Abstract
Background and Aims:
Infections in tropics often present as undifferentiated fevers with organ failures. We conducted this nationwide study to identify the prevalence, profile, resource utilization, and outcome of tropical fevers in Indian Intensive Care Units (ICUs).
Materials and Methods:
This was a multicenter prospective observational study done in 34 ICUs across India (July 2013–September 2014). Critically ill adults and children with nonlocalizing fever >48 h and onset < 14 days with any of the following: thrombocytopenia/rash, respiratory distress, renal failure, encephalopathy, jaundice, or multiorgan failure were enrolled consecutively.
Results:
Of 456 cases enrolled, 173 were children <12 years. More than half of the participants (58.7%) presented in postmonsoon months (August–October). Thrombocytopenia/rash was the most common presentation (60%) followed by respiratory distress (46%), encephalopathy (28.5%), renal failure (23.5%), jaundice (20%), and multiorgan failure (19%). An etiology could be established in 365 (80.5%) cases. Dengue (n = 105.23%) was the most common followed by scrub typhus (n = 83.18%), encephalitis/meningitis (n = 44.9.6%), malaria (n = 37.8%), and bacterial sepsis (n = 32.7%). Nearly, half (35% invasive; 12% noninvasive) received mechanical ventilation, a quarter (23.4%) required vasoactive therapy in first 24 h and 9% received renal replacement therapy. Median (interquartile range) ICU and hospital length of stay were 4 (3–7) and 7 (5–11.3) days. At 28 days, 76.2% survived without disability, 4.4% had some disability, and 18.4% died. Mortality was higher (27% vs. 15%) in patients with undiagnosed etiology (P < 0.01). On multivariate analysis, multiorgan dysfunction syndrome at admission (odds ratio [95% confidence interval]-2.8 [1.8–6.6]), day 1 Sequential Organ Failure Assessment score (1.2 [1.0–1.3]), and the need for invasive ventilation (8.3 [3.4–20]) were the only independent predictors of unfavorable outcome.
Conclusions:
Dengue, scrub typhus, encephalitis, and malaria are the major tropical fevers in Indian ICUs. The data support a syndromic approach, point of care tests, and empiric antimicrobial therapy recommended by Indian Society of Critical Care Medicine in 2014.
Keywords: Dengue, encephalitis, India, Intensive Care Unit, malaria, scrub typhus, tropical infections
INTRODUCTION
Infectious illnesses affect all age groups, particularly plaguing those living in tropical regions. Febrile infections that are prevalent in or unique to tropical and subtropical regions are collectively known as tropical fevers.[1] They are caused by a number of viruses, bacteria, and protozoa and often get transmitted by an insect bite. Dengue, malaria, leptospirosis, influenza A, Typhoid, rickettsiosis including scrub typhus, Japanese encephalitis (JE), and chikungunya were some of the common tropical fevers reported in patients from Asian countries.[2,3,4,5,6,7] Considerable variability exists in the proportion of these fevers in hospitalized patients as they are greatly influenced by season and geography.[8] A significant number of these patients also have one or more organ failures requiring intensive care. The challenge lies in clinically diagnosing them at the time of presentation as they often present as undifferentiated fever and with overlapping signs and symptoms. Laboratory confirmation is usually done by serology, which may not be available or reliable in the first few days. Regardless, it is important to treat them early as delay in institution of specific therapy may lead to increased morbidity and mortality. Recent Indian Society of Critical Care Medicine (ISCCM) consensus guidelines recommended a syndromic approach to management, comprising point of care diagnostic tests, and initial empiric treatment to cover most common treatable infections.[1] However, evidence base to support these recommendations came from limited epidemiological data from few centers.[9,10,11] Available literature is also limited to individual infections such as dengue, malaria, or scrub typhus rather than the profile of undifferentiated febrile illnesses and the proportion of differential diagnoses. Data on disease trends will guide management and help allocate health-care resources appropriately. Hence, we conducted this nation-wide study to identify the prevalence, clinical profile and etiology, utilization of Intensive Care Unit (ICU) resource, and outcome of a patient with tropical fevers in Indian ICUs.
MATERIALS AND METHODS
This was a multi-center prospective observational study conducted in 34 ICUs across India coordinated through the ISCCM research network. The study period was between July 2013 and September 2014 taking care that all participating centers enroll subjects to represent each distinct season (January–winter; May–dry summer; July–monsoon; and September–post rainy) at least once. Consecutive patients who presented with fever of at least 48 h duration with onset in the previous 14 days and one or more of the following (i) rash/thrombocytopenia, (ii) respiratory distress, (iii) renal failure, (iv) encephalopathy, (v) jaundice, or (vi) multi-organ dysfunction syndrome (MODS) were enrolled and followed up till death or discharge from hospital. We excluded children younger than 3 months, patients who were hospitalized or underwent surgery in the preceding 2 weeks, patients previously diagnosed with a medical condition posing increased risk for recurrent fever or infections (such as HIV, primary immunodeficiency, malignancy, autoimmune disorders, congenital lung or cardiac disorders, immunosuppressive therapy, indwelling hardware [shunt/prosthesis/catheters]), or history of travel outside India in the 4 weeks before onset of illness.
