ABSTRACT
The critically endangered elkhorn coral (Acropora palmata) is affected by white pox disease (WPX) throughout the Florida Reef Tract and wider Caribbean. The bacterium Serratia marcescens was previously identified as one etiologic agent of WPX but is no longer consistently detected in contemporary outbreaks. It is now believed that multiple etiologic agents cause WPX; however, to date, no other potential pathogens have been thoroughly investigated. This study examined the association of Vibrio bacteria with WPX occurrence from August 2012 to 2014 at Looe Key Reef in the Florida Keys, USA. The concentration of cultivable Vibrio was consistently greater in WPX samples than in healthy samples. The abundance of Vibrio bacteria relative to total bacteria was four times higher in samples from WPX lesions than in adjacent apparently healthy regions of diseased corals based on quantitative PCR (qPCR). Multilocus sequence analysis (MLSA) was used to assess the diversity of 69 Vibrio isolates collected from diseased and apparently healthy A. palmata colonies and the surrounding seawater. Vibrio species with known pathogenicity to corals were detected in both apparently healthy and diseased samples. While the causative agent(s) of contemporary WPX outbreaks remains elusive, our results suggest that Vibrio spp. may be part of a nonspecific heterotrophic bacterial bloom rather than acting as primary pathogens. This study highlights the need for highly resolved temporal sampling in situ to further elucidate the role of Vibrio during WPX onset and progression.
IMPORTANCE Coral diseases are increasing worldwide and are now considered a major contributor to coral reef decline. In particular, the Caribbean has been noted as a coral disease hot spot, owing to the dramatic loss of framework-building acroporid corals due to tissue loss diseases. The pathogenesis of contemporary white pox disease (WPX) outbreaks in Acropora palmata remains poorly understood. This study investigates the association of Vibrio bacteria with WPX.
KEYWORDS: Acropora palmata, Caribbean, Vibrio, bacterial bloom, coral disease, pathobiome, pathobiont, white pox
INTRODUCTION
The health and function of reef-building corals are intricately intertwined with their symbiotic microbial associates. Corals host a diverse array of archaea, bacteria, fungi, viruses, apicomplexans, and photosynthetic microalgae in their surface mucus layer (SML), tissue, and skeleton (1, 2). These microbial associates are collectively referred to as the microbiome and aid in nutrient cycling and host immunity. However, these symbiotic relationships can vary from mutualistic to potentially pathogenic, depending on environmental factors, such as sea temperature (3–9), water flow (10), and nutrient levels (6, 7). While bacterial etiological agents are believed to be associated with many coral diseases, only a few studies have shown that specific bacteria are capable of causing disease through fulfillment of Koch's and Hill's postulates (11). There is a growing realization that the application of these fundamental postulates in coral disease (11, 12) and other fields (13, 14) is limited, because they are largely based upon the “one pathogen, one disease” framework. For example, different pathogens may be capable of eliciting the same disease signs in a particular coral host (11). Additionally, many diseases that initiate on mucosal surfaces in coral (11, 12) and other organisms (13, 15) may be caused by polymicrobial consortia rather than monocultures of a single pathogen. These cases are particularly difficult to characterize because multiple pathogens act synergistically to cause disease signs.
There is an urgent need to develop new frameworks to investigate coral disease, as it is recognized as an increasing threat to coral reef ecosystems due to global climate change and anthropogenic stressors (16–19). In the Caribbean, disease has contributed to a >90% population decline of the framework-building elkhorn coral (Acropora palmata) (20, 21). Consequently, A. palmata is now listed as critically endangered on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species and as threatened under the U.S. Endangered Species Act. The loss of A. palmata, and the complex architecture it once provided, has led to a “flattening” of Caribbean reefs, with consequences for reef biodiversity and ecosystems functioning (22). Living cover of A. palmata in the upper Florida Keys declined by 50% from 2004 to 2010 (23). Thirty percent of this loss was attributable to partial mortality caused by white pox (WPX) and other tissue loss diseases. From 2009 to 2014, seasonal WPX prevalence rates ranged from 23% to 60% in a survey covering 7 reefs throughout the Florida Keys National Marine Sanctuary (11). Though the established WPX pathogen Serratia marcescens is still detected in A. palmata mucus and the reef environment, it is not consistently associated with contemporary WPX outbreaks in the Florida Keys (24). The bacterium has not been detected in A. palmata displaying white pox signs in St. John, U.S. Virgin Islands (25), and the Bahamas (26). This led to the presumption that WPX signs are likely caused by multiple pathogens (11); however, to date, no other microbial taxa have been thoroughly investigated in relation to WPX. The aim of this study was to examine the association between WPX lesions and the bacterial genus Vibrio, which contains many species known to be pathogenic to corals, other marine organisms, and humans (27).
