ABSTRACT
Mycotoxin contamination of cereal grains causes well-recognized toxicities in animals and humans, but the fate of plant-bound masked mycotoxins in the gut is less well understood. Masked mycotoxins have been found to be stable under conditions prevailing in the small intestine but are rapidly hydrolyzed by fecal microbiota. This study aims to assess the hydrolysis of the masked mycotoxin deoxynivalenol-3-glucoside (DON3Glc) by the microbiota of different regions of the porcine intestinal tract. Intestinal digesta samples were collected from the jejunum, ileum, cecum, colon, and feces of 5 pigs and immediately frozen under anaerobic conditions. Sample slurries were prepared in M2 culture medium, spiked with DON3Glc or free deoxynivalenol (DON; 2 nmol/ml), and incubated anaerobically for up to 72 h. Mycotoxin concentrations were determined using liquid chromatography-tandem mass spectrometry, and the microbiota composition was determined using a quantitative PCR methodology. The jejunal microbiota hydrolyzed DON3Glc very slowly, while samples from the ileum, cecum, colon, and feces rapidly and efficiently hydrolyzed DON3Glc. No further metabolism of DON was observed in any sample. The microbial load and microbiota composition in the ileum were significantly different from those in the distal intestinal regions, whereas those in the cecum, colon and feces did not differ.
IMPORTANCE Results from this study clearly demonstrate that the masked mycotoxin DON3Glc is hydrolyzed efficiently in the distal small intestine and large intestine of pigs. Once DON is released, toxicity and absorption in the distal intestinal tract likely occur in vivo. This study further supports the need to include masked metabolites in mycotoxin risk assessments and regulatory actions for feed and food.
KEYWORDS: deoxynivalenol-3-glucoside, pig, microbiota, masked mycotoxin, release, toxicity, trichothecene
INTRODUCTION
Mycotoxin contamination of agricultural commodities is an intractable problem globally. In temperate climates, Fusarium fungi comprise the most important mycotoxin producers and are particularly prevalent in small-grain cereals, such as wheat and barley, as well as maize. The major groups of Fusarium mycotoxins include trichothecenes, zearalenone, and fumonisins (1). In addition to the well-described trichothecenes deoxynivalenol (DON), nivalenol, T2 toxin, and HT2 toxin, cereals have been found to be cocontaminated with plant-derived mycotoxin metabolites, so-called masked mycotoxins. In response to fungal infection and mycotoxin production, the plant's own phase II metabolic enzymes conjugate mycotoxins with small molecules, such as glucose, glutathione, or sulfate, and sequester these masked mycotoxins into the plant cell vacuole (for a review, see references 2 to 4). Mycotoxins and masked mycotoxins are stable compounds withstanding processing into various cereal products and are carried over into finished food and feed. Once they are ingested, mycotoxins have been shown to be rapidly absorbed in the small intestine of humans and various animal species and exert their toxicities either locally on the gut epithelium (e.g., trichothecenes) or systemically (e.g., zearalenone) (1, 4–6). Masked mycotoxins, such as deoxynivalenol-3-glucoside (DON3Glc), on the other hand, are far less toxic than their free parent mycotoxins and are not absorbed intact (7–9). Hence, masked mycotoxins are transported into the distal parts of the intestine intact, where the intestinal microbiota (as studied using fecal samples) rapidly hydrolyze masked mycotoxins and release free mycotoxins (7, 10–12). Microbial metabolism experiments have also demonstrated further metabolism of DON to deepoxy DON (DOM-1) by microbiota samples derived from chickens, pigs, and some humans (10, 13, 14). This purely microbial metabolite, DOM-1, is not toxic (15) and can be found in the urine of some humans (10, 16, 17) and pigs (18), hence confirming its production and colonic absorption in vivo.
In pigs, the oral bioavailability and absorption of DON3Glc are significantly lower and slower than those of DON (18, 19). The delay in DON3Glc absorption and the fact that only free DON and no DON3Glc is found in plasma and urine confirm that hydrolysis and absorption occur in parts of the intestinal tract more distal from the parts where the hydrolysis and absorption of free DON occur. The microbial deepoxidation of DON or DON3Glc by the pig microbiota has been found in some studies (14, 18) but not in others (19).
All studies published to date have used fecal samples from pigs or humans to determine microbial hydrolysis and the metabolism of mycotoxins. However, the microbial metabolism of mycotoxins would need to occur in more proximal parts of the intestinal tract to release mycotoxin metabolites and allow intestinal absorption and/or potential colonic toxicity to occur. Therefore, the aim of this study was to investigate the capacity of the intestinal microbiota derived from different regions of the small and large intestines of pigs to degrade masked mycotoxins. For this study, DON3Glc was used as a model mycotoxin, as it is commercially available.
RESULTS
This study was conducted to assess the metabolism of DON and DON3Glc by porcine microbiota derived from different regions of the intestinal tract. The results show that detoxification of DON to DOM-1 did not occur in any animal or any gut region (Table 1). No trace of DOM-1 was detectable in any of the samples (data not shown), and the rate of recovery of DON ranged from 87 to 119% of the dose following incubation over 24 to 72 h.
TABLE 1.
