Abstract
Exercise capacity is reduced in prostate cancer patients concurrently treated with androgen deprivation therapy compared to healthy counterparts. We tested the hypothesis that prostate cancer independently reduces endurance exercise capacity in a preclinical orthotopic prostate tumor model. Male Copenhagen rats performed an initial treadmill running test to exhaustion. The rats’ prostates were subsequently injected with either prostate tumor cells (R-3327 AT-1, tumor bearing, n=9) or vehicle control (sham, n=9) and the treadmill tests were repeated four and eight weeks post-surgery. Left ventricle contractility (LV Δpressure/Δtime) was subsequently measured under anesthesia and the heart and select hindlimb muscles were dissected and weighed. Initial times to exhaustion were not different between groups (sham: 28.24±1.26, tumor bearing: 28.63±2.49 min, P=0.90). Time to exhaustion eight weeks post-surgery was reduced compared to initial values for both groups but was significantly lower in the tumor bearing (13.25±1.44 min) versus the sham (21.17±1.87 min, P<0.01) group. Within the tumor bearing group, LV Δpressure/Δtime was significantly negatively correlated with tumor mass (-0.71, P<0.05). Body mass at eight weeks post-surgery was not different between groups (P=0.26) but LV mass (↓17%, P<0.01), as well as the mass of select hindlimb skeletal muscles, was significantly lower in the tumor bearing versus sham group. Within the tumor bearing group, LV muscle mass was significantly negatively correlated with prostate tumor mass (r=-0.85, P<0.01). Prostate cancer reduced endurance exercise capacity in the rat and reductions in cardiac function and mass and skeletal muscle mass may have played an important role in this impairment.
Keywords: Prostate tumor, cardio-oncology, exercise tolerance, atrophy, cachexia
Introduction
Cancer patients have a reduced maximal exercise capacity (i.e., maximal oxygen consumption, VO2max) and report greater levels of fatigue when compared to age-matched healthy counterparts [1,2]. Specifically, fatigue is experienced by over 50% of cancer patients [3-7] and is related to the type of cancer [5,7,8] as well as the type [5,9] and duration [10] of treatment. Exaggerated fatigue in cancer patients impairs the ability to perform activities of daily living which has been shown to lead to a reduced quality of life [1,4,11]. Multiple mechanisms likely contribute to the reduced physical capacity and exercise intolerance in cancer patients. For example, reductions in cardiac function [12,13], anemia [8], reduced cardiac and skeletal muscle mass (i.e., cachexia) [14-17], and increased fat mass [18] have all been reported in cancer patients; typically during or after treatment(s). Given the clear link between fatigue and reduced quality of life in cancer patients [1], identification of its mechanistic underpinnings would have immense clinical value. One specific hindrance to identifying the causes and mechanisms of exaggerated fatigue in cancer patients is that various forms of cancer [14,19,20] and cancer treatments [12,21,22] independently inflict negative physiological consequences. Thus, pre-clinical animal models devoid of therapeutic cancer intervention may help elucidate the underlying link between cancer itself and exercise intolerance.
Prostate cancer is the most frequently diagnosed non-skin cancer in men [23]. One of the most common treatments for prostate cancer is surgical or pharmacological androgen deprivation therapy (ADT) which has been associated with multiple deleterious side-effects including reduced muscle mass and bone density as well as increased fat mass [24-26]. Such side-effects are thought to be responsible for the reduced 6-minute walk test performance and grip strength in prostate cancer patients receiving ADT compared to healthy controls [9]. Furthermore, prostate cancer patients receiving ADT for more than three months have a lower VO2max compared to those on ADT for less than three months [10]. While ADT is very likely to contribute to the reductions in exercise capacity found in prostate cancer patients, the effect of the prostate cancer itself may also hasten fatigue although this has not been investigated.
The purpose of this study was to investigate the effect of prostate cancer on endurance exercise capacity in an established rat prostate tumor model. We tested the hypothesis that the development of prostate cancer would reduce time to exhaustion during submaximal treadmill running. Additionally, to examine potential mechanisms by which prostate cancer may impact endurance exercise capacity in this model, we tested the hypotheses that the reductions in exercise capacity in rats with prostate cancer would be associated with impaired cardiac function and reductions in cardiac and skeletal muscle mass compared to sham-operated control rats.
Materials and methods
All procedures were approved by the Institutional Animal Care and Use Committee at Kansas State University and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council Committee, Washington, D.C., rev. 2011). Eighteen 6 month-old immunocompetent male Copenhagen rats (COP/CrCrl, Charles River, Wilmington, MA) were used. The rats were housed two per cage in AAALAC accredited temperature and light controlled facilities and provided standard rat chow and water ad libitum.
Experimental protocol
All rats were acclimated to treadmill running on a custom-built motor driven treadmill for approximately five days at 25 m/min at a 5% incline. Each acclimation run lasted <5 minutes to ensure the rats were not exercise trained. Within three days of the final acclimation, endurance exercise capacity was assessed in all rats by measuring time to exhaustion during a graded submaximal treadmill test according to the methods described in detail by Copp et al. [12] (see below).
After the initial endurance exercise capacity test, the rats were randomly assigned to either a sham (n=9) or prostate tumor bearing (n=9) group. At least 48 hours of recovery from the initial exercise capacity test was given until the sham or prostate tumor surgery was performed (see below). Three weeks after surgery, the rats were re-familiarized (~3 times, <5 min each) to treadmill running and the endurance exercise capacity test was repeated four weeks post-surgery. At seven weeks post-surgery, rats were again re-familiarized (~3 times, <5 min each) to treadmill running and the final endurance exercise capacity test was completed at eight weeks post-surgery.
