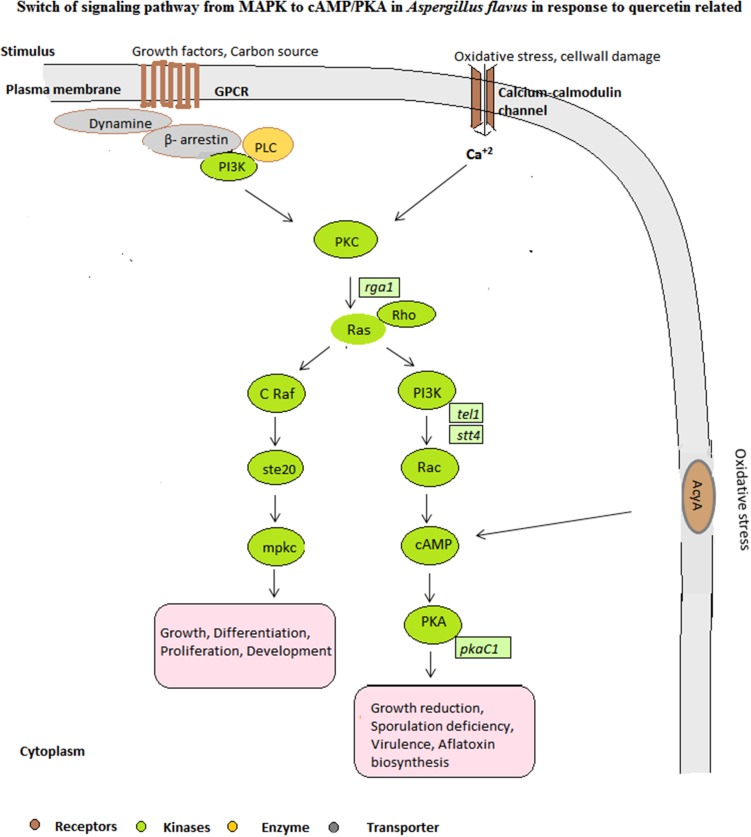
We observed MAPK signaling pathway in carbon source utilization such as corn in CF. Enzymes such as Mpkc, PKC, serine threonine protein kinase (Ste20, SepH, Kcc4 and Sky1), AcyA were observed in our study from signaling pathway. A remarkable switch was observed in signaling cascade when quercetin was introduced in CF. Out of 705 proteins, 24 proteins from signal transduction pathway in CFQ were majorly involved in GPCR-mediated cAMP/PKA signaling pathway and PKC signaling pathway as proteins related to both the pathways were reported, viz. AcyA, cAMP-dependent protein kinase catalytic subunit PKAC1, cAMP-specific phosphodiesterase, GTPase-activator protein for ras-like GTPas, guanyl-nucleotide exchange factor (Sec7), phosphoinositide phospholipase C, PKC, Ras GTPase-activating protein, Ras guanine nucleotide exchange protein, and Rho GTPase activator (Bem2). The absence of MAPK pathway-related proteins showed that Raf may be suppressed by cAMP leads to the inhibition of MAPK signaling pathway. These findings may be helpful in discovering signaling-mediated drug targets. The switch of pathway from MAPK to cAMP/PKA in CFQ is hypothetically represented in Fig. 2.
Fig. 2.