Recovery of DON from microbial incubations
| Time (h) | Recovery of DON (% of a dose of 2 nmol/ml)a |
||||
|---|---|---|---|---|---|
| Jejunum | Ileum | Cecum | Colon | Feces | |
| 0 | 100.3 ± 0.3 | 102.7 ± 1.2 | 98.6 ± 1.1 | 100.5 ± 0.3 | 99.9 ± 0.1 |
| 24 | 103.9 ± 6.7 | 97.2 ± 8.4 | 94.0 ± 9.3 | 99.6 ± 8.3 | 119.4 ± 5.7 |
| 48 | 87.3 ± 4.3 | 90.0 ± 6.1 | 113.9 ± 6.2 | 103.9 ± 9.3 | 114.6 ± 8.7 |
| 72 | 90.3 ± 6.2 | 91.8 ± 6.6 | 116.0 ± 6.5 | 108.1 ± 9.0 | 113.6 ± 7.8 |
The results presented as the averages for 5 animals ± SEM.
The microbial hydrolysis of DON3Glc was efficient in all pigs and occurred in all intestinal regions tested (Fig. 1). In the jejunum, DON3Glc hydrolysis was the slowest, and free DON was first observed after 24 h of incubation, increasing to a maximum of 1 to 41% of the added DON3Glc dose after 72 h. The ileal microbiota were more efficient in DON3Glc hydrolysis, releasing 60% ± 18% of the dose as free DON after 24 h of incubation. The microbiota of the large intestine hydrolyzed DON3Glc more rapidly, with 2 and 3% of the dose being detectable as free DON in cecum and colon incubations, respectively, after 2 h of incubation, increasing to 8 and 14%, respectively, after 6 h of incubation. The fecal microbiota were the most efficient in hydrolyzing DON3Glc, with only 4% ± 6% of the dose being left as DON3Glc after 9 h of incubation.
FIG 1.
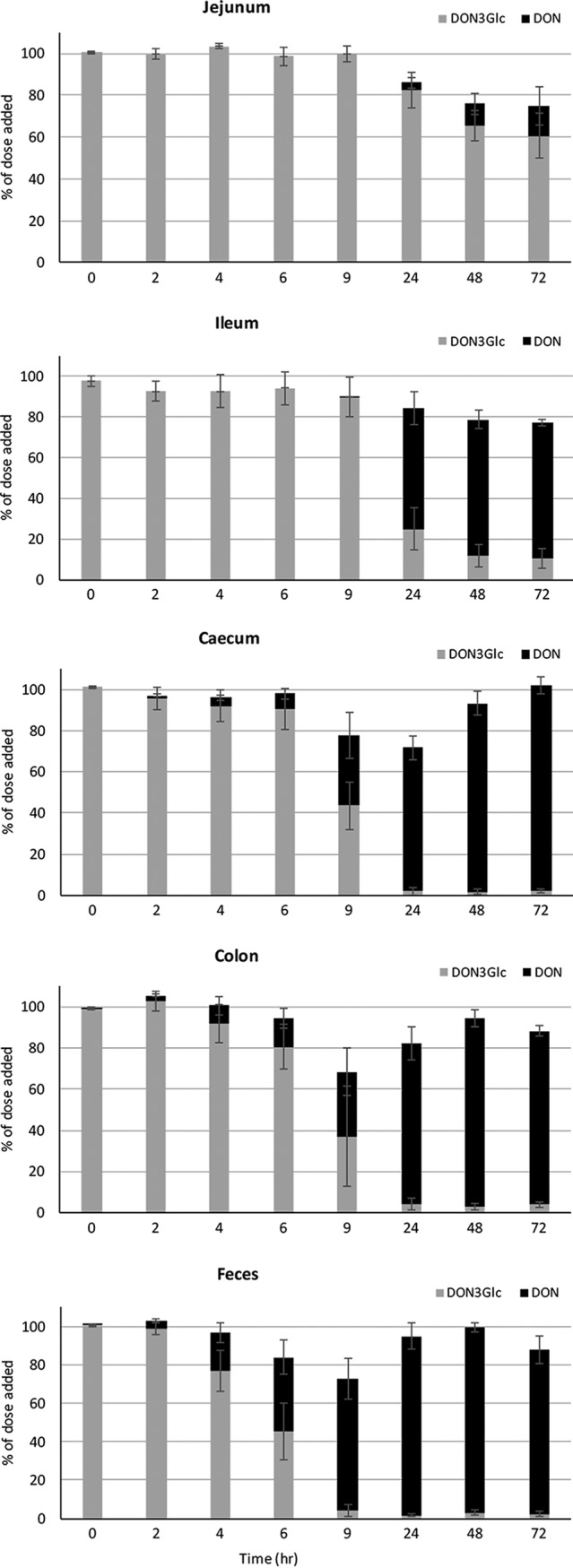
Hydrolysis of DON3Glc and release of free DON by porcine intestinal microbiota from different regions of the small and large intestines over 0 to 72 h. Results are presented as averages for 5 animals ± SEMs.
The results from the DON3Glc hydrolysis time course experiments (Fig. 1) were used to calculate the area under the curve (AUC) for each individual animal and each intestinal region for DON3Glc (Fig. 2, top) and DON (Fig. 2, bottom). DON3Glc hydrolysis rates were the slowest in the jejunal samples from all animals, as indicated by the highest AUC for DON3Glc curves and the lowest AUC for DON curves. In all animals, ileal DON3G hydrolysis was significantly (P < 0.05) faster than jejunal hydrolysis, but the rates were lower (P < 0.05) than the rates observed in the large intestine. No differences in the DON3Glc hydrolysis rates between cecum, colon, and fecal samples were observed.