Following the final endurance exercise capacity test, rats were anesthetized (2-3% isoflurane, oxygen balance) and the right carotid artery was isolated and cannulated for the advancement of a 2-Fr catheter-tipped pressure transducer (Millar Instruments, Houston, TX) into the left ventricle (LV) to measure the rate of LV pressure increase over time (∆pressure/∆time) and the LV end-diastolic pressure (LVEDP). The pressure transducer was then withdrawn from the LV and systolic blood pressure was measured when the catheter tip was in the aorta. Technical issues prevented cardiac measurements in one tumor bearing rat. Rats were then euthanized by a thoracotomy under anesthesia (5% isoflurane, oxygen balance) followed by removal of the heart to verify death. Subsequently, the left gastrocnemius, extensor digitorum longus (EDL), and soleus muscles, and the heart were dissected and weighed. The wall of the right ventricle (RV) was cut away and the RV wall and the LV (along with the intraventricular septum) were weighed separately. In addition, red and white portions of the gastrocnemius muscle were isolated and dissected. The left femur was also dissected and the length was measured. Select hindlimb skeletal muscles/muscle parts were frozen at -80°C for future determination of citrate synthase activity (see below).
Endurance exercise capacity protocol
The endurance exercise capacity test was conducted according to previously described methods [27]. Each test consisted of 15 minute stages starting at 25 m/min for 15 minutes at a 5% incline. The speed of the treadmill was increased by 5 m/min every 15 minutes (with the incline held constant) until the rat was unable or unwilling to run. Rats were motivated to run with bursts of high-pressure air aimed at the hind legs. An endurance exercise capacity test was deemed valid if a marked attenuation of the rat’s righting reflex and/or a noticeable change in gait that is indicative of exhaustion prior to termination of the test was present [27]. Time to exhaustion was measured to the nearest second. No endurance exercise capacity test was repeated more than once at a specific time point, and tests were repeated in the same proportion by sham rats and cancer rats. Each test was completed between 8 AM and 12 PM by the same investigators in a room with the temperature maintained between 21 and 23°C. No additional fans or cooling devices used. The investigators were blinded to the group and previous run times of the rat during each endurance exercise capacity test.
Orthotopic prostate tumor model
The AT-1 cell line from the Dunning R-3327 strain of Copenhagen rat prostate carcinoma cells was used [28]. These cells have a high growth rate, low metastatic potential, and are similar to the growth patterns of human prostate cancer [29]. The cells were grown in RPMI 1640 media with 10% Fetal Bovine Serum, 1% penicillin/streptomyocin, 2 mM L-glutamine, and 250 µM dexamethasone in a 37°C humidified incubator at 5% CO2. When cells reached ~90% confluence, a sample of the cells was counted in a hemocytometer, and the rest of the viable cells were used to prepare a tumor cell stock solution with Matrigel. Matrigel enhances the opportunity for the cancer cells to form a tumor and augments tumor growth [30]. This solution was aliquoted into 0.1 mL syringes that each contained 104-105 AT-1 cells. This model has been used previously to induce the development of prostate tumors [31,32].
All procedures were performed under aseptic conditions. Rats were anesthetized (2-4% isoflurane, oxygen balance) and the bladder/prostate complex was exposed through a small incision (<1 cm) lateral to the midline of the abdomen. The ventral lobe of the prostates in the tumor bearing group were injected with the cell stock solution with cancer cells and 0.1 ml of Matrigel using sterile insulin syringes (26G). The prostate of each rat in the sham group was injected with 0.1 ml of Matrigel without cancer cells. Following surgery, the incision was closed and rats were injected with buprenorphine (0.05 ml/kg) and acepromazine (0.04 ml/kg). Post-operative monitoring occurred daily for one week.
Skeletal muscle citrate synthase activity
Citrate synthase activity was measured in the red and white portions of the gastrocnemius and the soleus muscles as a marker of oxidative capacity. The muscles/muscle portions were mechanically homogenized and analysis was completed by a spectrophotometer using the methods of Srere [33]. Briefly, 15 µl and 30 µl samples were diluted using 210 µl and 195 µl of tris buffer, respectively, and 15 µl of acetyl coenzyme A and 30 µl of DTNB were added to each sample. All samples were incubated in a spectrophotometer (Fisher Scientific, accuSkan GO) for 5 minutes at 30°C. Readings were taken once per minute for five minutes and then 30 µl of oxaloacetate was added to all samples and immediately analyzed again. The citrate synthase activity was given in µmol/min/g wet weight. If the difference between the 15 µl and the 30 µl samples was larger than 15% the sample was re-analyzed until there was less than 15% difference. Citrate synthase activity measurements were not obtained in two sham rats.
Data analysis
Statistical analyses were completed with Prism (version 7.0, Graphpad) data analysis software. Data were compared between groups with mixed two-way ANOVAs and Holm-Sidak post hoc tests or unpaired Student’s t-tests as appropriate. Within the tumor bearing group, Pearson correlations and linear regression analyses were performed to quantify relationships between tumor mass and select variables. Statistical significance was accepted at P<0.05.
Results
The average prostate tumor mass eight weeks post-surgery in the tumor bearing group was 9.8±2.6 g (range: 0.2 to 19.5 g).
Body mass increased in both groups during the eight-week protocol and there was no difference in body mass between groups either four or eight weeks post-surgery (Figure 1A). When prostate tumor mass was subtracted from body mass at eight weeks post-surgery, the overall increase in “non-tumor” mass during the experimental protocol was significantly lower in the tumor bearing group compared to the increase in body mass in the sham group (Figure 1B). Within the tumor bearing group, the increase in “non-tumor” mass was significantly negatively correlated with prostate tumor mass (Figure 1C). There was no difference in femur length eight weeks post-surgery between the sham (40±1 mm) and tumor bearing groups (41±1 mm, P=0.59).
