Abstract
Purpose: MicroRNAs function through regulating specific target mRNA expression and then participate in the development and progression of diverse human cancers. MiR-98 shows aberrant expression and dysfunction in tumors. However, its clinical significance and exact role in squamous cell carcinoma of the head and neck (SCCHN) remain elusive. Methods: MiR-98 expression was examined by qRT-PCR and correlated with clinicopathological variables and prognosis in SCCHN patients. Effects of miR-98 on epithelial-mesenchymal transition (EMT) and the malignant phenotypes of SCCHN were studied. Finally, the role of target gene metadherin (MTDH) in miR-98 mediated effects were assayed. Results: Our results demonstrated that miR-98, as an endogenous inhibitor of MTDH via directly binding to its 3’-untranslated region (UTR) region, decreased significantly in SCCHN tissues. Decreased miR-98 expression was negatively correlated with T classification, clinical stage, lymph node metastasis and a shorter survival status in SCCHN patients. Loss-of-function and gain-of-function analyses confirmed that miR-98 inhibited cell proliferation, migration and invasion of SCCHN cells in vitro. Moreover, miR-98 repression led to increased MTDH expression and induced EMT alteration. Importantly, ectopic expression of MTDH partially reversed the effects caused by miR-98 overexpression. Conclusions: Our study identifies that miR-98 serves as a suppressor in SCCHN progression via targeting oncogene MTDH.
Keywords: Squamous cell carcinoma of the head and neck, metastasis, MicroRNA-98, MTDH, prognosis
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is an aggressive malignancy that originates from the nasal cavities, paranasal sinuses, oral cavity, nasopharynx, oropharynx, hypopharynx and larynx. Despite multimodal therapies including surgery, radiotherapy and chemotherapy having been applied in clinical settings for decades, the quality of life and survival rate in patients with SCCHN are far from satisfactory [1]. Mounting evidence suggests that cervical lymph node metastasis accounts for the frustrating prognosis in patients with SCCHN [1]. Therefore, it is urgent and necessary to clarify the mechanisms underlying metastasis [7], which will benefit the future surveillance and target therapy in patients with SCCHN.
MTDH, abbreviation of metadherin, also known as astrocyte elevated gene-1 (AEG-1) and lyric, is a novel oncogene that plays a crucial role in various human malignancies, including prostate carcinoma [28], breast carcinoma [10], non-small cell lung cancer [33] and cervical cancer [16]. Abundant functional investigations in vitro and in vivo indicate that MTDH is a valuable tumor biomarker and a potential therapy target in cancers [11,24]. Our previous study also showed that elevated expression of MTDH protein was tightly correlated with lymph node metastasis and negatively associated with an unfavourable prognosis in patients with SCCHN [17,38]. MTDH regulated the metastasis of SCCHN via epithelial mesenchymal transition (EMT) in vitro [34]. Therefore, it is worth clarifying upstream and downstream molecules involved in MTDH-mediated malignant alterations in SCCHN.
MicroRNAs (miRNAs) are a class of non-coding small regulated RNAs that have diverse functions in regulating cell activities, including cell proliferation, cell cycle progression, differentiation, invasion, tumor growth and metastasis [5,30]. Recent studies have shown that miRNAs contribute to carcinogenesis through directly interrupting their targeted mRNA expression or translation, and then serving as tumor suppressors or oncogenes [5,36]. MicroRNA 98 (miR-98) belongs to the mature let-7 family of miRNAs [22,23] and is initially found to be down-regulated in leukaemia cell lines [35]. Some reports have suggested that miR-98 expression is associated with diverse malignant behaviours of cancer cells, including growth and chemoresistance [13,31,32,37]. However, the effects of miR-98 on SCCHN cells remain unclear.
MiR-98 was putatively to interact with the 3’-untranslated region (3’-UTR) region of MTDH by online prediction algorithms, which may reduce the expression of MTDH mRNA. Therefore, based on our previous study in MTDH and the potential association between miR-98 and MTDH, our present study aims to confirm the exact association between miR-98 and MTDH, and illuminate the role of the interaction between miR-98 and MTDH in the metastasis of SCCHN.
Materials and methods
Cell lines and human tissues
SCCHN Tu686 cell line, established from a primary tongue tumor, was provided by Dr. Zhuo Chen (Emory University Winship Cancer Institute, Atlanta, Georgia, USA). The Nasopharyngeal carcinoma (NPC) 6-10B, 5-8F, and CNE-2 cell lines were purchased from the Cell Center of Central South University, Changsha, China. Tu686 cells and NPC cell lines were maintained in DMEM/F12 and RPMI 1640 medium at 37°C in 5% CO2 atmosphere, which was supplemented with 10% fetal bovine serum (FBS, Hyclone), 100 IU/mL penicillin and 100 IU/mL streptomycin (Gibco).
Two patient cohorts were included in our current investigation. Cohort 1 had 30 paired SCCHN specimens and adjacent noncancerous epithelial tissues, and cohort 2 included 83 SCCHN specimens. All tissues were collected from patients who received surgical therapy at the Department of Otolaryngology Head and Neck Surgery in Xiangya Hospital, Central South University, Changsha, Hunan, China. Patient cohort 1 ranged from January 2010 to October 2013, and cohort 2 ranged from January 2008 to December 2011. Pathological tumor-node-metastasis (TNM) stage was determined according to the 7th American Joint Committee on Cancer staging system in 2010. The main clinicopathological parameters of patient cohorts 1 and 2 are described in detail in Supplementary Table 1 and Table 1, respectively. Patient inclusion criteria of the current study is as follows: no history of radiotherapy or chemotherapy, and primary SCCHN without other malignancies. Informed consent was obtained from all patients before surgery. All experiments were approved by the Research Ethics Committee of Xiangya Hospital, Central South University, Changsha, China.
