Abstract
Overall survival of patients with hepatocellular carcinoma (HCC) remains poor, and the multidrug resistance of HCC cells contributes to the limited efficacy of anti-cancer drugs, and reduced time to recurrence. We systematically screened the expression of transporter genes in HCC samples and found that solute carrier family 29 member A1 (SLC29A1) expression was significantly elevated in human HCC cells compared with para-carcinoma cell samples. The results of tissues microarray showed that SLC29A1 was an independent prognostic factor for overall survival and tumor recurrence, especially for patients with AFP ≤ 20 ng/ml, no microvascular invasion and early staging. In vivo and vitro analyses showed that down-regulation of SLC29A1 expression could enhance tumor cell proliferation, invasion and reduced drug sensitivity. Further microarray-based gene expression profile indicated that low SLC29A1 expression may contribute to HCC progression by promoting the epithelial-mesenchymal transition through zinc finger E-box binding homeobox 2 and transforming growth factor beta receptor activation, modifying cell adhesion through up- or down-regulation of cell adhesion molecules, and activating the nuclear factor-kappaB pathway through tripartite motif-containing protein 9 inhibition. In conclusion, low SLC29A1 expression correlated with high recurrence risk and poor outcomes for patients with HCC after surgery. SLC29A1 might be a promising prognostic factor, a potential tumor suppressor, and a drug sensitizer for patients with HCC through its interaction with various signaling pathways involved in this disease.
Keywords: SLC29A1, hepatocellular carcinoma, drug resistance, epithelial-mesenchymal transition, cell adhesion, NF-κB
Introduction
Hepatocellular carcinoma (HCC) is one of the most commonly diagnosed liver cancers worldwide [1]. Despite improvements in monitoring programs and diagnostic tools, only 30-40% of patients with HCC are eligible for curative treatment [2,3]. The multikinase inhibitor sorafenib was approved as a standard treatment for patients with advanced HCC, but the survival benefit remains modest [4]. Because of its heterogeneity and multiple etiologies, HCC is highly refractory to conventional systemic chemotherapy and treatment with the kinase-targeting agent sorafenib [5]. Improved understanding HCC is urgently needed.
Cellular uptake of anti-cancer drugs is an important first step in the mechanism of drug action. Membrane transporters are responsible for anti-cancer drug uptake and export processes, which effect anti-tumor agent efficacy. Studies have investigated the multidrug resistance mechanism in HCC [6,7]. Two important proposed mechanisms of drug resistance, which act together or alone, are elevated drug efflux and decreased drug uptake in cancer cells. These processes are regulated by membrane transporters [8]. The two main transporter superfamilies are the solute carrier (SLC) transporters and the ATP-binding cassette transporters. The SLC transporter superfamily is important in endogenous compound homeostasis, xenobiotic disposition, and drug delivery [9]. The expression levels of drug transporters in HCC have been investigated [10-13]. However, the expression of the SLC transporter family in HCC has not been thoroughly researched, and the roles of the SLC gene family in HCC-associated drug resistance have not been well-elucidated.
Expression of SLC transporter-family genes was comprehensively investigated in HCC tissues and peritumor tissues. We demonstrate that low SLC29A1 expression correlated with high recurrence rates and poor outcomes for patients with HCC after surgery. Low SLC29A1 expression mechanistically enhanced tumor cell proliferation, invasion and reduced drug sensitivity through changing cell adhesion status, induction of the epithelial-mesenchymal transition (EMT) process, and nuclear factor-kappaB (NF-κB) pathway activation.
Materials and methods
Clinical materials
A total of 480 patients with HCC from the Liver Cancer Institute of Fudan University in Shanghai, China were recruited for this study. A cohort of 75 HCC patients who underwent surgery from 2000 to 2002 were screened by qPCR array for aberrant transporter genes in HCC. Additional 12 fresh tumors with matched peritumoral tissues from HCC patients who underwent surgery in 2010 were chosen for qRT-PCR and western blot analyses. Paraffin-embedded specimens from another cohort of 393 patients with HCC who underwent curative resection were collected and used to construct tissue microarrays (TMAs). TMAs were used to test the SLC29A1 expression in HCC and para-carcinoma tissues. The histopathological diagnoses were based on World Health Organization criteria [14]. Follow-up data were summarized at the end of May 2016; the median follow-up period was 65 months (range: 4 to 181 months). Patients with high risk of recurrence based on clinical features such as vascular invasion and microsatellite lesions, received prophylactic transcatheter arterial chemoembolization (TACE; doxorubicin, cisplatin, 5-fluorouracil, and iodized oil). Overall survival (OS) was defined as the interval between the date of surgery and death (or the last observation point taken). Time to recurrence (TTR) was defined as any diagnosed relapse (intrahepatic recurrence or extrahepatic metastasis). The follow-up procedures, postoperative treatment modalities, and surveillance followed previously-published uniform guidelines [15]. The clinicopathologic characteristics of patients are presented in Table 1. Ethical approval was obtained from the research ethics committee of Zhongshan Hospital. Each patient provided written informed consent.
Table 1.