FIG 2.
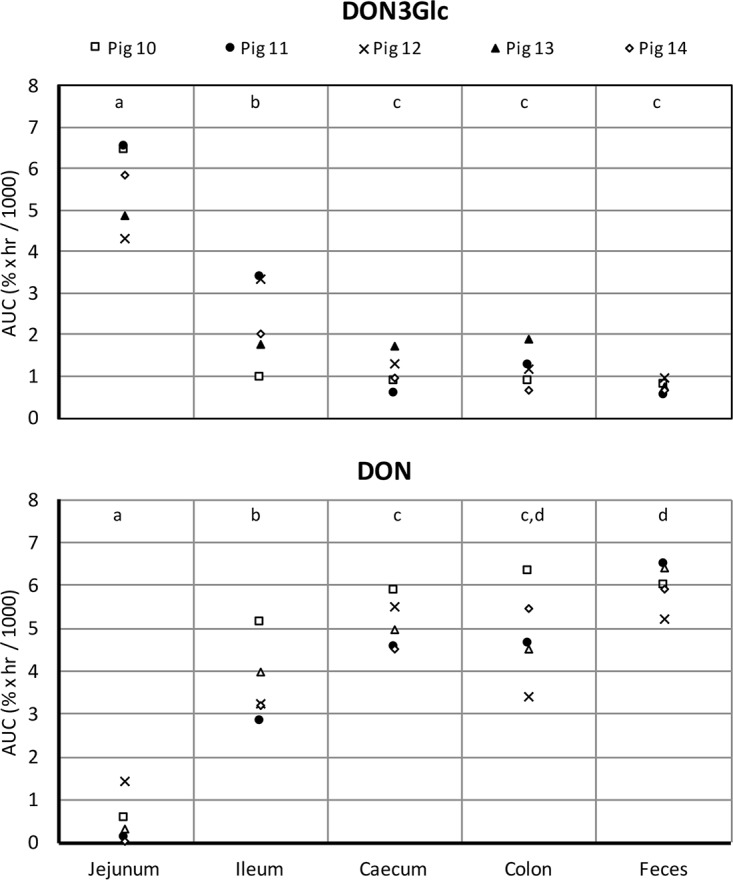
Individual differences in DON3Glc hydrolysis (top) and DON release (bottom) by the intestinal microbiota of 5 animals. Data from time course experiments were summarized by the area under the curve (AUC) for each individual animal and gut site. The effect of tissue was significant (P < 0.001, ANOVA) for both DON3Glc and DON. The results for tissues that do not share a letter at the top were statistically significantly different (P < 0.05, post hoc t test).
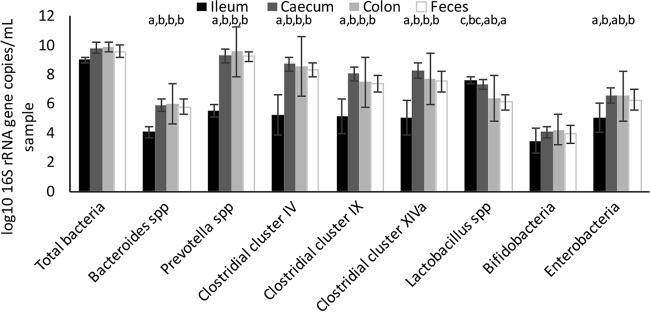
The microbiota composition was analyzed using DNA extracted from untreated digesta samples (without mycotoxin spiking) derived from the ileum, cecum, colon, and feces of experimental pigs. The total bacterial load showed a tendency (P = 0.057) toward differences between intestinal regions, with the log count in the ileum being lower than that in the cecum and colon (P < 0.05) (Fig. 3). The log counts of Bacteroides spp., Prevotella spp., Ruminococcaceae, Lachnospiraceae, and Negativicutes were all lower in samples from the ileum than the other samples (P < 0.05) but did not differ between the cecum, colon, and feces. In the ileum, members of the phylum Firmicutes dominated the microbiota, with lactobacilli forming the largest portion of bacteria. However, most bacteria in the ileum were not identified with the primers used, suggesting that the ileum harbors bacteria other than the groups covered here.
FIG 3.
Analysis of the microbial community in porcine digesta samples from different regions of the small and large intestines. Results are presented as the averages for 5 animals ± SEMs for the ileum, cecum, and feces and the averages for 4 animals ± SEMs for colon samples. Within those bacterial groups for which the effect of tissue was significant (P < 0.05, ANOVA), the results for tissues that do not share a letter at the top were statistically significantly different (P < 0.05).
DISCUSSION
The current study was conducted to assess the microbial metabolism of the masked mycotoxin DON3Glc and the free form, DON, by intestinal microbiota derived from different regions of the small and large intestines of pigs. We found no evidence of microbial deepoxidation of DON to DOM-1 in any digesta sample. Similarly, Eriksen and colleagues found no DOM-1 production in ileal or fecal samples from 5 experimental pigs, even though DOM-1 production was reported in pigs from commercial farms (14). Interestingly, 4 of these 5 animals acquired the microbiota capable of DOM-1 production after they were exposed to the feces of DOM-1-producing animals. This suggests that the microbes capable of DON deepoxidation are acquired from the environment and confirms that the ingestion of DON-contaminated feed may alter the intestinal microbiota (20, 21).