Table 1.
Correlations between the expression of miR-98 and clinicopathological parameters in patients with SCCHN
| Parameters | No. of patients | Relative expression | t value | P-value* |
|---|---|---|---|---|
| Age | ||||
| <59 | 41 | 0.509 ± 0.262 | 1.267 | 0.209 |
| ≥59 | 42 | 0.441 ± 0.220 | ||
| Gender | ||||
| Male | 79 | 0.481 ± 0.245 | 0.885 | 0.379 |
| Female | 4 | 0.371 ± 0.180 | ||
| Smoking history | ||||
| Yes | 58 | 0.443 ± 0.231 | -1.913 | 0.059 |
| No | 25 | 0.552 ± 0.258 | ||
| Histological grade | ||||
| G3 | 54 | 0.479 ± 0.239 | 0.154 | 0.878 |
| G1+G2 | 29 | 0.470 ± 0.254 | ||
| T classification | ||||
| T1+T2 | 46 | 0.539 ± 0.230 | 2.767 | 0.007 |
| T3+T4 | 37 | 0.396 ± 0.238 | ||
| Clinical stage | ||||
| I-II | 36 | 0.605 ± 0.196 | 4.779 | 0.000 |
| III-IV | 47 | 0.376 ± 0.230 | ||
| Lymph node status | ||||
| N0 | 54 | 0.572 ± 0.231 | 5.885 | 0.000 |
| N+ | 29 | 0.295 ± 0.141 |
P ≤ .05 was considered to be statistically significance.
& G1, G2 and G3 were defined as poorly-differentiated, moderately-differentiated and well-differentiated, respectively.
Follow-up information of patients after surgery was obtained by clinical examination, imaging evaluation, and pathological studies. Overall survival (OS) was calculated from the day of surgery to the date of death. Deaths from other causes were treated as censored cases.
Quantitative reverse transcription-polymerase chain reaction analysis (qRT-PCR)
Total RNA was isolated and purified from cells and tissues of SCCHN using the RNeasy Plus Mini Kit (QIAGEN, Germany) according to the manufacturer’s instructions. To quantify miR-98 expression, the All-in-OneTM miRNA qPCR Detection Kit (GeneCopoeia Inc., USA) was used for reverse transcription and PCR amplification. As to the expression of MTDH mRNA, the High Capacity RNA-to-cDNA Kit and SYBR® Green PCR Master Mix (Applied Biosystems, CA, USA) was used to reverse transcription and PCR amplification. All primers were designed as follows: MTDH primer, forward 5’-GAT GAT GAA TGG TCT GGG TTA AA-3’ and reverse 5’-GAC CTT TTG ATC ATC AGG AAT TG-3’; GAPDH primer, forward 5’-TCC AAA ATC AAG TGG GGC GA-3’ and reverse 5’-AGT AGA GGC AGG GAT GAT GT-3’. RNA U6 and GAPDH were used as endogenous controls for the detection of miR-98 and MTDH expression, respectively. Expression levels of miR-98 and MTDH were quantified using the methods of 2-ΔCt (miR-98/U6, MTDH/GAPHD) or 2-ΔΔCt (tumor/adjacent noncancerous epithelial tissues) [25].
SCCHN transfection
Tu686 and 6-10B cells were transfected with miR-98 mimic or inhibitor (Gene-Pharma Co. Shanghai, China) following the manufacturer’s protocol. For MTDH overexpression, a lentiviral vector encoding either MTDH cDNA without its 3’-untranslated regions (3’-UTR) or control eGFP was used (GeneCopoeia Inc., Guangzhou, China) at a multiplicity of infection (MOI) of 50 pfu/cell according to our previous studies [34,38]. Forty-eight to 72 hours post transfection, transfection efficiency of miR-98 and MTDH was assessed via qRT-PCR or/and western blotting.
Western blotting
Whole cell protein extracts were collected and western blot assays were performed as we previously described [19,34,38]. In brief, 50-100 μg were separated by 8-12% SDS-PAGE and then transferred onto polyvinylidene difluoride membrane (Millipore). The blotted membranes were then incubated with primary antibodies and subsequently incubated with an HRP-labelled secondary antibody. β-Actin was used as a loading control. Protein densitometry of western blotting bands was quantified by Image J. Details about antibody dilution, incubation time and temperature etc in our study are listed in Supplementary Table 2.
CCK-8 assay
All types of SCCHN cells were seeded in a 96-well plate at a concentration of 3000-5000 cells per well. Cells were then maintained at 37°C for 24, 48, 72 and 96 hours after transfection. The cells were treated with CCK-8 for 1 hour at 37°C. The absorbance was determined by a microplate reader at 450 nM. Averages were determined via triplicate experiments.
Colony formation assay
Forty-eight hours after transfection, all cells were seeded in a six-well plate at a density of 100 cells per well. After culture for 15 days, clones were fixed with 4% paraformaldehyde for 30 minutes and then stained with crystal violet dye for 5 minutes. Surviving colonies (a colony was defined as > 50 cells) were counted as in our previous study [14].
Flow cytometry analysis
Cell cycle distribution and apoptosis of SCCHN cells were examined by flow cytometry analyses, which were performed using the staining of annexin V/Cy5 and propidium iodide as we did previously [14,18].
Wounding-healing and transwell invasion assay
The wound-healing and invasion assays have been described previously [18,34,38]. For wound healing migration, SCCHN cells that grow to confluence were disrupted with 10-μl pipette tips, which were cultured in medium without serum for another 48 h and photographs were captured under phase-contrast microscope.
