Abstract
Long non-coding RNAs (lncRNAs) act as critical regulators of many malignant tumors cellular processes including cell proliferation, differentiation, apoptosis, invasion and metastasis. However, the functions and molecular mechanisms of lncRNA HOXD-AS1 in melanoma remain little known. In the present study, we observed that lncRNA HOXD-AS1 expression was remarkably higher in melanoma tissues compared to skin tissues with melanocytic nevus. Increased expression of lncRNA HOXD-AS1 correlated with poor survival of melanoma patients. Furthermore, functional experiments demonstrated that upregulated lncRNA HOXD-AS1 expression dramatically promoted cell proliferation and invasion of melanoma, while downregulation of lncRNA HOXD-AS1 showed a tumor inhibiting effects on melanoma cells in vitro. In vivo, data results showed that lncRNA HOXD-AS1 knockdown notably reduced tumor growth. Additionally, RNA immunoprecipitation (RIP) and Chromatin immunoprecipitation (ChIP) assays revealed that lncRNA HOXD-AS1 could epigenetically suppress the expression of RUNX3 via binding to EZH2. Downregulation of RUNX3 attenuated the proliferation and invasion-inhibiting effects induced by lncRNA HOXD-AS1 knockdown in melanoma cells. Therefore, these results indicated that HOXD-AS1 may serve as a potential therapeutic target of melanoma.
Keywords: Melanoma, long non-coding RNA, HOXD-AS1, RUNX3, EZH2
Introduction
Melanoma is the most aggressive skin cancer with increasing incidence worldwide and accounts for approximately 4% of skin cancer cases [1,2]. Due to high metastatic potential of melanoma, patients with late-stage metastatic disease represent poor prognosis and the 5-year survival rate is less than 15% [3,4]. Thus, to reveal molecular mechanisms underlying melanoma and investigate novel target of melanoma treatment is crucial.
Long non coding RNAs (lncRNAs) act as functional regulators of tumor development and progression in different types of cancer [5]. In melanoma, some studies have showed the importance of lncRNAs involved in molecular mechanisms underlying melanoma. Chen et al reported that lncRNA GAS5 is a critical regulator of metastasis phenotype of melanoma cells and inhibits tumor growth in vivo [6]. Downregulated long non-coding RNA BANCR promotes the proliferation of colorectal cancer cells via downregualtion of p21 expression [7]. EZH2-mediated epigenetic suppression of long non-coding RNA SPRY4-IT1 promotes NSCLC cell proliferation and metastasis by affecting the epithelial-mesenchymal transition [8].
HOXD-AS1, a novel lncRNA encoded in HOXD cluster, was revealed to control expression levels of clinically significant protein-coding genes involved in angiogenesis and inflammation, the hallmarks of metastatic cancer [9]. Zheng et al showed that knockdown of long non-coding RNA HOXD-AS1 inhibits gastric cancer cell growth via inactivating the JAK2/STAT3 pathway [10]. Lu et al revealed lncRNA HOXD-AS1 is a critical regulator of the metastasis and apoptosis phenotype in human hepatocellular carcinoma [11]. However, the functions and molecular mechanisms of lncRNA HOXD-AS1 in melanoma remain little investigated.
In the study, we observed that lncRNA HOXD-AS1 was remarkably higher in melanoma tissues and correlated with poor overall survival time of melanoma patients. Furthermore, upregulation of lncRNA HOXD-AS1 significantly enhanced cell proliferation and invasion in vitro and knockdown of lncRNA HOXD-AS1 in vivo reduced tumor growth. In addition, we revealed that lncRNA HOXD-AS1 could epigenetically inhibit the expression of RUNX3 by binding to EZH2. Therefore, these results indicated that lncRNA HOXD-AS1 may serve as a potential therapeutic target of melanoma.
Materials and methods
Patient tissue samples
A total of 25 human malignant melanoma tissues and 25 matched skin tissues with melanocytic nevus were obtained from patients who underwent surgery at Peking Union Medical College Hospital (Beijing, China). All samples were diagnosed by two professional pathologists. The study was approved by Review Board of Peking Union Medical College Hospital and written informed consent was obtained from all of the patients. None of patients had received radiotherapy or chemotherapy prior to operation. All tissue samples were stored at -80°C until RNA analyses.
Quantitative Real-time PCR (QRT-PCR)
Total RNA from melanoma tissues and matched skin tissues with melanocytic nevus was extracted using Trizol reagents (Takara, Dalian, China) according to the manufacturer’s instructions. RNA concentration was detected by a NanoDrop2000c spectrophotometer and was reversed transcription to DNA using M-MLV Reverse Transcriptase (Takara, Dalian, China). Quantitative RT-PCR was performed using the SYBR-Green PCR Master Mix kit (Takara, Dalian, China) on an ABI StepOne Plus system (Applied Biosystems, CA, USA) following the manufacturer’s instruction. The PCR reaction conditions was 95°C for 30 s, then followed 40 cycles of 95°C for 5 s and 60°C for 32 s. The lncRNA HOXD-AS1 and RUNX3 mRNA expression fold were calculated using 2-ΔΔCt method and normalized to GAPDH. The primer sequences used in the study were as follows: HOXD-AS1-forward: 5’-GGCTCTTCCCTAATGTGTGG-3’, HOXD-AS1-reverse: 5’-CAGGTCCAGCATGAAACAGA-3’; GAPDH-forward: 5’-CGCTCTCTGCTCCTCCTGTTC-3’, GAPDH-reverse: 5’-ATCCGTTGACTCCGACCTTCAC-3’.