Participant recruitment
The study was planned by ISCCM. All members were informed about the study through repeated E-mails and were invited to join. Approval was sought from Institute Ethics Committee. An exclusive password protected web-based program was developed for data collection. The individual investigators could access the program using their unique identity and enter data in case report form. During the study, participating sites identified all new patients admitted to the ICU and screened them for possible inclusion. ICU admissions were screened twice a day, and eligibility was determined by the most senior clinician caring for each patient. Enrollment occurred within 24 h of their ICU admission and all patients identified by the study criteria were assigned a study number and had a case report form completed. To determine inter-rater reliability of screening patients, a second investigator at each site was asked to independently screen the same list of patients for tropical fever. In recognition of a potential burden that could impede the ability for some sites to participate in this study, this process was optional for each site.
Data collection
Patient demographic details including age, gender, presenting symptoms and duration, associated comorbidities, and baseline organ dysfunction were recorded. Essential investigations including complete blood counts, electrolytes, renal function tests, liver function tests, and specific investigations for various etiologies such as thick and thin blood films and rapid diagnostic card test (RDT) for vivax and falciparum malaria, blood cultures, urine cultures, Typhidot, WIDAL, Weil Felix, IgM ELISA for scrub typhus, IgM for leptospirosis, NS1 antigen and IgM for dengue, IgM for herpes simplex virus (HSV) and JE virus, polymerase chain reaction (PCR) for HSV, JE and tuberculosis, cerebrospinal fluid (CSF) examination, latex agglutination for antigens, and other investigations that were performed according to the clinical indications by the treating physician were recorded. The assessment of cardiac function included electrocardiography, echocardiography, and cardiac enzymes.
Therapy-related variables such as drugs received (antimalarials, antibiotics, antivirals) and the duration of therapy, use of adjuvant therapies (osmolar agents, diuretics, insulin, corticosteroids, hyperimmune globulin, etc.), dose and duration of vasoactive agents infused, blood products transfusions, use of plasma exchange, and renal replacement therapy were recorded. In all patients who are mechanically ventilated, recording of ventilation related variables were carried out. Sequential Organ Failure Assessment (SOFA) score as a composite measure of organ dysfunction was calculated to determine the number of organ systems failing.[12] MODS was defined as ≥2 organ system failures.[13,14] Duration of mechanical ventilation, ICU, and hospital length of stay in days, outcome at discharge or at 28 days from study date were recorded.
Outcome measurement
The primary outcome was the prevalence of overall and specific tropical infections in critically ill patients. This was calculated from number of patients identified with laboratory confirmed specific infections. Confirmation of diagnosis was based on standard tests, namely, demonstration of IgM antibodies or NS1 antigen by ELISA for dengue, positive blood smears for malaria, detection of IgM by ELISA or PCR for scrub typhus, positive blood culture for typhoid, and Microscopic Agglutination Test or IgM ELISA for leptospirosis. Patients were categorized as meningitis/encephalitis based on CSF and neuroimaging findings. Gram stain, latex agglutination test, bacterial culture, and demonstration of IgM antibodies or nucleic acid by PCR in CSF for specific viruses determined the etiology. Patients were grouped into common clinical syndromes and most frequent etiologies for each syndrome were determined. Predictors of specific infections, proportion of patients treated with appropriate antimicrobials, proportions requiring mechanical ventilation, vasoactive agents and renal replacement therapy, length of ICU stay, and inhospital mortality were secondary outcomes. At 28 days, death, discontinuation of care and survival with disability were considered as unfavorable outcomes.
Statistical analysis
Patient demographic characteristics, therapies, and outcomes were summarized by standard descriptive statistics (e.g., means and standard deviations for continuous variables that are normally distributed, medians with interquartile ranges (IQR) for nonnormally distributed continuous variables, percentages for categorical variables). The primary analysis included all patients meeting the inclusion criteria and who were identified with tropical fever. The prevalence of individual tropical infections was calculated from the total number of patients identified with tropical fever. Treatment and outcome data were summarized as frequencies (percentages) for categorical data and means (± standard deviation) or medians (IQR) for continuous data with normal and nonnormal distributions, respectively. One-way ANOVA and Kruskal–Wallis test were used for comparing more than two groups. Bivariate logistic regression analysis was done to assess for predictors of unfavorable outcome. Those identified as significant in this analysis were incorporated into a multivariate logistic regression analysis to determine independent predictors of unfavorable outcome.
