Abstract
Background and Aims:
Hyponatraemia is frequent in post-operative patients and may be corrected with hypertonic saline (HTS). Oral tolvaptan is used to treat hypervolaemic or euvolaemic hyponatraemia. This study was performed to assess the efficacy of oral tolvaptan in correcting postoperative hyponatraemia compared to HTS.
Methods:
This prospective, randomised study was conducted in 40 symptomatic patients with serum sodium level ≤130 mEq/L. In Group H (n = 20), 3% HTS was infused at 20–30 mL/h aiming for correction of 6 mEq/L/day. Group T received oral tolvaptan 15 mg on the 1st day. If daily correction was <4 mEq/L, the dose was increased by 15 mg/day to a maximum of 45 mg. The primary outcome was serum sodium concentration 48 hours after starting treatment. Paired t-test was used to compare changes in sodium levels.
Results:
Baseline sodium and values at 12, 24 and 48 h were comparable in both groups. At 72 h, Group T had significantly higher sodium levels as compared to Group H (133.4 ± 1.9 vs. 131.3 ± 2.4 mEq/L). Intragroup analysis had shown a significant increase in sodium levels from baseline values in both groups at 12, 24, 48 and 72 h. Group H had a significantly lower potassium level and lower negative fluid balance on day 3.
Conclusion:
Oral tolvaptan and 3% HTS were equally effective in correcting hyponatraemia at 48 hours, but serum sodium levels were higher at 72 hours after oral tolvaptan.
Key words: Hyponatraemia, Inappropriate ADH Syndrome, tolvaptan, water–electrolyte balance
INTRODUCTION
Electrolyte abnormalities are frequent in post-operative patients and hyponatraemia is one of the most common. It is usually due to elevated levels of antidiuretic hormone (ADH) secondary to a number of non-osmotic stimuli such as subclinical volume depletion, pain and stress, as well as the administration of hypotonic fluids. ADH levels are universally elevated postoperatively when compared with pre-operative values.[1] The resultant hyponatraemia, secondary to volume expansion, is associated with poor patient outcome and longer hospital stay resulting in higher hospital costs[2,3] and is traditionally treated with hypertonic saline (HTS).[4] Tolvaptan, the first oral vasopressin V2 receptor antagonist approved by the Food and Drug Administration, directly combats elevated ADH levels and is used to treat hypervolaemic or euvolaemic hyponatraemia associated with congestive heart failure, hepatic cirrhosis or syndrome of inappropriate ADH secretion (SIADH).[5,6,7] We hypothesised that oral tolvaptan might be equally or more effective for the correction of post-operative hyponatraemia as compared to the use of HTS.
The primary objective of our study was to assess the efficacy of oral tolvaptan in correcting hyponatraemia in post-operative patients who had undergone major head and neck surgeries as compared to traditional management with intravenous administration of 3% HTS. The secondary objectives included assessment of fluid balance, changes in serum potassium levels and haemodynamics.
METHODS
This prospective, randomised study was conducted after approval from the institutional ethics committee and was conducted during June 2016 to July 2017. Post-operative patients aged 20–70 years, who had undergone major head and neck surgeries, who had a serum sodium level of ≤130 mEq/L and who were symptomatic (headache, nausea, vomiting, lethargy, confusion and disorientation) were included in the study. Patients with hypovolaemia and anuria and those receiving CYP3A inhibitors such as ketoconazole, fluconazole, clarithromycin or erythromycin were excluded from the study. Hypovolaemia was ruled out by the absence of signs of dehydration such as reduced skin turgor, dry mucosa, hypotension and elevated blood urea levels. Symptoms such as headache and nausea were initially managed with intravenous paracetamol and ondansetron, and if not relieved, with the background of hyponatraemia, they were considered for sodium correction. Consent to be included in the study was taken either from the patients or, if disoriented, from the relatives.
Patients were allocated into two Groups H and T based on computer-generated random sequence of numbers. In Group H (n = 20), 3% HTS (sodium chloride injection USP 3% W/V of Health Line Pharmaceuticals Pvt. Ltd.,) was started as an infusion at the rate of 20–30 mL/h; the dose was calculated using the following formula:[8]
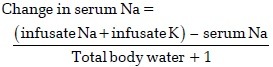
The rate of correction of serum sodium was aimed at 6 mEq/L in 24 h, 12 mEq/L in 48 h and 14–16 mEq/L in 72 h from the pre-intervention value, which was taken as the baseline. Group T patients (n = 20) received oral tolvaptan 15 mg (Shiokem™ of Alkem, HospiCare) on the 1st day. If the daily serum sodium correction was <4 mEq/L, dose of tolvaptan was increased by 15 mg on each subsequent day to a maximum of 45 mg on the 3rd day.[9] No diuretic was concurrently administered and there was no restriction of fluid intake.