For Transwell invasion assay, SCCHN cells were seeded into the upper 24-well Transwell chambers that coated with 200 μl Matrigel at a concentration of 200 μg/ml. SCCHN cells were allowed to invade through the pores under the chemotaxis of 12% FBS for 48-72 h. After the fixation of methanol and stained with crystal violet, invaded cells were counted and photographed under a microscope.
Luciferase reporter assays
The wild-type and mutated 3’-UTR of the MTDH gene were cloned into the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, USA). Tu686 cells seeded on a 24-well plate and co-transfected with 50 nM of either miR-98 mimics or NC oligos together with 200 ng reporter plasmid including either wild-type or mutant 3’-UTR using Lipofectamine 2000 Reagent (Invitrogen). The relative luciferase activity in each sample was measured using the Dual-Luciferase® Reporter Assay System (Promega, USA) 48 hours after transfection.
Statistical analysis
All data were evaluated using IBM SPSS version 13.0. Student’s t test or one-way analysis of variance (ANOVA) test was performed to analyse the significance of differences between samples obtained from three independent experiments. Clinical associations between miR-98 and clinicopathological parameters were compared by Student’s t test. Survival analyses were undertaken using the Kaplan-Meier method, and curves were compared by the log-rank test. The quantitative data in this study were expressed as the means ± standard deviation (SD). Differences were considered significant at the value of P < 0.05.
Results
MTDH is directly targeted by miR-98
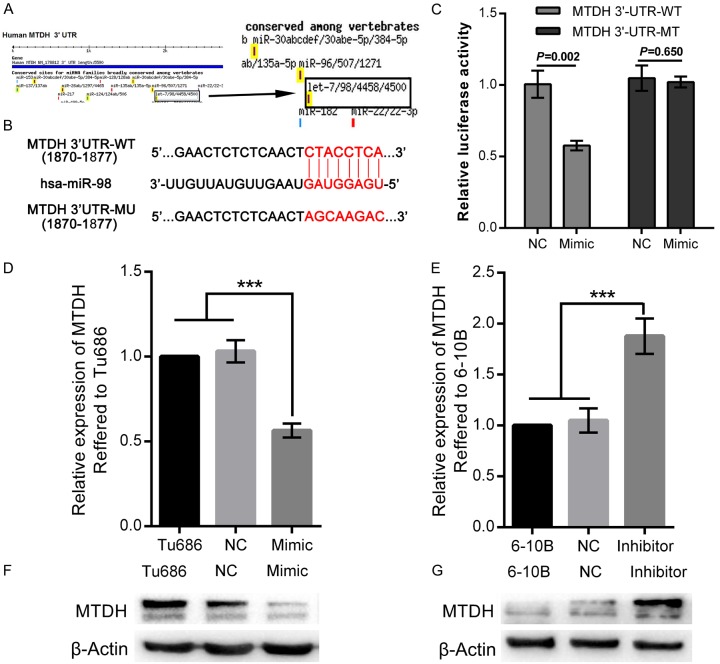
Our recent study suggested that elevated MTDH was an unfavourable factor in patients with SCCHN, and that MTDH regulated the metastasis of SCCHN in vitro [17,34,38]. To investigate how MTDH is aberrantly regulated by miRNAs in SCCHN, miR-98 is predicted to possibly bind to the 3’-UTR region at the position from 1870 to 1877 of MTDH mRNA, according to the results based on the online prediction algorithm of TargetScanHuman (Figure 1A and 1B). Therefore, luciferase reporter assays were applied to determine whether MTDH could be directly modulated by miR-98. Luciferase reporter plasmids encoding the wild type (WT) or mutant (MU) 3’-UTR domain of MTDH mRNA were designed (Figure 1B), and then miR-98 mimic was co-transfected with the reporter plasmid into SCCHN Tu686 cells. As shown in Figure 1C, luciferase activities in Tu686 cells co-transfected with MTDH 3’-UTR-WT and miR-98 mimic were significantly lower than those cells co-transfected with MTDH 3’-UTR-WT and NCs (P < 0.01). However, no significant differences in luciferase activities were observed between cells co-transfected with MTDH 3’-UTR-MU and NCs and cells co-transfected with MTDH 3’UTR-MU and miR-98 mimic (Figure 1C, P > 0.05). These data meant that miR-98 could regulate the expression of MTDH by directly binding to the 3’-UTR region of MTDH mRNA. Furthermore, qPCR and western blot analyses were performed after Tu686 cells were successfully transfected with miR-98 mimic (Supplementary Figure 1B). Our results demonstrated that up-regulation of miR-98 significantly reduced both mRNA and protein expression of MTDH (Figure 1D and 1F). Conversely, down-regulation of miR-98 in SCCHN 6-10B cells via miR-98 inhibitor contributed to the elevation of both mRNA and protein of MTDH (Supplementary Figure 1C, Figure 1E and 1G). Taken together, our results obviously show that miR-98 could directly combine the 3’-UTR domain of MTDH and then regulate its expression.
Figure 1.
MTDH is directly targeted by miR-98. A. MiRNAs that regulate MTDH mRNA are predicted by the online software TargetScanHuman, and the predicted miR-98 is highlighted by black arrow and black rectangle. B. Putative binding sites of the 3’UTR of MTDH mRNA and miR-98 and the mutant sequence of 3’UTR of MTDH mRNA. C. Tu686 cells that co-transfected with miR-98 mimic, MTDH 3’UTR-WT, or 3’UTR-MU were examined by luciferase reporter assays 48 hours after transfection. NC, negative control. D and F. MTDH mRNA and protein were quantified by qPCR and western blot in Tu686 cells 72 hours after the transfection of miR-98 mimic. E and G. MTDH mRNA and protein were quantified by qPCR and western blot in 6-10B cells 72 hours after the transfection of miR-98 inhibitor. ***P < 0.001.