With more than 110 recognized species (http://www.bacterio.net/vibrio.html), Vibrio is one of the most diverse marine bacterial genera and is globally distributed in the coastal environment (27). To date, 10 different Vibrio species have been implicated as etiologic agents in coral disease through infection trials and fulfillment of Koch's and Hill's postulates (Table 1). Vibrio mediterranei (synonymous with Vibrio shiloi) and various strains of Vibrio coralliilyticus have been documented to cause bacterial bleaching disease in Oculina patagonica in the Mediterranean Sea (28, 29) and Pocillopora damicornis in the Indian Ocean (30). Vibrio harveyi (synonymous with V. carchariae) is one causative agent of white band disease in Caribbean Acropora cervicornis (31, 32), and a consortium of four Vibrio spp. (V. alginolyticus, V. harveyi, V. proteolyticus, and V. rotiferianus) cause Caribbean yellow band disease in Orbicella faveolata (33). White syndromes affecting multiple Acropora, Porites, and Montipora corals throughout the Pacific have been associated with Vibrio spp. in the Coralliilyticus, Harveyi, and Orientalis clades (34–39).
TABLE 1.
Vibrio spp. previously shown to cause disease in coral through infection trials, with partial or complete fulfillment of Koch's postulates
| Species by clade | Coral disease or syndrome (reference) |
|---|---|
| Clade Coralliilyticus | |
| V. coralliilyticus | Bacterial bleaching disease, Pocillopora damicornis and Oculina patagonica (28, 30); Montipora white syndrome, acute, Montipora capitata (35)a; white syndrome, Acropora cytherea, Montipora aequituberculata, and Pachyseris speciosa (34)a |
| Clade Harveyi | |
| V. alginolyticus | Yellow band disease, Orbicella faveolata (33)b; Porites white patch syndrome, Porites andrewsi (39) |
| V. harveyi (synonym of V. carchariae) | White syndrome, Acropora spp. (38)a; white band disease, Acropora cervicornis (31, 32)a; yellow band disease, O. faveolata (33)b |
| V. natriegens | Porites ulcerative white spot disease, P. cylindrica (85) |
| V. owensii | Montipora white syndrome, chronic, M. capitata (36)a |
| V. parahaemolyticus | Porites ulcerative white spot disease, P. cylindrica (85) |
| V. rotiferianus | Yellow band disease, O. faveolata (33)b |
| Clade Mediterranei | |
| V. mediterranei (synonym of V. shiloi) | Bacterial bleaching disease, O. patagonica (29) |
| Clade Orientalis | |
| V. tubiashii | White syndrome, Acropora muricata (37)a |
| Clade Proteolyticus | |
| V. proteolyticus | Yellow band disease, O. faveolata (33)b |
Diseases in which additional non-Vibrio pathogens have been detected or are suspected.
Diseases caused by a consortium of Vibrio species.
To date, the association of Vibrio with WPX has not been thoroughly investigated (25). To further examine the relationship between Vibrio and WPX, we assessed the concentration and relative abundance of Vibrio spp. in WPX-affected A. palmata in the Florida Keys, USA.
RESULTS
Abundance of cultivable Vibrio spp.
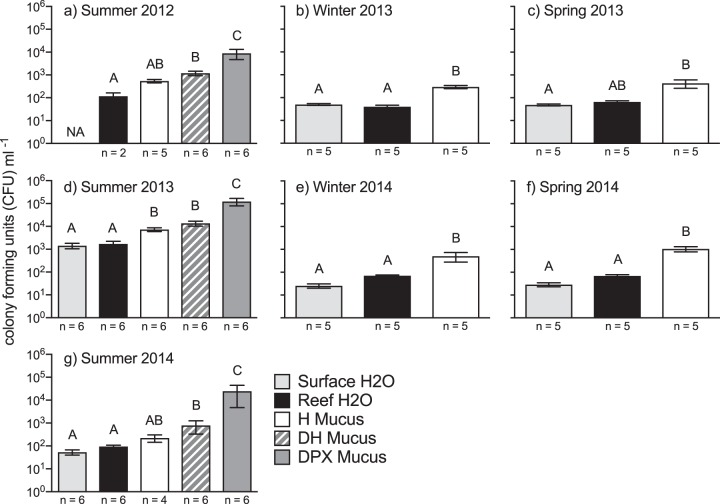