Heart rate, blood pressure and urine output were recorded hourly. More than 20% reduction in mean arterial pressure was considered as hypotension and was treated with 100–200 mL intravenous fluid bolus of a balanced salt solution like Ringer' lactate. Serum sodium level was checked at the beginning of intervention and thereafter 12, 24, 48 and 72 h later. The daily fluid balance and serum potassium levels were recorded at 24, 48 and 72 h following initiation of the treatment. Pre-operative use of diuretics, if any, and the sodium levels were documented. Development of thrombophlebitis, total dose of tolvaptan used and volume of HTS used in 72 h were also noted. Daily intake of 100 mL/h was maintained either orally or through Ryle's tube, and no additional salt supplementation was given. The primary objective of our study was to determine the efficacy of oral tolvaptan versus 3% HTS for correcting post-operative hyponatraemia as assessed 48h after initiation of therapy. The secondary outcome variables were daily fluid balance, changes in serum potassium levels and haemodynamics.
Based on a previous study by Vilapurathu and Rajarajan,[10] considering the change in serum sodium in patients receiving 3% HTS versus oral tolvaptan (134.1 ± 0.5 vs. 133.4 ± 0.3) at 48 h, with a confidence interval of 95% and power of 90%, the minimum sample size required to obtain statistically significant results was calculated as 16 (8 in each group). However, we were able to recruit 40 patients during our study period.
All normally distributed continuous variables were presented as mean with standard deviation and categorical variables as proportion. Chi-square test was used to compare the distribution of gender, American Society of Anesthesiologists (ASA) physical status and thrombophlebitis. Independent sample t-test was used to compare the continuous variables among Group H and Group T. Paired t-test was used to compare the change of sodium at different time points in each group. Statistical analyses were conducted using SPSS Version 20.0 for Windows (IBM Corporation ARMONK, NY, USA).
RESULTS
Demographics, distribution of sex and ASA physical status of the forty patients enrolled [Figure 1] were comparable between groups [Table 1]. The mean baseline sodium levels in both groups (128.4 ± 2.6 vs. 128.6 ± 2.4) as well as the values at 12, 24 and 48 h were comparable. However, at 72 h, Group H had significantly lower sodium level as compared to Group T [131.3 ± 2.4 vs. 133.4 ± 1.9, P = 0.005; Table 2 and Figure 2]. Intragroup analysis had shown a significant increase in sodium levels from baseline values in both groups at 12, 24, 48 and 72 h [P < 0.001, Table 2].
Figure 1.
CONSORT flow diagram
Table 1.
Comparison of demographics, sex and American Society of Anesthesiologists physical status
Table 2.
Changes in serum sodium and potassium levels between groups
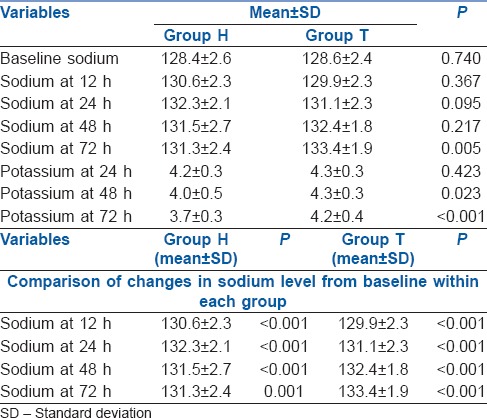
Figure 2.