Correlation between SLC29A1 expression and clinical characteristics of patients with HCC
| Low SLC29A1 expression (n=204) | High SLC29A1 expression (n=189) | P-value* | |
|---|---|---|---|
| Sex | |||
| Female | 26 | 24 | 1.000 |
| Male | 178 | 165 | |
| Age, years | |||
| ≤ 50 | 94 | 103 | 0.107 |
| > 50 | 110 | 86 | |
| HBsAg | |||
| Negative | 37 | 33 | 0.896 |
| Positive | 167 | 156 | |
| HCV | |||
| Negative | 202 | 182 | 0.094 |
| Positive | 2 | 7 | |
| Cirrhosis | |||
| No | 38 | 34 | 0.897 |
| Yes | 166 | 155 | |
| AFP, ng/mL | |||
| ≤ 20 | 89 | 52 | 0.001* |
| > 20 | 115 | 137 | |
| Tumor encapsulation | |||
| Complete | 122 | 111 | 0.838 |
| None | 82 | 78 | |
| Tumor size, cm | |||
| ≤ 5 | 111 | 120 | 0.081 |
| > 5 | 93 | 69 | |
| Tumor number | |||
| Single | 164 | 150 | 0.803 |
| Multiple | 40 | 39 | |
| Microvascular invasion | |||
| No | 145 | 151 | 0.047* |
| Yes | 59 | 38 | |
| Tumor differentiation | |||
| I-II | 140 | 117 | 0.169 |
| III-IV | 64 | 72 | |
| CLIP | |||
| 0 | 159 | 150 | 0.806 |
| 1 | 45 | 39 | |
| ALT | |||
| ≤ 75 U/L | 185 | 171 | 1.000 |
| > 75 U/L | 19 | 18 | |
| Child | |||
| A | 192 | 182 | 0.354 |
| B | 12 | 7 | |
| BCLC | |||
| A | 156 | 152 | 0.391 |
| B | 48 | 37 | |
Abbreviations: HCC, hepatocellular carcinoma; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; AFP, α-fetoprotein; CLIP, Cancer of the Liver Italian Program; ALT, alanine transaminase; BCLC, Barcelona Clinic Liver Cancer.
P-values < 0.05 were considered statistically significant.
The Pearson chi-square test was used for between-group comparisons.
Cell lines and animals
Human HCC MHCC97L and MHCC97H cell lines were established at the Liver Cancer Institute, Zhongshan Hospital, Fudan University. L02, Hep3B, and Huh-7 cell lines were obtained from the Shanghai Institute for Biological Science (China). Male, athymic, 8-week-old BALB/c nude mice (Chinese Academy of Science, Shanghai, China) were raised under specific-pathogen-free conditions. Animal care and experimental protocols were performed following the guidelines established by the Shanghai Medical Experimental Animal Care Commission.
qRT-PCR and western blot assays
The RT2 Profiler PCR Array System (Qiagen, Valencia, CA, USA) was used to screen the expression levels of SLC transporters in HCC and para-carcinoma tissues. This array contained 46 SLC transporter gene primers, 5 housekeeping gene primers (for normalization), 1 genomic DNA primer (for detecting genomic DNA contamination), 3 reverse transcription elements, and 3 positive PCR controls.
Thirty randomly selected HCC samples were used to investigate messenger RNA (mRNA) and protein expression levels. Expression of mRNA and protein in tissues was determined using qRT-PCR and western blot assays, respectively, as previously described [14]. The primers used are listed in Supplementary Table 1. Three independent repeats were performed. The primary antibodies used were against SLC29A1 (1:1000; Abcam, Cambridge, MA, USA), β-actin (1:3000; Abcam), E-cadherin, N-cadherin, and Vimentin (1:1000; Cell Signaling Technology, Danvers, MA, USA).
Immunohistochemistry
Tissue sections were treated with citrate buffer to retrieve antigen. After removing the endogenous peroxidase activity, the tissue sections were blocked with goat serum and subsequently incubated with the primary antibody, anti-SLC29A1 (1:500; Abcam, Cambridge, MA, USA), at 4°C. The samples were then incubated with secondary antibody, washed with PBS, and visualized using diaminobenzidine and hematoxylin re-staining.
Patients were divided into two subgroups for analysis of the associations between SLC29A1 expression and the OS and cumulative recurrence rates. A positive reaction for SLC29A1 was scored in four grade categories according to staining intensity (i.e., 0, 1, 2, and 3). The percentages of SLC29A1-positive cells were scored as 0 (0%), 1 (1 to 33%), 2 (34 to 66%), or 3 (67 to 100%). In cases with discrepancies between duplicated cores, the higher score of the two tissues was recorded as the final score. The sum of the intensity and percentage scores was used as the final staining score. The staining pattern was ranked by two independent investigators blinded of the clinicopathological data as previously defined [15]. Variations in enumeration within a range of 5% were re-evaluated to reach consensus.
Transfection and clone selection
The lentiviral construct PLKO-shRNA-SLC29A1 was generated with the target sequences sh1-GGAACTCTCTCAGTGCCATCT and sh2-GCCACTCTATCAAAGCCATCC. SLC29A1 in stably transfected clones was validated using qRT-PCR and western blot assays.
Cell proliferation and flat plate clone formation assays
Cell proliferation was analyzed using the CCK8 proliferation method. Briefly, 1×104 cells/well were seeded in triplicate in 96-well plates and incubated at 37°C and a 5% CO2 humidified atmosphere. After 24, 48, and 72 hours, the CCK8 assay was performed according to the manufacturer’s protocol. All experiments were performed in triplicate.
Cells in the logarithmic growth phase were seeded onto a 2-cm cell culture plate at a density of 500 cells/mL. The cells were cultured at 37°C in a well-humidified 95% air and 5% CO2 incubator for approximately 2 weeks. Colony formation was photographed and counted at 50× magnification. The assays were performed in triplicate for each cell line combination.
Cell invasion
The OrisTM Pro 96-well Invasion Assay was used to examine cell invasion. MHCC97-H-Mock, MHCC97-H-SLC29A1-sh1/2, Huh-7-Mock, and Huh-7-SLC29A1-sh1/2 cells. The OrisTM Pro Collagen I Overlay solution was added and incubated in a humidified chamber (37°C, 5% CO2) for 1 hour. Pre-invasion images of the Detection Zone were captured. Then, 100 µL complete medium (containing serum) was added on top of the overlay and incubated for 96 hours to permit invasion. Post-invasion images of the Detection Zone were taken at 24, 48, 72, and 96 hours.
The OrisTM Pro 96-well Invasion Assay results were evaluated using high-content screening/high-content imaging analysis.
Tumor models
Mice were manipulated and housed according to protocols approved by the Shanghai Medical Experimental Animal Care Commission. To evaluate in vivo tumor growth, 1×107 MHCC97-H-SLC29A1-sh2 and Huh-7-SLC29A1-sh2 cells were suspended in 100 μL serumfree Dulbecco’s Modified Eagle Medium and injected via the subcutaneous route into the left flank of each mouse (six in each group, 8-week-old male BALB/c-nu/nu); the control cells (MHCC97-H-Mock and Huh-7-Mock) were injected into the right flank. The tumor sizes were measured three times per week as soon as the tumors were measurable. The tumor volumes were calculated using the formula: (length × width2)/2. The mice were euthanized on day 22, and the tumors were weighed immediately after dissection.