Cell lines culture
Human malignant melanoma cell lines B16, A375 and A2508 cells and a human epidermal melanocytes (HEMn) cell were obtained from American Type Culture Collection (ATCC). Cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) at 37°C containing 5% CO2 in a humidified incubator.
Cells transfection
Two specifically targeting lncRNA HOXD-AS1 were constructed and synthesized by Ribobio (Guangzhou, China). The target sequences were used in the study was as follow: si-HOXD-AS1-1 (si-RNA-1): 5’-GAAAGAAGGACCAAAGTAA-3’, si-HOXD-AS1-2 (si-RNA-2): 5’-GCACAAAGGAACAAGGAAA-3’. Cells transfection was performed using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s instructions. The overexpression of pcDNA3.1-HOXD-AS1 plasmid was constructed and synthesized by Ribobio (Guangzhou, China) based on the sequence of lncRNA HOXD-AS1. The LV-GFP-shRNA-HOXD-AS1 was constructed and synthesized by Ribobio (Guangzhou, China) according to si-HOXD-AS1-2 sequences.
CCK8 cell proliferation assay
Melanoma cells were cultured in 96-well plates and the cell density was 2000 cells per well. Following melanoma cells were transfected with si-control, si-HOXD-AS1-1 (siRNA-1), and si-HOXD-AS1-2 (siRNA-2) or empty vector and pcDNA3.1-HOXD-AS1 plasmid. At indicated times (1, 2, 3, and 4 days), the 10 μL of CCK8 solution was added to each well, and cells were incubated for 2 h at 37°C in a humidified incubator containing 5% CO2. Cell viability was determined at the absorbance at 450 nm under a microplate reader (Thermo Labsystems, Waltham, MA, USA).
Cell invasion assay
Cell invasion was assessed using transwell chambers (8-μM pore size; Costar). The seeding transfected cell number (5 × 104) was resuspended in 400 μl DMEM medium without FBS and plated in the upper chamber and 500 μl of DMEM with 10% FBS was added to the lower chamber. After cell transfection at 48 h, cells were fixed for 20 min, stained with 0.1% crystal violet for 15 min, and then photographed using a photomicroscope. Cells were calculated in five random fields.
Western blot analysis
Total protein was extracted from transfected cells using RIPA buffer. Protein quantification was analyzed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Equal proteins (55 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bradford, MA, USA). The membrane was incubated with specific antibodies including anti-RUNX3 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-GAPDH (1:1,000; Cell Signaling Technology, Beverly, MA, USA). The membrane was detected using a chemiluminescent ECL reagent (Millipore).
Tumor xenograft model
A375 cells (1 × 106 cells) stably transfected with sh-control and sh-HOXD-AS1 were re-suspended using PBS. 3 weeks BALB/c nude mice were subcutaneously inoculated in the right flank using A375 cells stably transfected sh-control and sh-HOXD-AS1. Tumor volume was measured every 7 days, and mice were killed after 4 weeks.
RNA immunoprecipitation (RIP) assay
EZ-Magna RIP kit (Millipore, Billerica, MA) were used to perform RNA immunoprecipitation (RIP) assay according to the manufacturer’s protocol as previous describe [12]. Briefly, lysed cells were incubated with RIP buffer containing magnetic beads conjugated with antibodies against EZH2 and H3k27me3 (Cell Signaling Technology, Beverly, MA, USA). Immunoprecipitated RNA was purified and detected by qRT-PCR.
Chromatin immunoprecipitation (ChIP) assay
SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) was used to perform Chromatin immunoprecipitation assay following the manufacturer’s protocol. Precipitate protein/DNA complex were immunoprecipitated with EZH2 antibody and H3K27me3 antibody (Cell Signaling Technology, Beverly, MA, USA) and normalized to IgG antibody (Cell Signaling Technology, Beverly, MA, USA). Precipitated DNA was analyzed by qRT-PCR.
