Abstract
Background and Aims:
Hypotension during propofol induction is a common problem. Perfusion index (PI), an indicator of systemic vascular resistance, is said to be predictive of hypotension following subarachnoid block. We hypothesised that PI can predict hypotension following propofol induction and a cut-off value beyond which hypotension is more common can be determined.
Methods:
Fifty adults belonging to the American Society of Anesthesiologists' physical status I/II undergoing elective surgery under general anaesthesia were enrolled for this prospective, observational study. PI, heart rate, blood pressure (BP) and oxygen saturation were recorded every minute from baseline to 10 min following induction of anaesthesia with a titrated dose of propofol, and after endotracheal intubation. Hypotension was defined as fall in systolic BP (SBP) by >30% of baseline or mean arterial pressure (MAP) to <60 mm Hg. Severe hypotension (MAP of <55 mm Hg) was treated.
Results:
Within first 5-min after induction, the incidence of hypotension with SBP and MAP criteria was 30% and 42%, respectively, and that of severe hypotension, 22%. Baseline PI <1.05 predicted incidence of hypotension at 5 min with sensitivity 93%, specificity 71%, positive predictive value (PPV) 68% and negative predictive value (NPV) 98%. The area under the ROC curve (AUC) was 0.816, 95% confidence interval (0.699–0.933), P < 0.001
Conclusion:
Perfusion index could predict hypotension following propofol induction, especially before endotracheal intubation, and had a very high negative predictive value.
Key words: Blood pressure, general anaesthesia, hypotension, intravenous anaesthetics, perfusion index, plethysmography, propofol
INTRODUCTION
Perfusion index (PI) is a relatively new parameter estimating the pulsatility of blood in the extremities, calculated using infrared spectrum as part of plethysmography waveform processing. It is a simple, cost-effective and non-invasive method of assessing peripheral perfusion determined by the percentage of pulsatile to non-pulsatile blood flow in the extremities. PI indicates the status of the microcirculation which is densely innervated by sympathetic nerves, and therefore, is affected by multiple factors responsible for vasoconstriction or vasodilatation of the microvasculature.[1] It is also purported to be an indicator of systemic vascular resistance (SVR).[1] PI is said to be useful in monitoring depth of anaesthesia, hypothermia, successful epidural placement in parturients, adequate relief from ureteric obstruction, response to fluid therapy in critically ill and intraoperative patients and adequacy of circulation in newborn.[2,3,4] The value of PI is inversely related to the vascular tone, though not in a linear fashion. Therefore, vasodilatation reflecting higher baseline PI has been associated with reductions in blood pressure (BP) following spinal anaesthesia.[5] The resting SVR can influence incidence and severity of post-spinal hypotension in parturients.[5,6] It has been established that a positive correlation between pre-anaesthetic plethysmographic variability index (PVI) and reduction in BP following induction of anaesthesia using propofol in healthy adults, that is, higher PVI was associated with more mean arterial pressure (MAP) reductions.[7] Similarly, a significant proportion of hypotension after induction of anaesthesia with propofol can be attributed to the baseline SVR. Hence, we hypothesised that it is possible to define a threshold value of PI that predicts hypotension based on individual's pre-induction SVR. This study was conceived to obtain a cut-off value of pre-anaesthesia PI which may be useful for prediction of hypotension following anaesthetic induction with propofol.
