Abstract
Objectives:
To report the safety and clinical efficacy with the novel lumen-apposing metal stent (LAMS) with an electrocautery enhanced delivery system for the drainage of pancreatic fluid collections (PFCs).
Methods:
This was a retrospective analysis of all consecutive patients with PFCs who underwent endoscopic ultrasound (EUS)-guided drainage using the LAMS with an electrocautery enhanced delivery system in 2 US centers.
Results:
Thirteen patients with PFCs (69% with walled-off necrosis [WON]) underwent drainage using the study device. Successful stent placement was accomplished in all patients. Direct endoscopic necrosectomy was carried out in all nine patients with WON complete resolution of the PFC was obtained in all 13 cases, with no recurrence during follow-up. There was one procedure-related adverse event. In one patient, the LAMS was dislodged immediately after deployment, falling into the stomach where it was removed. A second electrocautery enhanced LAMS was placed in this patient immediately afterward.
Conclusions:
EUS-guided drainage using the LAMS with the electrocautery-enhanced delivery system is a safe, easily performed, and a highly effective for the drainage of PFCs.
Keywords: Endosonography, metal stents, pancreatic fluid collection
INTRODUCTION
In the current era, endoscopists are frequently called on to diagnose and more importantly, treat pancreatic fluid collections (PFCs). PFCs are known to develop as a consequence of pancreatic ductal injury following episodes of acute pancreatitis and can be seen in patients with chronic pancreatitis, iatrogenic causes (i.e., surgery), trauma, or in patients with the so-called disconnected duct syndrome.[1,2,3] PFCs can be delineated as either pancreatic pseudocysts (PPs) or walled-off necrosis (WON), with the former being fluid collections in the peripancreatic tissues that are surrounded by a well-defined wall and contain little to no solid material, the latter consist of necrotic tissue (often admixed with fluid), contained within a wall of reactive tissue. PFCs often produce symptoms including pain, gastric outlet obstruction, biliary obstruction, and can sometimes become infected.[4]
PFCs can be managed by a variety of approaches, including endoscopic, surgical, and percutaneous drainage; some patients require multiple modalities to treat these lesions.[5,6,7] The surgical approach is the most well-established means of draining PFCs can be performed laparoscopically but is invasive and carries a relatively high mortality and morbidity.[8] Percutaneous drainage of PFCs carries several risks including the risk of fistula formation, cyst recurrence, and infection although this approach is minimally invasive when compared to other treatment options.
Endoscopic drainage and debridement of PFCs, once rare, are now performed at many centers and have shown a high degree of both efficacy and safety.[9] The procedure is usually endoscopic ultrasound (EUS)-based. EUS-guided drainage has been shown to have a high success rate (87%–97%) with a low complication (6%–34%) and mortality (0%–1%) rate.[10,11]
Recently, lumen-apposing fully covered self-expanding lumen-apposing metal stent (LAMS) has become commercially available and has been demonstrated to be both safe and effective for endoscopic transmural drainage of PPs and WONs.[12,13,14]
Our group recently reported results of a large multicenter study using the first-generation version of one of these devices (Axios Stent, Boston Scientific, Natick MA).[15] This study included 82 patients with PP or WON. The mean size of the PFC was 11.8 cm. LAMSs were successfully placed in 80 patients (97.5%). Endoscopic debridement with the LAMS in WON was performed in 54 patients. The patency of the stent was maintained in 98.7% of the patients (77/78). Successful endoscopic therapy using the LAMS was successful in 12 of 12 patients (100%) with PP compared with 60 of 68 patients (88.2%) with WON. All stents were endoscopically removed from all patients after peri-PFC resolution. There was 1 PFC recurrence during the 3-month median follow-up period. Overall, adverse events were uncommon and clinical and technical success was high.
This study used the so-called “cold” version of this stent. This device, while highly effective, still requires the endoscopists to access the PFC with an EUS FNA needle, place a guidewire into the cyst, and dilate the cystgastrostomy tract before the LAMS can be placed. The “hot” version of this device allows direct access to the PFC without the need for these steps through a diathermic tip of the LAMS delivery catheter, thus simplifying the procedure considerably. We report in this study our initial multicenter experience with the “hot” version of this stent, so named for a diathermic tip on the stent deployment catheter used to create the cystenterostomy. The use of a diathermic tip speeds the procedure and potentially eliminates several steps from the stent deployment procedure, saving both time, equipment, and costs.
METHODS
We performed a multi-center, retrospective study conducted at 2 tertiary care centers. The study was approved by the Institutional Review Boards in both centers. The study concept, hypothesis, and design were investigator initiated and no financial support was received.