Chemosensitivity assay
MHCC97-H-Mock, Huh-7-Mock, MHCC97-H-SLC29A1-sh2, and Huh-7-SLC29A1-sh2 cells were plated in 96-well plates and treated with 0, 5, 10, 20, or 40 mg/L 5-fluorouracil (5-FU), with 0, 10, 15, 20, or 25 mg/L cisplatin, or with 0, 5, 10, 15, or 20 µM sorafenib for 48 hours, respectively. HCC cell viability was evaluated using a Cell Counting Kit-8 assay. The optical density OD was measured at 450 nm, and the relative viability was evaluated by normalizing the OD values from the test samples to the OD values of the control samples.
Microarray-based gene expression profile
We performed a microarray assay to compare the profiles of differentially expressed genes between SLC29A1 shRNA-treated and Mock Huh-7/MHCC97-H cells and to investigate the function of SLC29A1 and the potential mechanism by which the SLC29A1 shRNA promoted tumor cell proliferation. The 44K oligonucleotide microarray as constructed by Outdo Biotech (Shanghai, China). The pathways were analyzed by Gene Ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) using the DAVID tool.
Statistical analysis
The Student’s t test was used to evaluate the differences between SLC29A1 expression in HCC and para-carcinoma tissues. The correlation between SLC29A1 expression and clinical parameters was calculated using Pearson rank correlation coefficients. The Kaplan-Meier method and log-rank test were used to analyze survival curves according to SLC29A1 expression and clinical characteristics. Subsequently, all potential predictive factors were included in a Cox multivariate regression survival analysis. All statistical analyses were performed using SPSS 19.0 software (SPSS, Chicago, IL). A P-value < 0.05 was considered to indicate a statistically significant result.
Results
The SLC-family gene expression profile in HCC
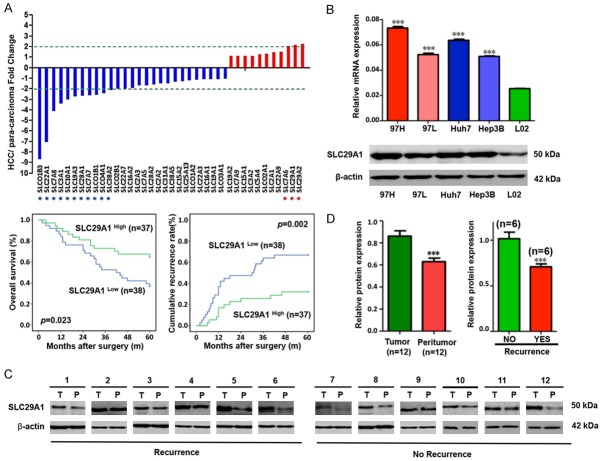
The mRNA expression levels of various SLC-family genes in a cohort of 75 frozen HCC specimens along with the corresponding para-carcinoma samples were analyzed by qPCR array to screen for aberrant expression of SLC-family genes in HCC. We identified 11 SLC-family genes that were down-regulated more than 2-fold in HCC samples, compared with the gene expression patterns in the para-carcinoma samples (Supplementary Table 2). Three genes (SLC7A6, SLC29A1, and SLC29A2) were up-regulated (Figure 1A). We divided the specimens into two groups according to the expression levels of the 14 identified genes and performed a survival analysis. SLC29A1 was the only gene correlated with prognosis (Supplementary Figure 1). Low SLC29A1 mRNA expression predicted poor survival (P=0.023) and high disease recurrence rates (P=0.002) after surgery. The qRT-PCR array also indicated that the SLC29A1 mRNA expression level was significantly elevated in HCC, compared with para-carcinoma tissues (P < 0.05).
Figure 1.
A. Solute carrier (SLC) family gene expression profile in hepatocellular carcinoma (HCC) samples. Eleven SLC genes (SLCO1B3, SLC22A1, SLC7A8, SLC3A1, SLC10A1, SLC19A3, SLC28A1, SLC7A7, SLCO1B1, SLCO4A1, and SLC38A2) were down-regulated more than two-fold in HCC samples. Three SLC genes (SLC7A6, SLC29A1 and SLC29A2) were up-regulated. B. SLC29A1 mRNA and protein levels in MHCC97H, Huh-7, MHCC97L, Hep3B, and L02 cells (P < 0.05). C. Western blot analysis of SLC29A1 expression in a cohort of 12 patients with HCC. D. Patients with HCC recurrence had lower SLC29A1 protein levels than those without recurrence.
SLC29A1 expression in HCC cell lines and HCC tissues
Expression of SLC29A1 was confirmed in HCC cell lines and HCC tissues using qRT-PCR and western blot analyses. The SLC29A1 mRNA and protein levels in MHCC97H and Huh-7 cells were higher than in MHCC97L and Hep3B cells and significantly higher than in L02 cells (P < 0.05; Figure 1B). A cohort of 12 HCC samples with corresponding para-carcinoma tissues and qRT-PCR were used to validate the aberrant high SLC29A1 transcription level. The functional SLC29A1 protein expression in HCC tissues was clearly greater than that in para-carcinoma tissues (Figure 1C). Western blot analysis of the tumor tissues revealed that patients without tumor recurrence exhibited higher SLC29A1 protein expression compared with those with recurrence (Figure 1D, ***P < 0.001).