METHODS
A prospective observational study was performed in a tertiary care hospital after clearance from institutional ethics committee (INST.EC/EC/113/2015-2016). Fifty adults aged between 18 and 60 years belonging to the American Society of Anesthesiologists' Physical Status I and II undergoing elective surgery under general anaesthesia were recruited after obtaining written informed consent. Patients with hypertension, vasoactive medications, difficult airway and pregnancy were excluded from the study. Premedication consisted of oral diazepam 0.1 mg/kg and ranitidine 150 mg on the morning of scheduled surgery. On reception in operation theatre, electrocardiograph, non-invasive BP, pulse oximeter (Intellivue MP40 Anaesthesia monitor, Philips Medizin Systeme, GmbH 71034, Boeblingen, Germany) were connected, and baseline values (heart rate [HR], PI, systolic [SBP], diastolic [DBP] and mean BPs [MAP]) were recorded. Intravenous infusion of Ringer's lactate was started at 100 ml/h. Intravenous (IV) fentanyl 2 μg/kg was administered followed by propofol injected slowly at a rate of 10 mg per every 5 s, titrated to loss of response to verbal communication and vecuronium 0.1 mg/kg IV was administered. The parameters were recorded every minute until 5 min. The lungs were ventilated with 100% O2 for 5 min before the trachea was intubated with the appropriate sized endotracheal tube by a consultant anaesthesiologist. Maintenance of anaesthesia was established with 50% N2O in oxygen along with isoflurane 0.6%. Haemodynamic parameters were recorded at 1-min intervals till 10 min after intubation. Hypotension was defined as a drop in SBP to <30% of baseline or absolute MAP <60 mmHg. MAP <55 mmHg (severe hypotension) was treated immediately by rapid intravenous fluid administration (10 ml/kg) and mephentermine 6 mg IV boluses. Bradycardia was defined as HR <50 bpm or decrease by more than 30% below baseline value, whichever was lower and was treated with atropine 0.6 mg IV boluses. The incidence of hypotension was calculated in 2 sets – 5 min after induction of anaesthesia (effect of induction agent) and first 15 min after induction (effect of induction process and endotracheal intubation). A cut-off value of baseline PI below which hypotension at 5 min post induction could be predicted was the primary outcome, while positive and negative predictive values at 15 minutes were secondary outcomes.
The sample size was calculated to observe effect size of at least 0.45 based on a study for correlation of PI and change in the MAP after propofol induction.[7] For an alpha error of 5% and 80% power, the sample size required was found to be 38. Factoring in attrition rate of 20%, 50 patients were enrolled. Data were collected and computed using Microsoft Excel 2007 (Microsoft Corporation, Redmond, Washington, 2007) and analysed using SPSS version 20 (IBM Corporation, New York, 2014). Data were represented as mean (±standard deviation) for quantitative variables and percentages for qualitative variables. Distribution of PI was subjected to normality test (P < 0.001). The point-biserial correlation was used for examining the association between baseline PI and hypotension incidence. If bivariate correlation was found to achieve statistical significance, binary logistic regression was conducted to identify independent predictability for predicting hypotension. Spearman's ρ was used for testing correlation between PI and all haemodynamic variables, and linear regression was applied to identify independent predictability if univariate correlation was found. Receiver operating characteristic (ROC) curves were constructed for values of baseline PI for predicting hypotension (SBP <30% below baseline). P < 0.05 was considered statistically significant.
RESULTS
All fifty patients completed the protocol and were available for data analysis [Table 1, consort diagram]. Demographic and baseline parameter data are represented in Table 2.
Table 1.
Consort diagram of recruitment
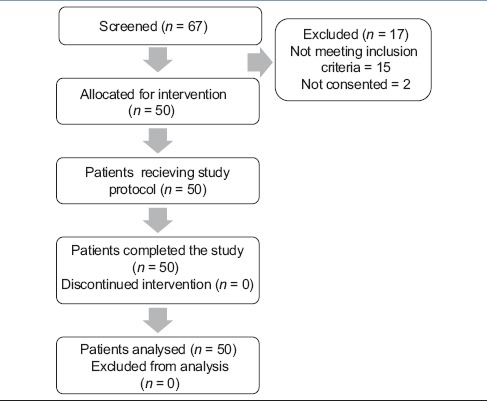
Table 2.
Demographic and baseline variables
Visual inspection of trend lines of variables over the time points shows a steep fall in blood pressure values after propofol injection till 5 min with a slight increase after endotracheal intubation and stable values thereafter. Mean SBP dropped by 18.2%, whereas diastolic BP (DBP) and MAP fell by 19.3% and 18.1%, respectively, from pre-induction levels. Laryngoscopy and intubation caused a clinically very insignificant change in the haemodynamic parameters. HR and PI values are relatively unchanged over the time points [Figure 1]. The changes have not been quantified statistically as they were not the objective of this study.
Figure 1.