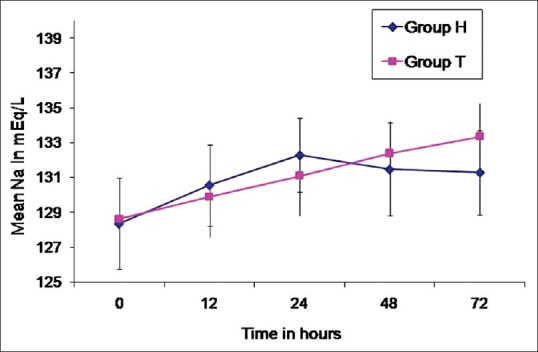
Changes in sodium level
Fluid balance was comparable in both the groups on day 1 and 2, but Group T had a significantly higher negative fluid balance on day 3 [Table 3]. Serum potassium levels also showed a similar trend with comparable values on day 1 and 2 with a significantly lower level on day 3 in Group H as compared to Group T [Table 2]. Fifty percent of patients in Group H developed thrombophlebitis. The average HTS consumption in Group H on day 1, 2 and 3 was 430, 227.5 and 137.5 mL, respectively. No patient in either group developed hypotension [Figure 3]. Pre-operative sodium levels were comparable in both groups (137.56 ± 4.29 vs. 135.86 ± 4.09, P = 0.155). The average time of onset of symptomatic hyponatraemia was 6th post-operative day. No patient in either group had received diuretics preoperatively.
Table 3.
Fluid balance in 72 h
Figure 3.
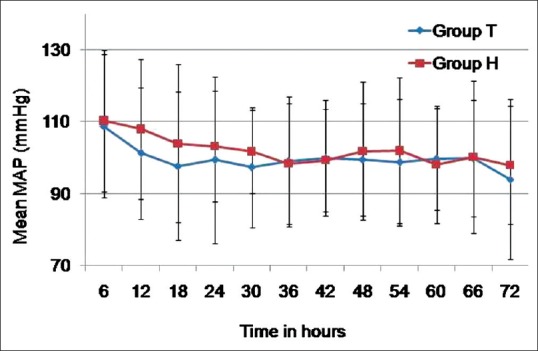
Changes in mean arterial pressure
DISCUSSION
In the present study, we observed a significant increase in serum sodium levels from 12 h till 72 h in both groups. The rise was comparable in both groups till 48 h, but at 72 h, Group T had a significant increase as compared to Group H. Fluid balance and serum potassium levels were comparable in both groups on day 1 and 2, but Group H had a significantly low potassium level and low negative fluid balance on day 3. No patient developed hypotension requiring intravenous fluid bolus during the study period.
Hyponatraemia is defined as serum sodium level <135 mEq/L. It usually implies a state of hypotonicity with a relative excess of body water compared to serum sodium level. It is common in post-operative patients who are elderly, and the outcome could be catastrophic if early warnings of hyponatraemia are not recognised on time and not managed effectively.[11,12] Postoperatively, it has been documented that the average length of hospital stay of hyponatraemic patients is between 1.44 and 9.2 days longer than normal patients with an increased mortality rate of 2.1%–28.1%.[13] As per our institutional protocol, during major surgeries, electrolytes are monitored at least every 4 h with arterial blood gas analysis. Daily electrolyte monitoring is mandatory in post-operative Intensive Care Units and is performed more frequently if there is dyselectrolytaemia or if patients present with symptoms suggestive of hyponatraemia.
The traditional management of post-operative hyponatraemia is fluid restriction and intravenous administration of HTS.[12] HTS is considered as a safe alternative to mannitol, especially for a long-term use or when multiple doses are needed in post-operative patients.[14] Acute-onset hyponatraemia (duration of <48 h) requires prompt correction, whereas chronic hyponatraemia should be corrected cautiously as there is a higher risk of development of central pontine myelinolysis following excessive and rapid correction.[15] Hence, it is recommended to limit daily correction to not more than 6 mEq/L, even in patients with extremely low serum sodium.[16,17] Even though the daily sodium correction in our study was approximately 3–4 mEq, patients were symptomatically better, probably because none of them had symptoms of severe hyponatraemia.
Vaptans such as conivaptan and tolvaptan are potent vasopressin receptor antagonists which are useful in the treatment of hypervolaemic or euvolaemic hyponatraemia associated with congestive heart failure, hepatic cirrhosis or SIADH.[18,19,20,21] Tolvaptan is a selective vasopressin V2 receptor antagonist that blocks arginine vasopressin (AVP) binding in the distal portions of the nephron and negates AVP's antidiuretic activity leading to electrolyte-free water diuresis (aquaresis) without significant electrolyte abnormalities. The recommended starting dose of oral tolvaptan is 15 mg/day, which may be increased at daily intervals to 30 mg/day, and to a maximum of 60 mg/day.[9] On initiation of therapy, fluid restriction should be avoided during the first 24 h, and patients should be advised to continue fluid ingestion in response to thirst. The most common adverse reactions are thirst, dry mouth, lethargy, constipation, polyuria and hyperglycaemia.[22]
Tolvaptan is mainly used for the treatment of hyponatraemia and autosomal dominant polycystic kidney disease.[23] Tolvaptan treatment in SIADH patients had shown a rapid normalisation of serum sodium levels, effectively reducing inpatient length of hospital stay. It has been shown that a section of hyponatraemic patients are discharged from hospital without fully correcting hyponatraemia. In this subset of patients, continuing the treatment with oral tolvaptan on an outpatient basis could be beneficial.