Decreased expression of miR-98 correlates with aggressive progression and poor survival in patients with SCCHN
To confirm the associations between miR-98 and clinical parameters of patients with SCCHN, qPCR was used to detect its expression in two cohorts of patients with SCCHN in our study. In patient cohort 1, qPCR data showed that miR-98 expression was significantly decreased in SCCHN tissues (Figure 2A and 2B). Due to the lack of prognostic information in patient cohort 1, we used patient cohort 2, which was consisted of 83 SCCHN patients with intact prognostic information, to validate the associations between miR-98 expression and patient prognosis. In our study, patients with SCCHN were divided into two groups, the high-miR-98 group (n = 41) and the low-miR-98 group (n = 42), in which miR-98 expression was greater than or less than the median value in the 83 patients with SCCHN, respectively. As summarised in Table 1 via Student’s t test, miR-98 expression levels were inversely correlated with T classification (P = 0.007), advanced clinical stages (P < 0.001) and lymph node metastasis (P < 0.001). However, no significant associations were found between miR-98 expression and other clinical parameters, including age (P = 0.209), gender (P = 0.379), smoking history (P = 0.059) and tumor histological grade (P = 0.878). Moreover, the Kaplan-Meier method and a log-rank test were applied to determine the prognostic value of miR-98 in this patient cohort. Our data showed that patients in the low-miR-98 group had significantly shorter overall survival time than that in patients with a high-miR-98 expression (Figure 2C, P = 0.006). Taken together, the above data show us that decreased miR-98 expression is tightly correlated with aggressive phenotypes and a poorer prognosis in patients with SCCHN.
Figure 2.
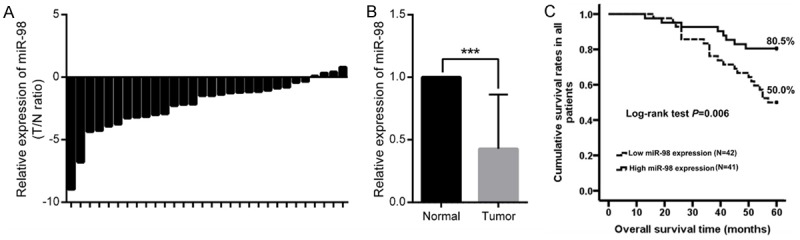
MiR-98 expression in SCCHN and its clinical significance. (A and B) Relative individual miR-98 expression was quantified by qPCR in patient cohort 1 comprising 30 patients with SCCHN (A), compared with corresponding adjacent noncancerous epithelial tissue (B). (C) Survival analysis was performed on patient cohort 2 comprising 83 patients with SCCHN according to the expression of miR-98. Data are shown as mean ± SD.
MiR-98 inhibits the proliferation of SCCHN cells in vitro
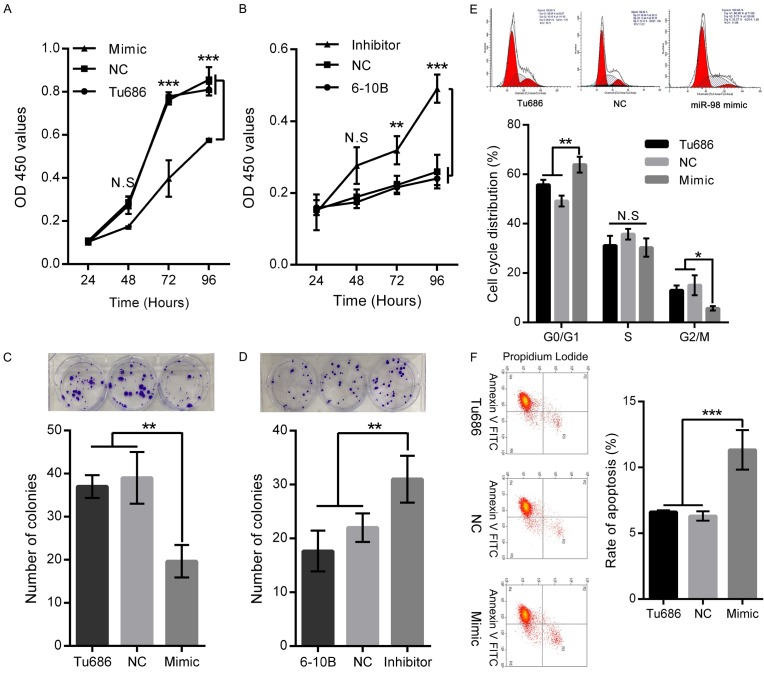
To further investigate the role of miR-98 in the tumorigenesis of SCCHN, mimic and inhibitor of miR-98 were designed to change its level in SCCHN cell lines with different expression of miR-98 (Supplementary Figure 1A). As shown in Supplementary Figure 1B, mimic successfully upregulated miR-98 expression by 10.8 ± 1.5-fold in Tu686 cells. At the same time, miR-98 inhibitor decreased its expression to a fold of 0.28 ± 0.4 in 6-10B cells (Supplementary Figure 1C). Subsequently, growth abilities of both Tu686 and 6-10B cells were examined by CCK-8 and plate colony formation assays. We found that miR-98 upregulation impeded the proliferation of Tu686 cells at day 3 and day 4 in vitro, and Tu686 cells also formed smaller and fewer colonies at day 15 after miR-98 upregulation (Figure 3A and 3C). Conversely, miR-98 downregulation promoted the proliferation of 6-10B cells at day 3 and day 4 in vitro, and led to more and larger colonies once miR-98 was inhibited at day 15 (Figure 3B and 3D). Importantly, flow cytometry assays displayed that miR-98 overexpression in Tu686 induced cell cycle arrest at the G0/G1 phase and decreased in G2/M phase (Figure 3E). As to apoptosis, Tu686 cells transfected with miR-98 mimic showed increased apoptotic rate (Figure 3F). Together, these results suggest that miR-98 overexpression could suppress the proliferation of SCCHN via promoting cell cycle progression and apoptosis.