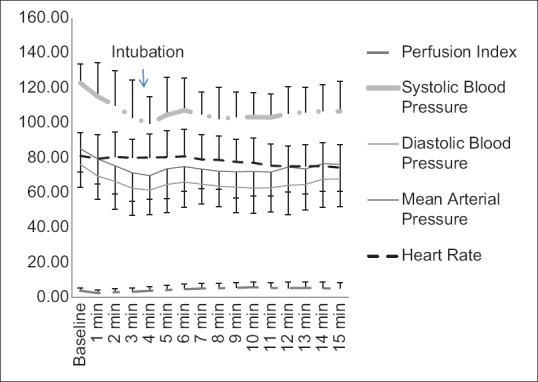
Trend of haemodynamic variables
PI showed a statistically significant, yet weak negative correlation (over all data points of all patients, n = 797) with HR (ρ = −0.305, P < 0.001), MAP (ρ = −0.106, P = 0.003), SBP (ρ = −0.092, P = 0.009) and DBP (ρ = −0.07, P = 0.048) [Figure 2]. Since MAP would have strong inter correlations with both SBP and DBP, only HR, SBP and DBP were entered into linear regression model for prediction of PI and only the fall in SBP was found to be independently predictive of PI with B = −0.025 (P = 0.01) and adjusted R2 = 0.012.
Figure 2.
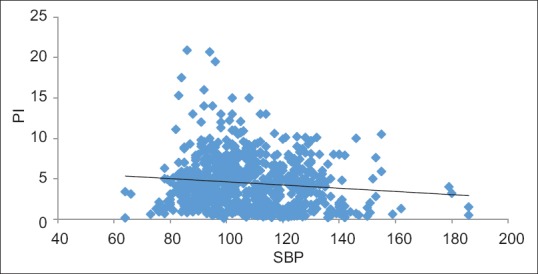
Scatter plot between perfusion index and systolic blood pressure
Within first 5-min post-induction, the incidence of hypotension by SBP criterion was 30%, and by MAP criterion, 42%. Severe hypotension necessitating mephentermine administration was observed in 22% patients. Similarly, within first 15 min, hypotension by SBP criterion was 38%, by MAP was 50% and severe hypotension was seen in 24% of patients.
PI did not show statistically significant correlation with incidence of hypotension (P = 0.283) or severe hypotension (P = 0.25) based on MAP criterion during first 5 min or first 15 min (hypotension, P = 0.514; severe hypotension, P = 0.412). However, hypotension based on SBP criterion showed a statistically significant correlation with PI, both during first 5 min (rpb= −0.503, P < 0.001) and 15 min (rpb= −0.296, P = 0.037).
In total, 12 (24%) patients had severe hypotension requiring a dose of mephentermine (6 mg). Furthermore, there was no incidence of bradycardia requiring atropine.
ROC curves were constructed for PI as a predictive test of hypotension at 5 min and 15 min. At 5 min, area under the ROC curve (AUC) was 0.816, 95% confidence interval (CI) (0.699–0.933), P < 0.001 [Figure 3a]. Baseline PI of 1.05 predicted any incidence of intraoperative hypotension at 5 min after propofol-based induction with sensitivity 93%, specificity 71%, positive predictive value (PPV) 68% and negative predictive value (NPV) 98%. At 15 min, the AUC was 0.676, 95% CI (0.517–0.834), P = 0.039 [Figure 3b]. Baseline PI of 1.25 predicted any incidence of intraoperative hypotension at 15 min with sensitivity 79%, specificity 48%, PPV 54% and NPV 86%.
Figure 3.