Figure 1.
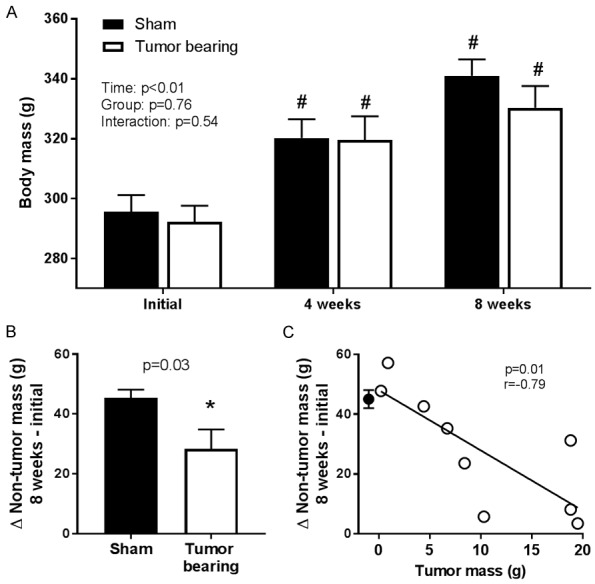
A. Body mass was not different between the sham (n=9) and prostate tumor bearing (n=9) groups at any time point (two-way ANOVA). B. The increase in “non-tumor” mass (body mass minus tumor mass for the tumor bearing group) during the eight week protocol was significantly lower in the tumor bearing group compared to the sham group (unpaired Student’s t-test). C. Within the tumor bearing group (open circles), the increase in “non-tumor” mass was significantly negatively correlated with tumor mass. The closed circle represents the mean and SEM of the sham group and is shown for comparison purposes only and is not factored into the correlation or regression calculation. “#” indicates a statistically significant difference versus the corresponding within-group initial value. “*” indicates a statistically significant difference versus the sham group.
Endurance exercise capacity
At four weeks post-surgery, there was no difference in mean time to exhaustion from its initial value in the sham group whereas time to exhaustion was significantly reduced (↓15%) compared to its initial value in the tumor bearing group (Figure 2). At eight weeks post-surgery, time to exhaustion was significantly reduced compared to initial values for both groups (sham: ↓26%, tumor bearing: ↓54%) but it was significantly lower in the tumor-bearing group compared to the sham group (Figure 2). Within the tumor bearing group, there were no significant correlations between prostate tumor mass and time to exhaustion at eight weeks post-surgery (r=-0.51, P=0.16) or the reduction in endurance capacity during the eight week protocol (r=-0.03, P=0.94).
Figure 2.
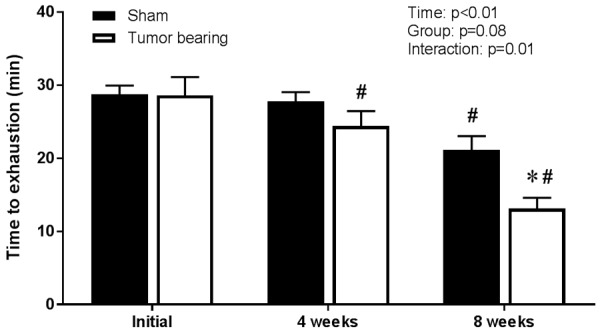
Time to exhaustion was lower at eight weeks post-surgery in the prostate tumor bearing group (n=9) compared to the sham (n=9) group (two-way ANOVA and Holm-Sidak post hoc tests). “#” indicates a statistically significant difference versus the corresponding within-group initial value. “*” indicates a statistically significant difference versus the sham group.
Cardiac function
Cardiac function was measured following the final endurance capacity test eight weeks post-surgery. There were no statistically significant differences between the group mean values for systolic blood pressure (sham: 122±2, tumor bearing: 114±6 mmHg, P=0.19), LV ∆pressure/∆time (sham: 7456±333, tumor bearing: 7038±311 mmHg/s, P=0.36), or LVEDP (sham: 12±1, tumor bearing: 11±2 mmHg, P=0.54). Within the tumor bearing group, however, systolic blood pressure and LV ∆pressure/∆time, but not LVEDP, were significantly negatively correlated with prostate tumor mass (Figure 3).
Figure 3.