Results
LncRNA HOXD-AS1 is significantly upregulated in melanoma tissues and associated with overall survival of melanoma patients
We first analyzed the HOXD-AS1 expression in 25 cases of human melanoma tissues and 25 matched skin tissues with melanocytic nevus. We observed that lncRNA HOXD-AS1 expression was significantly upregulated in human melanoma tissues compared with matched skin tissues with melanocytic nevus (Figure 1A). According to the median expression ratio, Patients were divided into two groups: lower lncRNA HOXD-AS1 group (n=12, lncRNA HOXD-AS1 expression ratio ≤ median ratio) and higher lncRNA HOXD-AS1 group (n=13, lncRNA HOXD-AS1 expression ratio ≥ median ratio). Kaplan-Meier survival analysis and log rank test indicated that higher lncRNA HOXD-AS1 expression group had significantly shorter overall survival time than those in lower lncRNA HOXD-AS1 group in melanoma patients (Figure 1B, log rank test, p<0.05). Then, we examined the lncRNA HOXD-AS1 expression from three human malignant melanoma cell lines B16, A375 and A2508 cells, and human epidermal melanocytes (HEMn) cells. We observed that lncRNA HOXD-AS1 expression was also upregulated in melanoma cells compared to HEMn cell (Figure 1C). These results indicated that lncRNA HOXD-AS1 may act as an oncogene involved in the tumorigenesis of melanoma.
Figure 1.
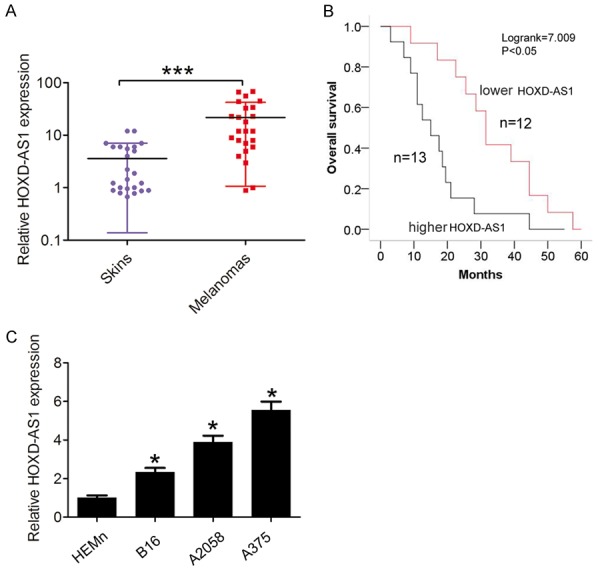
LncRNA HOXD-AS1 expression in melanoma tissues and cells was significantly upregulated. A. LncRNA HOXD-AS1 expression was examined using qRT-PCR in 25 cases of human melanoma tissues and 25 matched skin tissues with melanocytic nevus. The mRNA expression was normalized to GAPDH. B. Kaplan-Meier method with the log-rank test was applied to assess the overall survival in higher and lower lncRNA HOXD-AS1 expression groups in melanoma patients. C. LncRNA HOXD-AS1 expression was examined using qRT-PCR in human malignant melanoma cell lines B16, A375 and A2508 cells, and human epidermal melanocytes (HEMn) cells. The mRNA expression was normalized to GAPDH. Data represented the mean ± SD. *, P<0.05, ***, P<0.001.
LncRNA HOXD-AS1 promotes melanoma cell proliferation and invasion in vitro
To explore the functional relevance of lncRNA HOXD-AS1 in melanoma cells, we selected B16 cells for gaining function studies by transfection of pcDNA3.1-HOXD-AS1 plasmid and the A375 and A2058 cell lines were used for loss function studies by transfection of siRNAs based on they exhibited the expression levels of lncRNA HOXD-AS1 than that in HEMn cells (Figure 2A). CCK8 assay was used to determine the potential biological role of lncRNA HOXD-AS1 in melanoma cell proliferation. Results showed that cells growth viability was increased when B16 cells were transfected with pcDNA3.1-HOXD-AS1, compared with control group. However, cells growth viability was reduced when si-HOXD-AS1-1 or 2 were transfected into A375 cells and A2058 cells compared with control groups (Figure 2B, 2C). Then, we performed colony forming assays to detect the tumorigenicity of melanoma cells. Results showed that cell colony forming number was significantly decreased following lncRNA HOXD-AS1 knockdown in A375 cells and A2058 cells, compared to the control group. However, the cell colony number after HOXD-AS1 overexpression in B16 cells was formed more compared to control group (Figure 2D, 2E). Moreover, we also detected whether lncRNA HOXD-AS1 expression influenced cell invasion. The results showed that cell invasive number was significantly decreased following lncRNA HOXD-AS1 knockdown in A375 cells and A2058 cells, compared to the control group. However, lncRNA HOXD-AS1 overexpression promoted cell invasion in B16 cells (Figure 3A, 3B). These investigations indicated that lncRNA HOXD-AS1 exerted critical effects on melanoma cell proliferation and invasion.
Figure 2.