Figure 3.
MiR-98 regulates the proliferation, cell cycle and apoptosis of SCCHN cells in vitro. Tu686 cells and 6-10B cells were transfected with miR-98 mimic and inhibitor respectively. (A and B) CCK8 assays were applied to delineate the growth curves of Tu686 and 6-10B cells at indicated time points. (C and D) Representative images (Upper) and quantification (Down) of crystal violet stained cell colonies analysed by clongenic formation 15 days after plating. (E) Cell cycle phases and apoptotic rates (F) in Tu686 cells transfected with miR-98 mimic or NC were subjected to flow cytometry analyses. Data are shown as mean ± SD. N.S, nonsense. *P < 0.05, **P < 0.01, ***P < 0.001.
MiR-98 impedes in the migration and invasion of SCCHN cells in vitro
To further investigate the role of miR-98 in the migratory and invasive capacity of SCCHN, wound-healing assays and Transwell assays were employed. Our data clearly showed that the wound healing-ability of Tu686 cells transfected with miR-98 mimics significantly slowed down at 48 hours (Figure 4A, P < 0.05), and Tu686 cells transfected with miR-98 mimics invaded through the Transwell membrane were also significantly reduced (Figure 4C, P < 0.05). On the other hand, miR-98 inhibition in 6-10B cells correspondingly led to enhanced wound-healing ability at 48 hours (Figure 4B, P < 0.05) and increased the cell numbers that passed through the Transwell membrane at 48 hours (Figure 4D, P < 0.05). To explore the mechanism, we found that miR-98 upregulation increased the expression of E-cadherin and decreased the expression of vimentin in Tu686 cells (Supplementary Figure 2A), whereas miR-98 inhibition reduced the expression of E-cadherin and elevated the expression of vimentin in 6-10B cells (Supplementary Figure 2B). As E-cadherin and vimentin are canonical epithelial and mesenchymal biomarkers of epithelial-mesenchymal transition (EMT), these data indicate that miR-98 plays a critical role in the migration and invasion of SCCHN via regulating the EMT process.
Figure 4.
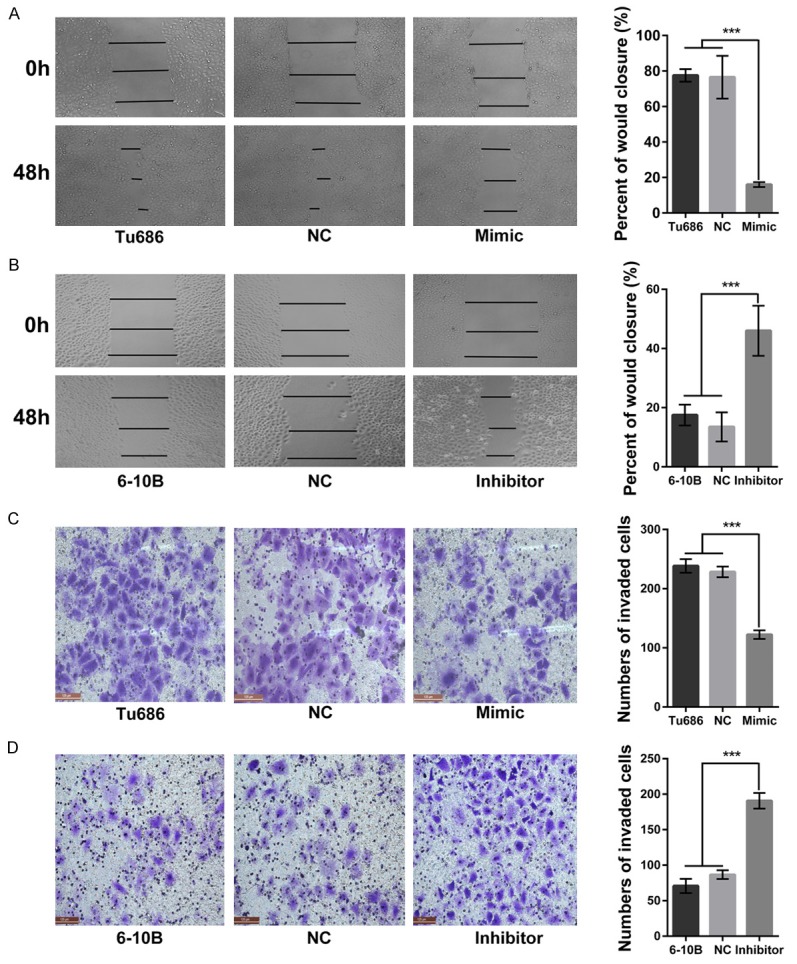
MiR-98 participates in the migration and invasion of SCCHN cell in vitro. Migratory abilities of Tu686 (A) and 6-10B (B) cells were examined by wound-healing assays 48 hours after transfection of miR-98 mimic or inhibitor. Invasive capacities of Tu686 (C) and 6-10B (D) cells were detected by Transwell assays. Data are shown as mean ± SD. ***P < 0.001.