Hypothesized signal transduction pathway at germinating A. flavus conidia with and without quercetin (stress). A. flavus grown on corn flour showed MAPK pathway, and a switch of signaling pathway to cAMP/PKA in response to quercetin
Quercetin treatment inhibits several important aflatoxin biosynthesis intermediates in aflatoxin gene cluster
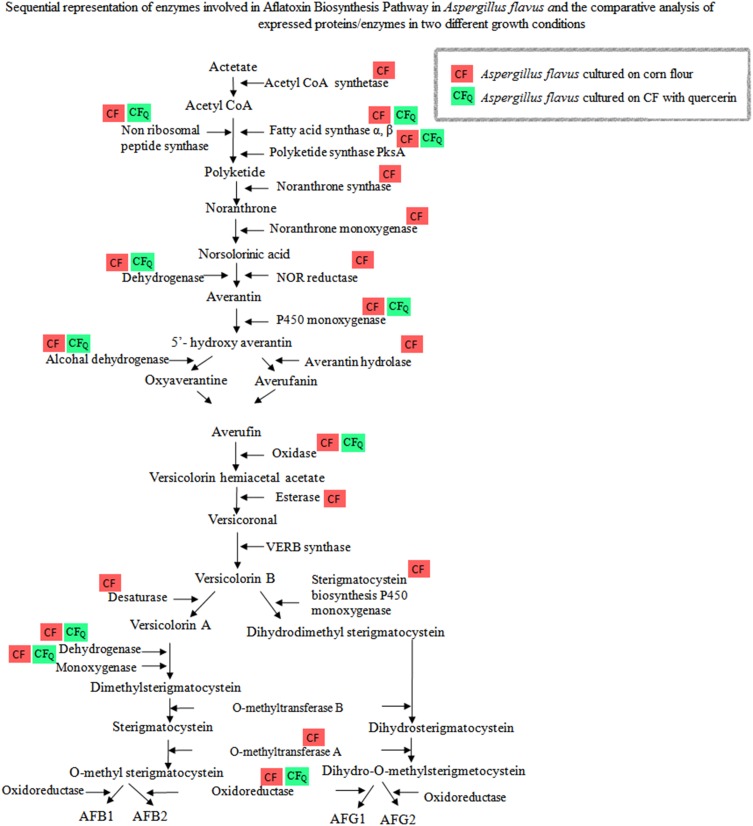
CFQ showed reduced number of expressed proteins related to aflatoxin B1 production when compared with CF. nLC-Q-TOF analysis of CFQ showed expression of polyketide synthase AflC/PksA/PksL1, and fatty acid synthase subunit-α and β. However, the control used in our study showed majority of enzymes involved in aflatoxin biosynthesis pathway (Shankar 2013) such as AflR (regulatory protein), nonribosomal peptide synthetase 10, subunit α and β of sterigmatocystin fatty acid synthase, sterigmatocystin biosynthesis polyketide synthase, polyketide synthase, noranthrone synthase, and noranthrone monooxygenase. (Fig. 3). The expression of ACoA synthetase involved in the synthesis of a precursor substrate, acetyl coenzyme A was observed in CF, whereas showed no expression in CFQ. Taking into account of aflatoxin biosynthetic pathway, there were no protein observed after PksA, required to form norsolorinic acid, a first product in aflatoxin biosynthesis pathway. This may suggest that mechanism of quercetin action may involve through inhibition of PksA enzyme.
Fig. 3.
Expressed level of aflatoxin pathway enzymes obtained in A. flavus grown on CF and CFQ
Quantitative HPLC analysis revealed quercetin-mediated aflatoxin B1 inhibition after 24 h of germination
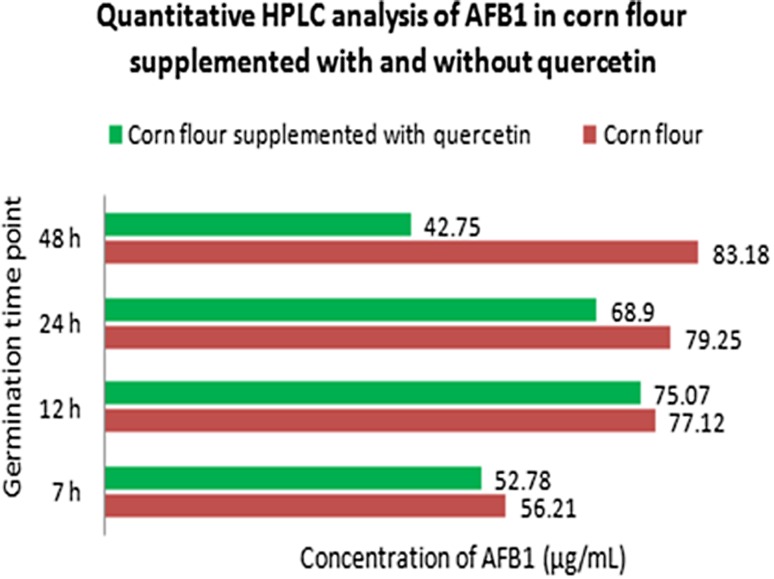
CFQ was compared with CF, for inhibited A. flavus AFB1 production through HPLC analysis. Using HPLC method, AFB1 peaks were efficiently resolved and were present in each sample, when compared with control (Figure S2). Results showed decrease in AFB1 (1%) at 7–24 h in CFQ. However, a significant decrease in AFB1 (51%) at 48 h time point was observed, suggesting quercetin as a potent inhibitor of aflatoxin production. This analysis showed the efficiency of A. flavus to form aflatoxin more vigorously in the presence of favorable substrate (CF). The comparative analysis of HPLC data is represented in Fig. 4.