The study presented here demonstrates that the microbiota derived from the porcine small intestine efficiently hydrolyze the masked mycotoxin DON3Glc and release free DON in vitro. Furthermore, the microbiota from porcine cecum, colon, and feces hydrolyze DON3Glc equally efficiently. Upon ingestion, DON3Glc has been found to be not toxic (in pig intestinal explants [8]) and is not absorbed intact in pigs, but free DON and further metabolites are detectable in plasma and urine. The absorption of DON3Glc in pigs (as DON) is less efficient than that of free DON (16% versus 81% of the dose is absorbed after 8 h [19]) and is also slower than that of DON (42 versus 84% of the dose is excreted in urine after 24 h [18]). These findings suggest the continuous, slow release of DON from DON3Glc prior to absorption, which would be in line with microbial hydrolysis beginning after 6 or 9 h incubation, as reported here.
This slow and continuous release of DON from DON3Glc exposure may result in toxicities in the distal regions of the intestine. There is some evidence that the binding of DON to a clay-based feed additive results in DON exerting its intestinal toxicity (disruption of the intestinal barrier function, induction of oxidative stress) in the more distal part of the small intestine in chickens compared to the location of the intestinal toxicity of free DON, although colonic tissue was not evaluated in this study (22). This suggests that the binding of DON can lead to the intestinal toxicity being shifted to more distal intestinal regions, and it can be hypothesized that the binding of DON3Glc to plants could act as a mechanism of delivery of DON3Glc to the ileum and colon, where microbial hydrolysis leads to DON exposure and potential toxicity. Upon the ingestion of DON3Glc, it would be interesting to determine the absorption and the effect of DON in the large intestine.
Microbiota profiling demonstrated that the microbiota from the cecum, colon, and feces were dominated by Prevotella spp., followed by Ruminococcaceae, Lachnospiraceae, and Negativicutes. This is in agreement with findings described in the literature suggesting that Bacteroidetes and Firmicutes are the dominant phyla in the large intestine and feces of pigs (23–25). Enterobacteria represented a substantial group in the small and large intestines of only one pig, whereas Bacteroides spp. and bifidobacteria did not represent major groups in any animal or gut site. This is in contrast to the findings of published work (26), which reported that Bacteroides spp. are a major group in porcine feces. The current study focused on a quantitative and qualitative analysis of the intestinal microbiota of the porcine intestine but did not identify specific bacterial groups involved in the hydrolysis of DON3Glc. Published work has identified bacteria from very different genera and phyla (lactobacilli, enterococci, bifidobacteria) that are capable of hydrolyzing DON3Glc and other masked mycotoxins (27, 28), and future studies are required to understand their contribution to hydrolysis in mixed microbial communities and in vivo.
The human intestinal microbiota possess several glycosyl hydrolase genes (29), and the human fecal microbiota are known to hydrolyze DON3Glc (7, 10, 11). It is therefore likely that DON3Glc hydrolysis occurs in the human intestine, but future experiments are required to provide evidence. The fact that the microbial metabolite DOM-1 is present in human urine (10, 16, 17) further supports the hypothesis that microbial mycotoxin metabolism and absorption occur in vivo in humans.
In conclusion, the present study demonstrates that masked mycotoxins can contribute to mycotoxin exposure following rapid, efficient, and nonspecific hydrolysis by the intestinal microbiota of the distal small intestine and the large intestine. The potential specific toxicities of microbial mycotoxin release in the distal intestine remain to be investigated in future studies.
MATERIALS AND METHODS
The following mycotoxin standards were used in this study: DON as a powder (Molekula, Gillingham, UK), DON and DON3Glc in acetonitrile (Romer Labs, Runcorn, UK), and DOM-1 in acetonitrile (Sigma-Aldrich Ltd., Poole, UK).
Animals and ethical approval.
Five crossbred castrated male pigs, weaned at 4 weeks of age, were bred in the animal facility of the INRA ToxAlim Laboratory (Toulouse, France). The experiment was conducted under the authorization of the French Ministry of Higher Education and Research after approval was provided by the Ethics Committee of Pharmacology-Toxicology of Toulouse-Midi-Pyrénées (Toxcométhique, no. TOXCOM/0163 PP), in accordance with the European Directive (2010/63/EU) on the protection of animals used for scientific purposes. Feed and water were provided ad libitum throughout the experimental period. Pigs were fed for 4 days with a starter diet and then with a commercial diet (Stimio) for growing pigs (Evialis, Longue Jumelles, France). The feed composition is summarized in Table 2. As the presence of antibiotics or probiotics in feed can alter the composition of the luminal and mucosa-associated microbiota (30), nonsupplemented feed was used. The pigs were maintained until 57 days of age, as the pig intestinal flora is stable between at least 48 and 70 days of age (31). Then, they were subjected to electronarcosis and euthanized by exsanguination (32). The intestinal tract was removed from each carcass, and sections of the jejunum, ileum, cecum, and colon were dissected. Five milliliters of the intestinal digesta content from each gut section was collected separately and placed into sterile Wheaton bottles. Feces (5 ml) were sampled directly from the pen. Ten milliliters of a sterile mixture of 70% phosphate-buffered saline (pH 7.4) and 30% glycerol bubbled with CO2 was added to each vial. The vials were sealed, and the headspace was flushed with CO2 before the vials were stored at −20°C.