MTDH is partially involved in the invasion of SCCHN caused by miR-98
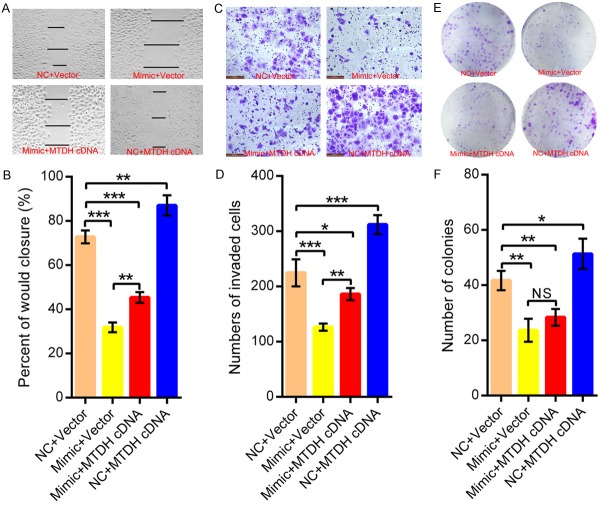
We have demonstrated that decreased miR-98 is responsible for the malignant progression of SCCHN and identified MTDH as a direct target of miR-98. Therefore, we studied the actual functional involvement of MTDH in the following rescue experiments. SCCHN Tu686 cells were transfected with miR-98 mimic and MTDH cDNA without its 3’-UTR region or corresponding empty vectors, and transfection efficiency was evaluated by western blot assays (Supplementary Figure 3). Subsequently, our assays showed that MTDH overexpression partially attenuated the inhibition of migration (Figure 5A and 5B) and invasion (Figure 5C and 5D) caused by miR-98 in Tu686 cells. Consistent with the phenotype alterations in these rescue experiments, elevated expression of E-cadherin and inhibited expression of Vimentin following miR-98 overexpression could also be reversed by MTDH overexpression (Supplementary Figure 3). However, colony formation assays indicated that ectopic overexpression of MTDH displayed no obvious effects on cell proliferation in Tu686 cells transfected with miR-98 mimic (Figure 5E and 5F). Collectively, our data show that MTDH could be at least partially implicated in the miR-98-dependent regulation of the invasion of SCCHN.
Figure 5.
MTDH implicates in the invasion of SCCHN cells caused by miR-98. SCCHN Tu686 cells were co-transfected with MTDH cDNA, vector, or miR-98 mimic, NC. 48 hours later, (A and B) wound-healing assays were used to assess alterations in migration. (C and D) Transwell invasive assays were used to evaluate changes in invasion. (E and F) Plate colony formation assays were performed at 15 days after plating. Data are shown as mean ± SD. N.S, nonsense. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
MTDH, as an oncogene, functions widely in numerous malignant behaviours in cancer cells, such as unlimited proliferation, invasion and migration [4,11]. Our previous study indicated that MTDH was overexpressed in SCCHN tissues and negatively correlated with the prognosis of patients with SCCHN [17]. Moreover, MTDH overexpression enhanced the migratory and invasive ability of SCCHN cells via promoting the occurrence of EMT [17,34,38]. Consistent with reports from other groups, MTDH gene copy also amplify in SCCHN patients [2]. To further answer the question of how MTDH is abnormally regulated in cancer, we used online algorithms to predict miRNAs that potentially regulate MTDH. As expected, numerous miRNAs, in which miRNA-375, miRNA-145 and miRNA-137 have been predicted and also confirmed to regulate MTDH in recent publications [6,8,9]. Therefore, we focused on evaluating the potential regulation of miR-98 on MTDH, which has not been studied. Our study initially confirmed that miR-98 could directly interact with the 3’-UTR region of MTDH, and then hinder the protein translation of MTDH.
Consistent with reports in other solid tumors, including oesophageal squamous cell carcinoma [12], oral squamous cell carcinoma [27] and non-small-cell lung cancer [21], the expression of miR-98 was also down-regulated in SCCHN, displaying an inverse expression style with its target gene MTDH [17,34]. Two patient cohorts were used in our study to find the associations between miR-98 expression and clinicopathological parameters, which obviously indicated that decreased miR-98 expression was tightly correlated with clinical stages and lymph node metastasis in patients with SCCHN. Especially in patient cohort 2, with intact clinical information, we found that SCCHN patients with decreased miR-98 expression were closely associated with a poorer survival status. The clinical significance of miR-98 has been scarcely investigated, except for in the case of melanoma, in which low miR-98 expression was also found to be associated with poor survival [13]. Consistent with the results found in tissues, our in vitro experiments demonstrated that up- or down-regulation of miR-98 could influence the capacities of proliferation, migration and invasion of SCCHN cells. Altogether, these data indicate the importance of miR-98 in SCCHN progression and metastasis after surgical resection.
MiRNAs exert their functions by focusing on different targets including oncogenes and/or suppressors. Specific miRNA can simultaneously target multiple genes [5]. Available evidence demonstrates that miR-98 can target the following oncogenes or suppressors in different tumors: ITGB3 [21], caspase-3 [15], IL-6 [13,29], HMGA2 [32], ALK4 [26] and MMP11 [26]. In our present study, we found that miR-98 could directly bind to the 3’-UTR region of MTDH mRNA, accelerate the degradation of MTDH mRNA, and eventually hinder the expression of MTDH protein. Also, miR-98 and MTDH have an opposite expression style in SCCHN, in which miR-98 is decreased and MTDH is overexpressed in SCCHN [17]. These data indicate that MTDH is a direct downstream target of miR-98. Together with the target of MTDH, we found that repressed expression of miR-98 in tumors led to abnormally elevated expression of these oncogenes and then promoted cancer progression. In our study, restored MTDH expression in miR-98 overexpressing cells partially abolished the inhibition of malignant behaviours caused by miR-98, which indicates that MTDH is at least partially implicated in the anti-tumor effects of miR-98, although we cannot exclude the possibility that other gene targets of miR-98 may be also involved in this process.