RESULTS
A total of 34 ICUs from different parts of India participated in the study [Figure 1]. Majority were medical ICUs (n = 27, 80%) and mixed medical surgical ICUs (n = 3, 9%) catering to adult patients. Four (11%) were exclusive Pediatric ICUs. Over the study period of 15 months from July 2013 to September 2014, a total of 456 patients with tropical fevers were enrolled. There was a clear seasonal trend as tropical fevers predominated in the postmonsoon months (August, September, and October), contributing to more than half (n = 268, 58.7%) of the total enrolled patients [Figure 2]. Median (IQR) age of the study population was 22 (7–42.8) years. Children younger than 12 years constituted 38% (173 patients) of the study participants. Males (295 out of 456) outnumbered females with a ratio of 1.8:1 which was maintained in pediatric (1.77:1) and adult (1.87:1) subgroups. Majority (n = 278, 61%) of the study population belonged to rural area and 35% were from urban background. Mixed residencies and migrant workers contributed to 4% of the study population. Most of the study patients admitted to ICUs were either transferred from Emergency Department (n = 218, 48%) or directly from other hospital ICUs and wards (n = 179, 41%). Requirement of respiratory support (n = 304, 66%) was the single most common indication for ICU transfer; half of them (n = 160) were transferred for invasive mechanical ventilation. A small proportion (6%, n = 27) had circulatory instability and required ICU admission for hemodynamic monitoring and/or continuous vasoactive drug therapy. In 41% (n = 187) of patients, the presence of more than one organ failures (including renal, central nervous system [CNS]) necessitated ICU transfer. Fever was noted in all; vomiting (48%), cough and/or rapid breathing (47%), headache (34%), and myalgia and backache (32%) were the other common symptoms. Preexisting renal disease (13%) and diabetes (10%) were present in some [Table 1].
Figure 1.
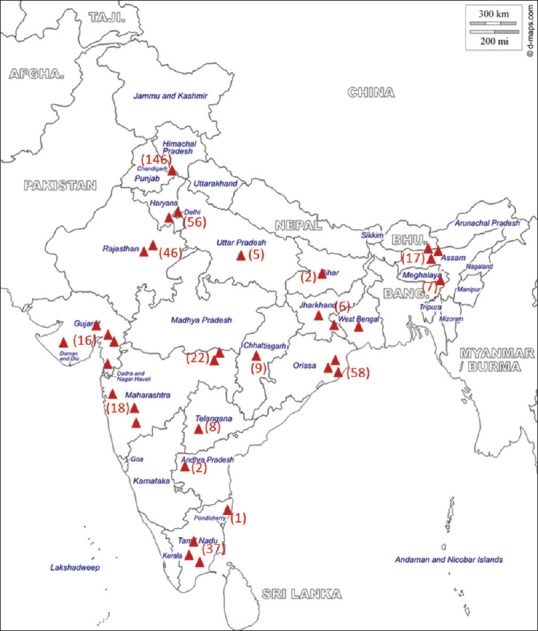
Map of India showing the distribution of study sites and participants.  Denotes individual recruitment center. The number of participants from each site/region is given in parenthesis
Denotes individual recruitment center. The number of participants from each site/region is given in parenthesis
Figure 2.
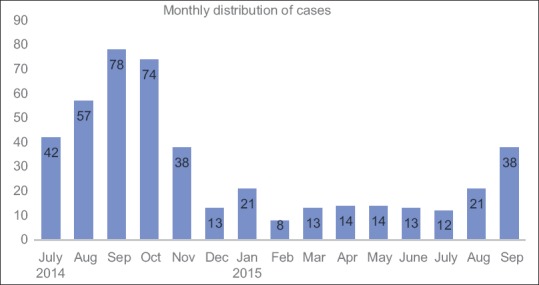
Monthly recruitment of cases depicting a seasonal trend
Table 1.
Presenting symptoms and syndromes
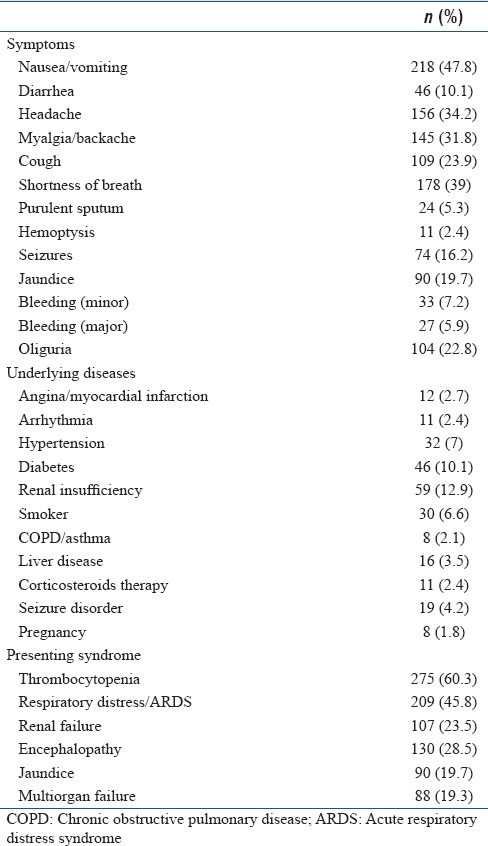
Presenting clinical syndrome
Thrombocytopenia and/or rash was the most common clinical syndrome (n = 275, 60%) observed among the enrolled subjects. Respiratory distress and/or acute respiratory distress syndrome (ARDS) was present in 209 (46%) study participants. Nearly a third (29%, n = 130) presented with encephalopathy and 88 patients (19%) had multiorgan failure at the time of enrollment [Table 1]. Etiology could be established in 367 (80.5%) patients. Dengue was the most common tropical fever diagnosed among study participants, contributing to 23% (n = 105) cases followed by scrub typhus (n = 83, 18%). Meningitis/Encephalitis syndrome accounted for 10% of cases but emerged as the most common tropical fevers group among pediatric patients (n = 37, 21%). Malaria, Leptospirosis, and bacterial sepsis were the other common diagnoses. Mixed infections (dengue and malaria) were seen in 3 patients. Nearly 20% (n = 89) of patients did not have a specific diagnosis at the time of discharge or death [Figure 3].