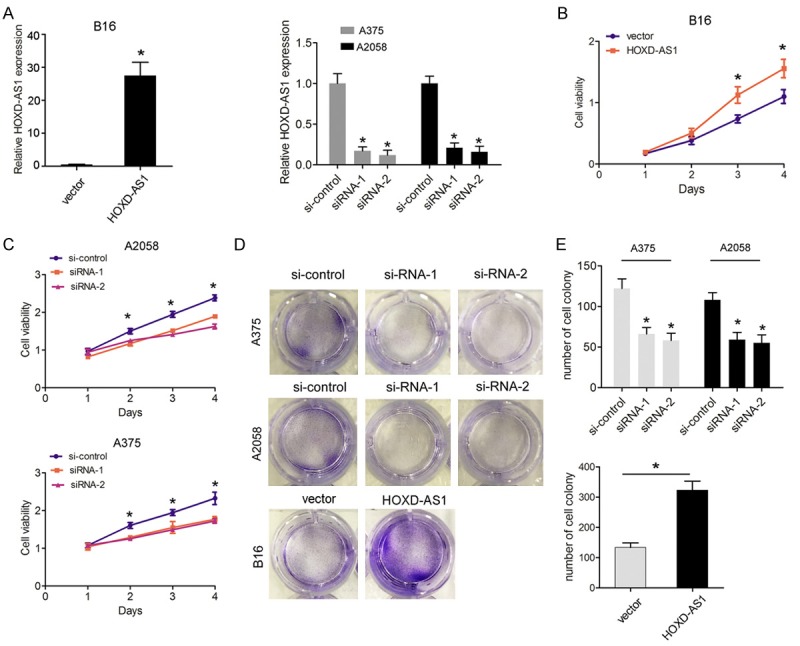
LncRNA HOXD-AS1 knockdown inhibited cell proliferation ability. (A). The lncRNA HOXD-AS1 mRNA expression in B16 cells transfected with pcDNA3.1-vector (vector) and pcDNA3.1-HOXD-AS1 (HOXD-AS1) or in A375 and A2508 cells transfected with si-control, si-HOXD-AS1-1 (siRNA-1) and HOXD-AS1-2 (siRNA-2) were measured by qRT-PCR. (B, C). CCK-8 assays were performed to determine cell growth in B16 cells transfected with pcDNA3.1-vector (vector) and pcDNA3.1-HOXD-AS1 (HOXD-AS1) or in A375 and A2508 cells transfected with si-control, si-HOXD-AS1-1 (siRNA-1) or HOXD-AS1-2 (siRNA-2). (D, E). Colony-forming assays were performed to determine the cloning ability in A375 and A2508 cells transfected with si-control, si-HOXD-AS1-1 (siRNA-1) or HOXD-AS1-2 (siRNA-2) or in B16 cells transfected with pcDNA3.1-vector (vector) and pcDNA3.1-HOXD-AS1 (HOXD-AS1). Data represented the mean ± SD. *, P<0.05.
Figure 3.
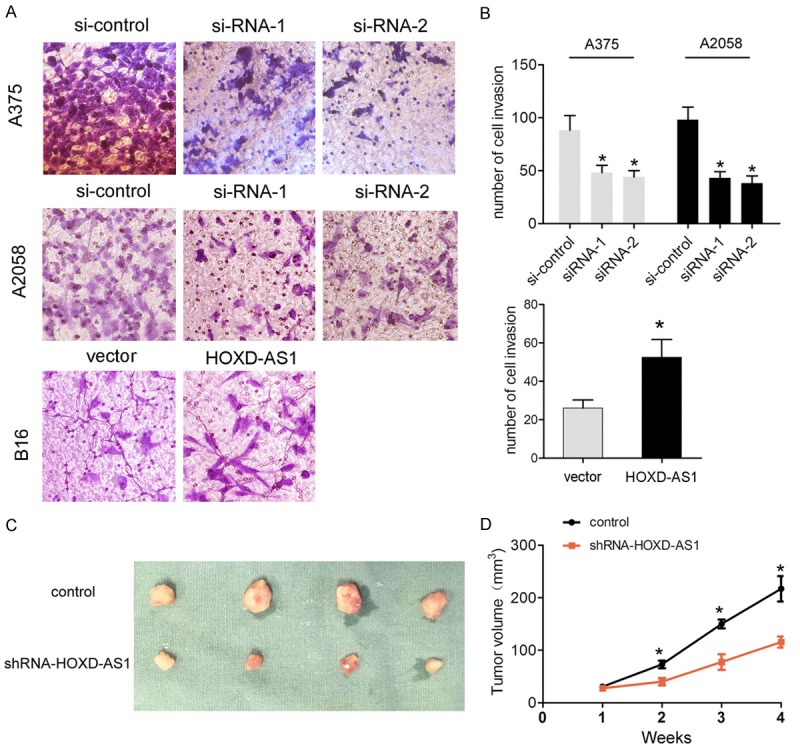
LncRNA HOXD-AS1 knockdown inhibited cell invasion ability in vitro and cell growth in vivo. (A, B) Transwell invasion assays were performed to determine the invasion ability in A375 and A2508 cells transfected with si-control, si-HOXD-AS1-1 (siRNA-1) and HOXD-AS1-2 (siRNA-2) or in B16 cells transfected with pcDNA3.1-vector (vector) and pcDNA3.1-HOXD-AS1 (HOXD-AS1). (C) The stable lncRNA HOXD-AS1 knockdown A375 cells were used for in vivo experiments. The nude mice carrying tumors from control and shRNA-HOXD-AS1 group were shown and (D) tumor volume curves were measured every 7 days after the injection of A375 cells. Data represented the mean ± SD. *, P<0.05.