Low SLC29A1 expression predicts poor prognosis in patients with HCC
We analyzed SLC29A1 localization in the cytoplasm in TMAs of 393 HCC specimens using immunohistochemistry. High SLC29A1 expression (score 3-6) was observed in 189 out of 393 HCC samples (48.1%), and low or negative SLC29A1 expression (score 0-2) in 204 HCC samples (51.9%). All peritumoral tissues showed negative or low SLC29A1 expression (score 0-2). There was a statistically significant difference in SLC29A1 expression between tumor and peritumoral tissues (P < 0.001). When comparing SLC29A1 expression in HCC samples and the corresponding para-carcinoma samples, higher SLC29A1 expression in HCC samples than in para-carcinoma samples was defined as being in the SLC29A1 high group; equal or lower SLC29A1 expression was defined as being in the SLC29A1 low group (Figure 2).
Figure 2.
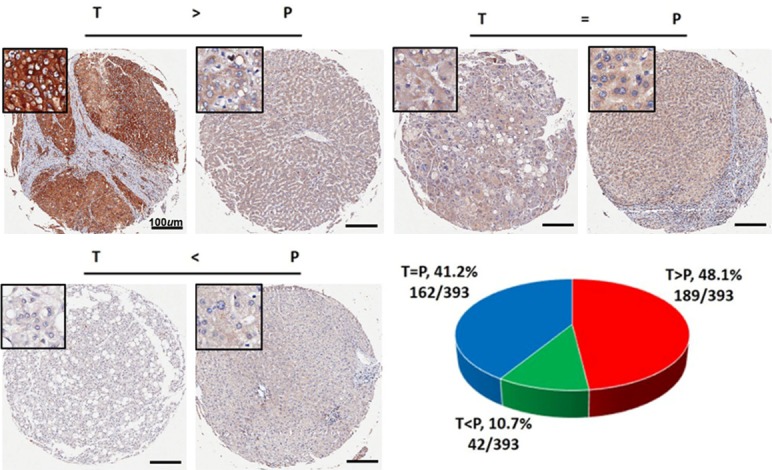
Immunohistochemistry results of 393 hepatocellular carcinoma specimens, by SLC29A1 expression.
At the last follow-up, 28.2% (111/393) of the patients experienced recurrence and 63.6% (250/393) had died. The 1-, 3-, 5-, 10-, and 15-year OS rates were 90.1%, 68.2%, 53.3%, 39.9%, and 34.1%, respectively. The corresponding cumulative recurrence rates were 18.6%, 46.6%, 58.5%, 69.2%, and 74.1%. The univariate survival analysis revealed that liver cirrhosis, tumor size, tumor encapsulation, microvascular invasion, tumor number, and low SLC29A1 expression were predictors of an unfavorable outcome for OS and tumor recurrence after surgery. The serum alpha-fetoprotein (AFP) level was only predictive of an unfavorable outcome for OS (Table 2). The results of further multivariate analysis indicated that SLC29A1 was an independent prognostic factor for OS (HR=0.691, 95% confidence interval (CI) 0.535-0.894, P=0.005) and tumor recurrence (HR=0.677, 95% CI 0.534-0.860, P=0.001) (Figure 3A, 3B). The median OS for SLC29A1 low patients was 50 months, compared with 78 months for the SLC29A1 high group, and the median TTR for SLC29A1 low patients was 38 months, compared with 64 months for the SLC29A1 high group. We further investigated the predictive value of SLC29A1 within clinical subgroups of AFP levels ≤ 20 ng/ml, without microvascular invasion (MVI) and Barcelona Clinic Liver Cancer (BCLC) Staging 0-A. The prognostic significance of SLC29A1 persisted in patients with HCC with normal AFP levels (P=0.041), without MVI (P=0.01) and BCLC 0-A (P=0.014) (Figure 3C-E). Forty-nine percent (145/296) of the no-MVI group patients expressed low levels of SLC29A1; these patients had a dismal prognosis. Most of the SLC29A1 low patients (59.7%) died of tumor recurrence within 5 years. In the AFP-normal group, 63.1% (89/141) expressed low levels of SLC29A1; the median TTR was 47 months and the 5-year recurrence rate was 57.8%. In the BCLC 0-A group patients, 50.6% (156/308) had low-level expression of SLC29A1; the median TTR was 40 months and the 5-year recurrence rate was 57.4%.
Table 2.
Univariate and multivariate analyses of factors associated with overall survival and recurrence in 393 patients with hepatocellular carcinoma
| Factors | OS | Cumulative recurrence | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
|
|
||||||||
| P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value | |
| Sex (female vs. male) | 0.205 | NA | 0.435 | NA | ||||
| Age, years (≤ 50 vs. > 50) | 0.868 | NA | 0.782 | NA | ||||
| HBsAg (positive vs. negative) | 0.216 | NA | 0.155 | NA | ||||
| HCVAb (positive vs. negative) | 0.820 | NA | 0.922 | NA | ||||
| Liver cirrhosis (yes vs. no) | 0.009* | 1.513 | 1.061-2.159 | 0.022* | 0.002* | 1.587 | 1.139-2.211 | 0.006* |
| Serum AFP, ng/mL (≤ 20 vs. > 20) | 0.011* | 1.347 | 1.028-1.765 | 0.031* | 0.061 | NA | ||
| Serum ALT, U/L (≤ 75 vs. > 75) | 0.855 | NA | 0.585 | NA | ||||
| Tumor size (diameter, cm) (> 5 vs. ≤ 5) | 0.031* | NS | 0.373 | NA | ||||
| Tumor encapsulation (absent vs. present) | 0.017* | NS | 0.074 | NA | ||||
| Microvascular invasion (yes vs. no) | < 0.001* | 1.351 | 1.011-1.805 | 0.042* | 0.001* | 1.356 | 1.037-1.773 | 0.026* |
| Tumor number (multiple vs. single) | < 0.001* | 1.785 | 1.322-2.412 | < 0.001* | < 0.001* | 1.831 | 1.381-2.427 | < 0.001* |
| Tumor differentiation (III/IV vs. I/II) | 0.316 | NA | 0.336 | NA | ||||
| SLC29A1 expression (high vs. low) | 0.026* | 0.691 | 0.535-0.894 | 0.005* | 0.003* | 0.677 | 0.534-0.860 | 0.001* |
Abbreviations and Notes: OS, overall survival; NA, not adopted; NS, not significant; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen; 95% CI, 95% confidence interval; HR, hazard ratio; Cox proportional hazards regression model.