Fig. 4.
Comparative analysis of aflatoxin B1 production measured by HPLC in culture filtrate of A. flavus grown on CF and CFQ at different time points (7, 12, 24 and 48 h)
Discussion
Comparative proteome analysis of CF and CFQ showed the majority of proteins were involved in transferase activity and the stress response activity. Transportation is the major biological function observed in our study in response to CFQ. Influx and efflux transporters come from two major super families, one is solute carrier family protein for influx of substrate and other one is ATP-binding cassette (ABC) and MFS superfamily (Natesan et al. 2013). In our study, ABC multidrug transporter proteins were induced, indicating the resistive action of A. flavus in CFQ. Solute carrier proteins include organic ion transporting peptide, organic cation transporter and organic ion transporters that are ATP independent (Girardin 2006). Sodium–hydrogen exchanger family protein (Nha1), aspartate aminotransferase, cation chloride co-transporter, importin-β N-terminal domain protein, phosphate transporter, plasma membrane zinc ion transporter, and siderophore iron transporter may involve in influx of quercetin in A. flavus. A docking study hypothesized that transport of quercetin across the membrane is mediated by glucose transporter, which involves efflux of glucose and influx of quercetin (Cunningham et al. 2006). Our study showed various glucose transporters such as UDP-glucose:glycoprotein glucosyltransferase, MFS sugar transporter, and monocarboxylate permease. These all proteins may participate in the influx of quercetin across the A. flavus plasma membrane. Comparative studies without phytochemical showed few ABC transporter proteins, and influx proteins were also not observed (Tiwari et al. 2016). This finding states that transporter protein is substrate and stress dependent. In coherence with Gautam et al. (2008) during the introduction of amphotericin B to Aspergillus fumigatus transcriptome, revealed the expression of cell stress proteins and transport proteins. In addition our data also showed the formation of quercetin 2,3-dioxygenase, which is involved in the degradation of quercetin-forming carboxylic acid instead of carbon monoxide, and hence inhibiting jasmonic acid pathway as suggested in the previous studies (El Hadrami et al. 2015; Walsh et al. 2004). These observations suggest that expression of trans-membrane transporter proteins is substrate and stress dependent.
