Abstract
The overall clinical outcome in T-cell acute lymphoblastic leukemia (T-ALL) can be improved by minimizing risk for treatment failure using effective pharmacological adjuvants. Phloridzin (PZ), a flavonoid precursor found in apple peels, was acylated with docosahexaenoic acid (DHA) yielding a novel ester known as phloridzin docosahexaenoate (PZ-DHA). Here, we have studied the cytotoxic effects of PZ-DHA on human leukemia cells using in vitro and in vivo models. The inhibitory effects of PZ-DHA were tested on human Jurkat T-ALL cells in comparison to K562 chronic myeloid leukemia (CML) cells and non-malignant murine T-cells. PZ-DHA, not PZ or DHA alone, reduced cell viability and ATP levels, increased intracellular LDH release, and caused extensive morphological alterations in both Jurkat and K562 cells. PZ-DHA also inhibited cell proliferation, and selectively induced apoptosis in Jurkat and K562 cells while sparing normal murine T-cells. The cytotoxic effects of PZ-DHA on Jurkat cells were associated with caspase activation, DNA fragmentation, and selective down-regulation of STAT3 phosphorylation. PZ-DHA significantly inhibited Jurkat cell proliferation in zebrafish larvae; however, the proliferation of K562 cells was not affected in vivo. We propose that PZ-DHA-induced cytotoxic response is selective towards T-ALL in the presence of a tumor-stromal microenvironment. Prospective studies evaluating the combinatorial effects of PZ-DHA with conventional chemotherapy for T-ALL are underway.
Keywords: Phloridzin, docosahexaenoic acid, flavonoid derivative, cytotoxicity, proliferation, apoptosis, STAT3, leukemia, zebrafish
Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer in children; however, it also affects adults of all ages. T-cell ALL (T-ALL) is a rare, but highly aggressive subtype of ALL accounting for about 12% of pediatric and 24% of adult ALL cases [1]. New insights into the pathogenesis of T-ALL and intensified combination chemotherapy regimens have markedly improved the overall prognosis and survival for patients; however, clinical challenges such as suboptimal response, resistance, morbidity from drug toxicities, and disease relapse often limit treatment efficacy in T-ALL [1,2]. In fact, patients with relapsed T-ALL face a dismal prognosis, and are highly resistant to chemotherapy [3]. Therefore, there is still clearly an unmet medical need for safe and effective single drug or adjuvant agents in combating T-ALL.
Many dietary phytochemicals have reportedly shown significant cytotoxic effects in malignant cells through interfering with the multistep process of carcinogenesis [4,5]. These phytochemicals are also capable of minimizing drug resistance and adverse side effects, thereby enhancing the overall efficiency of clinically-viable chemotherapeutics [6]. A recent study also showed that polyphenols synergistically increase the cytotoxicity of doxorubicin and etoposide in lymphoid leukemia cells [7]. However, the major disadvantages of phytochemicals are poor intestinal absorption and low metabolic stability; therefore, limiting their bioavailability and therapeutic applications in humans [8,9].
Phloridzin (PZ, also known as phlorizin or phloretin-2’-O-glucoside) is a major precursor of flavonoid glucoside largely found in apple peels. Previous studies showed that PZ significantly inhibit tumor cell growth in mice subcutaneously transplanted with mammary adenocarcinoma and bladder carcinoma [10]. However, further exploration of PZ as a cytotoxic agent was halted due to issues concerning poor bioavailability [8]. In efforts to address this problem, a novel fatty acid ester of PZ called phloridzin docosahexaenoate (PZ-DHA) was synthesized by conjugating PZ with docosahexaenoic acid (DHA) through a lipase B enzyme-catalyzed acylation [11].
Enzymatic acylation with long chain polyunsaturated fatty acids (PUFAs) has been used to increase penetrability of flavonoids into cells [11,12]. Besides providing acyl groups for esterification, PUFAs exhibit significant health benefits [13,14]. DHA, an omega-3 (ω-3) PUFA found abundantly in fish oil, was found to be the primary tumor-suppressing fatty acid in athymic mice bearing human colon carcinoma [15]. DHA is also an effective adjuvant agent, as it synergistically enhances the efficacy of numerous chemotherapeutics both in vitro and in vivo [16]. Despite the promising health benefits, the potential of PUFAs as a functional food ingredient is limited due to low stability and high susceptibility to oxidation [17]. Therefore, the enzymatic conjugation of PZ with DHA is mutually beneficial, as the modification not only improves flavonoid bioavailability, but it also increases the stability of the unsaturated fatty acid.
The potential individual ability of PZ and DHA to induce cytotoxic effects in malignant cells suggests that the single chemical entity, PZ-DHA, could be a potent and promising cancer therapeutic agent. Previous studies demonstrate the anti-oxidant, anti-tyrosinase, and anti-inflammatory effects of PZ-DHA [11,18]. PZ-DHA also showed inhibitory effects against HepG2 human hepatoma cells, MDA-MB-231 human breast carcinoma cells, and THP-1 human acute monocytic leukemia cells, while sparing normal human and rat hepatocytes [19].PZ-DHA also caused selective cytotoxicity in mammary carcinoma cells compared to human mammary epithelial cells and suppressed MDA-MB-231 xenograft growth in non-obese diabetic severe combined immune-deficient (NOD-SCID) female mice [20]. In the present study, we investigated the effects of PZ-DHA on the survival of a human T-ALL cell line (Jurkat) in comparison to a human chronic myeloid leukemia cell line (K562) and non-malignant murine T-cells, and in an in vivo model employing zebrafish human leukemia cell xenografts.
Materials and methods
Cell lines and culture conditions
Jurkat and K562 cells were cultured in RPMI-1640 and DMEM, respectively, supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were cultured in suspension and maintained at 37°C in a humidified incubator with 5% CO2.
Drug treatment
PZ-DHA; PZ, imatinib mesylate, and doxorubicin (Sigma, Oakville, ON, Canada); and DHA (Nu-Chekprep, Elysian, MN, USA) were dissolved in dimethyl sulfoxide (DMSO) (Sigma). Indicated treatment concentrations were generated by dilution in culture media such that the final concentration of DMSO did not exceed 0.05%.
MTS assay
Cell viability was measured using MTS calorimetric assay (Promega, Madison, WI, USA). Jurkat (3.5 × 104 cells/well) and K562 (5 × 103 cells/well) were seeded in 96-well plates and treated with vehicle or test compounds (PZ-DHA, PZ, DHA, imatinib, and doxorubicin) at 10, 25, 50, 75, and 100 µM for 12, 24, and 48 h at 37°C. At the end of each time-point, MTS/ phenazine methosulfate (PMS; Sigma) (333 μg/ml MTS and 25 μM PMS) was added into each well and incubated for 2.5 h at 37°C. The absorbance was measured at 490 nm using an Infinite® 200 PRO multimode microplate reader (Tecan Trading AG, Männedorf, Switzerland).