White pox disease (WPX) signs were noted at Looe Key Reef during late summer sampling events in August 2012, July 2013, and August 2014. No WPX occurred during winter and early summer sampling events. We enumerated the CFU of Vibrio spp. that grew on Vibrio-selective thiosulfate-citrate-bile salts-sucrose (TCBS) agar medium. The number of Vibrio CFU per milliliter was ∼9 times higher in mucus from apparently healthy colonies with no WPX signs (H mucus) than in seawater (Fig. 1). During periods of active WPX, the mean Vibrio CFU per milliliter was ∼19 times higher in mucus from WPX lesions (DPX mucus) than in both adjacent asymptomatic areas on the same A. palmata colonies (DH mucus; PAdj_Tukey ≤ 0.001 in all cases) and H mucus (PAdj_Tukey < 0.0001 in all cases) (Fig. 1). There was no difference between the mean CFU per milliliter in H mucus and DH mucus (PAdj_Tukey ≥ 0.31 in all cases). Comparing the mean Vibrio CFU per milliliter in late summer across the 3 years, Vibrio concentrations in all sample types were one to two orders of magnitude higher in 2013 (PAdj_Tukey ≤ 0.024 in all cases). Additionally, in 2013, Vibrio concentrations in apparently healthy samples (H mucus, 7,289 ± 1,351 CFU · ml−1; DH mucus, 13,524 ± 3,403 CFU · ml−1) reached levels measured in disease samples (DPX mucus) during other years (2012, 8,885 ± 4,196 CFU · ml−1 and 2014, 24,572 ± 19,857 CFU · ml−1; df = 55, q ≤ 2.15, PAdj_Tukey ≥ 0.913 in all cases). The prior 30-day mean water temperature, a metric previously found to be an important predictor of WPX (40), was lower for the disease sampling period in summer 2013 (28.89 ± 0.08°C) than in summer 2012 (30.85 ± 0.08°C; df = 87; q = 15.86; PAdj_Tukey < 0.0001) and 2014 (30.58 ± 0.08°C; df = 87; q = 13.39; PAdj_Tukey < 0.0001) (Table S2).
FIG 1.
The abundance of TCBS cultivable Vibrio spp. (mean ± standard error of the mean [SEM] CFU per milliliter) for A. palmata mucus and seawater collected from Looe Key Reef, FL. Bars with the same letters designate significant groupings based on Tukey's multiple-comparison tests following one-way ANOVA for each sampling period. Abbreviations: H, mucus from apparently healthy colonies with no WPX signs; DPX, mucus from WPX lesions; DH, mucus from asymptomatic areas on colonies with WPX lesions; reef H2O, seawater from approximately 1 m above the A. palmata colonies; surface H2O, seawater from the first 10 cm of the sea surface.
Quantitative PCR.
We examined the Vibrio relative abundance index (VAI) among coral mucus samples collected during August 2014 as the ratio of Vibrio species genome equivalents (GE) · ml−1 to total bacterial GE · ml−1, assessed by quantitative PCR (qPCR). The VAI of DPX mucus (0.24 ± 0.082) was ∼4.2 times higher than that of DH mucus (0.058 ± 0.0095, df = 9, q = 4.1, PAdj_Tukey = 0.042). However, this difference was not significantly significant for H mucus when accounting for multiple comparisons (0.082 ± 0.017, df = 9, q = 3.6, PAdj_Tukey = 0.096). There was no difference in the VAI values of H mucus and DH mucus (df = 9, q = 0.73, PAdj_Tukey = 0.86) (Fig. 2).
FIG 2.
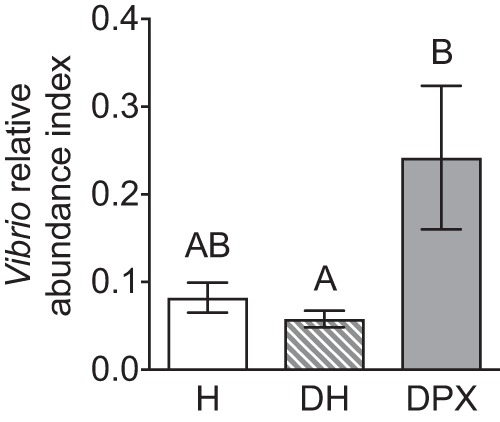
The Vibrio relative abundance index (VAI) of A. palmata mucus samples collected from Looe Key Reef, FL, during August 2014. The VAI (mean ± SEM) was calculated as the ratio of Vibrio species genome equivalents to total bacteria genome equivalents measured by qPCR. Bars with the same letters designate significant groupings assessed by Tukey's multiple-comparison tests following one-way ANOVA. Abbreviations: H, mucus from apparently healthy colonies with no WPX signs; DPX, mucus from WPX lesions; DH, mucus from asymptomatic areas on colonies with WPX lesions.
Isolate diversity assessed by multilocus sequence analysis.