Figure 3.
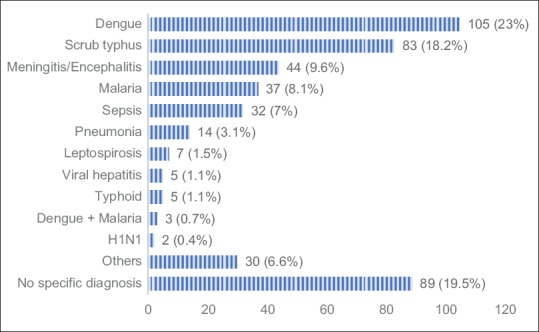
Bar chart showing specific diagnoses and their proportion
Presenting syndrome and final diagnosis
Clinical syndrome consisting of thrombocytopenia and/or rash was present in all patients with leptospirosis, 91% of cases with dengue and in about two-thirds of scrub typhus (66%) and malaria (62%) patients. Respiratory distress was a prominent clinical feature in patients with scrub typhus; 77% of patients with scrub typhus had respiratory distress/ARDS at admission. Renal failure was a less common syndrome overall, it was present in patients with leptospirosis (71%), malaria (35%), scrub typhus (22%), and dengue (20%). Most patients (91%) with acute CNS infections (Encephalitis syndrome) had presented with encephalopathy. Encephalopathy was also seen in patients with scrub typhus, being the presenting feature in 25% of cases. All cases with viral hepatitis and 43% of patients with malaria had jaundice at admission. Multi-organ failure was observed more commonly in patients presenting with sepsis (31%) and those without a specific diagnosis (30%) [Table 2].
Table 2.
Clinical syndromes and their distribution with respect to etiological diagnoses
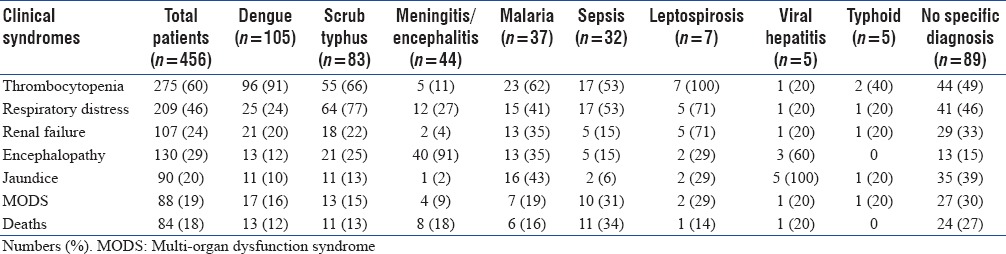
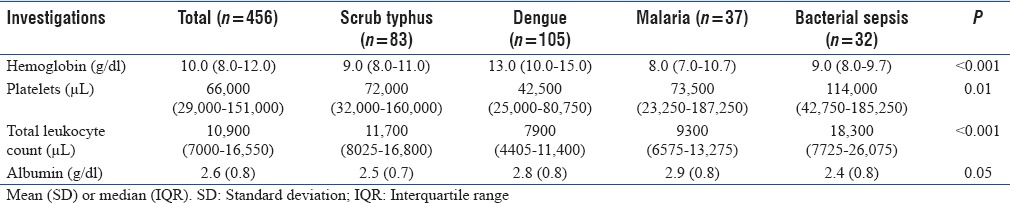
Table 3 shows the admission laboratory values of patients with various diagnoses. Patients with dengue had higher median (IQR) hemoglobin (13.0 [10–15] g/dl) at admission as compared to other diseases such as scrub typhus (9.0 [8–11] g/dl), malaria (8.0 [7–10.7] g/dl), and sepsis (9.0 [8–9.7] g/dl). Severe hypoalbuminemia (albumin <2.5 g/dl) was observed in 138 (30%) patients; more frequently in patients with sepsis (56%, n = 32) scrub typhus (44%, n = 37) and dengue (24%, n = 26). A higher (median [IQR] =18,300 [7725–26075]) total leukocyte count was noted in patients with bacterial sepsis.