Metastasis leads to the dissemination of primary tumor cells and then seeding at distant locations to form secondary tumors. The prerequisite of metastasis demands cancer cells to separate from the primary site and acquires enhanced migratory and invasive abilities. EMT has a critical role in the initiation of metastasis [3]. Recently, we found that MTDH was correlated with the promotion of EMT and metastasis [17,34]. Our current study also demonstrated that miR-98 could regulate the expression of epithelial marker E-cadherin and mesenchymal marker Vimentin, and subsequently participated in processes of EMT and metastasis. Ectopic expression of MTDH in Tu686 cells after miR-98 mimic transfection restored the changes of EMT and metastasis caused by miR-98, which indicates that MTDH and miR-98 can form a miR-98/MTDH axis pathway and together to influence the metastasis and EMT of SCCHN.
We have to mention that the mechanisms by which miR-98/MTDH axis contributes to the EMT and metastasis of SCCHN are still waiting for investigation. Available evidence indicates that MTDH facilitates the carcinogenesis via remodelling diverse signalling pathways including NF-kB, PI3K/Akt and Wnt/β-catenin pathways etc [11]. Among these pathways, PI3K/Akt pathway plays a central role in the regulation of cell proliferation, survival and metastasis [20]. Our previous studies have emphasised the importance of PI3K/Akt in the MTDH-mediated changes in SCCHN [34,38], which hints us that miR-98/MTDH axis and downstream PI3K/Akt may function together in the progression of SCCHN. However, the detailed signalling pathways underlying the effects of miR-98/MTDH axis on the EMT and metastasis are worth of systemic and deep study in the future.
In summary, our study indicates that repression of miR-98 leads to elevated MTDH, which in turn enhances the expression of effectors such as EMT molecules, and finally promotes the progression of SCCHN. MiR-98 serves as a suppressor miRNA and a potential therapeutic target for patients with SCCHN. However, in-depth molecular mechanism study and in vivo animal models are urgent and indispensable to further clarify the function of miR-98 in SCCHN.
Acknowledgements
This study was funded by grants from the National Natural Science Foundation of China (Nos. 81772903, 81773243, 81202128, 8160111661, 81372426, 8160102702 and 81472696), and the Natural Science Foundation of Hunan Province (No. 2015JJ3137).
Informed consent was obtained from all individual participants included in the study.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Allen CT, Law JH, Dunn GP, Uppaluri R. Emerging insights into head and neck cancer metastasis. Head Neck. 2013;35:1669–1678. doi: 10.1002/hed.23202. [DOI] [PubMed] [Google Scholar]
- 2.Baltaci E, Karaman E, Dalay N, Buyru N. Analysis of gene copy number changes in head and neck cancer. Clin Otolaryngol. 2016 doi: 10.1111/coa.12686. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Behbahani GD, Ghahhari NM, Javidi MA, Molan AF, Feizi N, Babashah S. MicroRNA-mediated post-transcriptional regulation of epithelial to mesenchymal transition in cancer. Pathol Oncol Res. 2017;23:1–12. doi: 10.1007/s12253-016-0101-6. [DOI] [PubMed] [Google Scholar]
- 4.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 5.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei Y, Li Y, Yang Q, Liu J, Wei JJ, Shao C, Liu Z, Kong B. miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma. Oncotarget. 2014;5:10816–10829. doi: 10.18632/oncotarget.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsheikh MN, Rinaldo A, Hamakawa H, Mahfouz ME, Rodrigo JP, Brennan J, Devaney KO, Grandis JR, Ferlito A. Importance of molecular analysis in detecting cervical lymph node metastasis in head and neck squamous cell carcinoma. Head Neck. 2006;28:842–849. doi: 10.1002/hed.20368. [DOI] [PubMed] [Google Scholar]
- 8.Guo J, Xia B, Meng F, Lou G. miR-137 suppresses cell growth in ovarian cancer by targeting AEG-1. Biochem Biophys Res Commun. 2013;441:357–363. doi: 10.1016/j.bbrc.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 9.He XX, Chang Y, Meng FY, Wang MY, Xie QH, Tang F, Li PY, Song YH, Lin JS. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31:3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- 10.Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, Reiss M, Au JL, Haffty BG, Kang Y. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15:9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15:5615–5620. doi: 10.1158/1078-0432.CCR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SD, Yuan Y, Zhuang CW, Li BL, Gong DJ, Wang SG, Zeng ZY, Cheng HZ. MicroRNA-98 and microRNA-214 post-transcriptionally regulate enhancer of zeste homolog 2 and inhibit migration and invasion in human esophageal squamous cell carcinoma. Mol Cancer. 2012;11:51. doi: 10.1186/1476-4598-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, Li XJ, Qiao L, Shi F, Liu W, Li Y, Dang YP, Gu WJ, Wang XG, Liu W. miR-98 suppresses melanoma metastasis through a negative feedback loop with its target gene IL-6. Exp Mol Med. 2014;46:e116. doi: 10.1038/emm.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Liu Y, Su Z, Ren S, Zhu G, Tian Y, Qiu Y. MicroRNA-324-3p regulates nasopharyngeal carcinoma radioresistance by directly targeting WNT2B. Eur J Cancer. 2013;49:2596–2607. doi: 10.1016/j.ejca.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Li HW, Meng Y, Xie Q, Yi WJ, Lai XL, Bian Q, Wang J, Wang JF, Yu G. miR-98 protects endothelial cells against hypoxia/reoxygenation induced-apoptosis by targeting caspase-3. Biochem Biophys Res Commun. 