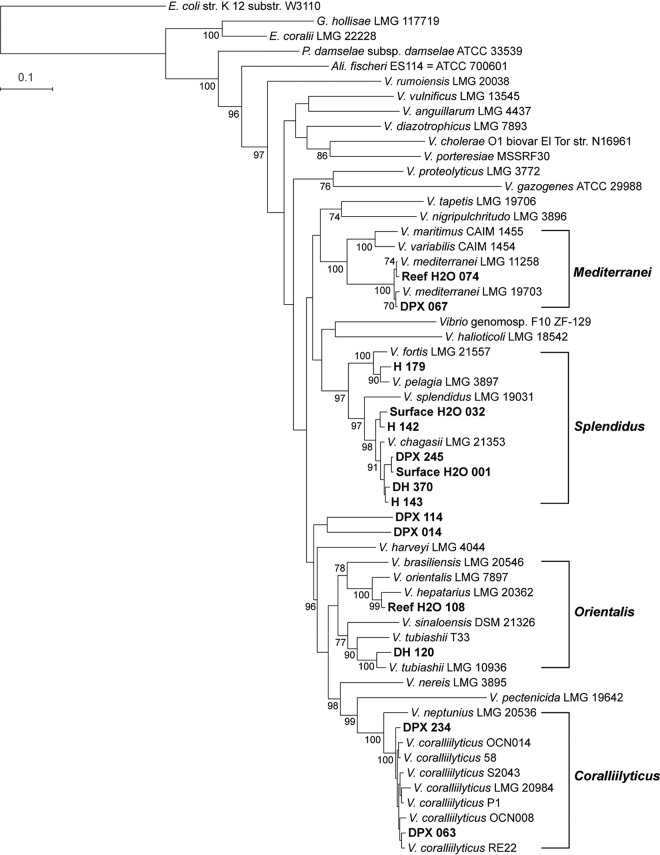
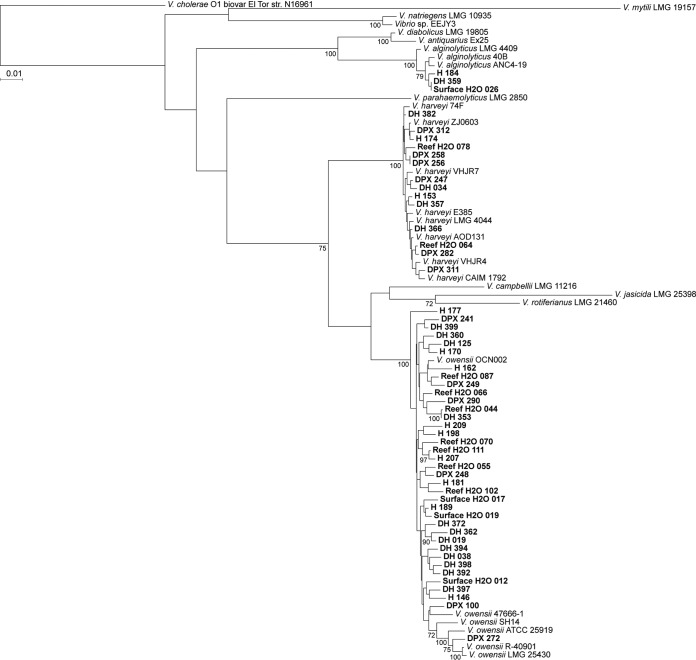
Using multilocus sequence analysis (MLSA) based on eight previously defined housekeeping genes, we analyzed the phylogenetic relatedness and diversity of 69 bacterial isolates that grew on Vibrio-selective TCBS medium (41, 42). The 69 Vibrio spp. utilized for MLSA were distributed across sample type: DPX mucus (n = 18), DH mucus (n = 19), H mucus (n = 15), surface H2O (n = 6), and reef H2O (n = 11) (Table 2). The isolates clustered within five of the 17 defined Vibrio clades (42), with the majority belonging to the Harveyi clade (Fig. 3 and 4). The species identity of these isolates was confirmed by calculating the average nucleotide identity (ANI) of the 8-gene loci concatenation with the reference strains listed in Table S1 in the supplemental material: V. alginolyticus (n = 3, 99.4 to 99.5% ANI), V. coralliilyticus (n = 2, 98.1 to 98.3% ANI), V. harveyi (n = 14, 99.4 to 99.6% ANI), V. mediterranei (n = 2, 98.9 to 99.0% ANI), V. owensii (n = 37, 98.1 to 98.8% ANI), and V. tubiashii (n = 1, 96.47% ANI) (Table 3). Of the 10 Vibrio species with known pathogenicity to corals (Table 1), three species (V. alginolyticus, V. harveyi, and V. owensii) were detected in mucus from apparently healthy corals, while four species (V. alginolyticus, V. coralliilyticus, V. mediterranei, and V. owensii) were detected in mucus from WPX lesions (Table 3).