TABLE 2.
Summary of feed composition
| Component | Value |
|---|---|
| Food constituent | |
| Raw proteins | 17% |
| Raw fat | 2.5% |
| Raw ash | 4.5% |
| Crude fiber | 4.5% |
| Phosphorus | 0.55% |
| Calcium | 0.65% |
| Sodium | 0.2% |
| Lysine | 11.9 g/kg |
| Methionine | 3.6 g/kg |
| Additives | |
| E672 A vitamin | 12,000 U/kg |
| E671 D3 vitamin | 2,000 U/kg |
| 3a700 E vitamin E | 60 U/kg |
| Oligoelements | |
| Iron | 86 mg/kg |
| Copper | 160 mg/kg |
| Manganese | 40 mg/kg |
| Zinc | 110 mg/kg |
| Iodine | 1 mg/kg |
| Selenium | 0.3 mg/kg |
| Enzymes | |
| Endo-1,3(4)-beta glucanase | 125 U/kg |
| Endo-1,4-beta-xylanase | 87 U/kg |
| Phytase | 1,880 U/kg |
Microbial batch culture experiments.
After the vials were defrosted and vortexed, 15 ml of slurry was centrifuged at 2,000 × g for 5 min. The supernatant was discarded, and the remaining pellet was purged with CO2. At this stage, two 1-ml aliquots were removed from each sample and stored in sample Matrix tubes at −70°C for subsequent DNA extraction. The remaining slurry was diluted 1/10 with anaerobic M2 medium as described before (10) and placed in a shaking water bath (37°C, 100 rpm) in a sealed Wheaton bottle for 1 h, and 1-ml aliquots were moved to sterile screw-cap Hungate tubes. Slurry aliquots were spiked with individual mycotoxins (2 nmol/ml of DON, DON3Glc, or DOM-1) and incubated anaerobically at 37°C for intervals of between 0 and 72 h. This wide range of incubation times was chosen to reflect both the short transit time in the small intestine (early time points) and the long transit time in the large intestine (late time points). Following incubation, 3 ml acetonitrile was added to each sample and the samples were centrifuged for 5 min at 2,000 × g. Supernatants were evaporated under N2 at 50°C, reconstituted with 1 ml of water, and passed through C18 solid-phase extraction columns (Agilent, Wokingham, UK). The samples were eluted with 3 ml methanol, evaporated under N2 at 50°C, reconstituted in 1 ml of 50% aqueous methanol, and analyzed for DON, DON3Glc, and DOM-1 using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Blank digesta incubations (in which spiking with mycotoxins was omitted) were included in each experiment to ensure that all digesta samples were free of mycotoxin residues. Furthermore, DON3Glc or DON (2 nmol/ml) was spiked individually into bacterium-free M2 culture medium (in duplicate) and incubated for 72 h to ensure the stability of DON3Glc and DON under the incubation conditions in the absence of bacteria. Both compounds were stable, with recoveries of 100.7% ± 4.7% and 102.8% ± 1.9%, respectively, after 72 h. To further assess the stability of DON during incubation, digesta samples were spiked with DON (2 nmol/ml) and incubated for 0 to 72 h. This experiment showed no loss of DON mass (level of recovery, up to 119% of the dose added), suggesting no binding of DON or further metabolism by microbes or any other digesta constituents. Each experiment also included digesta controls (in duplicate) individually spiked with DON3Glc, DON, or DOM-1, which were not incubated or immediately processed further (i.e., time zero), to account for potential matrix effects in mycotoxin detection. The levels of mycotoxins detected in the samples at time zero were set at a value of 100%, and all other results were calculated as a percentage of the value at time zero.
qPCR analysis of microbial composition.
The microbiota composition was analyzed using DNA extracted from untreated digesta samples (without mycotoxin spiking) derived from the ileum, cecum, colon, and feces of experimental pigs. Jejunal samples did not yield sufficient DNA to perform quantitative PCR (qPCR) analysis. DNA was extracted from 1 ml of the digesta slurry using a FastDNA kit for soil (MP Biomedicals, Santa Ana, CA, USA) following the manufacturer's instructions and quantified using a Qubit double-stranded DNA high-sensitivity assay kit (Thermo Fisher Scientific, Waltham, MA, USA). qPCR was performed using the primers described in Table 3. The quantification of total bacteria, Prevotella spp., Bacteroides spp., Ruminococcaceae, Lachnospiraceae, Negativicutes, Lactobacillus spp., enterobacteria, and bifidobacteria was performed as described before (33) using a Bio-Rad CFX384 real-time system and Bio-Rad CFX manager software (version 3.0; Bio-Rad Laboratories, Watford, UK). DNA concentrations were standardized to 1 ng per well, and standard curves were prepared with dilution series of amplified bacterial 16S rRNA genes from reference strains as described previously (34). Samples and standards were run in duplicate, and 5 ng/μl herring sperm DNA (Promega, Southampton, UK) was included in all reactions for stabilization. The efficiencies of the standard curves ranged from 92.6 to 104.7%, and R2 values ranged from 0.993 to 0.999 across all primers used.