P-values < 0.05 were considered statistically significant.
Figure 3.
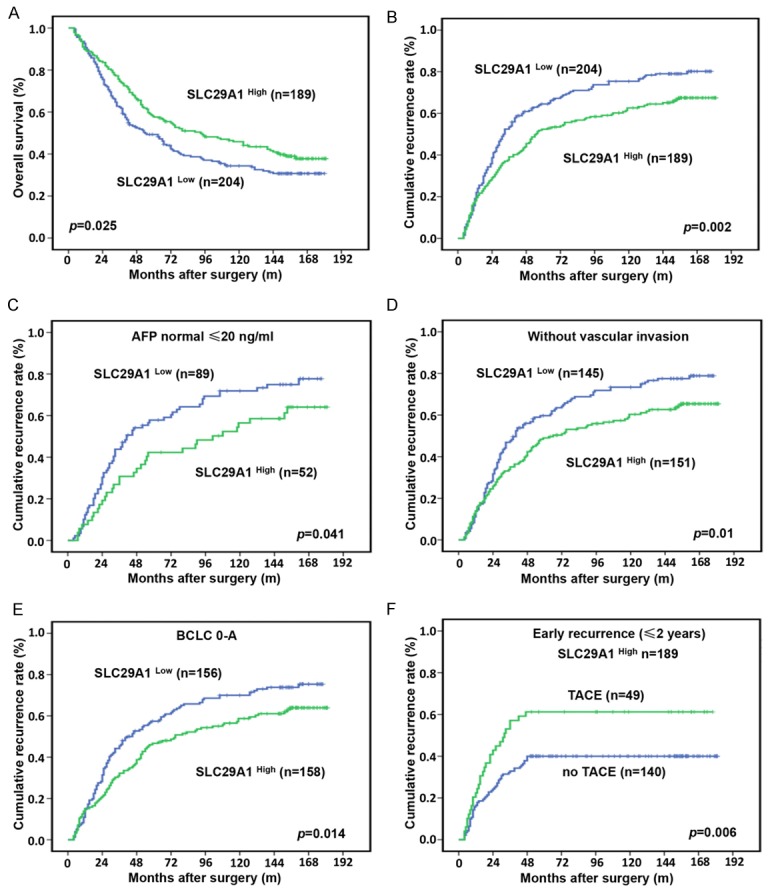
A and B. Kaplan-Meier analysis of overall survival (OS) and cumulative recurrence rates in 393 patients, by SLC29A1 expression. Significantly greater OS and lower cumulative recurrence rates occurred in the SLC29A1 high group, compared with the SLC29A1 low group. C-E. The prognostic significance of SLC29A1 in hepatocellular carcinoma (HCC) patients with normal alpha-fetoprotein (AFP) levels (P=0.041), without microvascular invasion (MVI; P=0.01), and Barcelona Clinic Liver Cancer (BCLC) Staging 0-A (P=0.014). F. Adjuvant transcatheter arterial chemoembolization (TACE) did not reduce the early recurrence rate in SLC29A1 high HCC patients (P=0.006).
Our previous studies found that TACE can improve the survival of patients with a high risk of residual tumor [16,17]. Here, we did not find that adjuvant TACE after surgery improved the OS rates and TTR in the study population (Supplementary Figure 2). Patients who received adjuvant TACE after surgery had higher early recurrence (≤ 2 year) rates, compared with those did not (43% vs. 30.8%) (Supplementary Figure 2). The SLC29A1 high group patients who received postoperative adjuvant TACE had significantly higher early recurrence rates, compared with those did not (P=0.020, Figure 3F). In the SLC29A1 low group patients, the difference in recurrence rate between patients who did or did not receive adjuvant TACE after surgery was not statistically significant (43.1% vs. 37%) (Supplementary Figure 2).
We found that patients with low SLC29A1 expression (score 0-2) were more likely to have MVI (P=0.047) and high serum AFP levels (P=0.001) (Table 1).
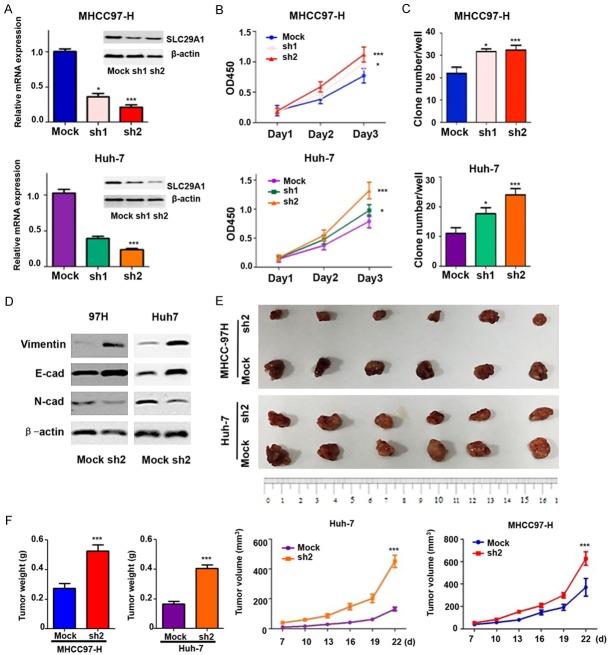
Down-regulation of SLC29A1 enhances tumor cells proliferation and invasion, induces EMT, and reduces drug sensitivity
We tested the hypothesis that SLC29A1 is involved in HCC tumorigenesis and progression (Figure 4A). SLC29A1 knockdown in MHCC97-H and Huh-7 cells promoted cell proliferation on day 3 (Figure 4B). The clonogenic ability of HCC cells was enhanced after SLC29A1 inhibition (Figure 4C). The in vitro invasion assay indicated that a greater proportion of the SLC29A1 knockdown cells had invaded, compared with the control cells (Supplementary Figure 3).
Figure 4.