Table 3.
Laboratory findings at the time of admission to Intensive Care Unit
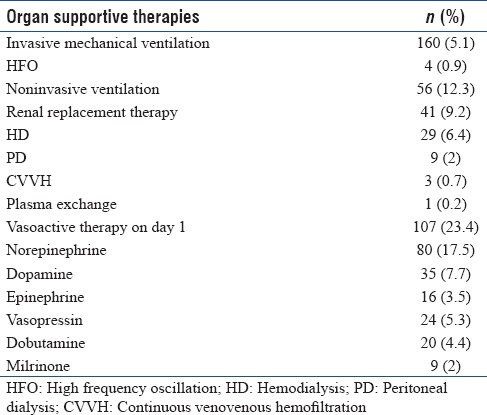
Ceftriaxone and doxycycline were the most common antibiotics used in patients with tropical fevers. 42% received ceftriaxone and 36% were administered doxycycline. Azithromycin was given in 15%. More than a quarter (27%) received antimalarials; artesunate being the most common (24%). Acyclovir was the most common antiviral drug used in 10%. More than a third of all patients (35%) received invasive mechanical ventilation and another 12% were treated with noninvasive ventilatory support. About 9% required renal replacement therapy and hemodialysis was the most common modality used. Nearly, a quarter (23.4%) required vasoactive therapy within 24 h of admission. Norepinephrine was used more commonly in adults while children received dopamine more often [Table 4]. Patients without a specific diagnosis had higher intensive care needs (mechanical ventilation [57%], vasoactive agents [40%], and renal replacement therapy [20%]). Median (IQR) ICU length of stay was 4 (3–7) days. Median (IQR) length of hospital stay was 7 (5–11.3) days. Eighty patients died and care was discontinued in 4 patients with an all-cause mortality rate of 18.4% at 28 days. Mortality was higher (27% vs. 15%, P = 0.011) in patients with undiagnosed etiology. Majority (76.2%) had good outcome and 4.4% survived with disability.
Table 4.
Details of supportive intensive care
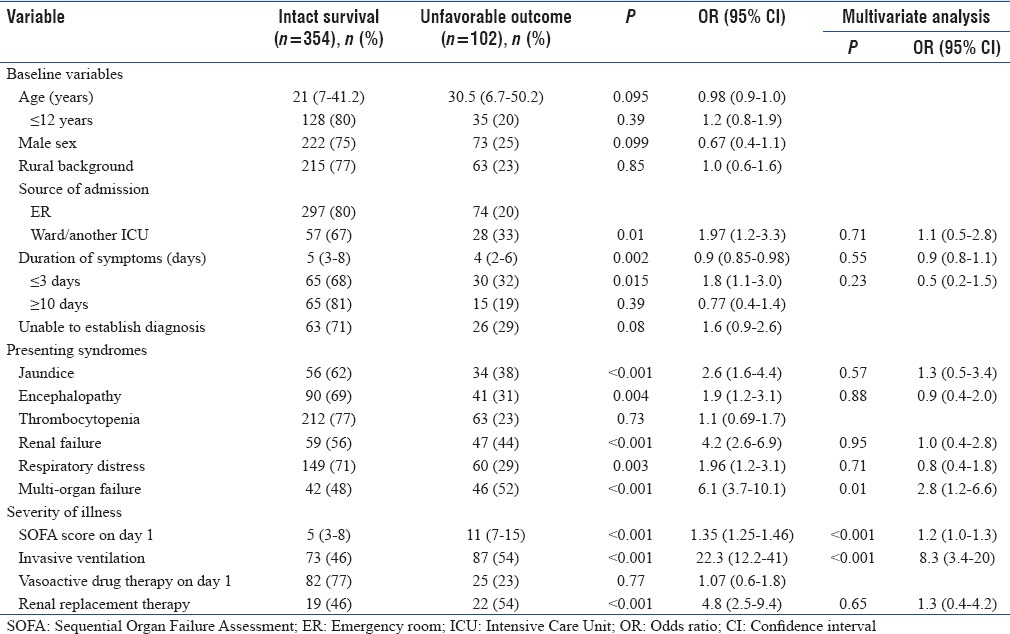
On bivariate analysis, shorter duration (<3 days) of symptoms, admission from another ICU or ward, presence of jaundice, encephalopathy, respiratory distress, renal failure, and multi-organ failure as presenting syndromes, SOFA score on day 1 and the need for invasive ventilation and renal replacement therapy were significant (P < 0.05) predictors of unfavorable outcome. Multivariate analysis showed that multi-organ failure at admission (odds ratio [OR] = 2.8 [1.8–6.6]), day 1 SOFA score (OR = 1.2 [1.0–1.3]) and the need for invasive ventilation (OR = 8.3 [3.4–20]) were the only independent predictors of unfavorable outcome [Table 5].