The endoscopy database at the University of Utah Hospitals and Clinics and Thomas Jefferson University Hospital was queried for all patients who had undergone EUS-guided drainage of PFCs (i.e., PP and WON) using the diathermic-tip LAMS between February 2012 and June 2014. Only patients with a 3-month or greater follow-up were included in the study.
PFCs were characterized by magnetic resonance imaging or computed tomography (CT) in concordance with EUS findings. WONs included in this study consisted of a mature, encapsulated collection of pancreatic, and/or peripancreatic necrotic tissue contained within an enhancing wall of reactive tissue. PPs were defined as an encapsulated collection of fluid with a well-defined inflammatory wall usually outside the pancreas with minimal or no necrosis (as per the revised Atlanta Classification).[1]
The indications for drainage of PFCs included (1) pain felt to be secondary to the PFC, (2) gastric outlet or biliary obstruction secondary to compression by the PFC, (3) ongoing systemic illness, anorexia, and weight loss, (4) rapidly enlarging PFCs, and/or (5) infected PFCs.[16] Some patients had more than one inclusion criteria. Data recorded from outpatient and hospital records to collect procedural details and overall clinical course of the patient.
Description of the lumen-apposing metal stent
The LAMS (Hot AXIOS™; Boston Scientific, Natick MA) is a saddle-shaped nitinol, braided flexible fully-covered stent. The stent has bilateral double-walled anchoring flanges designed to hold the stomach or duodenal wall in direct apposition to the inner wall of the PFC. The stent is available in 2 different lumen diameters (10 mm and 15 mm) and is 10 mm long.
Techniques
All patients underwent procedures by endoscopists with > 5 years of endosonography practice (DGA and AAS). PFC drainage was performed using the therapeutic linear array echoendoscope (Olympus; Center Valley, PA, USA). All procedures were performed under general anesthesia. Patients were given broad-spectrum antibiotics during and after the procedure to decrease the risk of secondary infection. Transgastric and transduodenal routes were evaluated in all patients before creation of a cystenterostomy. EUS imaging was used to determine the optimal puncture site of the cyst [Figure 1]. Color Doppler was used to exclude interposed vessels at the puncture site.
Figure 1.
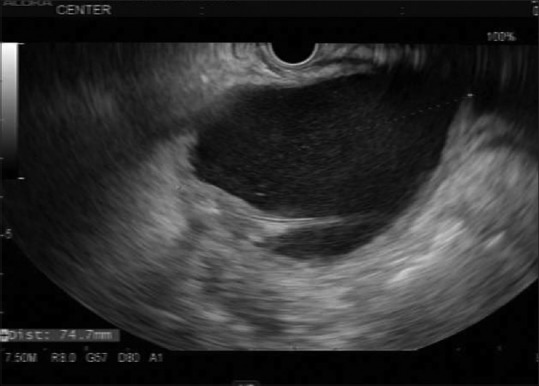
7.5 MHz EUS image of a pancreatic fluid collection prior to access and drainage
Under EUS guidance, the stent delivery system was advanced until it was in contact with the gastric or duodenal wall in optimal position for creation of a cystenterostomy. The stent delivery system was pressed against the gastric or duodenal lumen while electrocautery what delivered, essentially cutting and coagulating the cystenterostomy tract simultaneously. Cautery settings were as follows: auto cut, effect 4, 100 Watts on an ERBE ICC 200 electrocautery generator as recommended by the manufacturer of the LAMS (ERBE USA, Marietta Georgia). These settings were used for all procedures and were not adjusted for either transgastric or transduodenal placement.
The selection of stent diameter (10 mm or 15 mm) was at the discretion of the endoscopists based on the size and contents of the cyst but was not dependent on the route of placement of the LAMS. The distal flange of the stent was deployed under EUS guidance followed by positioning of this flange against the PFC wall. Deployment of the proximal flange was then performed under endoscopic and/or EUS guidance [Figures 2 and 3].
Figure 2.