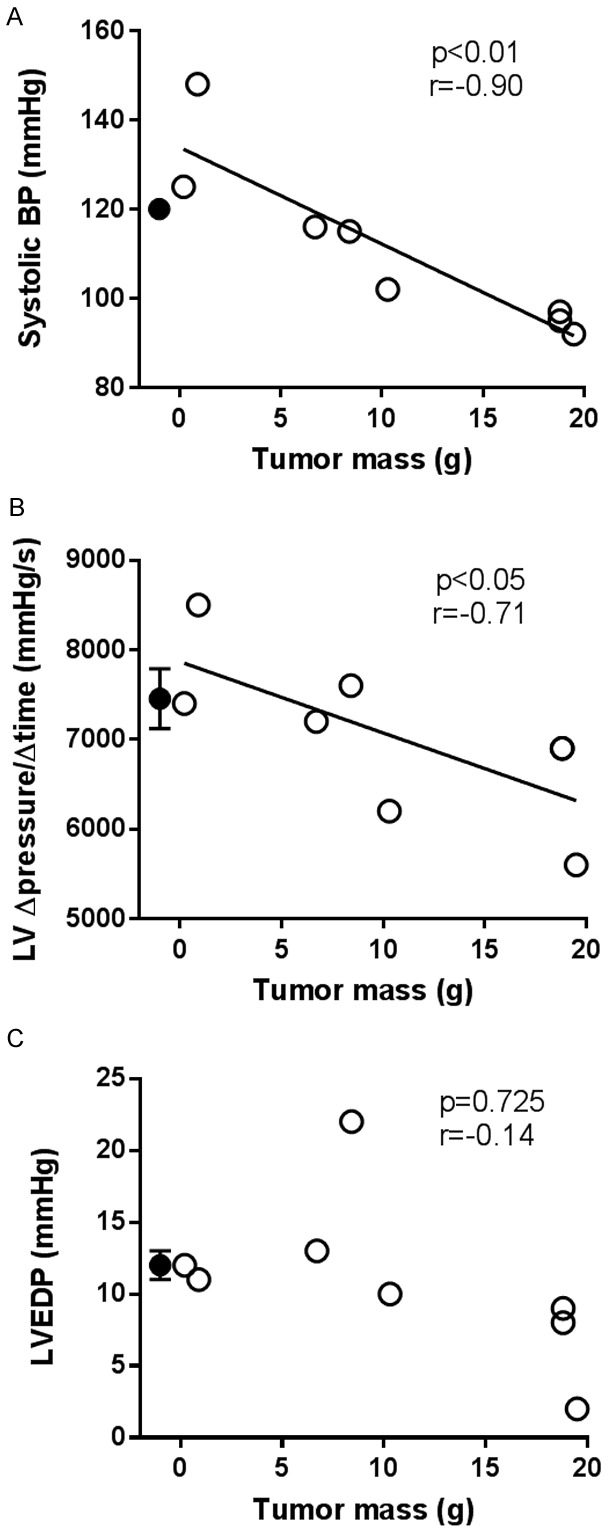
Within the tumor bearing group (open circles), tumor mass was significantly negatively correlated with systolic blood pressure (systolic BP, A) and left ventricle (LV) ∆pressure/∆time (B), but not LV end diastolic pressure (C). Closed circles represent the mean and SEM of the sham group (n=9, SEM in A is hidden by the mean circle) which are shown for comparison purposes only and are not factored into the correlation or regression calculations. Note that cardiac measurements were not obtained in one tumor bearing rat and that two tumor bearing rats had a tumor mass of 18.8 g and an LV ∆pressure/∆time of 6900 mmHg (i.e., two data points lie directly on top of one another) which is why there only appears to be seven data points in (B).
Cardiac and skeletal muscle mass and skeletal muscle citrate synthase activity
Cardiac and skeletal muscle mass and citrate synthase activity were measured eight weeks post-surgery. Heart, LV, gastrocnemius, and EDL muscle mass were significantly lower in the tumor bearing group compared to the sham group (P<0.05 for all, Table 1) and this was the case even when normalized to femur length. When normalized to body mass, heart, LV, and gastrocnemius mass, but not EDL mass, were significantly lower in the tumor bearing group compared to sham group. There was no significant difference in RV or soleus muscle mass between groups. Within the tumor bearing group, heart and LV mass, but not RV mass, were significantly negatively correlated with prostate tumor mass (Figure 4). There were no significant correlations between prostate tumor mass and gastrocnemius (r=-0.15, P=0.70), EDL (r=-0.58, P=0.10), or soleus (r=-0.36, P=0.34) muscle masses.
Table 1.
Cardiac and select hindlimb muscle mass
| Sham (n=9) | Tumor bearing (n=9) | p-value | |
|---|---|---|---|
| Absolute muscle mass (mg) | |||
| Heart | 854±26 | 723±30 | <0.01* |
| Left ventricle | 615±15 | 508±20 | <0.01* |
| Right ventricle | 239±17 | 215±18 | 0.35 |
| Gastrocnemius | 1843±28 | 1702±35 | <0.01* |
| Soleus | 164±6 | 166±4 | 0.80 |
| Extensor digitorum longus | 159±4 | 148±3 | 0.04* |
| Muscle mass normalized to femur length (mg/mm) | |||
| Heart/body mass | 2.47±0.07 | 2.19±0.08 | <0.01* |
| Left ventricle/femur length | 15.4±0.3 | 12.5±0.5 | <0.01* |
| Right ventricle/femur length | 6.0±0.4 | 5.3±0.4 | 0.22 |
| Gastrocnemius/femur length | 46.2±1.0 | 42.0±0.7 | <0.01* |
| Soleus/femur length | 4.1±0.1 | 4.1±0.1 | 0.94 |
| Extensor digitorum longus/femur length | 4.0±0.1 | 3.6±0.1 | 0.02* |
| Muscle mass normalized to body mass (mg/g) | |||
| Left ventricle/body mass | 1.80±0.03 | 1.54±0.05 | <0.01* |
| Right ventricle/body mass | 0.70±0.05 | 0.65±0.05 | 0.50 |
| Gastrocnemius/body mass | 5.46±0.07 | 5.16±0.10 | 0.03* |
| Soleus/body mass | 0.48±0.01 | 0.50±0.02 | 0.31 |
| Extensor digitorum longus/body mass | 0.47±0.01 | 0.45±0.01 | 0.11 |
Data are mean ± SEM and were compared with unpaired Student’s t-tests.
indicate a statistically significant difference between groups.
Figure 4.