Knockdown of lncRNA HOXD-AS1 inhibits melanoma growth in vivo
To assess whether lncRNA HOXD-AS1 expression could affect lncRNA HOXD-AS1 tumorigenesis in vivo, we applied A375 cells stably transfected with LV-GFP-control or LV-GFP-sh-HOXD-AS1 plasmids to subcutaneously inoculate into male nude mice. After 4 weeks, we observed that tumor volume in sh-HOXD-AS1 group was dramatically smaller and the tumor growth was notably slower compared to the control group (Figure 3C, 3D). Thus, our results indicated that knockdown of lncRNA HOXD-AS1 inhibited melanoma cell growth in vivo.
LncRNA HOXD-AS1 epigenetically silences RUNX3 transcription by interacting with EZH2
RUNX3 was reported to function as a tumor suppressor in some tumors including melanoma [13]. In the study, we found that the relative transcription levels of RUNX3 in melanoma tissues was significantly downregulated compared to matched skin tissues with melanocytic nevus (Figure 4A). Furthermore, lower RUNX3 expression in melanoma tissues negatively correlated with higher lncRNA HOXD-AS1 expression by Spearman correlation coefficient analysis (Figure 4B, r=-0.445, P<0.05). We selected the siRNA-2 for lncRNA HOXD-AS1 knockdown in the following experiments due to its highest knockdown efficiency. The results demonstrated that RUNX3 protein expression was significantly increased in si-HOXD-AS-treated A375 and A2058 cells, compared with the control group (Figure 4C, 4D), which suggested that lncRNA HOXD-AS1 affected RUNX3 expression.
Figure 4.
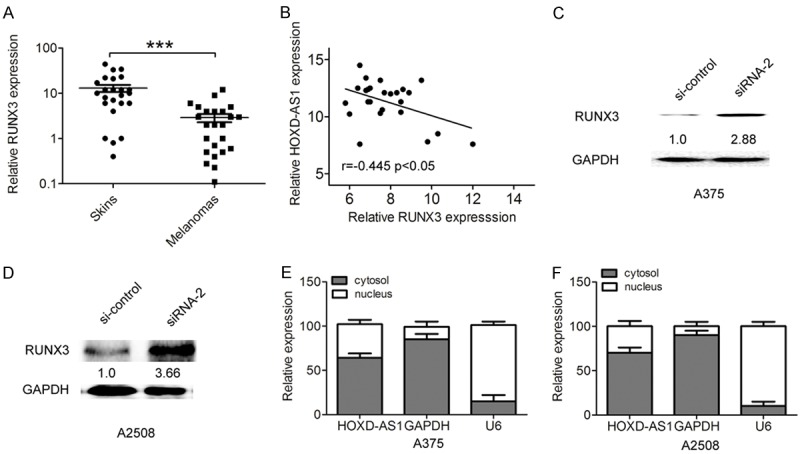
LncRNA HOXD-AS1 negatively regulated RUNX3 expression in melanoma. (A) RUNX3 expression was examined using qRT-PCR in 25 cases of human melanoma tissues and 25 matched skin tissues with melanocytic nevus. (B) Lower RUNX3 expression in melanoma tissues negatively correlated with higher lncRNA HOXD-AS1 expression by Spearman correlation coefficient analysis (r=-0.445, P<0.05). (C, D). The protein expression of RUNX3 was examined using western blot in A375 and A2508 cells transfected with si-control, si-HOXD-AS1-1(siRNA-2). (E, F) Relative lncRNA HOXD-AS1 expression in cell cytoplasm or nucleus of A375 and A2508 cells were determined by qRT-PCR. GAPDH was used as cytoplasm control and U1 was used as nucleus control. Data represented the mean ± SD. *, P<0.05.
To investigate the potential molecular mechanism and downstream targets of lncRNA HOXD-AS1 in melanoma progression, we detected the distribution of lncRNA HOXD-AS1 in A375 and A2058 cells. The qRT-PCR results found that lncRNA HOXD-AS1 was mostly located in nucleus (Figure 4E, 4F). EZH2 was found to mediate the lncRNAs function by epigenetically silencing downstream targets [14]. Furthermore, we performed the RIP assays to explore whether lncRNA HOXD-AS1 was related to EZH2 and H3k27me3. Our results showed that lncRNA HOXD-AS1 interacted with EZH2 and H3k27me3 in A375 and A2058 cells (Figure 5A, 5B).
Figure 5.