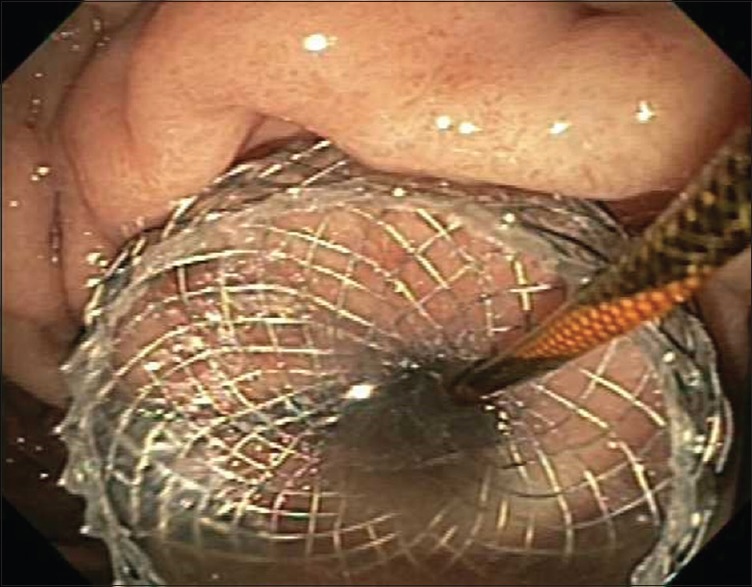
Endoscopic image of LAMS placed via electrocautery enhanced system immediately after deployment
Figure 3.
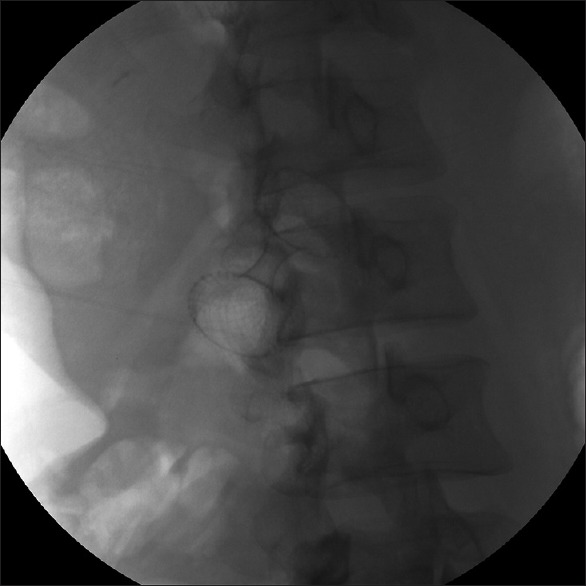
Fluoroscopic image of LAMS after deployment
In patients with WON, endoscopic necrosectomy sessions were performed using an upper endoscope advanced through the LAMS at intervals selected by the treating endoscopist, approximately every 3–14 days, until complete debridement of the necrotic cavity was performed as confirmed endoscopically and/or by cross-sectional imaging. Necrosectomy procedures involved a mixture of PFC lavage with diluted hydrogen peroxide mixed with sterile saline, blunt dissection, or necrotic PFC contents using endoscopic catheters, and capture and removal of necrotic tissue by a variety of devices including endoscopic forceps, snares, baskets, and retrieval net devices.
Complications including but not limited to perforation, bleeding, hypotension, or respiratory distress were recorded. The electronic medical records of hospital admissions and ambulatory office visits were also evaluated for any delayed complication (<30-day after procedure).
All patients were evaluated with periodic contrast-enhanced CT of the abdomen and pelvis 4–8 weeks after LAMS placement. Stent removal was undertaken through the use of rat-tooth forceps through simple traction when complete cyst decompression was achieved, i.e., the PFC had completely resolved without any residual solid or fluid contents remained. The cystogastrostomy site was left to close secondarily and was not clipped or sutured closed after LAMS removal.
RESULTS
Thirteen patients (5 male, 8 female) were included in the study. The mean age of the patients was 58.3 years (range 32–92). The etiology of the patient's pancreatitis was as follows: gallstones n = 6, alcohol n = 5, hypertriglyceridemia n = 1, idiopathic n = 1.
The mean size of the PFC was 138 mm (range 60–159 mm). Four lesions (31%) were felt to be pseudocysts, 9 lesions (69%) were felt to be WONs based on EUS and cross-sectional imaging. Two lesions were located in the region of the pancreatic head, 8 lesions were located in the region of the pancreatic body, and three lesions were located in the region of the pancreatic tail. The PFCs in the region of the pancreatic head were drained in a transduodenal manner, the remainder of the lesions was drained in a transgastric manner.
All but one patient received a 15 mm diameter Axios stent. Four patients had concomitant placement of a plastic double-pigtail stent through the Axios catheter to reduce the risk of clogging in patients with an excessive amount of solid debris and one patient underwent placement of a nasocystic tube through the Axios stent to allow the patient to perform saline lavage of the stent at home as an outpatient.
All procedures were technically successful. The mean procedure time was 15 min (range 8–32 min). There was one procedure-related adverse event. In one patient, the Axios stent was dislodged immediately after deployment, falling into the stomach where it was removed. A second hot Axios stent was placed in this patient immediately afterward without any adverse event. Of note, there was no bleeding at any of the cystenterostomy sites.