ATP assay
Cells were seeded in opaque-walled 96-well plates and treated with vehicle or test compounds (100 µM) for 24 h at 37°C. Cellular ATP levels were measured using CellTiter-Glo luminescent cell viability assay (Promega). CellTiter-Glo reagent was added and plates were incubated at room temperature for 10 min. Luminescence was measured using a microplate reader.
Lactate dehydrogenase (LDH) assay
LDH activity was measured using CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). Briefly, cells were seeded in 96-well plates and treated with vehicle or test compounds (100 µM) for 24 h at 37°C. After centrifugation, the supernatant was transferred to a fresh plate, followed by addition of an equal amount of CytoTox 96 reagent. After 30 min incubation at room temperature, acetic acid (1 M) was added to stop the reaction and absorbance at 490 nm was measured. Total cytotoxicity was calculated by comparing the levels of released LDH in the experimental samples to total levels of cellular LDH obtained by lysing 1 × 106 corresponding cells with lysis buffer (9% Triton X-100).
Oregon Green 488 staining
Serum-starved Jurkat and K562 cells were labelled with CellTrace Oregon Green 488 dye (1.25 μM) (Life Technologies Inc., Burlington, ON, Canada) in serum-free media for 45 min at 37°C. Cells were then resuspended in serum-containing growth media and 1 × 105 cells were fixed in 1% paraformaldehyde representing a non-proliferative control (baseline). The remaining wells were seeded in 6-well plates and treated with vehicle or test compounds (30 µM) at 37°C. After 72 h, flow cytometric analysis was performed using a FACSCalibur instrument (BD Bioscience, Mississauga, ON, Canada). The number of cell divisions (n) was calculated based on mean fluorescence (MF), where MFbaseline=(2n) (MFsample).
Phase contrast microscopy
Jurkat (1.4 × 105 cells/well) and K562 (2.5 × 104 cells/well) were seeded in 24-well plates and treated with vehicle or test compounds (50 µM) for 24 h at 37°C. The morphology of cells was observed under an inverted phase contrast Nikon Eclipse E 100 microscope (Nikon, Mississauga, ON, Canada) and images were captured at 100 × magnification using Infinity digital microscopy camera (Lumenera corporation, Ottawa, ON, Canada).
Annexin/PI dual staining
Jurkat, K562, and normal murine T-cells were seeded in 6-well plates at a density of 1 × 105 cells/well and treated with vehicle or test compounds for 24 h at 37°C. Treated cells were stained with Annexin V/propidium iodide (PI) using Annexin-V-FLUOS Staining Kit (Roche Diagnostics, Laval, QC, Canada) according to the manufacturer’s instructions. Briefly, cells were washed with 1 × phosphate-buffered saline (PBS) and subjected to centrifugation at 200 g for 5 min. Cell pellets were then resuspended in pre-mixed Annexin-V-FLUOS labeling solution. A flow cytometric analysis was performed after 15 min incubation at room temperature.
Caspase activation assay
Jurkat and K562 cells were seeded in opaque-walled 96-well plates and treated with vehicle or test compounds (50 µM) for 18 h at 37°C. Staurosporine (2.5 µM) was used as positive control for caspase 3 activation. The Caspase-Glo 3/7 assay (Promega) was performed according to manufacturer’s protocol. Briefly, Caspase-Glo® 3/7 reagent was added to each well and luminescence was measured after 2 h incubation.
DNA fragmentation assay
DNA fragmentation was quantified using Cellular DNA fragmentation ELISA kit (Sigma) according to the manufacturer’s protocol. Briefly, Jurkat and K562 cells were labelled with 5’-bromo-2’-deoxy-uridine (BrdU) solution (10 µM) and treated with vehicle or test compounds (50 µM) for 24 h at 37°C. Cells were then lysed with lysis buffer and transferred to 96-well plates pre-coated with anti-DNA antibody. Exonuclease III solution was added to induce DNA denaturation, followed by the addition of anti-BrdU-peroxidase conjugate and substrate (tetramethylbenzidine, TMB) solution. The absorbance was measured at 370 nm.
Western blot analysis
Jurkat and K562 were seeded at a density of 1 × 105 cells/ml and treated with vehicle or PZ-DHA (10, 15, and 30 µM) for 24 h at 37°C. Jurkat cells were stimulated with interferon alpha-2b (IFNα-2b) (Sigma) at a final concentration of 5 × 104 units/ml for 1 h at 37°C. Cells were then harvested and lysates were prepared in ice-cold radioimmunoprecipitation (RIPA) lysis buffer (1 × PBS containing 1% IGEPAL, 0.5% sodium deoxycholate, and 0.1% SDS; and freshly added protease inhibitor cocktail (Sigma), 1 mM phenylmethylsulfonylfluoride, and 1 mM sodium orthovanadate). Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, Mississauga, ON, Canada). Samples (20 μg/lane) were resolved by SDS-PAGE), transferred onto BioTrace NT nitrocellulose membranes (Pall Life Sciences, Pensacola, FL, USA) and blocked in 10% nonfat milk (w/v) in Tris-buffered saline-Tween 20 (TBS-T) (0.02 M Tris-HCl, pH 7.6, 0.2 M NaCl, 0.05% Tween 20) for 1 h at room temperature. The nitrocellulose blots were then incubated with primary antibodies [anti-pSTAT1, anti-pSTAT3, anti-pSTAT5, anti-STAT1, anti-STAT3, and anti-STAT5 (Cell Signaling Technology, Danvers, MA, USA); and anti-actin (Sigma)] overnight at 4°C. The blots were washed thoroughly with TBS and incubated with secondary antibody [goat anti-rabbit IgG horse radish peroxidase (HRP) conjugate (Abcam Inc, Toronto, ON, Canada)] prepared in 10% nonfat milk in TBS-T for 1 h. Immunoreactive signals were detected using Immun-Star™ WesternC™ Chemiluminescence (Bio-Rad, Mississauga, ON, Canada) and visualized by exposure to X-ray film.
Zebrafish (Danio rerio) husbandry
Casper zebrafish were maintained in 28.5°C water at pH 6-8, and exposed to light for 14 h [21]. The larvae were collected, and incubated at 28.5°C in egg water (5 mM NaCl, 0.17 mM KCl, 0.4 mM CaCl2 and 0.16 mM MgSO4). Use of zebrafish in this study was approved by the Dalhousie University Committee for Laboratory Animals (UCLA) (Protocol 15-126).