Aspergillus species respond to environmental stimuli such as nutrients, physical/chemical stimuli, and various stress conditions such as oxidative and osmotic stress. Heat shock proteins play important role in response to stress in fungi (Tiwari et al. 2015). Hsp70 and Hsp60 expressed in our studies showed that Hsps are key stress-related proteins. Regulation of germination, mycotoxin production, sporulation and stress tolerance has been reported by components of signaling pathways, viz. cAMP/PKA or MAPK pathway in Aspergillus species (Brodhagen and Keller 2006). The first line of defense against oxidative stress is cell wall integrity and signaling pathway. The signal transduction pathway majorly involves MAPK signaling pathway when the growth conditions is favorable and also in oxidative stress response (Kim et al. 2006; Tiwari et al. 2016). None of the proteins related to MAPK pathway was observed in CFQ, showing quercetin suppresses MAPK pathway in A. flavus in early germination stage. Supporting to this study, Chen and Dickman (2005) showed that cAMP pathway inhibits MAPK pathway, which is involved in the formation of sclerotium. They also revealed that PKA-independent cAMP inhibits Ras, which is an upstream activator of MAPK pathway, hence involved in Ras mediated MAPK inhibition by cAMP. In another study by Dumaz et al. (2002) showed that cAMP was found to inhibit the cell growth by phosphorylating Raf1, hence blocking its activity in NIH 3T3 cells (Dumaz et al. 2002). PKC signaling was observed in response to caspofungin treatment in Saccharomyces cerevisiae and Candida albicans which involves calcineurin function and activation of downstream MAPK pathway, hence provides drug resistance (LaFayette et al. 2010). Hence, we suggest that specificity of the signaling transduction pathway may depend on the fungal species and the type of stress condition. Alterations in signaling pathways in CFQ were observed which showed cAMP/PKA pathway in response to quercetin-mediated stress.
It has been observed that the quercetin inhibits aflatoxin production (Choi et al. 2010) and exhibits anti-aspergillus properties (Tiwari et al. 2017). Our study revealed that A. flavus expressed limited set of enzymes including PksA in CFQ. This showed that inhibition of aflatoxin biosynthetic pathway in A. flavus in CFQ. In addition, A. flavus produces aflatoxin at germination or post-germination stage. Similar studies on protein profile of Aspergillus terreus at germination stage showed 10 enzymes related to geodin (mycotoxin) production (Thakur and Shankar 2017). HPLC data in CFQ showed AFB1 production have decreased post germination. In other similar study, the decrease in expression of genes from aflatoxin pathway was observed due to 2-phenylethanol at 24 h time point, which further decreased at 48 and 72 h (Chang et al. 2015). However, CF showed most of the important enzymes in aflatoxin biosynthesis pathway including transcriptional factors aflR. Our study also showed similarity with the work of Zhou et al. (2015) indicating inhibitory effect on AFB1 production by A. flavus under the influence of quercetin at 800 µg/mL using HPLC analysis. Hence, quercetin can be a potential compound and can be used with various corn products to make it anti-aflatoxigenic and safe.
Identification of an effective and eco-friendly approach inhibiting biosynthesis of AFB1 in A. flavus is a critical area of research for mitigation of aflatoxin contamination in human foods and feeds. In this study, we showed that quercetin is an efficient anti-aflatoxigenic agent in A. flavus. Based on the results from nLC-Q-TOF and quantitative HPLC analysis approach, we also attempt to elucidate the inhibition of AFB1 by quercetin, expression of proteins and enzymes at germination stage of A. flavus, and the possible mechanism of inhibition of AFB1 biosynthesis. Results revealed the inability of A. flavus to form aflatoxin biosynthesis enzymes in the presence of quercetin. Comparative proteomics of CFQ v/s CF showed switch of cAMP/PKA from MAPK pathway, activation of various transport proteins involved in influx of quercetin and efflux ABC transporter in CFQ at early stage of germination in A. flavus. Results also suggest that MAPK pathway is the dominant pathway active in aflatoxin biosynthesis. Hence, we suggest that quercetin has potential as anti-aflatoxigenic agent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology, Solan, Himachal Pradesh, India, for providing facilities and financial support to Ph.D. student ST.
Abbreviations
- CF
Aspergillus flavus cultured in corn flour alone (corn flour powder; 20 g/L, peptone; 5 g/L)
- CFQ
Aspergillus flavus cultured in CF with quercetin (MIC50: 113 μg/ml)
- nLC-Q-TOF
Nano liquid chromatography-quadrupole time-of-flight mass spectrometry
- PLGS
Protein Lynx Global Services
- GOSC
Gene Ontology Slim Category
- SDS-PAGE
Sodium dodecyl sulfate Polyacrylamide Gel Glectrophoresis
- HPLC
High-Performance Liquid Chromatography
Authors’ contributions
ST and JS conceived and designed the experiments. ST performed the experiments. ST and JS analyzed the data. JS contributed reagents/materials/analysis tools. ST and JS contributed in writing the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1067-0) contains supplementary material, which is available to authorized users.