TABLE 3.
Summary of group-specific qPCR primers
| Target group | Sequence | Annealing temp (°C) | Amplicon size (bp) | Reference strain | Reference |
|---|---|---|---|---|---|
| Universal | GTGSTGCAYGGYYGTCGTCA | 60 | 141 | Ruminococcus bromii L2-63 | 35 |
| ACGTCRTCCMCNCCTTCCTC | |||||
| Prevotella spp. | CRCRCRGTAAACGATGGATG | 65 | 105 | Prevotella copri DSM18205 | 33 |
| TTGAGTTTCACCGTTGCCGG | |||||
| Bacteroides spp. | GCTCAACCKTAAAATTGCAGTTG | 63 | 110 | Bacteroides thetaiotaomicron B5482 | 33 |
| GCAATCGGRGTTCTTCGTG | |||||
| Lactobacillus spp. | AGCAGTAGGGAATCTTCCA | 60 | 341 | Lactobacillus reuteri DSM20016 | 36 |
| CACCGCTACACATGGAG | 37 | ||||
| Bifidobacteria | TCGCGTCYGGTGTGAAAG | 60 | 128 | Bifidobacterium adolescentis DSM20083 | 35 |
| GGTGTTCTTCCCGATATCTACA | |||||
| Enterobacteria | GACCTCGCGAGAGCA | 60 | 180 | Escherichia coli XL1-Blue | 38 |
| CCTACTTCTTTTGCAACCCA | |||||
| Cluster IV Ruminococcaceae family | GCACAAGCAGTGGAGTa | 60 | 241 | R. bromii L2-63 | 39 |
| GCACAAGCGGTGGATTa | |||||
| CTTCCTCCGTTTTGTCAA | |||||
| Cluster IX Negativicutes class | GTTGTCCGGAATYATTGGGC | 63 | 321 | Megasphaera elsdenii LC1 | 40 |
| ATTGCGTTAACTCCGGCACAb | |||||
| ATTGCGTTAACTCCGGCACGb | |||||
| Cluster XIVa Lachnospiraceae family | CGGTACCTGACTAAGAAGC | 60 | 429 | Roseburia hominis A2-183 | 34 |
| AGTTTYATTCTTGCGAACG |
Both forward primers for cluster IV were used together at an equimolar concentration.
Both reverse primers for cluster IX primers were used together at an equimolar concentration.
LC-MS/MS analysis.
The liquid chromatography analysis of the mycotoxins was performed on an Agilent 1200 high-performance liquid chromatography system (Agilent Technologies, Wokingham, UK) fitted with an Agilent Zorbax C18 column (particle size, 5 μm; 150 mm by 4.6 mm). The method parameters were described previously (10). Mycotoxins were detected on a Q-Trap 4000 triple-quadrupole mass spectrometer (AB Sciex, Warrington, UK) fitted with a turbo ion spray (TIS) source. The transitions for DON, DOM-1, and DON3Glc from the microbial incubations were 355.1 → 265.1, 339.1 → 249.1, and 517.3 → 427.3, respectively. The concentrations on the calibration curves for each metabolite ranged from 0.25 to 2 nmol/ml.
Statistical analysis.
The time course over 72 h of the DON and DON3Glc hydrolysis results from Fig. 1 (expressed as a percentage of the dose) were used to calculate the area under the curve (AUC; percent · hour) for each animal and each intestinal section individually. Bacterial count data were log transformed to meet the requirements of constant variance and normality (on the basis of visual inspection of residual plots). These data were then analyzed by analysis of variance (ANOVA) with animal as the random effect and tissue as the fixed effect. When the effect of tissue was significant (P < 0.05), tissues were compared by use of a post hoc t test based on the ANOVA output. The colon sample from one animal was excluded from the statistical analyses due to failure of the qPCR assay. All analyses were carried out using the GenStat program, version 17, release 17.1 (Lawes Agricultural Trust, VSN International Ltd., Hemel Hempstead, UK). A P value of <0.05 was considered significant. Results are presented as the mean ± standard error of the mean (SEM) on the basis of the spread between animals.
ACKNOWLEDGMENTS
This study was supported by the Scottish Government Rural and Environment Science and Analytical Services Division (RESAS) and by the French Agence Nationale de la Recherche (project ANR-13-CESA-0003-03).
We thank Anne-Marie Cossalter for her excellent technical assistance with the pigs.