Table 5.
Predictors of outcome (Bivariate and multivariate regression analysis)
DISCUSSION
We found that in patients admitted to ICU with acute febrile illness and systemic manifestations, dengue and scrub typhus to be the most common etiological diagnoses. Our results are similar to those found in studies from India and other tropical regions of the developing world, although the relative incidence of specific etiologies varied considerably.[9,10,15,16,17,18] A report from south India suggested that scrub typhus (47.5%), malaria (17.1%), enteric fever (8%), and dengue (7%) were the most prevalent tropical fevers among adult patients.[10] Similarly, in a large cohort of 2547 patients with acute undifferentiated febrile illnesses dengue (37.54%), enteric fever (16.5%), scrub typhus (14.42%), bacterial sepsis (10.3%), and malaria (6.8%) were the main infections identified.[19] However, both these studies were single center experiences, included non-ICU patients and the latter was also retrospective. A large multicenter study by Leelarasamee et al. from Thailand in 1137 patients older than 2 years of age presenting with acute undifferentiated febrile illnesses identified scrub typhus (7.5%), influenza (6.0%), and dengue fever (5.7%) as the most common infections though etiologies could be found only in 38% of the cases.[16] We were able to identify the etiology in majority (80.5%) of enrolled subjects. This may in part be due to our study cohort who comprised of subjects with advanced illness requiring ICU care but more importantly due to the wider diagnostic methods employed such as molecular diagnostic methods for viruses, ELISA for scrub typhus combined with RDTs for dengue, malaria, and typhoid.
It is increasingly recognized that tropical fevers present with overlapping clinical features. Hence, knowledge of local epidemiology and etiology is very important in the early management; laboratory investigations and empirical treatment both are guided by the local prevalence. Recently, ISCCM published consensus guidelines for management of tropical fevers which envisage a syndromic approach to management, comprising point of care diagnostic tests for dengue, malaria and typhoid, and initial empiric treatment with ceftriaxone and doxycycline and optional addition of acyclovir in patients with encephalopathy.[1] The results from our study supplements this approach by providing evidence base. In accordance with the etiologies identified from our study, it appears to be reasonable to initiate ceftriaxone and doxycycline as empirical antimicrobials as they would adequately cover common diagnoses including scrub typhus, enteric fever, leptospirosis, meningitis, and most cases of bacterial sepsis. Dengue can be diagnosed at admission using NS1 antigen and IgM antibody based RDTs. However, in situations where RDT is not available or falsely negative, there may be difficulties in discriminating dengue and scrub typhus at the outset as both infections present with thrombocytopenia, signs of capillary leak, and circulatory abnormalities. A Thai study evaluated simple criteria to differentiate these two infections and found that the presence of bleeding manifestations, low platelet (<1.4 Lakhs) and white cell count (<5000/mm3) were associated with dengue.[20] In this study, thrombocytopenia, bleeding, and hypoalbuminemia were common to both infections but patients with dengue presented more often with hemoconcentration (median [IQR] Hb 13 [10–15 g/dl]) and a Hb >11 g/dl was associated with a diagnosis of dengue (OR and 95% confidence interval [CI] 10.1 [5.5–18.40], P < 0.001). The clinical syndrome of thrombocytopenia/rash along with respiratory distress/ARDS predicted scrub typhus.
Severe malaria accounted for 8% of tropical fever admissions to ICU. Our finding of predominant nonmalarial etiology also reinforces the fact that there is limited role for empiric artesunate in patients presenting with fever even from endemic areas. Moreover, malaria can be easily ruled out in the initial hours with the help of RDTs and peripheral smears. Mixed infections were uncommon and only 3 cases of malaria with dengue were noted in our cohort.
Acute encephalopathy syndrome comprised of 10% of cases of tropical fevers; 84% (37 out of 44) of all cases were noted in children younger than 12 years. Pyogenic meningitis accounted for 39% of cases. JE (33%) and herpes encephalitis (21%) were the other major diagnoses. Patients with fever and encephalopathy had a low Glasgow Coma Scale Score (median [IQR] = 7 [4–10]) and a Glasgow Coma Scale Score <10 was associated with the diagnosis of acute CNS infections (OR and 95% CI - 9.0 [3.5–23.1], P < 0.001). It is important to note that one fourth of those diagnosed with scrub typhus had presented in acute encephalopathy syndrome. CNS involvement presenting as mononuclear meningitis and/or encephalitis is increasingly recognized in patients with scrub typhus.[21,22] The presence of thrombocytopenia can be a useful distinguishing feature between scrub typhus and other acute CNS infections. Given the diagnostic spectrum among patients presenting with fever and encephalopathy in this study, in Indian ICUs in most circumstances, a combination of ceftriaxone with or without doxycycline and acyclovir can be a wise empirical choice for such patients.