TABLE 2.
Sequenced Vibrio isolates and accession numbers
| Isolation yr | Isolation sourcea | Isolate namea | BioSample accession no. |
|---|---|---|---|
| 2012 | DH | CDDH_019 | SAMN06921107 |
| 2012 | DH | CDDH_034 | SAMN06921108 |
| 2012 | DH | CDDH_038 | SAMN06921109 |
| 2012 | DH | CDDH_120 | SAMN06921110 |
| 2012 | DH | CDDH_125 | SAMN06921111 |
| 2013 | DH | CDDH_353 | SAMN06921112 |
| 2013 | DH | CDDH_357 | SAMN06921113 |
| 2013 | DH | CDDH_359 | SAMN06921114 |
| 2013 | DH | CDDH_360 | SAMN06921115 |
| 2013 | DH | CDDH_362 | SAMN06921116 |
| 2013 | DH | CDDH_366 | SAMN06921117 |
| 2013 | DH | CDDH_370 | SAMN06921118 |
| 2013 | DH | CDDH_372 | SAMN06921119 |
| 2013 | DH | CDDH_382 | SAMN06921120 |
| 2013 | DH | CDDH_392 | SAMN06921121 |
| 2013 | DH | CDDH_394 | SAMN06921122 |
| 2013 | DH | CDDH_397 | SAMN06921123 |
| 2013 | DH | CDDH_398 | SAMN06921124 |
| 2013 | DH | CDDH_399 | SAMN06921158 |
| 2012 | DPX | CDPX_014 | SAMN06921125 |
| 2012 | DPX | CDPX_063 | SAMN06921126 |
| 2012 | DPX | CDPX_067 | SAMN06921127 |
| 2012 | DPX | CDPX_100 | SAMN06921128 |
| 2012 | DPX | CDPX_114 | SAMN06921129 |
| 2013 | DPX | CDPX_234 | SAMN06921130 |
| 2013 | DPX | CDPX_241 | SAMN06921131 |
| 2013 | DPX | CDPX_245 | SAMN06921132 |
| 2013 | DPX | CDPX_247 | SAMN06921133 |
| 2013 | DPX | CDPX_248 | SAMN06921134 |
| 2013 | DPX | CDPX_249 | SAMN06921135 |
| 2013 | DPX | CDPX_256 | SAMN06921136 |
| 2013 | DPX | CDPX_258 | SAMN06921137 |
| 2013 | DPX | CDPX_272 | SAMN06921138 |
| 2013 | DPX | CDPX_282 | SAMN06921139 |
| 2013 | DPX | CDPX_290 | SAMN06921140 |
| 2013 | DPX | CDPX_311 | SAMN06921141 |
| 2013 | DPX | CDPX_312 | SAMN06921142 |
| 2013 | H | CH_142 | SAMN06921143 |
| 2013 | H | CH_143 | SAMN06921144 |
| 2013 | H | CH_146 | SAMN06921145 |
| 2013 | H | CH_153 | SAMN06921146 |
| 2013 | H | CH_162 | SAMN06921147 |
| 2013 | H | CH_170 | SAMN06921148 |
| 2013 | H | CH_174 | SAMN06921149 |
| 2013 | H | CH_177 | SAMN06921150 |
| 2013 | H | CH_179 | SAMN06921151 |
| 2013 | H | CH_181 | SAMN06921152 |
| 2013 | H | CH_184 | SAMN06921153 |
| 2013 | H | CH_189 | SAMN06921154 |
| 2013 | H | CH_198 | SAMN06921155 |
| 2013 | H | CH_207 | SAMN06921156 |
| 2013 | H | CH_209 | SAMN06921157 |
| 2013 | Surface H2O | H2O_001 | SAMN06921159 |
| 2013 | Surface H2O | H2O_012 | SAMN06921160 |
| 2013 | Surface H2O | H2O_017 | SAMN06921161 |
| 2013 | Surface H2O | H2O_019 | SAMN06921162 |
| 2013 | Surface H2O | H2O_026 | SAMN06921163 |
| 2013 | Surface H2O | H2O_032 | SAMN06921164 |
| 2013 | Reef H2O | H2O_044 | SAMN06921165 |
| 2013 | Reef H2O | H2O_055 | SAMN06921166 |
| 2013 | Reef H2O | H2O_064 | SAMN06921167 |
| 2013 | Reef H2O | H2O_066 | SAMN06921168 |
| 2013 | Reef H2O | H2O_070 | SAMN06921169 |
| 2013 | Reef H2O | H2O_074 | SAMN06921170 |
| 2013 | Reef H2O | H2O_078 | SAMN06921171 |
| 2013 | Reef H2O | H2O_087 | SAMN06921172 |
| 2013 | Reef H2O | H2O_102 | SAMN06921173 |
| 2013 | Reef H2O | H2O_108 | SAMN06921174 |
| 2013 | Reef H2O | H2O_111 | SAMN06921175 |
Abbreviations for isolation source: H, mucus from apparently healthy A. palmata colonies with no white pox disease (WPX) signs; DPX, mucus from WPX lesions; DH, mucus from asymptomatic areas on A. palmata colonies with WPX lesions; reef H2O, seawater from approximately 1 m above the A. palmata colonies; surface H2O, seawater from the first 10 cm of the sea surface.