REFERENCES
- 1.Nesic K, Ivanovic S, Nesic V. 2014. Fusarial toxins: secondary metabolites of Fusarium fungi. Rev Environ Contam Toxicol 228:101–120. doi: 10.1007/978-3-319-01619-1_5. [DOI] [PubMed] [Google Scholar]
- 2.Berthiller F, Crews C, Dall'Asta C, Saeger SD, Haesaert G, Karlovsky P, Oswald IP, Seefelder W, Speijers G, Stroka J. 2013. Masked mycotoxins: a review. Mol Nutr Food Res 57:165–186. doi: 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broekaert N, Devreese M, De Baere S, De Backer P, Croubels S. 2015. Modified Fusarium mycotoxins unmasked: from occurrence in cereals to animal and human excretion. Food Chem Toxicol 80:17–31. doi: 10.1016/j.fct.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Payros D, Alassane-Kpembi I, Pierron A, Loiseau N, Pinton P, Oswald IP. 2016. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch Toxicol 90:2931–2957. doi: 10.1007/s00204-016-1826-4. [DOI] [PubMed] [Google Scholar]
- 5.Pestka JJ. 2010. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84:663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- 6.Pinton P, Oswald IP. 2014. Effect of deoxynivalenol and other type B trichothecenes on the intestine: a review. Toxins 6:1615–1643. doi: 10.3390/toxins6051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gratz SW, Dinesh R, Yoshinari T, Holtrop G, Richardson AJ, Duncan G, MacDonald S, Lloyd A, Tarbin J. 2017. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol Nutr Food Res 61:1–10. doi: 10.1080/16546628.2017.1265324. [DOI] [PubMed] [Google Scholar]
- 8.Pierron A, Mimoun S, Murate LS, Loiseau N, Lippi Y, Bracarense APFL, Schatzmayr G, Berthiller F, Moll WD, Oswald IP. 2016. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-d-glucoside. Arch Toxicol 90:2037–2046. doi: 10.1007/s00204-015-1592-8. [DOI] [PubMed] [Google Scholar]
- 9.Wu W, He K, Zhou HR, Berthiller F, Adam G, Sugita-Konishi Y, Watanabe M, Krantis A, Durst T, Zhang H, Pestka JJ. 2014. Effects of oral exposure to naturally-occurring and synthetic deoxynivalenol congeners on proinflammatory cytokine and chemokine mRNA expression in the mouse. Toxicol Appl Pharmacol 278:107–115. doi: 10.1016/j.taap.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gratz SW, Duncan G, Richardson A. 2013. Human fecal microbiota metabolize deoxynivalenol and deoxynivalenol-3-glucoside and may be responsible for urinary de-epoxy deoxynivalenol. Appl Environ Microbiol 79:1821–1825. doi: 10.1128/AEM.02987-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dall'Erta A, Cirlini M, Dall'Asta M, Del Rio D, Galaverna G, Dall'Asta C. 2013. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem Res Toxicol 26:305–312. doi: 10.1021/tx300438c. [DOI] [PubMed] [Google Scholar]
- 12.McCormick SP, Kato T, Maragos CM, Busman M, Lattanzio VMT, Galaverna G, Dall-Asta C, Crich D, Price NP, Krutzman CP. 2015. Anomericity of T-2 toxin-glucoside: masked mycotoxin in cereal crops. J Agric Food Chem 63:731–738. doi: 10.1021/jf504737f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H, Zhou T, Gong J, Young C, Su X, Li XZ, Zhu H, Tsao R, Yang R. 2010. Isolation of deoxynivalenol-transforming bacteria from the chicken intestines using the approach of PCR-DGGE guided microbial selection. BMC Microbiol 10:182. doi: 10.1186/1471-2180-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksen GS, Pettersson H, Johnsen K, Lindberg JE. 2002. Transformation of trichothecenes in ileal digesta and faeces from pigs. Arch Tierernahr 56:263–274. doi: 10.1080/00039420214343. [DOI] [PubMed] [Google Scholar]
- 15.Pierron A, Mimoun S, Murate LS, Loiseau N, Lippi Y, Bracarense APFL, Schatzmayr G, He J, Zhou T, Moll WD, Oswald IP. 2016. Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci Rep 6:29105. doi: 10.1038/srep29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner PC, Hopton RP, Lecluse Y, White KL, Fisher J, Lebailly P. 2010. Determinants of urinary deoxynivalenol and de-epoxy deoxynivalenol in male farmers from Normandy, France. J Agric Food Chem 58:5206–5212. doi: 10.1021/jf100892v. [DOI] [PubMed] [Google Scholar]
- 17.Heyndrickx E, Sioen I, Huybrechts B, Callebaut A, De Henauw S, De Saeger S. 2015. Human biomonitoring of multiple mycotoxins in the Belgian population: results of the BIOMYCO study. Environ Int 84:82–89. doi: 10.1016/j.envint.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Nagl V, Woechtl B, Schwartz-Zimmermann HE, Hennig-Pauka I, Moll WD, Adam G, Berthiller F. 2014. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol Lett 229:190–197. doi: 10.1016/j.toxlet.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Broekaert N, Devreese M, Van Bergen T, Schauvliege S, De Boevre M, De Saeger S, Vanhaecke L, Berthiller F, Michlmayr H, Malachova A, Adam G, Vermeulen A, Croubels S. 2017. In vivo contribution of deoxynivalenol-3-beta-d-glucoside to deoxynivalenol exposure in broiler chickens and pigs: oral bioavailability, hydrolysis and toxicokinetics. Arch Toxicol 91:699–721. doi: 10.1007/s00204-016-1710-2. [DOI] [PubMed] [Google Scholar]