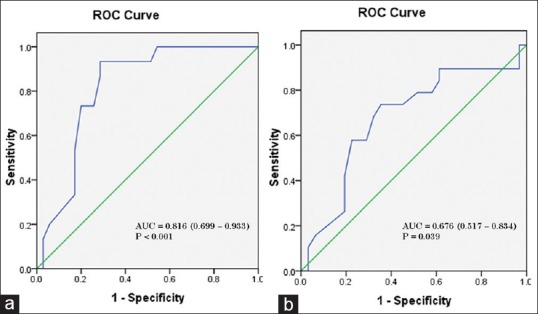
(a) Receiver operating characteristic curve of baseline perfusion index as predictive test of hypotension within first 5 min after propofol induction. (b) Receiver operating characteristic curve of baseline perfusion index as predictive test of hypotension within first 15 min after propofol induction. AUC – Area under curve, 95% confidence intervals in parenthesis
DISCUSSION
We hypothesised that baseline PI will be useful in predicting hypotension following propofol and looked for a cutoff value that predicted hypotension. Our observations support this notion as there was a correlation between PI and incidence of hypotension. PI <1.05 was associated with a higher incidence of hypotension. The overall association of PI and haemodynamic variables revealed a weak negative correlation with SBP, DBP, MAP and HR. However, SBP was the only independent predictive variable with correlation coefficient of rpb= −0.503 (P < 0.001). This presumably reflects the genesis of PI variable as the percentage of pulsatile component (as an indicator of SBP) of the plethysmographic waveform. The weak linear correlation can be explained by the fact that the relationship between PI and SBP is not linear [Figure 2].[1] Scatter plot of baseline PI shows a bell-shaped curve with very high and very low SB P values being associated with low PI. Although curve estimation revealed linear model can be adequately fitted for this relationship (F = 9.91, P = 0.002), caution should be used before determining a causality. Low PI with low SBP is easily explainable as loss of pulsatility. It is seen with hypovolaemia and use of vasopressors.[8] However, high SBP with low PI may be due to concomitant increases in the non-pulsatile component.
Baseline PI was not found to be associated with hypotension by absolute MAP criterion but showed a weak to moderate significant negative correlation with SBP criterion. Defining hypotension as a function of baseline SBP is probably more physiological in this context as PI is a derivative of vascular contractile state, whereas absolute MAP does not consider only pre-operative vasomotor tone of the patient. The negative association has been echoed by other authors who found low baseline PI to be associated with greater reductions in MAP after propofol-based induction.[7] Compensatory vasoconstriction due to relative hypovolemia may result in low PI values and propofol, by virtue of vasodilatation can cause hypotension in such patients. The difference of magnitude of correlation between first 5 and 15 min is probably due to the haemodynamic effect of endotracheal intubation causing BP to normalise, thus reducing the incidence of hypotension.
These findings were confirmed by the ROC analysis, showing baseline PI cut-off of 1.05 to be highly predictive of SBP-based hypotension following propofol (5 min) with AUC 0.816 (95% CI, 0.699–0.933, P < 0.001) and sensitivity 93%, specificity 71%, PPV 68% and NPV 98%. After intubation, the predictive power reduced, with PI of 1.25 predicting hypotension with sensitivity 79%, specificity 48%, PPV 54%, NPV 86% and AUC 0.676 (95% CI, 0.517–0.834, P = 0.039). The high NPV would potentially help in clinical scenarios to rule out hypotension occurrence 5 minutes after induction of anaesthesia with propofol.
Baseline PI >3.5 in pregnant patients was predictive of hypotension following spinal anaesthesia.[5] This contradiction to our findings can be explained by the relationship between PI and vascular sympathetic tone. The high PI was the result of an overall low sympathetic tone, predisposing to post-spinal hypotension, whereas in our study, the lower PI was probably indicative of hypovolemia and compensatory vasoconstriction. They reported reduction in PI following spinal anaesthesia during periods of hypotension which was explained as compensatory vasoconstriction in non-anaesthetised dermatomes.[5] Observations similar to our study were reported with baseline PI ≤0.82 predicting hypotension in response to progressive fluid withdrawal by continuous venovenous hemofiltration in critically ill patients with acute kidney injury.[9] However, when circulatory volume was decreased steadily in healthy volunteers, a gradual reduction in PI from 2.2 to 1.2 was observed without change in BP. Also, hypotension occurred after PI stabilised around 1.2 and authors suggested a median value of 1.4 as normality for healthy individuals.[1] ROC analysis in current work predicted hypotension below a threshold value of 1.05 which is close to the values obtained by above work.[1] However, the cut-off value is higher than that found predicting hypotension during fluid withdrawal by continuous veno-venous haemofiltration in critically ill patients. Those patients had acute illness in the form of acute kidney injury.[9] Acutely ill patients are often on multiple inotropes (vasopressors) resulting in significant vasoconstriction of systemic and peripheral vascular beds, as indicated by low PI.