The efficacy of 3% HTS for the correction of hyponatraemia is well documented.[10] It had shown a slightly superior efficacy in raising the serum sodium concentration at both 24-h and 48-h periods in hyponatraemic patients as compared with oral tolvaptan 15–30 mg daily.[10] At the same time, bolus dose of conivaptan 20 mg alone as well as that followed by an infusion of 40 mg over 24 h for the next 72 h was found to be superior than HTS in this respect.[21,24]
Although less expensive, use of HTS carries a risk of volume overload in oliguric or anuric patients in addition to central pontine myelinolysis following rapid overcorrection. The 2014 European Society of Endocrinology guidelines for the correction of severe symptomatic hyponatraemia recommends infusion of 150 mL of 3% HTS over 20 min with monitoring at regular intervals till improvement of symptoms or till maximum of 5 mmol/L increase in serum sodium is achieved.[25] Nevertheless, in our institute, due to concern over rapid correction and its associated morbidity, a slower infusion rate has been used as a protocol. However, this has to be given as an infusion, and the duration of drug administration may extend over many hours or even days depending on the condition of the patient. While giving an intravenous infusion, to avoid the risk of an accidental administration of a bolus dose, it is always safer to administer the drug using an infusion pump. The advantages of using oral tolvaptan over HTS are the ease of administration and avoidance of an infusion which requires careful monitoring by nursing staff or use of an infusion pump, which adds to the cost of treatment, for safe administration.
The major differences between the study by Vilapurathu and Rajarajan[10] and ours were that in their study, the daily dose of tolvaptan used varied between 15 and 30 mg and changes in sodium levels were monitored for 48 h only. We used escalating doses of tolvaptan starting with 15 mg. As daily correction was <6 mEq/L in all patients, dose was increased by 15 mg on a daily basis till 3rd day. This could have been the reason for the significant increase in sodium observed in our study in the tolvaptan group persisting up to 72 h.
The major drawbacks of our study were that it was an open-label study and measurement of daily body weight of the patients was not possible as many of them were bedridden. Another shortcoming was that the urine osmolality was not analysed. In patients with hyponatraemia presenting with concentrated urine, with the exception of hypovolemic hyponatraemia, vaptans are the primary agents of choice which result in production of dilute urine. However, in hyponatraemic patients presenting with dilute urine or those who develop urinary dilution after saline infusion, desmopressin should be administered as an infusion which leads to urinary concentration.[26] Hence, not taking urine osmolality into consideration during the study might have influenced our choice of treatment and thereby the results also.
CONCLUSION
Both oral tolvaptan and 3% HTS were effective in correcting hyponatraemia after 48 hours of treatment in post-operative patients. Serum sodium levels were significantly higher at 72 h after initiation of oral tolvaptan.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Moritz ML, Ayus JC. The pathophysiology and treatment of hyponatraemic encephalopathy: An update. Nephrol Dial Transplant. 2003;18:2486–91. doi: 10.1093/ndt/gfg394. [DOI] [PubMed] [Google Scholar]
- 2.Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170:294–302. doi: 10.1001/archinternmed.2009.513. [DOI] [PubMed] [Google Scholar]
- 3.Shea AM, Hammill BG, Curtis LH, Szczech LA, Schulman KA. Medical costs of abnormal serum sodium levels. J Am Soc Nephrol. 2008;19:764–70. doi: 10.1681/ASN.2007070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vivo P, Del Gaudio A, Ciritella P, Puopolo M, Chiarotti F, Mastronardi E, et al. Hypertonic saline solution: A safe alternative to mannitol 18% in neurosurgery. Minerva Anestesiol. 2001;67:603–11. [PubMed] [Google Scholar]
- 5.Dixon MB, Lien YH. Tolvaptan and its potential in the treatment of hyponatremia. Ther Clin Risk Manag. 2008;4:1149–55. doi: 10.2147/tcrm.s3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reilly T, Chavez B. Tolvaptan (Samsca) for hyponatremia: Is it worth its salt? P T. 2009;34:543–7. [Google Scholar]