Inhibition of SLC29A1 expression promotes hepatocellular carcinoma (HCC) cell growth in vitro and in vivo. Functional analysis, in vitro: A. SLC29A1 expression in MHCC97-H and Huh-7 cells transfected with an shRNA. B. SLC29A1 knockdown in MHCC97-H and Huh-7 cells promoted cell proliferation on day 3. C. The clonogenic ability of HCC cells was enhanced after SLC29A1 inhibition. Functional analysis, in vivo: D. Differences in the expression of epithelial and mesenchymal markers were compared between cancer cells with high and low SLC29A1 expression (MHCC97-H-Mock vs. MHCC97-H-SLC29A1-sh2 and Huh-7-Mock vs. Huh-7-SLC29A1-sh2 cells) using western blot assay. E. The morphological characteristics of tumors in the SLC29A1 shRNA and control groups. F. The differences in tumor weights and volumes between the SLC29A1 shRNA group and control group were statistically significant.
EMT is a dominant characteristic of most cancers and has a crucial role in cancer metastasis and invasion. Therefore, we compared the expression of epithelial and mesenchymal markers between knockdown groups and Mock cells. MHCC97-H-SLC29A1-sh2 and Huh-7-SLC29A1-sh2 cells expressed lower levels of the epithelial gene E-cadherin than MHCC97-H-Mock and Huh-7-Mock cells (Figure 4D). Mesenchymal gene (Vimentin and N-cadherin) expression was significantly up-regulated in MHCC97-H-SLC29A1-sh2 and Huh-7-SLC29A1-sh2 cells, compared with MHCC97-H-Mock and Huh-7-Mock cells.
Using a successful subcutaneous xenograft tumor model constructed with HCC cells, we found that down-regulation of SLC29A1 expression significantly enhanced tumor growth in vivo (Figure 4E). The weights and volumes of Mock cell-derived xenografts were significantly lower than those of tumor xenografts from MHCC97-H-SLC29A1-sh2 (MHCC97-H Mock: 0.275 ± 0.031 g vs. sh2: 0.525 ± 0.042 g and 387.72 ± 120.86 mm3 vs. 627.29 ± 148.46 mm3) and Huh-7-SLC29A1-sh2 hepatoma cells (Huh-7 Mock: 0.167 ± 0.180 g vs. sh2: 0.405 ± 0.023 g and 131.18 ± 30.26 mm3 vs. 451.27 ± 100.14 mm3) (Figure 4F).
The chemosensitivity assay was performed in Huh-7, MHCC97-H, Huh-7-SLC29A1-sh2, and MHCC97-H-SLC29A1-sh2 cells, which were treated with 0, 5, 10, 20, or 40 mg/L 5-FU or 0, 10, 15, 20, or 25 mg/L cisplatin or 0, 5, 10, 15, or 20 µM sorafenib for 48 hours; a CCK8 assay was used to evaluate cell viability (*, all P < 0.05). shRNA-mediated SLC29A1 knockdown significantly decreased sensitivity of HCC cells to 5-FU, cisplatin, and sorafenib (Figure 5, ***, P < 0.001).
Figure 5.
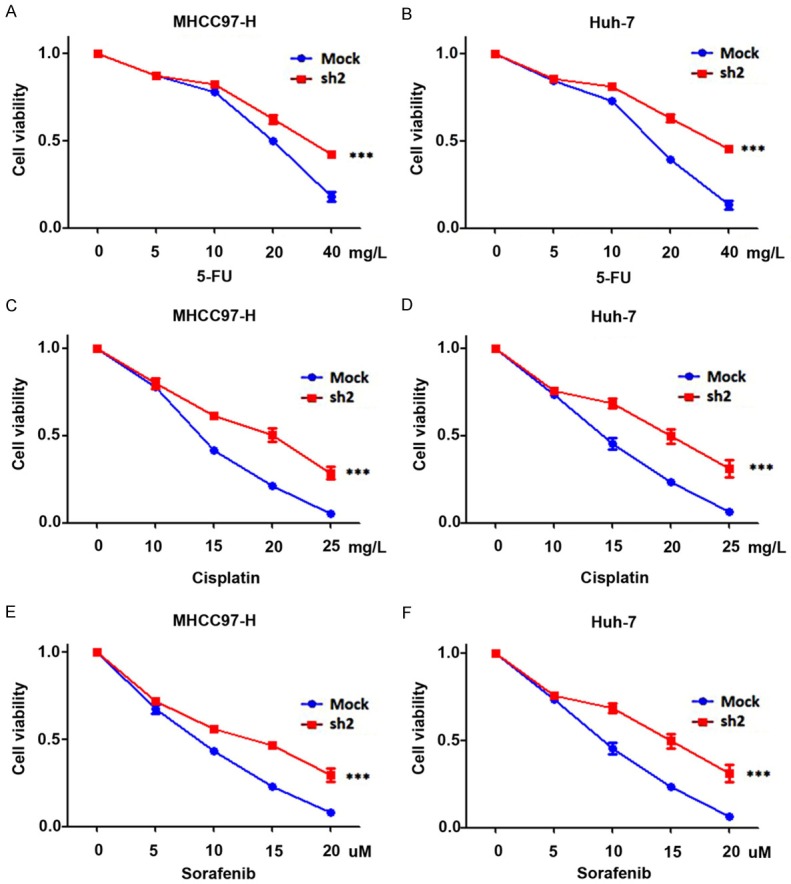
Inhibition of SLC29A1 expression promotes drug resistance in vitro. ShRNA-mediated SLC29A1 down-regulation inhibited the sensitivity of hepatocellular carcinoma cells to 5-FU (A and B), Cisplatin (C and D), and Sorafenib (E and F).