FIG 3.
Maximum likelihood phylogenetic analysis based on eight gene loci. Boldface type indicates study isolates. Brackets indicate Vibrio clades containing isolates from A. palmata colonies. Bootstrap values (≥70) for 500 iterations are shown. Abbreviations for isolation source: H, mucus from apparently healthy A. palmata colonies with no WPX signs; DPX, mucus from WPX lesions; DH, mucus from asymptomatic areas on colonies with WPX lesions; reef H2O, seawater from approximately 1 m above the A. palmata colonies; surface H2O, seawater from the first 10 cm of the sea surface.
FIG 4.
Maximum likelihood phylogenetic analysis based on eight gene loci for the Harveyi clade. Boldface type indicates study isolates. Bootstrap values (≥70) for 500 iterations are shown. Abbreviations for isolation source: H, mucus from apparently healthy A. palmata colonies with no WPX signs; DPX, mucus from WPX lesions; DH, mucus from asymptomatic areas on colonies with WPX lesions; reef H2O, seawater from approximately 1 m above the A. palmata colonies; surface H2O, seawater from the first 10 cm of the sea surface.
TABLE 3.
Detection of Vibrio species with known pathogenicity to corals by sample type and year isolated
| Species by clade | 8-gene ANI (%)a | No. of isolates/total no. of isolates characterized, by yr isolatedb |
|||||
|---|---|---|---|---|---|---|---|
| 2012 |
2013 |
||||||
| DH | DPX | H2O | H | DH | DPX | ||
| Clade Coralliilyticus | |||||||
| V. coralliilyticus | 98.1–98.3 | 1/5 | 1/13 | ||||
| Clade Harveyi | |||||||
| V. alginolyticus | 99.4–99.5 | 1/17 | 1/15 | 1/14 | |||
| V. harveyi | 99.4–99.6 | 1/5 | 2/17 | 2/15 | 3/14 | 6/13 | |
| V. owensii | 98.1–98.8 | 3/5 | 1/5 | 10/17 | 10/15 | 8/14 | 5/13 |
| Clade Mediterranei | |||||||
| V. mediterranei | 98.9–99.0 | 1/5 | 1/17 | ||||
| Clade Orientalis | |||||||
| V. tubiashii | 96.5 | 1/14 | |||||
| Vibrio spp. with no known pathogenicity to corals | 1/5 | 2/5 | 3/17 | 2/15 | 1/14 | 1/13 | |
Range in average nucleotide identity (ANI) of the 8-gene concatenation of isolates.
H2O, combined surface and reef water; H, mucus from apparently healthy coral; DH, mucus from an asymptomatic region of a diseased coral; DPX, mucus from white pox lesions.
DISCUSSION
To date, 10 Vibrio species have been shown to cause disease in coral through infection trials, with partial or complete fulfillment of Koch's postulates (Table 1). In this study, the relative abundance of Vibrio spp. increased from ∼5% of the total bacteria in apparently healthy regions on diseased A. palmata to ∼25% of total bacteria in WPX lesions. Elevated Vibrio abundance in the disease state has been documented in A. cervicornis with white band disease (43) and multiple coral species affected by white syndromes in the Indo Pacific and Hawaii (34, 36, 44); however, an association between WPX and Vibrio has not been previously documented. Multilocus sequence analysis revealed that potentially pathogenic Vibrio species were present in samples from both diseased and apparently healthy A. palmata. These findings are similar to those of a previous study, which found potentially pathogenic Vibrio spp. in diseased and apparently healthy corals in at the Abrolhos reef bank in Brazil (45).