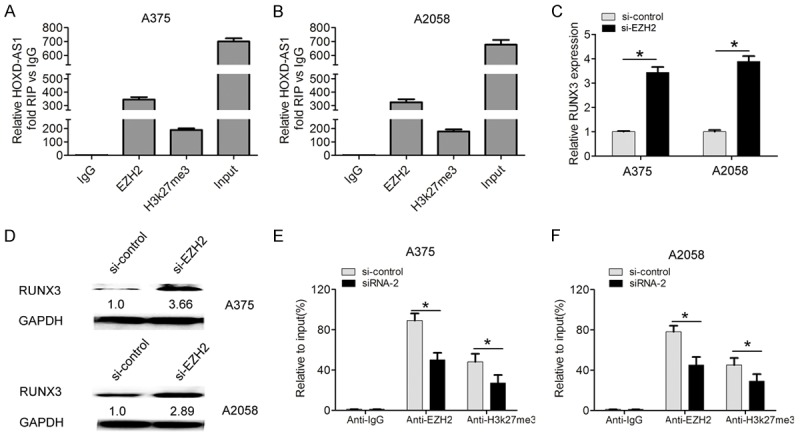
LncRNA HOXD-AS1 epigenetically silences RUNX3 transcription by interacting with EZH2. (A, B) Relative RNA levels in immunoprecipitates with EZH2 and H3k27me3 were determined in A375 and A2508 cells by qRT-PCR. Expression of lncRNA HOXD-AS1 RNA was shown as fold enrichment relative to IgG immunoprecipitate. (C, D) The mRNA and protein expression of RUNX3 was examined using western blot in A375 and A2508 cells transfected with si-control and si-EZH2. (E, F) ChIP-qPCR analysis of EZH2 occupancy and H3K27me3 binding to the RUNX3 promoter regions in A375 and A2508 cells, and IgG was used as a negative control. Data represented the mean ± SD. *, P<0.05.
EZH2 was reported to mediate epigenetic silencing of neuroblastoma suppressor gene RUNX3 [15]. We also found that RUNX3 mRNA and protein expression levels were increased in si-EZH2-treated A375 and A2058 cells (Figure 5C, 5D). Moreover, the results of chromatin immunoprecipitation (ChIP)-qRT-PCR assays showed that knockdown of lncRNA HOXD-AS1 resulted in reduced EZH2 binding and H3K27me3 occupancy of RUNX3 the promoter in A375 and A2058 cells, respectively (Figure 5E, 5F). Thus, these data indicated that lncRNA HOXD-AS1 epigenetically silenced RUNX3 transcription by interacting with EZH2.
RUNX3 potentially mediates the oncogenic function of lncRNA HOXD-AS in melanoma
To validate whether lncRNA HOXD-AS1 regulated melanoma cell proliferation and invasion by silencing RUNX3 expression, we performed the rescue assays. A375 and A2058 cells were cotransfected with si-HOXD-AS1 and si-RUNX3. The CCK8 assay and cell forming assays results suggested that cell proliferation was suppressed after lncRNA HOXD-AS1 knockdown, but was increased after RUNX3 knockdown. However, co-transfecting si-HOXD-AS1 and si-RUNX3 partially rescued the inhibiting effects induced by lncRNA HOXD-AS1 knockdown in A375 cells (Figure 6A, 6D). Consistent with above results, cell invasion capacity was suppressed after HOXD-AS knockdown, but was increased after RUNX3 knockdown, whereas, co-transfecting si-HOXD-AS1 and si-RUNX3 partially rescued the inhibiting effects induced by lncRNA HOXD-AS1 knockdown in A375 cells (Figure 6E, 6F). These results indicated that RUNX3 potentially mediated the oncogenic function of lncRNA HOXD-AS in melanoma.
Figure 6.
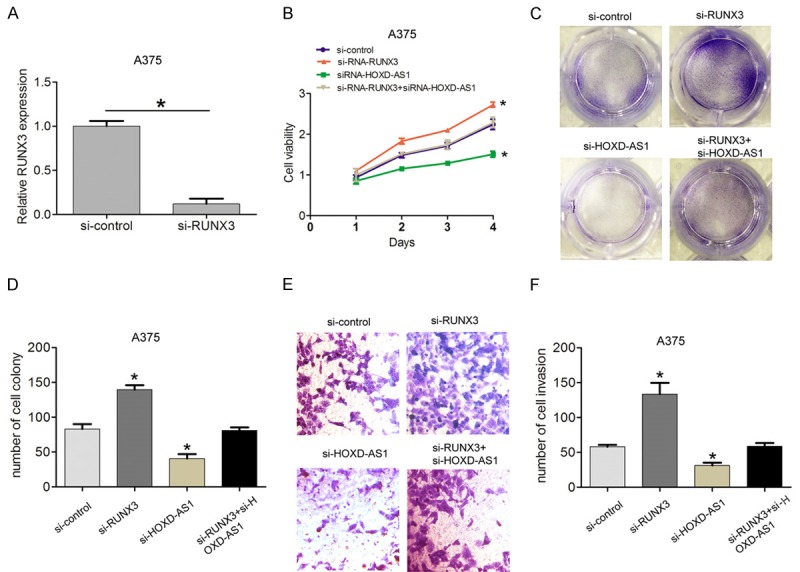
Knockdown of RUNX3 partly mediated the oncogenic function induced by lncRNA HOXD-AS1. (A) Relative mRNA expression of RUNX3 in A375 cells transfected with si-control and si-RNA-RUNX3. (B-D) CCK8 assays and colony-forming assays were performed to detected the cell viability and cloning ability in A375 cells transfected with si-control, si-RNA-RUNX3, siRNA- HOXD-AS1, and siRNA-HOXD-AS1+siRNA-RUNX3. (E, F) Transwell assays were performed to detected the cell invasion ability in A375 cells transfected with si-control, si-RNA-RUNX3, siRNA-HOXD-AS1, and siRNA-HOXD-AS1+siRNA-RUNX3. Data represented the mean ± SD. *, P<0.05.