In vivo cell proliferation assay
Jurkat and K562 cells were labelled with CellTracker™ Orange CMTMR fluorescent dye (Life Technologies, Burlington, ON, Canada). Xenotransplantation was carried out as described by Corkery et al., (2011). Briefly, casper larvae at 48-hour post fertilization (hpf) were anesthetized with tricaine methanesulfonate (Sigma) at a final concentration of 200 μg/mL prior to injection. Approximately 50-100 labelled-cells were injected into the yolk sac of each larvae. At 24 h post injection (hpi), larvae were screened for the presence of a consistent fluorescent mass within the yolk sac. Positively injected larvae were divided into two groups; one sacrificed at 24 hpi (baseline), and the other sacrificed at 96 hpi after being treated with vehicle or PZ-DHA (50 and 75 µM). The treatment doses did not exceed 50% of the maximum tolerated dose (MTD), where MTD indicates 100% survival of non-xenotransplanted larvae in the presence of drug. PZ-DHA was added directly to the fish water, and larvae were incubated at 35°C during treatment. At indicated time-points, live cell imaging of larvae was performed and both brightfield and accompanying fluorescent images were captured using an inverted Axio Observer Z1 microscope equipped with a Colibri LED light source (Carl Zeiss, Westlar, Germany). Twenty larvae from each treatment groups were also dissociated into single-cell suspension, imaged and analyzed using a semi-automated macro (Image J computer software, NIH, Bethesda, MD) where the relative number of fluorescent cells per larvae was determined.
Statistical analysis
All the data were analyzed statistically using Minitab 16 at 5% or 0.1% significance level. Results were expressed as mean ± SEM of three independent experiments conducted in triplicates. Differences among means were analysed using 2-sample Student’s t-test or one-way analysis of variance (ANOVA) using Tukey’s or Dunnent’s tests. Differences were considered statistically significant at *P < 0.05 and **P < 0.001.
Results
PZ-DHA suppresses the viability of leukemia cells
The preliminary inhibitory effects of PZ-DHA on the survival of human leukemia cells was evaluated using MTS assay. PZ-DHA resulted in a dose-dependent reduction in the viability of both Jurkat and K562 cells (Figure 1A). As we predicted, PZ-DHA was found to be more potent than its parent compounds, PZ or DHA, with the exception that DHA caused a marked inhibitory effect on Jurkat and K562 cells at the highest treatment concentration (100 µM). Both doxorubicin and imatinib, the respective positive controls for Jurkat and K562 cell lines, suppressed the viability of cells in a dose- and time-dependent manner as expected. Consistently, ATP levels, as an indication of cell viability, were found to be significantly reduced following PZ-DHA treatment; PZ or DHA alone were less effective (Figure 1B). Further evaluation of cell viability using the LDH assay confirmed that PZ-DHA caused leukemia cell cytotoxicity. PZ-DHA induced the greatest release of lactate dehydrogenase, a stable cytosolic enzyme, into the culture medium indicating compromised membrane integrity upon treatment (Figure 1C).
Figure 1.
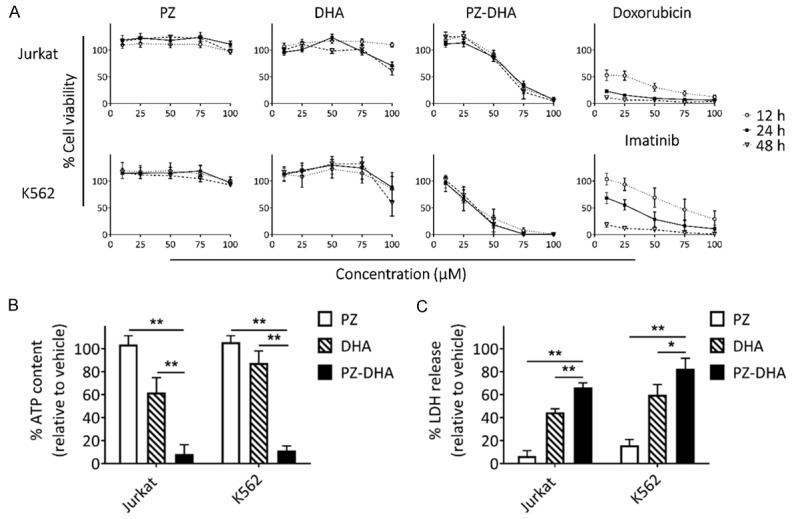
PZ-DHA is more potent than the parent compounds, PZ and DHA. A. Cell viability was measured using MTS assay after treating Jurkat and K562 cells with 10, 25, 50, 75, and 100 µM PZ, DHA, or PZ-DHA for 12, 24, and 48 h. Doxorubicin and imatinib were used as positive controls for Jurkat and K562 cell lines, respectively. B. Cellular ATP levels were measured using CellTiter-Glo luminescent assay following treatment with test compounds (100 µM) for 24 h. C. The release of LDH into culture media was measured following treatment with test compounds (100 µM) for 24 h. Data represent mean ± SEM (n=3). *P < 0.05 and **P < 0.001, compared among means (ANOVA, Tukey’s test).
PZ-DHA inhibits proliferation and selectively induces apoptosis in leukemia cells
PZ-DHA suppressed the proliferation of Jurkat and K562 cells, thereby significantly reducing the number of cell divisions (Figure 2A and 2B). In the course of cell death, a series of typical morphological changes, such as shrinkage of cells, membrane blebbing, and fragmentation into apoptotic bodies occurs. PZ-DHA-treated Jurkat and K562 cells displayed morphological alterations, with membrane blebbing and formation of apoptotic bodies being evident (Figure 3A). These extensive morphological changes suggested PZ-DHA-induced apoptotic cell death, which was confirmed by Annexin-V-FLUOS/PI dual staining-based flow cytometric analysis. In comparison to vehicle, PZ-, or DHA-treated Jurkat and K562 cells, the percent of early and/or late apoptotic/necrotic cells were significantly increased following treatment with 50 μM PZ-DHA (Figure 3B and 3C). It is also important to note that PZ-DHA exerted a minimal inhibitory effect on normal murine T-cells at doses that were effective at inducing cell death in leukemia cells (Figure 3D). Together, these results demonstrate that the cytotoxic activity of PZ-DHA is not selective towards a specific type of leukemia, where PZ-DHA significantly killed both T-ALL (Jurkat) and CML (K562) cells. Moreover, PZ-DHA effectively induced apoptosis in leukemia cells, while sparing normal T-cells.