- 7.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 8.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–9. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 9.Berl T, Quittnat-Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21:705–12. doi: 10.1681/ASN.2009080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilapurathu JK, Rajarajan S. A prospective study to compare the clinical efficacy of tolvaptan with 3% hypertonic saline solution in hospitalized patients having hyponatremia. J Res Pharm Pract. 2014;3:34–6. doi: 10.4103/2279-042X.132710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton J. Detection of hyponatremia in the PACU. J Perianesth Nurs. 2003;18:392–7. doi: 10.1016/j.jopan.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Mujtaba B, Sarmast AH, Shah NF, Showkat HI, Gupta RP. Hyponatremia in postoperative patients. Gen Med (Los Angel) 2016;4:224. [Google Scholar]
- 13.García Segura A, Gadea Ruiz C, Oliva Fanlo B, Ruiz Rodríguez R, Antón Botella F, Pinilla Moraza J, et al. Hyponatremia upon admission in patients over 65 years of age. Relation with medium length of stay and hospital mortality. An Med Interna. 1994;11:487–9. [PubMed] [Google Scholar]
- 14.Thongrong C, Kong N, Govindarajan B, Allen D, Mendel E, Bergese SD, et al. Current purpose and practice of hypertonic saline in neurosurgery: A review of the literature. World Neurosurg. 2014;82:1307–18. doi: 10.1016/j.wneu.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 15.Biswas M, Davies JS. Hyponatraemia in clinical practice. Postgrad Med J. 2007;83:373–8. doi: 10.1136/pgmj.2006.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterns R. Treatment of Hyponatremia. In: Simon E, editor. Hyponatremia. New York, NY: Springer; 2013. pp. 221–250. [Google Scholar]
- 17.Sterns RH, Hix JK, Silver SM. Management of hyponatremia in the ICU. Chest. 2013;144:672–9. doi: 10.1378/chest.12-2600. [DOI] [PubMed] [Google Scholar]
- 18.Jahangiri A, Wagner J, Tran MT, Miller LM, Tom MW, Kunwar S, et al. Factors predicting postoperative hyponatremia and efficacy of hyponatremia management strategies after more than 1000 pituitary operations. J Neurosurg. 2013;119:1478–83. doi: 10.3171/2013.7.JNS13273. [DOI] [PubMed] [Google Scholar]
- 19.Vinod P, Krishnappa V, Chauvin AM, Khare A, Raina R. Cardiorenal syndrome: Role of arginine vasopressin and vaptans in heart failure. Cardiol Res. 2017;8:87–95. doi: 10.14740/cr553w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aditya S, Rattan A. Vaptans: A new option in the management of hyponatremia. Int J Appl Basic Med Res. 2012;2:77–83. doi: 10.4103/2229-516X.106347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajan S, Srikumar S, Paul J, Kumar L. Effectiveness of single dose conivaptan for correction of hyponatraemia in post-operative patients following major head and neck surgeries. Indian J Anaesth. 2015;59:416–20. doi: 10.4103/0019-5049.160943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zmily HD, Daifallah S, Ghali JK. Tolvaptan, hyponatremia, and heart failure. Int J Nephrol Renovasc Dis. 2011;4:57–71. doi: 10.2147/IJNRD.S7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horie S. Will introduction of tolvaptan change clinical practice in autosomal dominant polycystic kidney disease? Kidney Int. 2015;88:14–6. doi: 10.1038/ki.2015.143. [DOI] [PubMed] [Google Scholar]
- 24.Reddy SN, Rangappa P, Jacob I, Janakiraman R, Rao K. Efficacy of conivaptan and hypertonic (3%) saline in treating hyponatremia due to syndrome of inappropriate antidiuretic hormone in a tertiary Intensive Care Unit. Indian J Crit Care Med. 2016;20:714–8. doi: 10.4103/0972-5229.195708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014;170:G1–47. doi: 10.1530/EJE-13-1020. [DOI] [PubMed] [Google Scholar]
- 26.Tzamaloukas AH, Shapiro JI, Raj DS, Murata GH, Glew RH, Malhotra D, et al. Management of severe hyponatremia: Infusion of hypertonic saline and desmopressin or infusion of vasopressin inhibitors? Am J Med Sci. 2014;348:432–9. doi: 10.1097/MAJ.0000000000000331. [DOI] [PMC free article] [PubMed] [Google Scholar]