References
- Bai Y, Wang S, Zhong H, Yang Q, Zhang F, Zhuang Z, Yuan J, Nie X, Wang S. Integrative analyses reveal transcriptome-proteome correlation in biological pathways and secondary metabolism clusters in A. flavus in response to temperature. Sci Rep. 2015;5:14582. doi: 10.1038/srep14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bbosa GS, Kitya D, Odda J, Ogwal-Okeng J. Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health. 2013;5:14–34. doi: 10.4236/health.2013.510A1003. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brodhagen M, Keller NP. Signalling pathways connecting mycotoxin production and sporulation. Mol Plant Pathol. 2006;7:285–301. doi: 10.1111/j.1364-3703.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- Chang P-K, Hua SST, Sarreal SBL, Li RW. Suppression of aflatoxin biosynthesis in Aspergillus flavus by 2-phenylethanol is associated with stimulated growth and decreased degradation of branched-chain amino acids. Toxins. 2015;7:3887–3902. doi: 10.3390/toxins7103887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Dickman MB. cAMP blocks MAPK activation and sclerotial development via Rap-1 in a PKA-independent manner in Sclerotinia sclerotiorum. Mol Microbiol. 2005;55:299–311. doi: 10.1111/j.1365-2958.2004.04390.x. [DOI] [PubMed] [Google Scholar]
- Choi K-C, et al. Inhibitory effects of quercetin on aflatoxin B 1-induced hepatic damage in mice. Food Chem Toxicol. 2010;48:2747–2753. doi: 10.1016/j.fct.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Cunningham P, Afzal-Ahmed I, Naftalin RJ. Docking studies show that d-glucose and quercetin slide through the transporter GLUT1. J Biol Chem. 2006;281:5797–5803. doi: 10.1074/jbc.M509422200. [DOI] [PubMed] [Google Scholar]
- Domijan A-M, Peraica M, Jurjević Ž, Ivić D, Cvjetković B. Fumonisin B1, fumonisin B2, zearalenone and ochratoxin A contamination of maize in Croatia. Food Addit Contam. 2005;22:677–680. doi: 10.1080/02652030500132927. [DOI] [PubMed] [Google Scholar]
- Du L-Y, et al. Identification of the metabolites of myricitrin produced by human intestinal bacteria in vitro using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Expert Opin Drug Metab Toxicol. 2014;10:921–931. doi: 10.1517/17425255.2014.918954. [DOI] [PubMed] [Google Scholar]
- Dumaz N, Light Y, Marais R. Cyclic AMP blocks cell growth through Raf-1-dependent and Raf-1-independent mechanisms. Mol Cell Biol. 2002;22:3717–3728. doi: 10.1128/MCB.22.11.3717-3728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hadrami A, Islam MR, Adam LR, Daayf F. A cupin domain-containing protein with a quercetinase activity (VdQase) regulates Verticillium dahliae’s pathogenicity and contributes to counteracting host defenses. Front Plant Sci. 2014;6:440. doi: 10.3389/fpls.2015.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farthing D, Karnes T, Gehr TW, March C, Fakhry I, Sica DA. External-standard high-performance liquid chromatographic method for quantitative determination of furosemide in plasma by using solid-phase extraction and on-line elution. J Pharm Sci. 1992;81:569–571. doi: 10.1002/jps.2600810621. [DOI] [PubMed] [Google Scholar]