Figure 2.
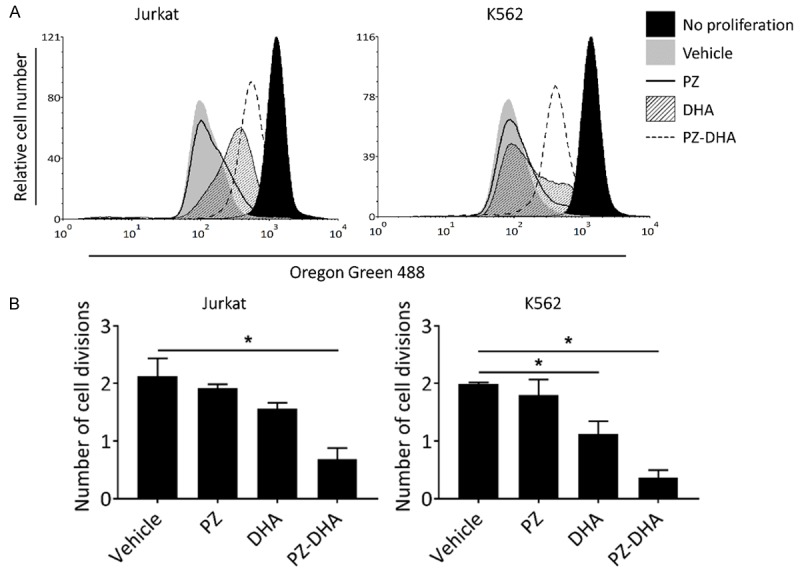
PZ-DHA suppresses leukemia cell proliferation. A. Oregon Green 488 fluorescent dye-stained Jurkat and K562 cells were treated with vehicle or test compounds (30 µM) for 72 h. Cells were then harvested and fluorescence intensity was quantified by flow cytometry relative to the non-proliferative control (baseline). Histograms from a representative experiment are shown. B. Bar graphs (derived from A show mean number of cell division in each treatment normalized to the non-proliferative control. Data represent mean ± SD (n=3). *P < 0.05, compared to vehicle control (ANOVA, Dunnett’s test).
Figure 3.
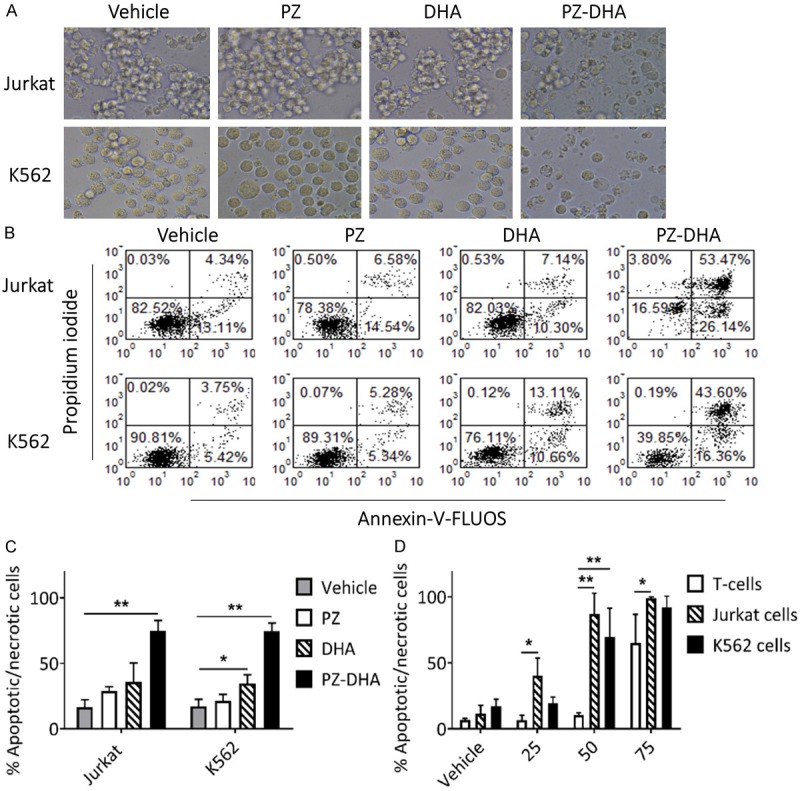
PZ-DHA induces apoptosis in leukemia cells while sparing normal murine T-cells. A. Jurkat and K562 cells were treated with vehicle or test compounds (50 µM) for 24 h and photographed using phase contrast microscopy at 400 × magnification. Photos are representative of three independent experiments. B. Flow cytometry analysis with Annexin V/PI dual staining was used to evaluate apoptosis induction in Jurkat and K562 cell lines treated with vehicle or test compounds (50 µM) for 24 h. Cytograms are representative of one of three independent experiments. C. Bar graphs (derived from A show % of apoptotic/necrotic (Annexin V, PI and Annexin V/PI positive) cells. D. Bar graphs showing % apoptotic/necrotic normal murine T-cells, Jurkat, and K562 cells treated with vehicle or test compounds at 25, 50, and 75 µM treatment doses. Data represent mean ± SD (n=3). *P < 0.05 and **P < 0.001, compared among means (ANOVA, Tukey’s test).
PZ-DHA exerts differential effects on caspase activation and DNA fragmentation
Caspases are frequently activated during cell death and are associated with the typical hallmarks of apoptosis such as formation of apoptotic bodies and DNA fragmentation. Since caspase activation is an early event in apoptosis, a shorter drug exposure time (< 24 h) was required to detect caspase activity. Staurosporine (STS; 2.5 µM), which induces apoptosis via caspase 3, was used as a positive control. Although 50 µM PZ-DHA significantly induced caspase 3/7 activation in Jurkat cells at the 18 h treatment time point, no caspase activity was detected in K562 cells (Figure 4A). Similarly, DNA fragmentation primarily activated by caspases was present in PZ-DHA-treated Jurkat cells, but not in K562 cells (Figure 4B). The ability of PZ-DHA to cleave chromosomal DNA into multiples of internucleosomal 180-bp fragments, rendering DNA laddering, was also detected in Jurkat cells by agarose gel electrophoresis (data not shown).
Figure 4.