The mechanism of propofol-induced hypotension involves action on autonomic nervous system with effect on the sympathetic as well as parasympathetic components.[10] There is also evidence for the direct effect of propofol through endothelium-dependent and endothelium-independent pathways.[11] Both culminate in vasodilatation. If the patient has significant peripheral vasoconstriction that is, low PI, they are more likely to have hypotension. Similarly, if the patient is already having low vascular tone (vasodilated and relatively compensated blood volume), indicated by higher PI, there is less possibility of hypotension as demonstrated with current work.
Although the PPV was lower for first 5 min (68%), the NPV was higher (98%). Thus, PI is clinically very useful in predicting likelihood of no hypotension in a given set of patients. Use of propofol among patients with high PI is likely to cause less haemodynamic disturbances than those with PI < 1.05. However, if whole duration of induction and intubation is considered, predictability/non-predictability of hypotension by PI was less accurate though the incidence of hypotension was higher during this period (50% vs. 42% respectively by MAP criterion).
A multicentre study on haemodynamic effects of propofol in 25000 patients reported the occurrence of hypotension well beyond 10-min post-induction.[12] More than 20% of hypotensive episodes occurred beyond 10-min post-induction. Our findings are also in agreement with this, as the observation period was 15-min post-induction, emphasising the need for continued vigilance well into maintenance period. Nevertheless, evidence supports association between post-induction hypotension and higher incidence of post-operative mortality and morbidity.[13] Further, occurrence of post-operative acute kidney injury following transient intraoperative hypotension with MAP <55 mmHg has been confirmed.[14] Hence, predicting, preventing and effectively treating any haemodynamic instability especially hypotension is very vital in ensuring best patient outcome after surgery. PI greater than 1.05 suggests that propofol induction is unlikely to result in hypotension.
Therefore, it may be safely stated that PI depends on SBP, and their association is bimodal (low PI was present with both high and low SBP). Also, PI can predict hypotension better based on baseline SBP rather than MAP. Finally, If PI is >1.05 at baseline, 98% patients will not experience significant hypotension (reduction in SBP <30%) within 5 minutes after induction of anaesthesia with propofol.
There is a need to verify the cut-off value of PI for predictability by studying adequate number of patients in general as well as subcategory of patients such as specific age group, obese, hypertensive patients, etc. Further, comparison between PI and PVI could have indicated superiority of one index over the other. Available literature does not mention effect of age on PI. Hence, cut-off value inferred in the current work may not be universally applicable, considering the possibility of increasing vascular tone with advancing age, which may be one of the risk factors for propofol-induced hypotension in the elderly. Future research may also focus on incorporating advanced warning systems among clinical monitors in preventing hypotension at the time of anaesthesia.
The current study was adequately powered to observe the differences noted and further studies with larger sample size are probably not warranted. We are able to explain the relationship between changes in blood pressure and baseline PI adequately with available information pertaining to PI and mechanism of hypotension following propofol injection. As the dosing of the drug was based on titration, the amount of drug used was precise as demanded by each patient, making the results more generalisable. A fixed-dose would have suffered the bias due to age and other factors affecting dose requirement of the induction agent. We treated severe hypotension promptly (MAP <55 mmHg) as it has been reported that such hypotension, even if it was transient can cause significant organ dysfunction.[14] A MAP of 70 mmHg and above is considered physiological for perfusion of tissues. Earlier, a MAP of <60 mmHg was taken as cut-off for hypotension.[12] Similarly, hypotension was defined as MAP <60 mmHg and treated when it was less than 55 mmHg by others as well.[15] A MAP below 55 mmHg is known to produce deleterious outcomes even if it lasts for a short while.[14] Therefore, we adopted same methodology for our study.
This study is limited by lack of direct evidence for the explanations proposed, and they are based on hypotheses. The total dose of propofol was not recorded, which would have given idea regarding total amount of drug required for individual. A more rigorous study with cardiac output monitoring, invasive BP monitoring and dynamic indices of hypovolaemia would be required to confirm or refute the explanations and further refine knowledge in this regard.
CONCLUSION
Perfusion index could predict hypotension following propofol induction. However, it has a very high negative predictive value in predicting hypotension following induction of anaesthesia with propofol, especially before endotracheal intubation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We acknowledge the assistance rendered by Dr Dhritiman Chakrabarti, Assistant Professor, Department of Neuroanaesthesia and Critical Care, NIMHANS, Bengaluru, Karnataka in carrying out statistical analysis and interpretation.