- Fountain JC, Bajaj P, Pandey M, Nayak SN, Yang L, Kumar V, Jayale AS, Chitikineni A, Zhuang W, Scully BT, Lee RD, Kemerait RC, Varshney RK, Guo B. Oxidative stress and carbon metabolism influence Aspergillus flavus transcriptome composition and secondary metabolite production. Sci Rep. 2016;6:38747. doi: 10.1038/srep38747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P, Shankar J, Madan T, Sirdeshmukh R, SundaramCS Gade WN, Basir SF, Sarma PU. Proteomic and transcriptomic analysis of Aspergillus fumigatus on exposure to amphotericin B. Antimicrob Agents Chemother. 2008;52:4220–4227. doi: 10.1128/AAC.01431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin F. Membrane transporter proteins: a challenge for CNS drug development. Dialogues Clin Neurosci. 2006;8:311. doi: 10.31887/DCNS.2006.8.3/fgirardin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giray B, Girgin G, Engin AB, Aydın S, Sahin G. Aflatoxin levels in wheat samples consumed in some regions of Turkey. Food Control. 2007;18:23–29. doi: 10.1016/j.foodcont.2005.08.002. [DOI] [Google Scholar]
- Guchi E. Implication of aflatoxin contamination in agricultural products. Am J Food Nutr. 2015;3:12–20. [Google Scholar]
- Kim JH, Campbell B, Mahoney N, Chan K, May G. Targeting antioxidative signal transduction and stress response system: control of pathogenic Aspergillus with phenolics that inhibit mitochondrial function. J Appl Microbiol. 2006;101:181–189. doi: 10.1111/j.1365-2672.2006.02882.x. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Ghadevaru S, Manimehalai N, Athmaselvi K, Padmavati R. Determination of aflatoxin B1 in corn flour using high performance liquid chromatography. Int J Adv Biol Res. 2015;5:172–176. [Google Scholar]
- Kumar VP, Chauhan NS, Padh H, Rajani M. Search for antibacterial and antifungal agents from selected Indian medicinal plants. J Ethnopharmacol. 2006;107:182–188. doi: 10.1016/j.jep.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LaFayette SL, Collins C, Zaas AK, Schell WA, Betancourt-Quiroz M, Gunatilaka AA, Perfect JR, Cowen LE. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 2010;6:e1001069. doi: 10.1371/journal.ppat.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnussen A, Parsi MA. Aflatoxins, hepatocellular carcinoma and public health. World J Gastroenterol. 2013;19:1508–1512. doi: 10.3748/wjg.v19.i10.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musungu BM, Bhatnagar D, Brown RL, Payne GA, OBrian G, Fakhoury AM, Geisler M. A network approach of gene co-expression in the Zea mays/Aspergillus flavus pathosystem to map host/pathogen interaction pathways. Front Gen. 2016;7:206. doi: 10.3389/fgene.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwalwayo DS, Thole B. Prevalence of aflatoxin and fumonisins (B1 + B2) in maize consumed in rural Malawi. Toxicol Rep. 2016;3:173–179. doi: 10.1016/j.toxrep.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan SK, Lamichchane A, Swaminathan S, Wu W. Differential expression of ATP-binding cassette and/or major facilitator superfamily class efflux pumps contributes to voriconazole resistance in Aspergillus flavus. Diagn Microbiol Infect Dis. 2013;76:458–463. doi: 10.1016/j.diagmicrobio.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Obrian G, Georgianna DR, Wilkinson JR, Yu J, Abbas HK, Bhatnagar D, Cleveland TE, Nierman W, Payne GA. The effect of elevated temperature on gene transcription and aflatoxin biosynthesis. Mycologia. 2007;99:232–239. doi: 10.1080/15572536.2007.11832583. [DOI] [PubMed] [Google Scholar]
- Patel TK, Anand R, Singh AP, Shankar J, Tiwary BN. Evaluation of aflatoxin B1 biosynthesis in A. flavus isolates from central india and identification of atoxigenic isolates. Biotechnol Bioproc Eng. 2014;19:1105–1113. doi: 10.1007/s12257-014-0464-z. [DOI] [Google Scholar]
- Pechanova O, Pechan T, Rodriguez JM, Paul Williams W, Brown AE. A two-dimensional proteome map of the aflatoxigenic fungus Aspergillus flavus. Proteomics. 2013;13:1513–1518. doi: 10.1002/pmic.201100659. [DOI] [PubMed] [Google Scholar]