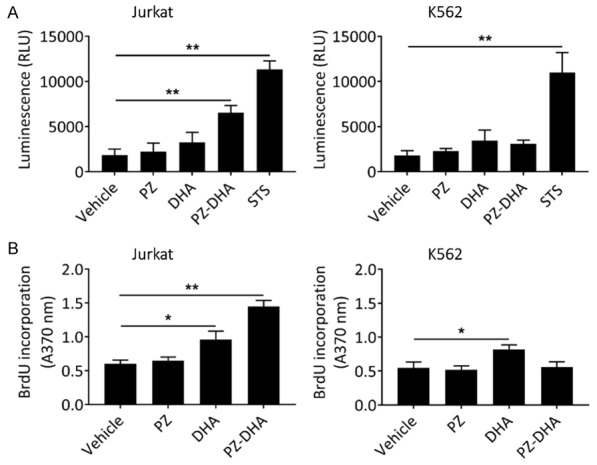
PZ-DHA-induced apoptosis is associated with caspase activation and DNA fragmentation in Jurkat cells. A. Jurkat and K562 cells were treated with vehicle, test compounds (50 µM), or 2.5 µM staurosporine, STS (positive control) for 18 h and caspase activity was detected using Caspase-Glo 3/7 assay. Luminescence units were recorded. B. Jurkat and K562 cells were treated with vehicle or test compounds (50 µM) for 24 h and DNA fragmentation was quantified by ELISA assay. Absorbance values were measured at 370 nm. Data represent mean ± SD (n=3). *P < 0.05 and **P < 0.001, compared to vehicle control (ANOVA, Dunnett’s test).
PZ-DHA selectively inhibits the activation of STAT3 in Jurkat cells
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway is often aberrantly activated in hematological malignancies reflecting the critical role of JAK and STAT proteins in hematopoiesis [23,24], a superfamily of more than 30 transmembrane proteins that recognize specific cytokines, and is critical in blood formation and immune response. Many of those receptors transmit anti-apoptotic, proliferative and differentiation signals, and their expression and functions are critical for the formation of blood lineages. Several cancers, including blood malignancies, have been associated with constitutive activation of members of the STAT family, which normally require JAK-mediated tyrosine phosphorylation for transcriptional activation. In the events of persistent JAK or STAT activation, for example, through mutations that may cause constitutive ligand/receptor coupling or abnormal tyrosine kinase activity, aberrant JAK/STAT signaling stimulates the proliferation and survival pathways of leukemia cells. Among the STAT family members, STAT1, STAT3, and STAT5, are commonly associated with malignant transformation [25]. Active STAT3, in particular, is an oncogene that could potentially be an attractive drug target in leukemia [26]. We evaluated the effect of PZ-DHA on STAT1, STAT3, and STAT5 expression and activation levels (Figure 5). Jurkat cells constitutively express phosphorylated STAT1; however, STAT3 and STAT5 activation requires stimulation by IFNα-2b. PZ-DHA selectively inhibited inducible-phosphorylation of STAT3 at 30 µM (Figure 5B), while not affecting the phosphorylation of STAT1 or STAT5 in Jurkat cells (Figure 5A and 5C, respectively). No significant suppression of STAT3 phosphorylation was observed in PZ-DHA-treated K562 cells (data not shown).
Figure 5.
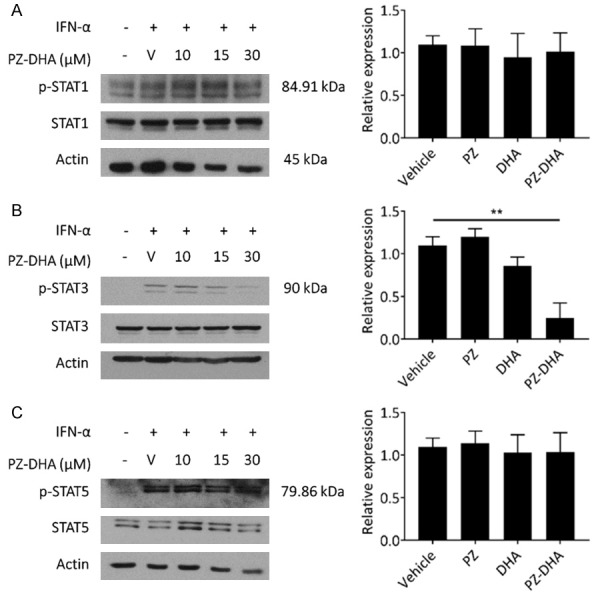
PZ-DHA selectively inhibits inducible phosphorylation of STAT3 protein in Jurkat cells. Jurkat cells were treated with vehicle (V) or indicated concentrations of PZ-DHA for 24 h before 1 h IFN-α incubation. Relative expression levels of (A) phospho-STAT1 (p-STAT1) and STAT1, (B) phospho-STAT3 (p-STAT3) and STAT3, and (C) phospho-STAT5 (p-STAT5) and STAT5 were measured by Western blot analysis. Actin was used as loading control. Relative graphical representations of p-STAT expression (normalized to STAT) determined using densitometry are shown. Data represent mean ± SD (n=3). **P < 0.001, compared to vehicle control (ANOVA, Dunnett’s test).
PZ-DHA suppresses Jurkat cell proliferation in zebrafish xenografts
Testing of drug efficacy in vivo is an important aspect of drug discovery. Following the specific responses of Jurkat and K562 cells to PZ-DHA in in vitro cell-based assays, we further assessed the anti-proliferative effect of PZ-DHA in a previously established zebrafish xenograft model (Bentley et al., 2015; Corkery et al., 2011). Figure 6A shows representative brightfield and fluorescent images of larvae injected with Jurkat cells from vehicle- and PZ-DHA-treated groups. The proliferation rate of cells in treated larvae was determined by quantifying fluorescent cells at baseline (denoted 0 h post-treatment, hpt) and at 72 hpt following exposure to either vehicle or PZ-DHA added to the larval water. Jurkat xenotransplanted larvae exposed to 75 µM PZ-DHA showed significant reduction in cell growth compared to vehicle-treated control larvae (Figure 6B). However, no significant difference was observed between control and treated larvae xenotransplanted with K562 cells (data not shown).
Figure 6.