Discussion
Recent reporters revealed that lncRNA HOXD-AS1 was identified as potentially diagnostic biomarker and therapeutic target in a variety of tumors. Knockdown of lncRNA HOXD-AS1 suppressed cell proliferation/migration and increased the rate of apoptotic cell in bladder cancer cells [16]. Overexpression of lncRNA HOXD-AS1 competitively bound to miR-130a-3p that prevented SOX4 from miRNA-mediated degradation, thus activated the expression of EZH2 and MMP2 and facilitated HCC metastasis [17]. In the study, we demonstrated that lncRNA HOXD-AS1 was remarkably higher in melanoma tissues and increased lncRNA HOXD-AS1 expression correlated with poor prognosis of melanoma patients. Upregulation of lncRNA HOXD-AS1 dramatically increased melanoma cell proliferation and invasion abilities in vitro, while lncRNA HOXD-AS1 knockdown had reverse effects on cell proliferation and invasion. In vivo, we demonstrated that lncRNA HOXD-AS1 knockdown suppressed tumor growth.
In previous study, some lncRNAs regulated tumor development and progression by interacting with EZH2 to affect downstream targets. For instance, long non-coding RNA CRNDE promotes colorectal cancer cell proliferation via epigenetically silencing DUSP5/CDKN1A expression [18]. LncRNA HOXA11-AS Exerts Oncogenic Functions by Repressing p21 and miR-124 in Uveal LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27 [19]. Long intergenic non-coding RNA 00673 promotes non-small-cell lung cancer metastasis by binding with EZH2 and causing epigenetic silencing of HOXA5 [20]. In the study, RNA immunoprecipitation and chromatin immunoprecipitation assays revealed that lncRNA HOXD-AS1 could epigenetically inhibited the expressions of RUNX3 by binding to EZH2. lncRNA HOXD-AS1 knockdown increased the RUNX3 expression in melanoma cells.
RUNX3 was identified as tumor suppressor in some tumors including melanoma, Kitago et al showed that RUNX3 expression was down-regulated in metastatic melanoma lines relative to normal melanocytes [13]. Loss of RUNX3 expression was also correlated with a poor 5-year disease-specific survival in primary melanoma [21]. Runx3 inhibits melanoma cell migration through regulation of cell shape change [22]. Our results indicated that cell proliferation and invasion capacities were suppressed after lncRNA HOXD-AS1 knockdown, but were increased after RUNX3 knockdown, whereas, co-transfecting si-HOXD-AS1 and si-RUNX3 partially rescued the inhibiting effects induced by lncRNA HOXD-AS1 knockdown in A375 cells. These results indicated that lncRNA HOXD-AS1 epigenetically silenced RUNX3 transcription by interacting with EZH2.
In conclusion, we observed that lncRNA HOXD-AS1 was remarkably higher in melanoma tissues and correlated with poor prognosis of melanoma. Reduced lncRNA HOXD-AS1 inhibited cell proliferation and invasion in vitro and in vivo reduced the tumor growth. Moreover, we revealed that lncRNA HOXD-AS1 could epigenetically inhibit the expressions of RUNX3 by binding to EZH2. Thus, these results indicated that lncRNA HOXD-AS1 serves as target of melanoma treatment.
Disclosure of conflict of interest
None.