Case fatality in this study was 18.4%. The outcome data highlight the importance of reaching a diagnosis in patients with undifferentiated fevers as those without a specific diagnosis more often required organ supportive therapies and had poor outcome. In addition to morbidity and mortality, tropical fevers also pose a significant burden on ICU health-care resources. Nearly, a fifth of all ICU patient resources were consumed by these illnesses. In one of the centers (146 cases), 16% of ICU days (841 out of 5251 days) and 21% of total ventilation days (426 out of 2088 days) were utilized for tropical fevers.
This study has several strengths. The study population consists of all age groups including adults and children and etiology could be identified in majority of the cases. Nation-wide multicenter data collection and yearlong consecutive enrollment representing all seasons makes our results generalizable to most ICU settings in India. There are also some limitations of the paper. First, this study is based in intensive care settings, and may not reflect the true prevalence of tropical infections pattern in the community as nonserious, non-ICU requiring patients with tropical infections were not included in the study. Second limitation is related to epidemiology of tropical infections. These infections occur in outbreaks and therefore their frequency may not be similar every year. In the year that data were collected dengue outbreak was on, whereas influenza/H1N1 outbreak from the previous years had died down and remained underrepresented in this study. Periodic update with follow-up studies is required to identify the changing trends in epidemiology and antimicrobial susceptibility.
CONCLUSIONS
Dengue, scrub typhus, encephalitis, and malaria are common causes of tropical fevers presenting to Indian ICUs, with organ involvement in postmonsoon season. Point of care testing for dengue, malaria, and typhoid can rule in or rule out these diagnoses at admission and help in instituting specific therapy. Ceftriaxone with doxycycline or azithromycin would be appropriate for empirical therapy and acyclovir may be considered in addition for patients presenting with encephalopathy while waiting for laboratory reports.
Financial support and sponsorship
This study was supported by Indian Society of Critical Care Medicine.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
List of Participating units and Site Investigators: 1. Sunit Singhi, Karthi Nallasamy, and Ashish Bhalla, PGIMER, Chandigarh; 2. Prakash Shastri, Sir Ganga Ram Hospital, New Delhi; 3. Samir Sahu, Apollo Hospitals, Bhubaneswar; 4. Narendra Rungta, Jeevan Rekha Critical Care & Trauma Hospital, Jaipur; 5. Vijayanand Palaniswamy, G Kuppuswamy Naidu Memorial Hospital, Coimbatore; 6. Deepak Jeswani, Criticare Hospital and Research Institute, Nagpur; 7. Balaso Khot, Ruby Hall Clinic, Pune; 8. Vandana Sinha, GNRC Hospitals, Dispur, Guwahati; 9. Surya Prakash Sahu, Shri Balaji superspeciality Hospital, Raipur; 10. Kalpesh Joshi, Lotus Hospital, Valsad; 11. Habib Md Reazaul Karim, North Eastern Indira Gandhi Regional Institute of Health & Medical Sciences, Shillong; 12. Madhusudan Jaju, Srinivas Samavedam, CARE Hospital, Hyderabad; 13. Sanghamitra Mishra, Institute of Medical Sciences & Sum Hospital, Bhubaneswar; 14. Dipak Aghara, Mangalam Hospital, Rajkot; 15. Arvind Baronia, SGPGIMS, Lucknow; 16. Anand Dongre, Swastik Critical Care Hospital, Nagpur; 17. Brajendra Lahkar, Dispur Hospital, Guwahati; 18. Sultana Teslima, Nemcare Hospital, Guwahati; 19. Kumkum Srivastava, Santevita Hospital, Ranchi; 20. Vivek Nangia, Fortis Hospital, New Delhi; 21. Amiya Kumar Mandal, TATA Main Hospital, Jamshedpur; 22. Mohammed Ibrahim, Apollo Speciality Hospital, Madurai; 23. Asit Behera, Ashwini Hospital, Cuttack; 24. Tapan Sarkar, Bellevue Clinic, Kolkata; 25. Ravi K Periasamy, Kovai Medical Center, Erode; 26. Rajesh Mishra, Medilink Hospital, Ahmedabad; 27. Meraj Alam, Paras HMRI Hospital, Patna; 28. Vivekanand Singh, Prachin Health Care Pvt Ltd, Panvel; 29 Subhal Dixit, Sanjeevan Hospital, Pune; 30. Pradip Ghadge, Satara Hospital & Research Center, Satara; 31. Sudhir Khunteta, Shubh Hospital, Jaipur; 32. Ankur Bhavsar, SpandanMultispeciality Hospital, Vadodara; 33. Revathi Aiyer, Sterling Hospitals, Vadodara; 34. Susovan Mitra, Sundaram Medical Foundation, Chennai.