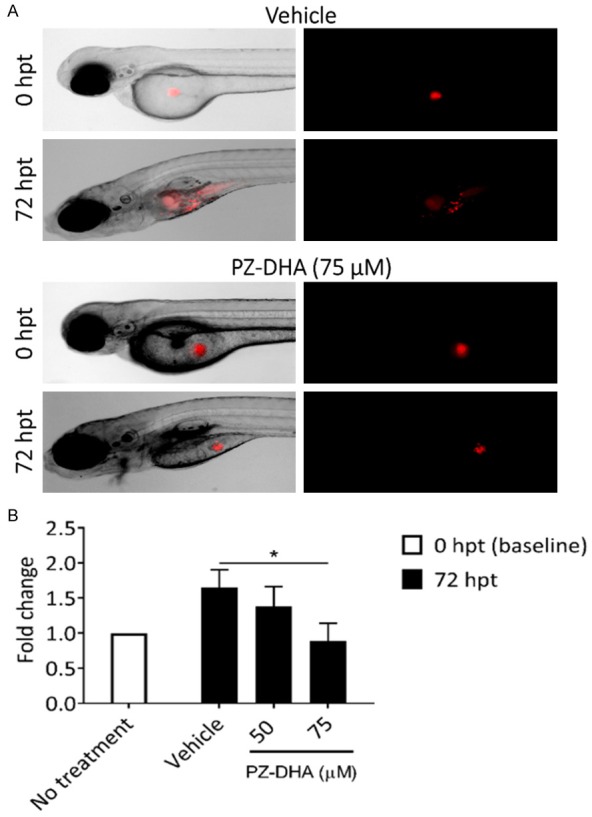
PZ-DHA suppresses in vivo proliferation of Jurkat cells in xenotransplanted zebrafish larvae. A. Representative brightfield and fluorescent images (5 × objective) of zebrafish larvae injected with fluorescently labeled Jurkat cells and monitored in the presence of vehicle or 75 µM PZ-DHA for 72 h. B. The effect of PZ-DHA on Jurkat cell proliferation was quantified by dissociating untreated and treated groups of larvae at baseline (0-hour post-treatment, hpt) and 72 hpt. Bar graph depicts fold change in cell number at 72 hpt relative to baseline. Data represent mean ± SD (n=3). *P < 0.05, compared to vehicle control (ANOVA, Dunnett’s test).
Discussion
Historically, T-ALL is associated with a worse prognosis than B-cell precursor disease. Risk-oriented treatment tailored specifically for T-ALL has improved the survival of these patients in recent years [27] . Despite this progress, treatment failure in T-ALL is persistent, largely due to disease relapse [3,28] which underlies the importance and necessity of developing novel drugs as a single agent and/or in combination with other agents for T-ALL treatment. Since the 1960s, plant-derived anti-cancer drugs like vincristine sulfate (Oncovin®) and vinblastine sulfate (Velban®) have been approved by the U.S. Food and Drug Administration (FDA) for treating hematological malignancies [29]. Here, for the first time, we show the selective cytotoxic effects of a novel plant-derived ester, PZ-DHA, against human T-ALL cells both in vitro and in vivo. We examined the cytotoxic properties of PZ-DHA on Jurkat T-ALL cells, in comparison with K562 CML cells. Our findings clearly show that the enzymatic conjugation of PZ with DHA is indeed advantageous as PZ-DHA was found to be more potent in killing Jurkat and K562 cells in vitro than the parent compounds. This is consistent with our previous studies reporting the cytotoxic effects of PZ-DHA on hepatocellular carcinoma cells, and both triple negative and estrogen receptor (ER)-positive mammary carcinoma cells [19,20]. Similarly, lipase B enzymatic acylation of other flavonoids, like rutin and naringin, have shown more significant anti-tumor effects compared to their parental molecules [30]. It is also important to note that the enzymatically-conjugated PZ-DHA had much greater effect on breast cancer cells than a mere mixture of PZ and DHA [20].
PZ-DHA significantly reduced cell viability of Jurkat and K562 cells in a dose-dependent manner (Figure 1), inhibited cell proliferation at a sub-cytotoxic treatment dose (Figure 2), and selectively induced apoptosis while sparing normal murine T-cells (Figure 3). The ability of PZ-DHA to target both T-ALL and CML cells was not surprising because most flavonoids are effective against various cancer types, attributing to their ability to interfere with multiple molecular targets and signaling pathways [31]. PZ-DHA also significantly increased caspase activation and DNA fragmentation (Figure 4), as compared to vehicle control, providing a clear mechanism of action for its pro-apoptotic against Jurkat cells. The common biochemical features of apoptotic pathway involve the initiation of caspase-3 cleavage, followed by DNA fragmentation and formation of apoptotic bodies, which are then phagocytosed [32]. Other flavonoids such as acacetin, myricetin, and EGCG have shown similar cytotoxic effects on Jurkat cells by inducing caspase activation and DNA fragmentation [33-35]. Interestingly, PZ-DHA showed no effect on caspase levels nor on DNA fragmentation in K562 cells. Two plausible explanations for these findings are: (1) PZ-DHA-induced apoptosis may not be caspase-dependent or (2) PZ-DHA may induce atypical cell death in K562 cells. Apoptosis is primarily, but not exclusively, mediated by caspase activation [36]. There is increasing evidence that cytotoxic agents can initiate cell death without relying on caspase activation [37,38]. For example, staurosporine was shown to induce both caspase-dependent and independent apoptotic cell death in two different carcinogen-induced acute lymphoid leukemia lines in murine cells [37]. The absence of caspase activity and DNA fragmentation could also be associated with atypical cell death like autophagy, necrosis, or necroptosis [39]. Other flavonoids and their derivatives such as flavopiridol, luteolin-7-O-glucoside, EGCG also triggered a caspase independent death pathway in human cancer cells [40-42]. Knowing that the cytotoxic effect of PZ-DHA is not restricted to Jurkat cells, it would be interesting to further characterize PZ-DHA-induced apoptosis in K562 cells in the future.
Importantly, PZ-DHA selectively kills leukemia cells, while sparing normal murine T-cells. The highest tested dose of PZ-DHA (75 µM) in vivo did not impact the survival, development or behavior of zebrafish larvae. In addition, no adverse side effects based on behavioral observations, food intake, and body weight measurements, were observed in non-obese diabetic/severe combined immunodeficiency (NOD-SCID) female mice intraperitoneally administered with 100 mg/kg PZ-DHA [20]. We further evaluated the effect of PZ-DHA on the activation levels of STAT proteins, particularly STAT3, which are often overexpressed in many types of leukemia [43]. Novel STAT inhibitors are progressively making their way into the arsenal of therapeutics for cancer [44,45]. Despite significant promise in pre-clinical studies, only a limited number of STAT inhibitors have been suitable for clinical development and the search for a specific and selective, yet potent, anti-STAT agent remains a challenging goal. Many flavonoids and their derivatives have shown cytotoxic effects against malignant cells via interrupting JAK/STAT signaling [46]. In line with this, PZ-DHA selectively inhibited IFNα-2b-induced STAT3 phosphorylation in Jurkat cells, without suppressing activation of STAT1 and STAT5 (Figure 5). The constitutively activated STAT3 in K562 cells remained unaffected by PZ-DHA (data not shown). This emphasizes the survival dependency of Jurkat cells on STAT3 activation, which can be selectively inhibited by PZ-DHA. An elegant study of STAT3 function employing STAT3-deficient mice showed that STAT3 activation is responsible for bypassing apoptosis and enhancing T-cell proliferation, thereby suggesting that inhibition of STAT3 activation could be an effective therapeutic approach for T-ALL [47].