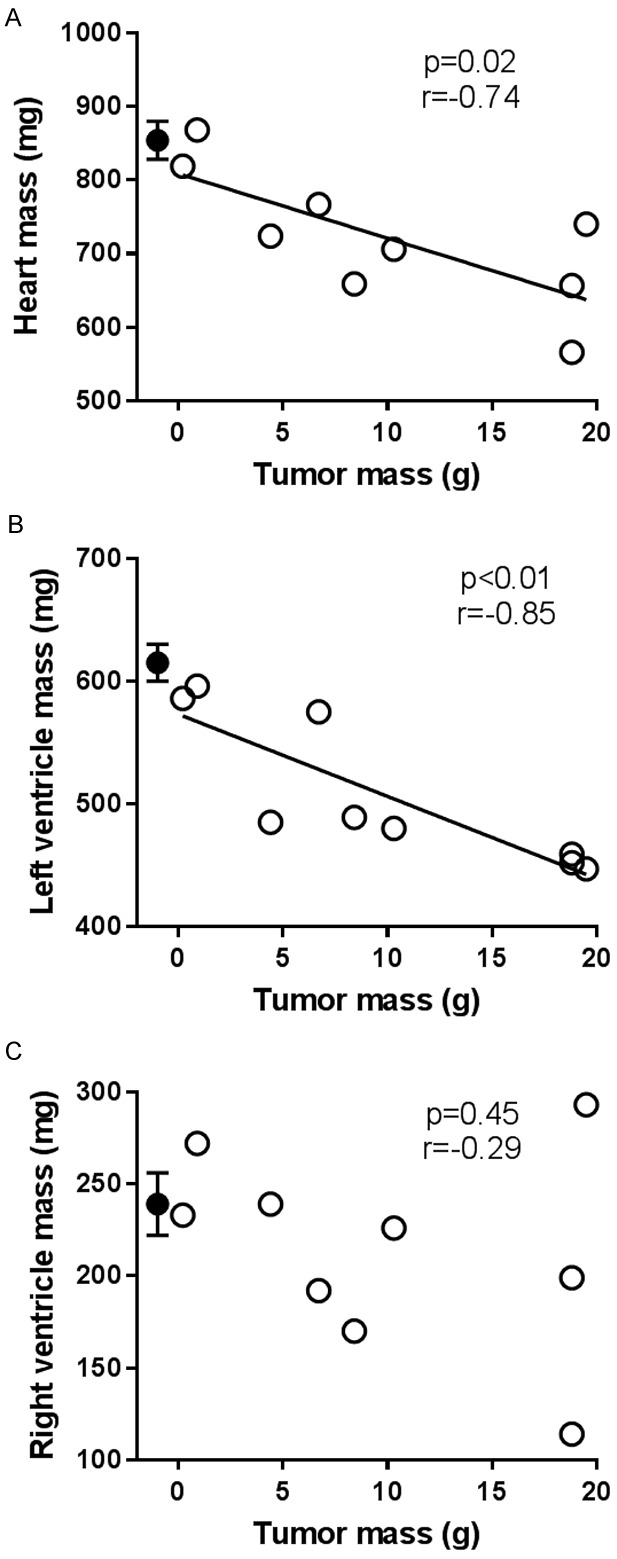
Within the tumor bearing group (n=9, open circles), tumor mass was significantly negatively correlated with heart mass (A) and left ventricle (LV) mass (B), but not right ventricle mass (C). Closed circles represent the mean and SEM of the sham group (n=9) which are shown for comparison purposes only and are not factored into the correlation or regression calculations.
There were no differences in citrate synthase activity between the sham and tumor bearing groups for any muscle or muscle part analyzed (Table 2).
Table 2.
Skeletal muscle citrate synthase activity (µmol/min/g)
| Sham (n=7) | Tumor bearing (n=9) | p-value | |
|---|---|---|---|
| Soleus | 19.9±1.7 | 17.0±1.2 | 0.17 |
| Red gastrocnemius | 24.1±2.7 | 25.4±2.1 | 0.66 |
| White gastrocnemius | 8.5±0.4 | 7.4±0.3 | 0.11 |
Date are mean ± SEM and were compared with unpaired Student’s t-tests. There were no statistically significant differences between groups.
Discussion
The primary novel finding of this investigation is that time to exhaustion during treadmill running was significantly lower in rats with prostate cancer compared to sham-operated control rats. Moreover, the reduced time to exhaustion in the tumor bearing group may be linked mechanistically to significant reductions in cardiac, specifically the LV, and select hindlimb skeletal muscle mass. We also found evidence that untreated prostate cancer impaired cardiac function as evidenced by the fact that both systolic blood pressure and LV Δpressure/Δtime (i.e., LV contractility) were negatively correlated with prostate tumor mass. These results are important because they indicate, for the first time, that exercise intolerance in prostate cancer patients may be attributable, at least in part, to the effects of prostate cancer itself and cannot be ascribed entirely to the effects of prostate cancer treatments such as ADT [9,10].
We measured time to exhaustion using a standardized submaximal treadmill running protocol which has been shown to have high within-rat reproducibility for up to five weeks [27]. In the present investigation, we found that time to exhaustion decreased over the course of the eight week experimental protocol in the sham group but to a greater degree in the tumor bearing group. The reduction in endurance capacity in the sham group was expected and is likely attributable to the effects of increasing age and body mass on exercise capacity in the absence of chronic exercise training (which was specifically avoided herein) as a countermeasure. The present protocol was initiated in 6 month old rats which, based upon strain longevity curves, would represent a middle age-human. The ~two-month experimental protocol reflects ~10-15% of the lifespan of the Copenhagen rat, or ~7 years in a human. Thus, the reduction in endurance capacity in the sham group demonstrates the necessity of including this group in our experimental design and the difference in time to exhaustion between groups eight weeks post-surgery discriminates the effect of untreated prostate cancer. We did not find a significant correlation between prostate tumor mass and reductions in time to exhaustion eight weeks post-surgery which is likely attributable to the multi-factorial nature of the determinants of exercise capacity [34] and the relatively small sample size of the tumor bearing group. It is also noteworthy that the prostate cancer-induced reduction in time to exhaustion was evident by four weeks post-surgery which was before any obvious tumors were present. That finding suggests that the reductions in endurance capacity in the prostate tumor group are not attributable to tumor-induced alterations in gait. Additional studies are needed to investigate whether reductions in cardiac and skeletal muscle mass follow a similar time course to the effects of prostate cancer on time to exhaustion.