REFERENCES
- 1.Singhi S, Chaudhary D, Varghese GM, Bhalla A, Karthi N, et al. The Indian Society of Critical Care Medicine Tropical Fever Group. Tropical fevers: Management guidelines. Indian J Crit Care Med. 2014;18:62–9. doi: 10.4103/0972-5229.126074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, et al. Causes of acute, undifferentiated, febrile illness in rural Thailand: Results of a prospective observational study. Ann Trop Med Parasitol. 2006;100:363–70. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- 3.Punjabi NH, Taylor WR, Murphy GS, Purwaningsih S, Picarima H, Sisson J, et al. Etiology of acute, non-malaria, febrile illnesses in Jayapura, Northeastern Papua, Indonesia. Am J Trop Med Hyg. 2012;86:46–51. doi: 10.4269/ajtmh.2012.10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phuong HL, de Vries PJ, Nga TT, Giao PT, Hung le Q, Binh TQ, et al. Dengue as a cause of acute undifferentiated fever in Vietnam. BMC Infect Dis. 2006;6:123. doi: 10.1186/1471-2334-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmerman JM, Uyeki TM. The burden of influenza in east and South-East Asia: A review of the English language literature. Influenza Other Respir Viruses. 2008;2:81–92. doi: 10.1111/j.1750-2659.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung HC, Chon SB, Oh WS, Lee DH, Lee HJ. Etiologies of acute undifferentiated fever and clinical prediction of scrub typhus in a non-tropical endemic area. Am J Trop Med Hyg. 2015;92:256–61. doi: 10.4269/ajtmh.14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susilawati TN, McBride WJ. Acute undifferentiated fever in Asia: A review of the literature. Southeast Asian J Trop Med Public Health. 2014;45:719–26. [PubMed] [Google Scholar]
- 8.Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395–405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamsen SK, Haugen CN, Rupali P, Mathai D, Langeland N, Eide GE, et al. Fever in the tropics: Aetiology and case-fatality – A prospective observational study in a tertiary care hospital in South India. BMC Infect Dis. 2013;13:355. doi: 10.1186/1471-2334-13-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, Thomas EM, et al. Acute undifferentiated febrile illness in adult hospitalized patients: The disease spectrum and diagnostic predictors – An experience from a tertiary care hospital in South India. Trop Doct. 2010;40:230–4. doi: 10.1258/td.2010.100132. [DOI] [PubMed] [Google Scholar]
- 11.John TJ, Dandona L, Sharma VP, Kakkar M. Continuing challenge of infectious diseases in India. Lancet. 2011;377:252–69. doi: 10.1016/S0140-6736(10)61265-2. [DOI] [PubMed] [Google Scholar]
- 12.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 13.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American college of chest physicians/Society of critical care medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 15.Ellis RD, Fukuda MM, McDaniel P, Welch K, Nisalak A, Murray CK, et al. Causes of fever in adults on the Thai-Myanmar border. Am J Trop Med Hyg. 2006;74:108–13. [PubMed] [Google Scholar]
- 16.Leelarasamee A, Chupaprawan C, Chenchittikul M, Udompanthurat S. Etiologies of acute undifferentiated febrile illness in Thailand. J Med Assoc Thai. 2004;87:464–72. [PubMed] [Google Scholar]
- 17.Murdoch DR, Woods CW, Zimmerman MD, Dull PM, Belbase RH, Keenan AJ, et al. The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu, Nepal. Am J Trop Med Hyg. 2004;70:670–5. [PubMed] [Google Scholar]
- 18.Sripanidkulchai R, Lumbiganon P. Etiology of obscure fever in children at a university hospital in Northeast Thailand. Southeast Asian J Trop Med Public Health. 2005;36:1243–6. [PubMed] [Google Scholar]
- 19.Mittal G, Ahmad S, Agarwal RK, Dhar M, Mittal M, Sharma S, et al. Aetiologies of acute undifferentiated febrile illness in adult patients – An experience from a tertiary care hospital in Northern India. J Clin Diagn Res. 2015;9:DC22–4. doi: 10.7860/JCDR/2015/11168.6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watt G, Jongsakul K, Chouriyagune C, Paris R. Differentiating dengue virus infection from scrub typhus in Thai adults with fever. Am J Trop Med Hyg. 2003;68:536–8. doi: 10.4269/ajtmh.2003.68.536. [DOI] [PubMed] [Google Scholar]
- 21.Kar A, Dhanaraj M, Dedeepiya D, Harikrishna K. Acute encephalitis syndrome following scrub typhus infection. Indian J Crit Care Med. 2014;18:453–5. doi: 10.4103/0972-5229.136074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viswanathan S, Muthu V, Iqbal N, Remalayam B, George T. Scrub typhus meningitis in South India – A retrospective study. PLoS One. 2013;8:e66595. doi: 10.1371/journal.pone.0066595. [DOI] [PMC free article] [PubMed] [Google Scholar]