Following the specific responses of Jurkat and K562 cells to PZ-DHA, we next evaluated the efficacy of PZ-DHA in a zebrafish xenograft model [22,50]. The in vitro anti-proliferative effect of PZ-DHA was recapitulated in vivo, where PZ-DHA significantly inhibited Jurkat cell growth in xenotransplanted zebrafish larvae (Figure 6). However, K562 cell proliferation in larvae was not affected by PZ-DHA (data not shown). The differential response in vivo suggests that the PZ-DHA-induced cytotoxic response in human leukemia cell lines may be impacted by the tumor microenvironment. Resveratrol has shown similar disparate in vitro and in vivo effects, where no significant antileukemic effect was detected in mouse xenografts, albeit inducing significant caspase-dependent apoptosis in a murine myeloid leukemia cell line [48]. There is increasing evidence that the interaction between non-malignant stromal cells within the tumor microenvironment may affect tumor cell response to therapeutics [49]. However, stroma-induced resistance is not common to all types of cancer; it may vary depending on the particular type of tumor or its microenvironment, thereby implicating PZ-DHA as a novel and potent therapeutic agent selective for T-ALL.
Taken together, we provide evidence that PZ-DHA inhibits the proliferation of leukemia cells, and more specifically T-ALL cells, using both in vitro and in vivo approaches. The potential use of PZ-DHA as an adjuvant agent for the treatment of T-ALL is currently being evaluated by studying the synergistic effect of PZ-DHA when used in combination with doxorubicin and other chemotherapeutic agents. It would be worthwhile to study the effect of PZ-DHA in animal models xenotransplanted with primary patient-derived leukemia samples. This is feasible in the zebrafish xenotransplantation assay [50] and may provide new insights on the interaction of PZ-DHA with tumor cells in a clinically relevant time frame and the prospects of employing PZ-DHA to personalize patient treatment.
Acknowledgements
This study was supported by NSERC Discovery Grant (327056) and Canada Research Chair Funds (220744). We are grateful to Wasundara Fernando, Lynn Thomas, and Simon Gebremeskel of Dalhousie University for their intellectual and technical assistance throughout various stages of this research.
References
- 1.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 2.Aifantis I, Raetz E, Buonamici S. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat Rev Immunol. 2008;8:380–390. doi: 10.1038/nri2304. [DOI] [PubMed] [Google Scholar]
- 3.Kunz JB, Rausch T, Bandapalli OR, Eilers J, Pechanska P, Schuessele S, Assenov Y, Stütz AM, Kirschner-Schwabe R, Hof J, Eckert C, von Stackelberg A, Schrappe M, Stanulla M, Koehler R, Avigad S, Elitzur S, Handgretinger R, Benes V, Weischenfeldt J, Korbel JO, Muckenthaler MU, Kulozik AE. Pediatric T-cell lymphoblastic leukemia evolves into relapse by clonal selection, acquisition of mutations and promoter hypomethylation. Haematologica. 2015;100:1442–50. doi: 10.3324/haematol.2015.129692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, Kong AN. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12:1281–305. doi: 10.2174/187152012803833026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernando W, Rupasinghe HPV. Anticancer properties of phytochemicals present in medicinal plants of North America. In: Kulka M, editor. Using Old Solut. to New Probl. - Nat. Drug Discov. 21st Century. InTech; 2013. pp. 159–180. [Google Scholar]
- 6.Sak K. Chemotherapy and dietary phytochemical agents. Chemother Res Pract. 2012;2012:282570. doi: 10.1155/2012/282570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahbub AA, Le Maitre CL, Haywood-Small SL, Cross NA, Jordan-Mahy N. Polyphenols act synergistically with doxorubicin and etoposide in leukaemia cell lines. Cell Death Discov. 2015;1:15043. doi: 10.1038/cddiscovery.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crespy V, Aprikian O, Morand C, Besson C, Manach C, Demigné C, Rémésy C. Remesy, Bioavailability of phloretin and phloridzin in Rats. J Nutr. 2001;131:3227–3230. doi: 10.1093/jn/131.12.3227. [DOI] [PubMed] [Google Scholar]
- 9.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 10.Nelson JA, Falk RE. The efficacy of phloridzin and phloretin on tumor cell growth. Anticancer Res. 1993;13:2287–92. [PubMed] [Google Scholar]
- 11.Ziaullah , Bhullar KS, Warnakulasuriya SN, Rupasinghe HPV. Biocatalytic synthesis, structural elucidation, antioxidant capacity and tyrosinase inhibition activity of long chain fatty acid acylated derivatives of phloridzin and isoquercitrin. Bioorg Med Chem. 2013;21:684–92. doi: 10.1016/j.bmc.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson DE, Wibisono R, Jensen DJ, Stanley RA, Cooney JM. Direct acylation of flavonoid glycosides with phenolic acids catalysed by Candida antarctica lipase B (Novozym 435®) Enzyme Microbial Technology. 2006;39:1236–1241. [Google Scholar]
- 13.Kinsella JE. Food components with potential therapeutic benefits: the n-3 polyunsaturated fatty acids of fish oils. Food Technol. 1986;40:89–97. [Google Scholar]
- 14.Ruxton C, Reed S, Simpson M, Millington K. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet. 2004;17:449–459. doi: 10.1111/j.1365-277X.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Hancock RL, Mohammadpour H, McGregor B, Manalo P, Khaiboullina S, Hall MR, Pardini L, Pardini RS. Influence of omega-3 fatty acids on the growth of human colon carcinoma in nude mice. Cancer Lett. 2002;187:169–77. doi: 10.1016/s0304-3835(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui RA, Harvey KA, Xu Z, Bammerlin EM, Walker C, Altenburg JD. Docosahexaenoic acid: a natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors. 2011;37:399–412. doi: 10.1002/biof.181. [DOI] [PubMed] [Google Scholar]
- 17.Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In Vivo. 1999;13:295–309. [PubMed] [Google Scholar]