2015;467:595–601. doi: 10.1016/j.bbrc.2015.09.058. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Wang D, Liu H, Feng Y, Zhu T, Zhang L, Zhu B, Zhang Y. Knockdown of astrocyte elevated gene-1 (AEG-1) in cervical cancer cells decreases their invasiveness, epithelial to mesenchymal transition, and chemoresistance. Cell Cycle. 2014;13:1702–1707. doi: 10.4161/cc.28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Su Z, Li G, Yu C, Ren S, Huang D, Fan S, Tian Y, Zhang X, Qiu Y. Increased expression of metadherin protein predicts worse diseasefree and overall survival in laryngeal squamous cell carcinoma. Int J Cancer. 2013;133:671–679. doi: 10.1002/ijc.28071. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Yu C, Qiu Y, Huang D, Zhou X, Zhang X, Tian Y. Downregulation of EphA2 expression suppresses the growth and metastasis in squamous-cell carcinoma of the head and neck in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138:195–202. doi: 10.1007/s00432-011-1087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Zhang X, Qiu Y, Huang D, Zhang S, Xie L, Qi L, Yu C, Zhou X, Hu G, Tian Y. Clinical significance of EphA2 expression in squamous-cell carcinoma of the head and neck. J Cancer Res Clin Oncol. 2011;137:761–769. doi: 10.1007/s00432-010-0936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [DOI] [PubMed] [Google Scholar]
- 21.Ni R, Huang Y, Wang J. miR-98 targets ITGB3 to inhibit proliferation, migration, and invasion of non-small-cell lung cancer. Onco Targets Ther. 2015;8:2689–2697. doi: 10.2147/OTT.S90998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizos E, Siafakas N, Katsantoni E, Skourti E, Salpeas V, Rizos I, Tsoporis JN, Kastania A, Filippopoulou A, Xiros N, Margaritis D, Parker TG, Papageorgiou C, Zoumpourlis V. Let-7, mir-98 and mir-181 as biomarkers for cancer and schizophrenia. PLoS One. 2015;10:e0123522. doi: 10.1371/journal.pone.0123522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: clinical significance. Adv Cancer Res. 2013;120:39–74. doi: 10.1016/B978-0-12-401676-7.00002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Siragam V, Rutnam ZJ, Yang W, Fang L, Luo L, Yang X, Li M, Deng Z, Qian J, Peng C, Yang BB. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget. 2012;3:1370–1385. doi: 10.18632/oncotarget.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterenczak KA, Eckardt A, Kampmann A, Willenbrock S, Eberle N, Länger F, Kleinschmidt S, Hewicker-Trautwein M, Kreipe H, Nolte I, Murua Escobar H, Gellrich NC. HMGA1 and HMGA2 expression and comparative analyses of HMGA2, Lin28 and let-7 miRNAs in oral squamous cell carcinoma. BMC Cancer. 2014;14:694. doi: 10.1186/1471-2407-14-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan L, Hu G, Wei Y, Yuan M, Bronson RT, Yang Q, Siddiqui J, Pienta KJ, Kang Y. Genetic ablation of metadherin inhibits autochthonous prostate cancer progression and metastasis. Cancer Res. 2014;74:5336–5347. doi: 10.1158/0008-5472.CAN-14-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Shu C, Su J, Li X. A crosstalk triggered by hypoxia and maintained by MCP-1/miR-98/IL-6/p38 regulatory loop between human aortic smooth muscle cells and macrophages leads to aortic smooth muscle cells apoptosis via Stat1 activation. Int J Clin Exp Pathol. 2015;8:2670–2679. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Zhang E, Lin C. MicroRNAs in tumor angiogenesis. Life Sci. 2015;136:28–35. doi: 10.1016/j.lfs.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Wendler A, Keller D, Albrecht C, Peluso JJ, Wehling M. Involvement of let-7/miR-98 microRNAs in the regulation of progesterone receptor membrane component 1 expression in ovarian cancer cells. Oncol Rep. 2011;25:273–279. [PubMed] [Google Scholar]
- 32.Xiang Q, Tang H, Yu J, Yin J, Yang X, Lei X. MicroRNA-98 sensitizes cisplatin-resistant human lung adenocarcinoma cells by up-regulation of HMGA2. Pharmazie. 2013;68:274–281. [PubMed] [Google Scholar]
- 33.Yao Y, Gu X, Liu H, Wu G, Yuan D, Yang X, Song Y. Metadherin regulates proliferation and metastasis via actin cytoskeletal remodelling in non-small cell lung cancer. Br J Cancer. 2014;111:355–364. doi: 10.1038/bjc.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C, Liu Y, Tan H, Li G, Su Z, Ren S, Zhu G, Tian Y, Qiu Y, Zhang X. Metadherin regulates metastasis of squamous cell carcinoma of the head and neck via AKT signalling pathway-mediated epithelial-mesenchymal transition. Cancer Lett. 2014;343:258–267. doi: 10.1016/j.canlet.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Wang F, Yang GH, Wang FL, Ma YN, Du ZW, Zhang JW. Human microRNA clusters: genomic organization and expression profile in leukemia cell lines. Biochem Biophys Res Commun. 2006;349:59–68. doi: 10.1016/j.bbrc.2006.07.207. [DOI] [PubMed] [Google Scholar]
- 36.Yu X, Li Z. The role of microRNAs expression in laryngeal cancer. Oncotarget. 2015;6:23297–23305. doi: 10.18632/oncotarget.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Zhang C, Li Y, Wang P, Yue Z, Xie S. miR-98 regulates cisplatin-induced A549 cell death by inhibiting TP53 pathway. Biomed Pharmacother. 2011;65:436–442. doi: 10.1016/j.biopha.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Zhu GC, Yu CY, She L, Tan HL, Li G, Ren SL, Su ZW, Wei M, Huang DH, Tian YQ, Su RN, Liu Y, Zhang X. Metadherin regulation of vascular endothelial growth factor expression is dependent upon the PI3K/Akt pathway in squamous cell carcinoma of the head and neck. Medicine (Baltimore) 2015;94:e502. doi: 10.1097/MD.0000000000000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.