References
- 1.Mueller DW, Bosserhoff AK. Role of miRNAs in the progression of malignant melanoma. Br J Cancer. 2009;101:551–556. doi: 10.1038/sj.bjc.6605204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 3.Streicher KL, Zhu W, Lehmann KP, Georgantas RW, Morehouse CA, Brohawn P, Carrasco RA, Xiao Z, Tice DA, Higgs BW, Richman L, Jallal B, Ranade K, Yao Y. A novel oncogenic role for the miRNA-506-514 cluster in initiating melanocyte transformation and promoting melanoma growth. Oncogene. 2012;31:1558–1570. doi: 10.1038/onc.2011.345. [DOI] [PubMed] [Google Scholar]
- 4.Pacheco I, Buzea C, Tron V. Towards new therapeutic approaches for malignant melanoma. Expert Rev Mol Med. 2011;13:e33. doi: 10.1017/S146239941100202X. [DOI] [PubMed] [Google Scholar]
- 5.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Yang H, Xiao Y, Tang X, Li Y, Han Q, Fu J, Yang Y, Zhu Y. LncRNA GAS5 is a critical regulator of metastasis phenotype of melanoma cells and inhibits tumor growth in vivo. Onco Targets Ther. 2016;9:4075–4087. doi: 10.2147/OTT.S98203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Liu Y, Wang J, Jie D, Yun T, Li W, Yan L, Wang K, Feng J. Downregulated Long Noncoding RNA BANCR Promotes the Proliferation of Colorectal Cancer Cells via Downregualtion of p21 Expression. PLoS One. 2015;10:e0122679. doi: 10.1371/journal.pone.0122679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun M, Liu XH, Lu KH, Nie FQ, Xia R, Kong R, Yang JS, Xu TP, Liu YW, Zou YF, Lu BB, Yin R, Zhang EB, Xu L, De W, Wang ZX. EZH2-mediated epigenetic suppression of long noncoding RNA SPRY4-IT1 promotes NSCLC cell proliferation and metastasis by affecting the epithelial-mesenchymal transition. Cell Death Dis. 2014;5:e1298. doi: 10.1038/cddis.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarmishyn AA, Batagov AO, Tan JZ, Sundaram GM, Sampath P, Kuznetsov VA, Kurochkin IV. HOXD-AS1 is a novel lncRNA encoded in HOXD cluster and a marker of neuroblastoma progression revealed via integrative analysis of noncoding transcriptome. BMC Genomics. 2014;15(Suppl 9):S7. doi: 10.1186/1471-2164-15-S9-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng L, Chen J, Zhou Z, He Z. Knockdown of long non-coding RNA HOXD-AS1 inhibits gastric cancer cell growth via inactivating the JAK2/STAT3 pathway. Tumour Biol. 2017;39:1010428317705335. doi: 10.1177/1010428317705335. [DOI] [PubMed] [Google Scholar]
- 11.Lu S, Zhou J, Sun Y, Li N, Miao M, Jiao B, Chen H. The noncoding RNA HOXD-AS1 is a critical regulator of the metastasis and apoptosis phenotype in human hepatocellular carcinoma. Mol Cancer. 2017;16:125. doi: 10.1186/s12943-017-0676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen QN, Chen X, Chen ZY, Nie FQ, Wei CC, Ma HW, Wan L, Yan S, Ren SN, Wang ZX. Long intergenic non-coding RNA 00152 promotes lung adenocarcinoma proliferation via interacting with EZH2 and repressing IL24 expression. Mol Cancer. 2017;16:17. doi: 10.1186/s12943-017-0581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitago M, Martinez SR, Nakamura T, Sim MS, Hoon DS. Regulation of RUNX3 tumor suppressor gene expression in cutaneous melanoma. Clin Cancer Res. 2009;15:2988–2994. doi: 10.1158/1078-0432.CCR-08-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Sun M, Zang C, Ma P, He J, Zhang M, Huang Z, Ding Y, Shu Y. Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells. Cell Death Dis. 2016;7:e2225. doi: 10.1038/cddis.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang B, Du J, Li Y, Tang F, Wang Z, He S. EZH2 elevates the proliferation of human cholangiocarcinoma cells through the downregulation of RUNX3. Med Oncol. 2014;31:271. doi: 10.1007/s12032-014-0271-6. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Zhuang C, Liu Y, Chen M, Chen Y, Chen Z, He A, Lin J, Zhan Y, Liu L, Xu W, Zhao G, Guo Y, Wu H, Cai Z, Huang W. Synthetic tetracycline-controllable shRNA targeting long noncoding RNA HOXD-AS1 inhibits the progression of bladder cancer. J Exp Clin Cancer Res. 2016;35:99. doi: 10.1186/s13046-016-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Huo X, Yang XR, He J, Cheng L, Wang N, Deng X, Jin H, Wang N, Wang C, Zhao F, Fang J, Yao M, Fan J, Qin W. STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol Cancer. 2017;16:136. doi: 10.1186/s12943-017-0680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding J, Li J, Wang H, Tian Y, Xie M, He X, Ji H, Ma Z, Hui B, Wang K, Ji G. Long noncoding RNA CRNDE promotes colorectal cancer cell proliferation via epigenetically silencing DUSP5/CDKN1A expression. Cell Death Dis. 2017;8:e2997. doi: 10.1038/cddis.2017.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F, Peng H. LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. J Orthop Surg Res. 2017;12:103. doi: 10.1186/s13018-017-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma C, Wu G, Zhu Q, Liu H, Yao Y, Yuan D, Liu Y, Lv T, Song Y. Long intergenic noncoding RNA 00673 promotes non-small-cell lung cancer metastasis by binding with EZH2 and causing epigenetic silencing of HOXA5. Oncotarget. 2017;8:32696–32705. doi: 10.18632/oncotarget.16158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Chen G, Cheng Y, Martinka M, Li G. Prognostic significance of RUNX3 expression in human melanoma. Cancer. 2011;117:2719–2727. doi: 10.1002/cncr.25838. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Wang L, Zeng X, Fujita T, Liu W. Runx3 inhibits melanoma cell migration through regulation of cell shape change. Cell Biol Int. 2017;41:1048–1055. doi: 10.1002/cbin.10824. [DOI] [PubMed] [Google Scholar]