The lack of association between particular Vibrio spp. and disease status while overall relative abundance of Vibrio increased suggested that Vibrio might be a part of an opportunistic nonspecific heterotrophic bloom. The composition of organic compounds in coral mucus has been proposed to regulate microbial communities in the SML (46, 47), and heterotrophic bacterial blooms may be a response to changes in mucus composition during stress or substrate availability in dead and decaying tissue (48). For example, thermally driven shifts in the dominant monosaccharaides in A. muricata mucus have been shown to coincide with a change in bacterial community composition and an increase in potentially pathogenic Vibrio and other heterotrophic bacteria (49). It is also likely that tissue lysis caused by a primary pathogen or some other means (e.g., fish or snail predation) may lead to an increase in substrate availability for opportunistic heterotrophic bacteria. It is unclear whether the Vibrio blooms documented here are a cause or consequence of changes in microbiome homeostasis and host health. Regardless of the mechanism of occurrence, heterotrophic Vibrio blooms may contribute to further tissue breakdown through the induction of localized hypoxia. Microgradients of O2, driven by microbial activity, are known to be important in the progression of other coral diseases, such as black band disease (50). However, to determine the course of the WPX disease process, it is critical to know the age of lesions sampled, which can be difficult to determine in the field without very high-resolution monitoring (e.g., at least daily). The lesions sampled here were active (tissue was sloughing and skeleton was bright white), but we did not know when disease signs started with respect to when we collected our samples. It is likely that our results reflect secondary disease stages while missing initial pathogenesis. Future studies, with higher temporal resolution, are needed to understand whether certain Vibrio spp. are directly involved in WPX onset and progression.
It is also important to note that corals harbor different microbial communities in their SML from their tissue and skeleton (51). Due to sampling restrictions for this protected species, we were only able to analyze coral mucus and may have missed species-specific trends in other compartments (i.e., tissue and skeleton). Vibrio spp. may shift from the coral SML into coral tissue under temperature stress (5). It is likely that some Vibrio species are able to make this transition (due to traits such as chemotactic motility, attachment mechanisms, and oxidative stress defense systems [52, 53]), while other Vibrio species cannot. For instance, Lee and colleagues (5) found that under thermal stress, the relative abundance of Vibrio spp. increased first in coral mucus and then in coral tissue, and the majority of the Vibrio sequences recovered from coral tissues belonged to V. coralliilyticus. Our data, showing an increase in the relative abundance of Vibrio in mucus, suggest that it would be valuable to examine possible transitions of Vibrio populations between the A. palmata SML and tissue during late-summer WPX outbreaks. Fluorescence in situ hybridization (FISH) is needed to further elucidate how Vibrio bacteria are spatially located in both diseased and healthy tissue from A. palmata.
Our results highlight the need to understand triggers that allow Vibrio spp. to bloom in the coral SML. Seawater temperature is one well-described factor known to influence Vibrio dynamics in the coral microbiome (3, 4, 6–9); however, the effects of other stressors, such as increased nutrients and sedimentation, are not understood. In this study, we measured 1 to 2 orders of magnitude more Vibrio bacteria in all sample types during late summer 2013 than in other years. Late-summer sea temperatures were cooler in 2013 than in 2012 and 2014 (Table S2); however, this sampling event on 25 July 2013 coincided with a significant influx of Saharan dust aerosols that deposited iron and other nutrients into the oligotrophic coastal waters of the Florida Keys (54). Westrich et al. (54) reported that in the surface waters of Looe Key Vibrio spp. increased from less than 1% to 20% of the total microbial community within 24 h of the arrival and deposition of this Saharan dust event (54). Our data show that during the 2013 sampling event, Vibrio concentrations from apparently healthy A. palmata reached levels typically measured in disease samples (Fig. 1). This suggests that Saharan dust deposition may serve to tip iron and nutrient availability above certain thresholds, thus promoting Vibrio growth in the coral microbiome and potentially disrupting microbial community dynamics. Further studies are warranted to investigate whether African dust storms influence disease dynamics in A. palmata and other Caribbean corals by promoting the expansion or colonization of potentially pathogenic bacteria in the coral SML.