- 18.Sekhon-Loodu S, Ziaullah , Rupasinghe HPV. Docosahexaenoic acid ester of phloridzin inhibit lipopolysaccharide-induced inflammation in THP-1 differentiated macrophages. Int Immunopharmacol. 2015;25:199–206. doi: 10.1016/j.intimp.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Nair SV, Ziaullah , Rupasinghe HPV. Fatty acid esters of phloridzin induce apoptosis of human liver cancer cells through altered gene expression. PLoS One. 2014;9:e107149. doi: 10.1371/journal.pone.0107149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernando W, Coombs MRP, Hoskin DW, Rupasinghe HPV. Docosahexaenoic acid-acylated phloridzin, a novel polyphenol fatty acid ester derivative, is cytotoxic to breast cancer cells. Carcinogenesis. 2016;37:1004–1013. doi: 10.1093/carcin/bgw087. [DOI] [PubMed] [Google Scholar]
- 21.White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE, Zon LI. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–9. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corkery DP, Dellaire G, Berman JN. Leukaemia xenotransplantation in zebrafish-chemotherapy response assay in vivo. Br J Haematol. 2011;153:786–9. doi: 10.1111/j.1365-2141.2011.08661.x. [DOI] [PubMed] [Google Scholar]
- 23.Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32:2601–13. doi: 10.1038/onc.2012.347. [DOI] [PubMed] [Google Scholar]
- 24.Ward AC, Touw I, Yoshimura A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood. 2000;95:19–29. [PubMed] [Google Scholar]
- 25.Coffer PJ, Koenderman L, de Groot RP. The role of STATs in myeloid differentiation and leukemia. Oncogene. 2000;19:2511–2522. doi: 10.1038/sj.onc.1203479. [DOI] [PubMed] [Google Scholar]
- 26.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr. Stat3 as an Oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 27.Raetz EA, Teachey DT. T-cell acute lymphoblastic leukemia. Hematology. 2016;2016:580–588. doi: 10.1182/asheducation-2016.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elreda L, Sandhu M, Sun X, Bekele W, Cohen AJ, Shah M. T-cell lymphoblastic leukemia/lymphoma: relapse 16 years after first remission. Case Rep Hematol. 2014;2014:359158. doi: 10.1155/2014/359158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster S. From Herbs to Medicines: the Madagascar Periwinkle’s impact on childhood leukemia: a serendipitous discovery for treatment. Altern Complement Ther. 2010;16:347–350. [Google Scholar]
- 30.Mellou F, Loutrari H, Stamatis H, Roussos C, Kolisis FN. Enzymatic esterification of flavonoids with unsaturated fatty acids: effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochemistry. 2006;41:2029–2034. [Google Scholar]
- 31.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8:122–46. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa H, Hasumi K, Woo JT, Nagai K, Wachi M. Generation of hydrogen peroxide primarily contributes to the induction of Fe(II)-dependent apoptosis in Jurkat cells by (-)-epigallocatechin gallate. Carcinogenesis. 2004;25:1567–1574. doi: 10.1093/carcin/bgh168. [DOI] [PubMed] [Google Scholar]
- 34.Ko CH, Shen SC, Hsu CS, Chen YC. Mitochondrial-dependent, reactive oxygen species-independent apoptosis by myricetin: roles of protein kinase C, cytochrome c, and caspase cascade. Biochem Pharmacol. 2005;69:913–927. doi: 10.1016/j.bcp.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K, Kanno S, Tomizawa A, Yomogida S, Ishikawa M. Acacetin induces apoptosis in human T cell leukemia Jurkat cells via activation of a caspase cascade. Oncol Rep. 2012;27:204–9. doi: 10.3892/or.2011.1498. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–42. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 37.Belmokhtar CA, Hillion J, Ségal-Bendirdjian E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene. 2001;20:3354–62. doi: 10.1038/sj.onc.1204436. [DOI] [PubMed] [Google Scholar]
- 38.Chipuk JE, Green DR. Do inducers of apoptosis trigger caspase-independent cell death? Nat Rev Mol Cell Biol. 2005;6:268–75. doi: 10.1038/nrm1573. [DOI] [PubMed] [Google Scholar]
- 39.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, V Blagosklonny M, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alonso M, Tamasdan C, Miller DC, Newcomb EW. Flavopiridol induces apoptosis in glioma cell lines independent of retinoblastoma and p53 tumor suppressor pathway alterations by a caspase-independent pathway. Mol Cancer Ther. 2003;2:139–50. [PubMed] [Google Scholar]
- 41.Hwang YJ, Lee EJ, Kim HR, Hwang KA. Molecular mechanisms of luteolin-7-O-glucoside-induced growth inhibition on human liver cancer cells: G2/M cell cycle arrest and caspase-independent apoptotic signaling pathways. BMB Rep. 2013;46:611–6. doi: 10.5483/BMBRep.2013.46.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwasaki R, Ito K, Ishida T, Hamanoue M, Adachi S, Watanabe T, Sato Y. Catechin, green tea component, causes caspase-independent necrosis-like cell death in chronic myelogenous leukemia. Cancer Sci. 2009;100:349–356. doi: 10.1111/j.1349-7006.2008.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrajoli A, Faderl S, Ravandi F, Estrov Z. The JAK-STAT pathway: a therapeutic target in hematological malignancies. Curr Cancer Drug Targets. 2006;6:671–9. doi: 10.2174/156800906779010227. [DOI] [PubMed] [Google Scholar]
- 44.Hayakawa F, Sugimoto K, Harada Y, Hashimoto N, Ohi N, Kurahashi S, Naoe T. A novel STAT inhibitor, OPB-31121, has a significant antitumor effect on leukemia with STAT-addictive oncokinases. Blood Cancer J. 2013;3:e166. doi: 10.1038/bcj.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fagard R, Metelev V, Souissi I, Baran-Marszak F. STAT3 inhibitors for cancer therapy: have all roads been explored? JAKSTAT. 2013;2:e22882. doi: 10.4161/jkst.22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arumuggam N, Bhowmick NA, Rupasinghe HPV. A review: phytochemicals targeting JAK/STAT signaling and IDO expression in cancer. Phytother Res. 2015;29:805–17. doi: 10.1002/ptr.5327. [DOI] [PubMed] [Google Scholar]
- 47.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 48.Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076–81. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- 49.McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov. 2013;12:217–28. doi: 10.1038/nrd3870. [DOI] [PubMed] [Google Scholar]
- 50.Bentley VL, Veinotte CJ, Corkery DP, Pinder JB, LeBlanc MA, Bedard K, Weng AP, Berman JN, Dellaire G. Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia. Haematologica. 2015;100:70–6. doi: 10.3324/haematol.2014.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]