As highlighted recently by Murphy [15], compared to skeletal muscle cachexia, much less is known regarding the mechanisms and treatment of cancer-induced cardiac cachexia and it is generally underappreciated in the clinical and research settings. Previous studies have reported cancer-induced cardiac cachexia in humans [17] and pre-clinical rodent models [14,19,35-40] but, to our knowledge, the present investigation is the first to link prostate cancer and cardiac cachexia. In the present investigation, the lower cardiac mass likely contributed importantly to the reduced time to exhaustion in the prostate tumor bearing group given the clear association between maximal cardiac output and maximal exercise capacity [41]. Specifically, the smaller LV mass likely resulted in a reduced stroke volume and therefore cardiac output during exercise (if one assumes there was no compensatory increase in heart rate). A particularly interesting finding of this investigation is the strength of the inverse relationship between tumor size and cardiac, specifically LV, mass especially considering the lack of such an inverse relationship between tumor mass and select hindlimb skeletal muscle mass. This may reflect the fact that distinct molecular pathways regulate cardiac atrophy compared to skeletal muscle atrophy in various pre-clinical cancer models [35,36].
The pumping performance of the heart (i.e., stroke volume) is determined by the preload of the LV (LVEDP), afterload on the LV (systolic blood pressure), and contractility of the LV (LV Δpressure/Δtime). Although we did not find any statistical differences between group means of any of these indexes of cardiac function in the present study, we did find that, within the tumor bearing group, systolic blood pressure and LV Δpressure/Δtime were negatively correlated with tumor mass. Those findings suggest that prostate tumors have the potential to impair cardiac function, especially as tumor burden increases, which is consistent with prior studies that have reported cardiac dysfunction in various pre-clinical tumor models [13,20,40]. In our study, the fact that LV contractility was negatively correlated with tumor mass even under anesthetized, unstressed conditions suggests that impaired cardiac function likely contributed to the reduction in exercise capacity in the tumor bearing group; particularly in the rats with the largest prostate tumors.
Whereas the cachectic effects of ADT on total lean body and skeletal muscle mass are well-documented [18,42-44], to our knowledge, the present investigation is the first to report that prostate cancer in an orthotopic model reduces skeletal muscle mass. Maturo et al. [45], however, did report that prostate cancer patients that were not on ADT had significantly less total lean body mass than aged-matched healthy control subjects. Our finding that EDL (comprised of 76% type IIb+d/x muscle fibers, [46]) and gastrocnemius (a predominately mixed fiber-type muscle with distinct glycolytic and oxidative portions [46]) muscle mass, but not soleus muscle mass (comprised of 91% type I+IIa muscle fibers [46]), was lower in the tumor-bearing group compared to the sham group is consistent with previous reports that cancer cachexia preferentially targets type II glycolytic muscle fibers [47-49]. A limitation of our study is that we did not measure food or water consumption and we cannot determine whether possible differences between groups contributed to our findings. However, the fiber-type selective muscle-wasting evident herein suggests that prostate cancer played the predominant role. The lower EDL and gastrocnemius mass likely contributed to the reduced exercise capacity in the tumor bearing group in the present investigation because the greater work per unit of EDL and gastrocnemius muscle mass is expected to have increased muscle fiber recruitment due to the greater energetic requirement per unit muscle mass. In contrast to the effect on hindlimb skeletal muscle mass, there was no effect of prostate cancer on citrate synthase activity (index of muscle oxidative capacity) in hindlimb skeletal muscles/muscle portions that spanned the range of fiber-type composition. Thus, prostate cancer likely did not impact the inherent oxidative capacity of the hindlimb muscles/muscle portions investigated presently, although potential effects on other oxidative enzymes were not measured. During exercise, however, oxidative ATP synthesis may potentially be impaired by any possible prostate cancer-induced derangements in peripheral vascular function and, therefore, oxygen delivery to contracting skeletal muscles.
There are several limitations of this investigation. First, as indicated above we did not measure food consumption. Reduced food consumption has been reported in other murine tumor models [37,40,50,51] and reduced protein synthesis may have contributed to cardiac and skeletal muscle cachexia in the tumor bearing group herein. Importantly, however, tumor-induced cachexia has also been reported in rats with colon-26 tumors despite no difference in food intake from healthy controls [52]. Second, we did not measure cage activity and spontaneous cage activity has been shown to be reduced in a murine tumor model [50]. Prostate tumor-induced reductions in cage activity and associated deconditioning may have contributed to the lower endurance exercise capacity in the tumor bearing group compared to the sham group in our study. However, soleus muscle citrate synthase activity is reduced by chronic immobilization [53], but was not different between the sham and tumor bearing groups in our study which suggests that any differences in cage activity, if they occurred, were minimal.
In summary, prostate cancer reduced endurance exercise capacity in an established pre-clinical rat orthotopic prostate tumor model. Moreover, heart and, more specifically, LV mass were significantly lower in the tumor bearing group compared to the sham group and within the tumor bearing group, heart and LV mass were significantly negatively correlated to tumor mass. The significantly lower EDL and gastrocnemius muscle mass in the tumor bearing group compared to the sham group were not significantly correlated with tumor mass. Although multiple mechanisms likely contributed to the reduced endurance exercise capacity in the prostate tumor bearing group, the deleterious effects of prostate cancer on cardiac and skeletal muscle mass, as well as cardiac function, likely played an important role. The present findings have important implications for prostate cancer patients and their clinicians given that the bulk of the available literature has focused on the effects of prostate cancer treatment (namely ADT) and its role in the exaggerated fatigue and reduced exercise capacity in this population. Additionally, the present findings open the door to a new area of research investigating the mechanisms by which prostate cancer independently reduces exercise capacity and cardiac and skeletal muscle mass.