- 20.Waché YJ, Valat C, Bougeard S, Burel C, Oswald IP, Fravalo P. 2009. Impact of deoxynivalenol on the intestinal microflora of pigs. Int J Mol Sci 10:1–17. doi: 10.3390/ijms10010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert H, Payros D, Pinton P, Théodorou V, Mercier-Bonin M, Oswald IP. 2017. Impact of mycotoxins on the intestine: are mucus and microbiota new targets? J Toxicol Environ Health B Crit Rev 16:3489–3507. doi: 10.1080/10937404.2017.1326071. [DOI] [PubMed] [Google Scholar]
- 22.Osselaere A, Santos R, Hautekiet V, De Backer P, Chiers K, Ducatelle R, Croubels S. 2013. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS One 8:e69014. doi: 10.1371/journal.pone.0069014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isaacson R, Kim HB. 2012. The intestinal microbiome of the pig. Animal Health Res Rev 13:100–109. doi: 10.1017/S1466252312000084. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Huang X, Fang S, Xin W, Huang L, Chen C. 2016. Uncovering the composition of microbial community structure and metagenomics among three gut locations in pigs with distinct fatness. Sci Rep 6:27427. doi: 10.1038/srep27427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao W, Wang Y, Liu S, Huang J, Zhai Z, He C, Ding J, Wang J, Wang H, Fan W, Zhao J, Meng H. 2015. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One 10:e0117441. doi: 10.1371/journal.pone.0117441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinritz SN, Mosenthin R, Weiss E. 2013. Use of pigs as a potential model for research into dietary modulation of the gut microbiota. Nutr Res Rev 26:119–209. doi: 10.1017/S0954422413000152. [DOI] [PubMed] [Google Scholar]
- 27.Berthiller F, Krska R, Domig KJ, Kneifel W, Juge N, Schuhmacher R, Adam G. 2011. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol Lett 206:264–267. doi: 10.1016/j.toxlet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michlmayr H, Varga E, Malachova A, Nguyen NT, Lorenz C, Haltrich D, Berthiller F, Adam GA. 2015. Versatile family 3 glycoside hydrolase from Bifidobacterium adolescentis hydrolyzes beta-glucosides of the Fusarium mycotoxins deoxynivalenol, nivalenol, and HT-2 toxin in cereal matrices. Appl Environ Microbiol 81:4885–4893. doi: 10.1128/AEM.01061-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrascu O, Beguet-Crespel F, Marinelli L, Le Chatelier E, Abraham A-L, Leclerc M, Klopp C, Terrapon N, Henrissat B, Blottiere HM, Dore J, Bera-Maillet C. 2017. A fibrolytic potential in the human ileum mucosal microbiota revealed by functional metagenomics. Sci Rep 7:40248. doi: 10.1038/srep40248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Looft T, Allen HK, Cantarel BL, Levine UY, Bayles DO, Alt DP, Henrissat B, Stanton TB. 2014. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J 8:1566–1576. doi: 10.1038/ismej.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mach N, Berri M, Estelle J, Levenez F, Lemonnier G, Denis C, Leplat J-J, Chevaleyre C, Billon Y, Dore J, Rogel-Gaillard C, Lepage P. 2015. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ Microbiol Rep 7:554–569. doi: 10.1111/1758-2229.12285. [DOI] [PubMed] [Google Scholar]
- 32.Grenier B, Bracarense AP, Schwartz HE, Trumel C, Cossalter AM, Schatzmayr G, Kolf-Clauw M, Moll WD, Oswald IP. 2012. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochem Pharmacol 83:1465–1473. doi: 10.1016/j.bcp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Chung WSF, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D, Duncan SH, Flint HJ. 2016. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol 14:3. doi: 10.1186/s12915-015-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. 2009. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr 101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 35.Fuller Z, Louis P, Mihajlovski A, Rungapamestry V, Ratcliffe B, Duncan AJ. 2007. Influence of cabbage processing methods and prebiotic manipulation of colonic microflora on glucosinolate breakdown in man. Br J Nutr 98:364–372. doi: 10.1017/S0007114507709091. [DOI] [PubMed] [Google Scholar]
- 36.Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammes WP. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol 67:2578–2585. doi: 10.1128/AEM.67.6.2578-2585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heilig HGHJ, Zoetendal EG, Vaughan EE, Marteau P, Akkermans ADL, de Vos WM. 2002. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl Environ Microbiol 68:114–123. doi: 10.1128/AEM.68.1.114-123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen R, Russell RK, Reiff C, Louis P, McIntosh F, Berry S, Mukhopadhya I, Bisset WM, Barclay AR, Bishop J, Flynn DM, McGrogan P, Loganathan S, Mahdi G, Flint HJ, El-Omar EM, Hold GL. 2012. Microbiota of de-novo pediatric IBD: increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn's but not in ulcerative colitis. Am J Gastroenterol 107:1913–1922. doi: 10.1038/ajg.2012.335. [DOI] [PubMed] [Google Scholar]
- 39.Vollmer M, Schröter D, Esders S, Neugart S, Farquharson F, Duncan SH, Schreiner M, Louis P, Maul R, Rohn S. 2017. Chlorogenic acid versus amaranth's caffeoylisocitric acid—gut microbial degradation of caffeic acid derivatives. Food Res Int 100(Pt 3):375–384. doi: 10.1016/j.foodres.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam-Leitch C, Scott KP, Flint HJ, Louis P. 2014. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 8:1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]