All patients with WON underwent endoscopic necrosectomy with a mean of 3 sessions per patient (range 1–6 sessions). All Axios stents were removed without difficulty. Mean stent indwell time was 2 months (range 2–4 months), and patients had recurrence of their PFCs in a mean duration of follow-up of 2.5 months (range 2–6 months).
DISCUSSION
Traditional cystenterostomy for PFC drainage involves several steps, including accessing the PFC with a EUS FNA needle (typically 19-gauge) to allow guidewire advancement and looping, dilation of the cystenterostomy with a dilation balloon, and then the placement of one or more plastic or metal stents across the cystenterostomy. Although simple in concept, the procedure can be technically very demanding with limited endoscopic visualization, easy loss of access to the PFC at any step of the procedure, and risks of bleeding and perforation from the creation of the cystenterostomy.
The diathermic-tipped device used in this series eliminates the need for the endoscopist to use an EUS FNA needle to puncture the PFC as well as eliminates the need for a guidewire at all although one can be inserted through the stent delivery catheter if desired. Furthermore, as the diathermic tip creates an initial cystgastrostomy identical in diameter to the stent delivery catheter, no dilation is needed before stent deployment. The stent gradually dilates the cystenterostomy after deployment, ultimately reaching its final inner diameter.
This device potentially eliminates the need for three other devices at the time of deployment, potentially resulting in significant cost savings although the “hot” version of the device is more expensive than the “cold” version. In addition, the elimination of these other steps in the creation of the cystenterostomy reduces time.
CONCLUSION
The electrocautery enhanced LAMS device proved safe, effective, and efficient for the access and drainage of pancreatic fluid collections.
Financial support and sponsorship
This study was funded entirely by existing intramural funds and salary support in the respective institutions.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 2.Baillie J. Pancreatic pseudocysts (Part I) Gastrointest Endosc. 2004;59:873–9. doi: 10.1016/s0016-5107(04)00354-2. [DOI] [PubMed] [Google Scholar]
- 3.Brun A, Agarwal N, Pitchumoni CS. Fluid collections in and around the pancreas in acute pancreatitis. J Clin Gastroenterol. 2011;45:614–25. doi: 10.1097/MCG.0b013e318213ef3e. [DOI] [PubMed] [Google Scholar]
- 4.Yeo CJ, Bastidas JA, Lynch-Nyhan A, et al. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet. 1990;170:411–7. [PubMed] [Google Scholar]
- 5.Tsiotos GG, Sarr MG. Management of fluid collections and necrosis in acute pancreatitis. Curr Gastroenterol Rep. 1999;1:139–44. doi: 10.1007/s11894-996-0013-9. [DOI] [PubMed] [Google Scholar]
- 6.Baron TH, Harewood GC, Morgan DE, et al. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7–17. doi: 10.1067/mge.2002.125106. [DOI] [PubMed] [Google Scholar]
- 7.Nealon WH, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage) Ann Surg. 2002;235:751–8. doi: 10.1097/00000658-200206000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitas GJ, Sarr MG. Selected management of pancreatic pseudocysts: Operative versus expectant management. Surgery. 1992;111:123–30. [PubMed] [Google Scholar]
- 9.Binmoeller KF, Seifert H, Walter A, Soehendra N. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:219–24. doi: 10.1016/s0016-5107(95)70095-1. [DOI] [PubMed] [Google Scholar]
- 10.Varadarajulu S, Bang JY, Phadnis MA, et al. Endoscopic transmural drainage of peripancreatic fluid collections: Outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080–8. doi: 10.1007/s11605-011-1621-8. [DOI] [PubMed] [Google Scholar]
- 11.Varadarajulu S, Rana SS, Bhasin DK. Endoscopic therapy for pancreatic duct leaks and disruptions. Gastrointest Endosc Clin N Am. 2013;23:863–92. doi: 10.1016/j.giec.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy. 2011;43:337–42. doi: 10.1055/s-0030-1256127. [DOI] [PubMed] [Google Scholar]
- 13.Shah RJ, Shah JN, Waxman I, et al. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015;13:747–52. doi: 10.1016/j.cgh.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 14.Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastrointest Endosc. 2012;75:870–6. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui AA, Adler DG, Nieto J, et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: A large retrospective, multicenter U. S. experience (with videos) Gastrointest Endosc. 2016;83:699–707. doi: 10.1016/j.gie.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson BC, Baron TH, Adler DG, et al. ASGE guideline: The role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc. 2005;61:363–70. doi: 10.1016/s0016-5107(04)02779-8. [DOI] [PubMed] [Google Scholar]