Acknowledgements
This work was supported by the American Cancer Society (RSG-14-150-01-CCE to BJB).
Disclosure of conflict of interest
None.
References
- 1.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, Vogelzang NJ. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 2.Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc. 2014;3:e000432. doi: 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cella D, Davis K, Breitbart W, Curt G, Fatigue C. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J. Clin. Oncol. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 4.Charalambous A, Kouta C. Cancer related fatigue and quality of life in patients with advanced prostate cancer undergoing chemotherapy. Biomed Res Int. 2016;2016:3989286. doi: 10.1155/2016/3989286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 6.Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. Br J Cancer. 2004;91:822–828. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XS, Zhao F, Fisch MJ, O’Mara AM, Cella D, Mendoza TR, Cleeland CS. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120:425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birgegard G, Aapro MS, Bokemeyer C, Dicato M, Drings P, Hornedo J, Krzakowski M, Ludwig H, Pecorelli S, Schmoll H, Schneider M, Schrijvers D, Shasha D, Van Belle S. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3–11. doi: 10.1159/000083128. [DOI] [PubMed] [Google Scholar]
- 9.Alibhai SM, Breunis H, Timilshina N, Naglie G, Tannock I, Krahn M, Warde P, Fleshner NE, Canning SD, Tomlinson G. Long-term impact of androgen-deprivation therapy on physical function and quality of life. Cancer. 2015;121:2350–2357. doi: 10.1002/cncr.29355. [DOI] [PubMed] [Google Scholar]
- 10.Wall BA, Galvao DA, Fatehee N, Taaffe DR, Spry N, Joseph D, Newton RU. Reduced cardiovascular capacity and resting metabolic rate in men with prostate cancer undergoing androgen deprivation: a comprehensive cross-sectional investigation. Adv Urol. 2015;2015:976235. doi: 10.1155/2015/976235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Support Care Cancer. 2002;10:389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- 12.Chicco AJ, Schneider CM, Hayward R. Voluntary exercise protects against acute doxorubicin cardiotoxicity in the isolated perfused rat heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R424–R431. doi: 10.1152/ajpregu.00636.2004. [DOI] [PubMed] [Google Scholar]
- 13.Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Potsch M, von Websky K, Hocher B, Latouche C, Jaisser F, Morawietz L, Coats AJ, Beadle J, Argiles JM, Thum T, Foldes G, Doehner W, Hilfiker-Kleiner D, Force T, Anker SD. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur Heart J. 2014;35:932–941. doi: 10.1093/eurheartj/eht302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuyama T, Ishikawa T, Okayama T, Oka K, Adachi S, Mizushima K, Kimura R, Okajima M, Sakai H, Sakamoto N, Katada K, Kamada K, Uchiyama K, Handa O, Takagi T, Kokura S, Naito Y, Itoh Y. Tumor inoculation site affects the development of cancer cachexia and muscle wasting. Int J Cancer. 2015;137:2558–2565. doi: 10.1002/ijc.29620. [DOI] [PubMed] [Google Scholar]
- 15.Murphy KT. The pathogenesis and treatment of cardiac atrophy in cancer cachexia. Am J Physiol Heart Circ Physiol. 2016;310:H466–477. doi: 10.1152/ajpheart.00720.2015. [DOI] [PubMed] [Google Scholar]
- 16.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 17.Burch GE, Phillips JH, Ansari A. The cachetic heart. A clinico-pathologic, electrocardiographic and roentgenographic entity. Dis Chest. 1968;54:403–409. doi: 10.1378/chest.54.5.403. [DOI] [PubMed] [Google Scholar]
- 18.Galvao DA, Spry NA, Taaffe DR, Newton RU, Stanley J, Shannon T, Rowling C, Prince R. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102:44–47. doi: 10.1111/j.1464-410X.2008.07539.x. [DOI] [PubMed] [Google Scholar]
- 19.Wysong A, Couch M, Shadfar S, Li L, Rodriguez JE, Asher S, Yin X, Gore M, Baldwin A, Patterson C, Willis MS. NF-kappaB inhibition protects against tumor-induced cardiac atrophy in vivo. Am J Pathol. 2011;178:1059–1068. doi: 10.1016/j.ajpath.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Crawford D, Hutchinson KR, Youtz DJ, Lucchesi PA, Velten M, McCarthy DO, Wold LE. Myocardial dysfunction in an animal model of cancer cachexia. Life Sci. 2011;88:406–410. doi: 10.1016/j.lfs.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–1792. [PubMed] [Google Scholar]
- 22.van Norren K, van Helvoort A, Argiles JM, van Tuijl S, Arts K, Gorselink M, Laviano A, Kegler D, Haagsman HP, van der Beek EM. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer. 2009;100:311–314. doi: 10.1038/sj.bjc.6604858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 24.Cheung AS, Zajac JD, Grossmann M. Muscle and bone effects of androgen deprivation therapy: current and emerging therapies. Endocr Relat Cancer. 2014;21:R371–394. doi: 10.1530/ERC-14-0172. [DOI] [PubMed] [Google Scholar]
- 25.Nelson AM, Gonzalez BD, Jim HS, Cessna JM, Sutton SK, Small BJ, Fishman MN, Zachariah B, Jacobsen PB. Characteristics and predictors of fatigue among men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Support Care Cancer. 2016;24:4159–4166. doi: 10.1007/s00520-016-3241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee H, Gunter JH, Heathcote P, Ho K, Stricker P, Corcoran NM, Nelson CC. Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int. 2015;115(Suppl 5):3–13. doi: 10.1111/bju.12964. [DOI] [PubMed] [Google Scholar]