- Pieragostino D, et al. Differential protein expression in tears of patients with primary open angle and pseudoexfoliative glaucoma. Mol Biosyst. 2012;8:1017–1028. doi: 10.1039/C1MB05357D. [DOI] [PubMed] [Google Scholar]
- Ranum P, Peña-Rosas JP, Garcia-Casal MN. Global maize production, utilization, and consumption. Ann N Y Acad Sci. 2014;1312:105–112. doi: 10.1111/nyas.12396. [DOI] [PubMed] [Google Scholar]
- Reddy KRN, Raghavender CR, Salleh B, Reddy CS, Reddy BN. Potential of aflatoxin B1 production by Aspergillus flavus strains on commercially important food grains. Int J Food Sci Tech. 2011;46:161–165. doi: 10.1111/j.1365-2621.2010.02468.x. [DOI] [Google Scholar]
- Ren S, et al. Global phosphoproteomic analysis reveals the involvement of phosphorylation in aflatoxins biosynthesis in the pathogenic fungus Aspergillus flavus. Sci Rep. 2016 doi: 10.1038/srep34078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverberi M, Punelli M, Scala V, Scarpari M, Uva P, Mentzen WI, Dolezal AL, Woloshuk C, Pinzari F, Fabbri AA, Fanelli C, Payne GA. Genotypic and phenotypic versatility of Aspergillus flavus during maize exploitation. PLoS ONE. 2013;8:e68735. doi: 10.1371/journal.pone.0068735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim AA, Chin Y-W, Kinghorn AD. Bioactive molecules and medicinal plants. Columbus: Springer; 2008. pp. 1–24. [Google Scholar]
- Shankar J. An overview of toxins in Aspergillus associated with pathogenesis. Int J Life Sci Biotechnol Pharma Res. 2013;2:16–31. [Google Scholar]
- Thakur R, Shankar J. Proteome profile of Aspergillus terreus conidia at germinating stage: identification of probable virulent factors and enzymes from mycotoxin pathways. Mycopathologia. 2017 doi: 10.1007/s11046-017-0161-5. [DOI] [PubMed] [Google Scholar]
- Thakur R, Anand R, Tiwari S, Singh AP, Tiwary BN, Shankar J. Cytokines induce effector T-helper cells during invasive aspergillosis; what we have learned about T-helper cells? Front Microbiol. 2015;6:429. doi: 10.3389/fmicb.2015.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Thakur R, Shankar J. Role of heat-shock proteins in cellular function and in the biology of fungi. Biotechnol Res Int. 2015;2015:132635. doi: 10.1155/2015/132635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Thakur R, Goel G, Shankar J. Nano-LC-Q-TOF analysis of proteome revealed germination of Aspergillus flavus conidia is accompanied by MAPK signalling and cell wall modulation. Mycopathologia. 2016;181:769–786. doi: 10.1007/s11046-016-0056-x. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Gupta N, Udaybanu M, Shankar J. Anti-aspergillus properties of phytochemicals against aflatoxin producing Aspergillus flavus and Aspergillus parasiticus. Natl Acad Sci Lett. 2017;40:267–271. doi: 10.1007/s40009-017-0569-y. [DOI] [Google Scholar]
- Van Egmond HP, Jonker MA. Aflatoxin and food safety. London: CRC Press; 2005. Worldwide regulations on aflatoxins; pp. 77–94. [Google Scholar]
- Van Egmond HP, Schothorst RC, Jonker MA. Regulations relating to mycotoxins in food. Anal Bioanal Chem. 2007;389:147–157. doi: 10.1007/s00216-007-1317-9. [DOI] [PubMed] [Google Scholar]
- Walsh J, Long J, Nivens D, Lynch W. Isolation and purification of quercetin 2,3-dioxygenase from Aspergillus flavus via lectin affinity chromatography. J Undergrad Chem Res. 2004;2:51. [Google Scholar]
- Zhou W, Hu L-B, Zhao Y, Wang M-Y, Zhang H, Mo H-Z. Inhibition of fungal aflatoxin B1 biosynthesis by diverse botanically-derived polyphenols. Trop J Pharm Res. 2015;14:605–609. doi: 10.4314/tjpr.v14i4.7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.