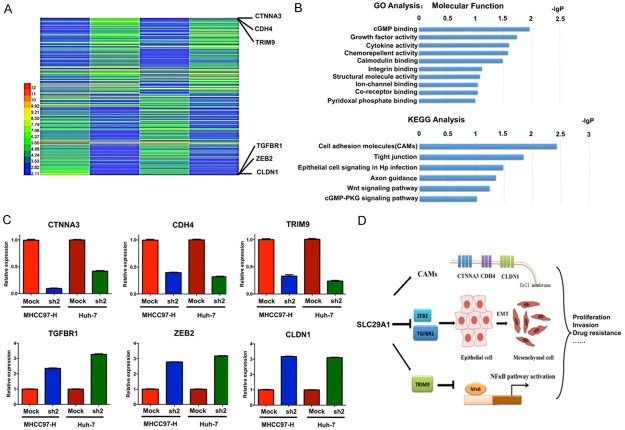
Gene expression profiling using a microarray analysis
The results of the microarray analysis indicated that 235 genes were differentially expressed between SLC29A1 shRNA-treated Huh-7/MHCC97-H cells and Mock cells. Of these genes, 112 were up-regulated (ratio > 2.0) and 123 were down-regulated (ratio < 0.5) (Figure 6A). The significant signaling pathways identified using the DAVID GO and KEGG databases are presented in Figure 6B. Many of these genes were related to growth factor activity (Figure 6B).
Figure 6.
Microarray-based gene expression profiles after SLC29A1 down-regulation. A. One hundred twelve genes were up-regulated (ratio > 2.0), and 123 genes were down-regulated (ratio < 0.5) between SLC29A1 shRNA-treated and Mock Huh-7/MHCC97-H cells. B. GO and KEGG analysis results. C. SLC29A1 expression was positively correlated with CAM and TRIM expression, thus increasing cell adhesion and inhibiting the NF-κB pathway, and negatively correlated with ZEB2 and TGFBR expression, thus inhibiting the epithelial-mesenchymal transition (EMT). D. Schematic diagram of the mechanisms of SLC29A1 involvement in hepatocellular carcinoma.
Six differentially expressed genes were further examined using qRT-PCR. Up-regulated and down-regulated genes were validated (Figure 6C). The possible functional relationship between SLC29A1 and its target genes is presented in Figure 6D. SLC29A1 might promote tumor cell proliferation, invasion, and drug resistance by regulating cell adhesion molecules (CLDN1, CTNNA3, and CDH4), the EMT (ZEB2 and TGFBR1), and the NF-κB pathway (TRIM9 is a negative regulator).
Discussion
Aberrant expression of drug transporters is associated with HCC drug resistance, and SLC transporters have important roles in tumor drug resistance [11]. We investigated the expression of SLC-superfamily genes in HCC tissues and peritumoral tissues to explore aberrantly expressed genes. The SLC29A1 mRNA was significantly elevated in HCC tissues. Low expression of SLC29A1 was also correlated with tumor recurrence after surgery and confirmed by western blot. TMA immunohistochemistry results from a larger series of human HCC specimens indicated that low SLC29A1 expression was correlated with high recurrence rates and poor OS. Down-regulation of SLC29A1 expression in HCC cells promoted tumor cell proliferation, invasion, EMT, and drug resistance, based on in vitro and in vivo studies.
SLC29A1 participates in cellular uptake of anti-cancer drugs and nucleosides [18]. SLC29A1 enhances the cytotoxicity of some anti-cancer drugs and is a useful predictive marker of drug efficacy in cancer cells. Single nucleotide polymorphisms in SLC29A1 contribute to the cytotoxic effects of AraC, an effective treatment for acute myeloid leukemia [19]. SLC29A1 is positively associated with chemotherapy efficacy in Asian pancreatic cancer patients [20]. We found that SLC29A1 knockdown enhanced drug resistance to 5-FU, cisplatin, and sorafenib in vitro. However, further studies of the mechanism by which SLC29A1 increases anti-cancer drug efficacy are needed.
The anti-tumor effects of SLC29A1 involve multiple signaling pathways. CTNNA3, CDH4, and CLDN1 are cell adhesion molecules that are essential for the development and progression of cancer [21,22]. ZEB2 and TGFBR1 regulate the EMT process [23,24], and TRIM9 inhibits the NF-κB pathway [25]. SLC29A1 knockdown may result in decreased CAM and TRIM9 function in HCC cells or increased ZEB2 and TGFBR1 function, which subsequently inhibits cell adhesion, and promotes the EMT and activation of the NF-κB pathway. These changes will increase tumor cell proliferation and invasion, and induce EMT and drug resistance. SLC29A1-induced changes in the expression of any one of these proteins has critical effects on carcinogenesis, progression, and drug resistance.
The prognostic significance of SLC29A1 was present in patients with BCLC 0-A HCC: the 5-year tumor recurrence rates for SLC29A1 high and SLC29A1 low patients were 46.7% vs. 57.4%, respectively. AFP is the most widely used tumor marker; however, approximately 40% to 60% of patients with HCC have normal serum AFP levels [26]. Patients with normal AFP could be stratified according to SLC29A1 expression into two groups with substantially different TTR (36.9% vs. 63.1% for SLC29A1 high and SLC29A1 low, respectively, P < 0.05). Our results indicated that SLC29A1 might be a useful prognostic marker for HCC, especially in AFP-normal patients and those with low recurrence risk [27]. We also found that the risk of early recurrence for SLC29A1 high HCC patients with adjuvant TACE was high and there was not beneficial for TACE in SLC29A1 high HCC patients after surgery (Figure 3F). SLC29A1 might be a useful index for choosing suitable adjuvant treatment for HCC patients after surgery.
To the best of our knowledge, our study is the first to reveal the correlation between SLC29A1 expression and the prognosis of patients with HCC, and its role in drug resistance of HCC. Most patients with HCC in China are positive for the hepatitis B virus; 84.4% of our study population was hepatitis B virus-positive, which differs greatly from populations in the United States, Europe, and Japan [28]. The prognostic significance of SLC29A1 should be validated in HCC patients from these countries. Prospective studies with large patient cohorts and additional studies into the mechanism of SLC29A1 in HCC are needed.
In conclusion, SLC29A1 has important roles in tumor cell proliferation and invasion, and induces EMT and drug resistance by interacting with various signaling pathways in HCC cells. Low expression of SLC29A1 in tumor tissues was correlated with a high recurrence rate and poor outcomes for patients with HCC after surgery. SLC29A1 might be a promising prognostic factor, a potential tumor suppressor, and a drug sensitizer for patients with HCC.