- 27.Copp SW, Davis RT, Poole DC, Musch TI. Reproducibility of endurance capacity and VO2peak in male Sprague-Dawley rats. J Appl Physiol (1985) 2009;106:1072–1078. doi: 10.1152/japplphysiol.91566.2008. [DOI] [PubMed] [Google Scholar]
- 28.Dunning WF. Prostate cancer in the rat. Natl Cancer Inst Monogr. 1963;12:351–369. [PubMed] [Google Scholar]
- 29.Isaacs JT, Heston WD, Weissman RM, Coffey DS. Animal models of the hormone-sensitive and -insensitive prostatic adenocarcinomas, Dunning R-3327-H, R-3327-HI, and R-3327-AT. Cancer Res. 1978;38:4353–4359. [PubMed] [Google Scholar]
- 30.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Garcia E, Becker VG, McCullough DJ, Stabley JN, Gittemeier EM, Opoku-Acheampong AB, Sieman DW, Behnke BJ. Blood flow responses to mild-intensity exercise in ectopic vs. orthotopic prostate tumors; dependence upon host tissue hemodynamics and vascular reactivity. J Appl Physiol (1985) 2016;121:15–24. doi: 10.1152/japplphysiol.00266.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst. 2014;106:dju036. doi: 10.1093/jnci/dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sere PA. Citrate synthase. Methods Emzymol. 1969;13:3–11. [Google Scholar]
- 34.Bassett DR Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res. 2011;71:1710–1720. doi: 10.1158/0008-5472.CAN-10-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shum AM, Fung DC, Corley SM, McGill MC, Bentley NL, Tan TC, Wilkins MR, Polly P. Cardiac and skeletal muscles show molecularly distinct responses to cancer cachexia. Physiol Genomics. 2015;47:588–599. doi: 10.1152/physiolgenomics.00128.2014. [DOI] [PubMed] [Google Scholar]
- 37.Tessitore L, Costelli P, Bonetti G, Baccino FM. Cancer cachexia, malnutrition, and tissue protein turnover in experimental animals. Arch Biochem Biophys. 1993;306:52–58. doi: 10.1006/abbi.1993.1479. [DOI] [PubMed] [Google Scholar]
- 38.Schafer M, Oeing CU, Rohm M, Baysal-Temel E, Lehmann LH, Bauer R, Volz HC, Boutros M, Sohn D, Sticht C, Gretz N, Eichelbaum K, Werner T, Hirt MN, Eschenhagen T, Muller-Decker K, Strobel O, Hackert T, Krijgsveld J, Katus HA, Berriel Diaz M, Backs J, Herzig S. Ataxin-10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia. Mol Metab. 2016;5:67–78. doi: 10.1016/j.molmet.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian M, Asp ML, Nishijima Y, Belury MA. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. Int J Oncol. 2011;39:1321–1326. doi: 10.3892/ijo.2011.1150. [DOI] [PubMed] [Google Scholar]
- 40.Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer-induced cachexia in mice. Int J Oncol. 2010;37:347–353. doi: 10.3892/ijo_00000683. [DOI] [PubMed] [Google Scholar]
- 41.Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38:VII1–78. [PubMed] [Google Scholar]
- 42.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63:742–745. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 43.Berruti A, Dogliotti L, Terrone C, Cerutti S, Isaia G, Tarabuzzi R, Reimondo G, Mari M, Ardissone P, De Luca S, Fasolis G, Fontana D, Rossetti SR, Angeli A Gruppo Onco Urologico Piemontese (G.O.U.P.), Rete Oncologica Piemontese. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–2367. [PubMed] [Google Scholar]
- 44.Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TL, Ke C, Leder BZ, Goessl C. Sarcopenia during androgen-deprivation therapy for prostate cancer. J. Clin. Oncol. 2012;30:3271–3276. doi: 10.1200/JCO.2011.38.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maturo G, Vespasiani G, Mohamed EI, Maiolo C, Finazzi Agro E, Forte F, De Lorenzo A. Evaluating body composition of Italian prostate cancer patients without metastases. Acta Diabetol. 2003;40(Suppl 1):S168–170. doi: 10.1007/s00592-003-0056-4. [DOI] [PubMed] [Google Scholar]
- 46.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol (1985) 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 47.Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, Muscarella P, Burghes AH, Rafael-Fortney JA, Guttridge DC. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8:421–432. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Mendell JR, Engel WK. The fine structure of type II muscle fiber atrophy. Neurology. 1971;21:358–365. doi: 10.1212/wnl.21.4.358. [DOI] [PubMed] [Google Scholar]
- 49.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–2199. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Elkina Y, Palus S, Tschirner A, Hartmann K, von Haehling S, Doehner W, Mayer U, Coats AJ, Beadle J, Anker SD, Springer J. Tandospirone reduces wasting and improves cardiac function in experimental cancer cachexia. Int J Cardiol. 2013;170:160–166. doi: 10.1016/j.ijcard.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 51.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T, Taguchi T. Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res. 1990;50:2290–2295. [PubMed] [Google Scholar]
- 53.Fell RD, Steffen JM, Musacchia XJ. Effect of hypokinesia-hypodynamia on rat muscle oxidative capacity and glucose uptake. Am J Physiol. 1985;249:R308–312. doi: 10.1152/ajpregu.1985.249.3.R308. [DOI] [PubMed] [Google Scholar]