REFERENCES
- 1.van Genderen ME, Bartels SA, Lima A, Bezemer R, Ince C, Bakker J, et al. Peripheral perfusion index as an early predictor for central hypovolemia in awake healthy volunteers. Anesth Analg. 2013;116:351–6. doi: 10.1213/ANE.0b013e318274e151. [DOI] [PubMed] [Google Scholar]
- 2.Krishnamohan A, Siriwardana V, Skowno JJ. Using a pulse oximeter to determine clinical depth of anesthesia-investigation of the utility of the perfusion index. Paediatr Anaesth. 2016;26:1106–11. doi: 10.1111/pan.13000. [DOI] [PubMed] [Google Scholar]
- 3.De Felice C, Latini G, Vacca P, Kopotic RJ. The pulse oximeter perfusion index as a predictor for high illness severity in neonates. Eur J Pediatr. 2002;161:561–2. doi: 10.1007/s00431-002-1042-5. [DOI] [PubMed] [Google Scholar]
- 4.Huang HS, Chu CL, Tsai CT, Wu CK, Lai LP, Yeh HM, et al. Perfusion index derived from a pulse oximeter can detect changes in peripheral microcirculation during uretero-renal-scopy stone manipulation (URS-SM) PLoS One. 2014;9:e115743. doi: 10.1371/journal.pone.0115743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyama S, Kakumoto M, Morioka M, Matsuoka K, Omatsu H, Tagaito Y, et al. Perfusion index derived from a pulse oximeter can predict the incidence of hypotension during spinal anaesthesia for caesarean delivery. Br J Anaesth. 2013;111:235–41. doi: 10.1093/bja/aet058. [DOI] [PubMed] [Google Scholar]
- 6.Duggappa DR, Lokesh M, Dixit A, Paul R, Raghavendra Rao R S, Prabha P. Perfusion index as a predictor of hypotension following spinal anaesthesia in lower segment caesarean section. Indian J Anaesth. 2017;61:649–54. doi: 10.4103/ija.IJA_429_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuchiya M, Yamada T, Asada A. Pleth variability index predicts hypotension during anesthesia induction. Acta Anaesthesiol Scand. 2010;54:596–602. doi: 10.1111/j.1399-6576.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Dong J, Xu Z, Shen H, Zheng J. The pleth variability index as an indicator of the central extracellular fluid volume in mechanically ventilated patients after anesthesia induction: Comparison with initial distribution volume of glucose. Med Sci Monit. 2014;20:386–92. doi: 10.12659/MSM.890073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klijn E, Groeneveld AB, van Genderen ME, Betjes M, Bakker J, van Bommel J, et al. Peripheral perfusion index predicts hypotension during fluid withdrawal by continuous veno-venous hemofiltration in critically ill patients. Blood Purif. 2015;40:92–8. doi: 10.1159/000381939. [DOI] [PubMed] [Google Scholar]
- 10.Kanaya N, Hirata N, Kurosawa S, Nakayama M, Namiki A. Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology. 2003;98:34–40. doi: 10.1097/00000542-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kassam SI, Lu C, Buckley N, Lee RM. The mechanisms of propofol-induced vascular relaxation and modulation by perivascular adipose tissue and endothelium. Anesth Analg. 2011;112:1339–45. doi: 10.1213/ANE.0b013e318215e094. [DOI] [PubMed] [Google Scholar]
- 12.Hug CC, Jr, McLeskey CH, Nahrwold ML, Roizen MF, Stanley TH, Thisted RA, et al. Hemodynamic effects of propofol: Data from over 25,000 patients. Anesth Analg. 1993;77:S21–9. [PubMed] [Google Scholar]
- 13.Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–8. doi: 10.1213/01.ANE.0000175214.38450.91. [DOI] [PubMed] [Google Scholar]
- 14.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–23. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 15.Möller Petrun A, Kamenik M. Bispectral index-guided induction of general anaesthesia in patients undergoing major abdominal surgery using propofol or etomidate: a double-blind, randomized, clinical trial. Br J Anaesth. 2013 Mar;110(3):388–96. doi: 10.1093/bja/aes416. [DOI] [PubMed] [Google Scholar]