Acknowledgements
This study was supported by grants from the National Key Research and Development Program of China (2016YFF01400 and 2016YFC0902400), the National High Technology Research and Development Program (863 Program) of China (2015AA020401), the State Key Program of National Natural Science of China (81530077), the National Natural Science Foundation of China (81472676, 81572823, 81602543, 81602581, and 81672839), the Projects from the Shanghai Science and Technology Commission (14DZ1940300, 14411970200 and 14140902301).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Han KH, Park JY. Chemotherapy for advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:682–684. doi: 10.1111/j.1440-1746.2008.05444.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhao XY, Li L, Wang XB, Fu RJ, Lv YP, Jin W, Meng C, Chen GQ, Huang L, Zhao KW. Inhibition of snail family transcriptional repressor 2 (SNAI2) enhances multidrug resistance of hepatocellular carcinoma cells. PLoS One. 2016;11:e0164752. doi: 10.1371/journal.pone.0164752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibasaki Y, Sakaguchi T, Hiraide T, Morita Y, Suzuki A, Baba S, Setou M, Konno H. Expression of indocyanine green-related transporters in hepatocellular carcinoma. J Surg Res. 2015;193:567–576. doi: 10.1016/j.jss.2014.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 9.Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. 2015;14:543–560. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaeffeler E, Hellerbrand C, Nies AT, Winter S, Kruck S, Hofmann U, van der Kuip H, Zanger UM, Koepsell H, Schwab M. DNA methylation is associated with downregulation of the organic cation transporter OCT1 (SLC22A1) in human hepatocellular carcinoma. Genome Med. 2011;3:82. doi: 10.1186/gm298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heise M, Lautem A, Knapstein J, Schattenberg JM, Hoppe-Lotichius M, Foltys D, Weiler N, Zimmermann A, Schad A, Grundemann D, Otto G, Galle PR, Schuchmann M, Zimmermann T. Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer. 2012;12:109. doi: 10.1186/1471-2407-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namisaki T, Schaeffeler E, Fukui H, Yoshiji H, Nakajima Y, Fritz P, Schwab M, Nies AT. Differential expression of drug uptake and efflux transporters in Japanese patients with hepatocellular carcinoma. Drug Metab Dispos. 2014;42:2033–2040. doi: 10.1124/dmd.114.059832. [DOI] [PubMed] [Google Scholar]
- 13.Zollner G, Wagner M, Fickert P, Silbert D, Fuchsbichler A, Zatloukal K, Denk H, Trauner M. Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver Int. 2005;25:367–379. doi: 10.1111/j.1478-3231.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 14.Ishak KG, Anthony PP, Sobin LH. Histological typing of tumours of the liver. Berlin Heidelberg: Springer; 1994. [Google Scholar]
- 15.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 16.Ren ZG, Lin ZY, Xia JL, Ye SL, Ma ZC, Ye QH, Qin LX, Wu ZQ, Fan J, Tang ZY. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol. 2004;10:2791–2794. doi: 10.3748/wjg.v10.i19.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XR, Xu Y, Yu B, Zhou J, Li JC, Qiu SJ, Shi YH, Wang XY, Dai Z, Shi GM, Wu B, Wu LM, Yang GH, Zhang BH, Qin WX, Fan J. CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin Cancer Res. 2009;15:5518–5527. doi: 10.1158/1078-0432.CCR-09-0151. [DOI] [PubMed] [Google Scholar]
- 18.Senyavina NV, Gerasimenko TN, Fomicheva KA, Tonevitskaya SA, Kaprin AD. Localization and expression of nucleoside transporters ENT1 and ENT2 in polar cells of intestinal epithelium. Bull Exp Biol Med. 2016;160:771–774. doi: 10.1007/s10517-016-3306-5. [DOI] [PubMed] [Google Scholar]
- 19.Wan H, Zhu J, Chen F, Xiao F, Huang H, Han X, Zhong L, Zhong H, Xu L, Ni B, Zhong J. SLC29A1 single nucleotide polymorphisms as independent prognostic predictors for survival of patients with acute myeloid leukemia: an in vitro study. J Exp Clin Cancer Res. 2014;33:90. doi: 10.1186/s13046-014-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao JC, Zhang TP, Zhao YP. Human equilibrative nucleoside transporter 1 (hENT1) predicts the Asian patient response to gemcitabinebased chemotherapy in pancreatic cancer. Hepatogastroenterology. 2013;60:258–262. doi: 10.5754/hge12687. [DOI] [PubMed] [Google Scholar]
- 21.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 22.Zigler M, Dobroff AS, Bar-Eli M. Cell adhesion: implication in tumor progression. Minerva Med. 2010;101:149–162. [PubMed] [Google Scholar]
- 23.Dimitrova Y, Gruber AJ, Mittal N, Ghosh S, Dimitriades B, Mathow D, Grandy WA, Christofori G, Zavolan M. TFAP2A is a component of the ZEB1/2 network that regulates TGFB1-induced epithelial to mesenchymal transition. Biol Direct. 2017;12:8. doi: 10.1186/s13062-017-0180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun J, Hoang-Vu C, Dralle H, Huttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 25.Shi M, Cho H, Inn KS, Yang A, Zhao Z, Liang Q, Versteeg GA, Amini-Bavil-Olyaee S, Wong LY, Zlokovic BV, Park HS, García-Sastre A, Jung JU. Negative regulation of NF-kappaB activity by brain-specific TRIpartite Motif protein 9. Nat Commun. 2014;5:4820. doi: 10.1038/ncomms5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127(Suppl 1):S108–112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Hsu YC, Fu HH, Jeng YM, Lee PH, Yang SD. Proline-directed protein kinase FA is a powerful and independent prognostic predictor for progression and patient survival of hepatocellular carcinoma. J. Clin. Oncol. 2006;24:3780–3788. doi: 10.1200/JCO.2005.03.7499. [DOI] [PubMed] [Google Scholar]
- 28.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.