Vibrio spp. can be characterized as conditionally rare taxa (CRT) (55) in coastal seawater because they typically make up a minor portion of the total microbial assemblage but are capable of blooming in response to nutrient availability and other environmental factors (54). Likewise, Vibrio spp. are commonly detected, but at low abundance, in the coral microbiome (28, 45, 56, 57), and are proposed to play a role in sulfur and nitrogen cycling in the coral holobiont (58–62). Strains of Vibrio alginolyticus, V. harveyi, V. campbellii, and V. parahaemolyticus have been isolated from coral mucus that are capable of fixing N2, and in doing so may function as coral mutualists under nonstressful conditions (58, 63). However, Vibrio relative abundance can increase significantly in the coral microbiome during disease (34, 36, 44), temperature stress (3, 4, 6–9), and low water flow (10) and could affect its function in the coral microbial community. Vibrio spp. have a relatively large two-chromosome genome encoding an expansive metabolic repertoire that enables a competitive and quick response to new resources (50), and several Vibrio spp. display some of the highest known bacterial growth rates, at ∼10 to 12 min per doubling (51). Thus, Vibrio spp. may exist as CRT in the healthy coral microbiome, primed to respond rapidly to changing environmental conditions. Furthermore, the nature of the interactions between Vibrio and the coral host may change as Vibrio spp. transition from the mucus into host tissue (5) and/or with the activation and expression of Vibrio virulence mechanisms (64). Thus, Vibrio spp. may shift along the symbiotic spectrum from functioning as commensals or mutualists to functioning as potential pathogens during environmental stress or when hosts are immunosuppressed.
Sweet and Bulling (12) recently proposed that taking the coral pathobiome (members within the microbiome that are directly involved in pathogenesis) into consideration offers a new framework for advancing coral disease research beyond the “one pathogen, one disease” paradigm. Given that Vibrio spp. are often found in low abundance in apparently healthy coral hosts, it may be useful to examine conditionally rare Vibrio spp. as potential pathobionts in WPX and other tissue loss diseases. The concept is based in part on evidence that several gastrointestinal inflammatory disorders in humans are caused by bacterial species found in most healthy hosts (65, 66). Several well-described examples from human microbiome studies include the bacteria Clostridium difficile and Helicobacter pylori, which are present in low abundance in healthy hosts but are able to bloom and cause severe gastric disorders in some patients due to host genetics and immune function or environmental factors (such as long-term antibiotic use) (67, 68). While the pathobiont/pathobiome concept has not yet been widely applied to the study of coral disease (12), studies have begun to describe such observations. For example, Muller and van Woesik (40) found that WPX did not display properties of a contagious disease in situ. Instead, environmental conditions and host genetics were important predictors of WPX, consistent with the pathobiome model. The authors concluded that a common member of the A. palmata microbiome was the likely agent of WPX signs in A. palmata, but only with high seawater temperatures and in genetically susceptible hosts (40).
The etiology of contemporary WPX outbreaks in threatened elkhorn coral of the Florida Keys remains unknown (69). We documented a consistent pattern of Vibrio blooms in WPX lesions over a 3-year period. We also uncovered a large diversity of Vibrio bacteria, which encompassed six of the 10 Vibrio species with known pathogenicity to corals. These potentially pathogenic species were detected in both apparently healthy and diseased samples. This study lays the foundation to further examine Vibrio in WPX from a pathobiome/pathobiont framework. This framework should emphasize longitudinal studies to gain a mechanistic understanding of WPX onset and secondary stages, examination of environmental thresholds that trigger Vibrio blooms in the SML, immunological and genetic traits that may predispose certain hosts to Vibrio blooms, and comparative genomics to examine the metabolic and virulence potential of coral-associated Vibrio at the strain level.
MATERIALS AND METHODS
Field surveys and sample collection.
Acropora palmata colonies were sampled from Looe Key (3 m depth; 24°32.724′N 81°24.360′W), located in the Florida Keys National Marine Sanctuary (FKNMS), three times per year (winter, spring, and summer) from August 2012 to August 2014. At each sampling, colonies were examined visually for signs of WPX, defined as irregularly shaped lesions (at least 1 cm2) with sloughing tissue and exposure of bright white skeleton (11). Water temperature data were recorded hourly using a HOBO Temperature Pro version 2 data logger and attached to the substrate at the same depth as the A. palmata colonies. Late-summer temperature data were averaged for the 30 days prior to the sampling date, because this temperature window was previously found to be an important predictor of WPX (40). The 30-day average was compared for the three sampling years using a one-way analysis of variance (ANOVA), followed by Tukey's pairwise tests, accounting for multiple comparisons.
Nondestructive methods (7, 70) were used to collect the surface mucus layer (SML) of A. palmata with sterile needleless 12-ml syringes. During each sampling period, SML samples were taken from apparently healthy A. palmata colonies displaying no visual signs of disease (n = 4 to 6; denoted H mucus). We collected paired SML samples from colonies with WPX: one from the active margin of a disease lesion (n = 4 to 6; denoted DPX mucus) and one from an adjacent branch displaying no disease signs (n = 4 to 6; denoted DH mucus). Seawater was collected with 12-ml syringes from approximately 1 m above the A. palmata colonies (n = 2 to 6; denoted reef H2O) and from the first 10 cm of the sea surface (n = 4 to 6; denoted surface H2O). All samples were placed in bags, transported in a cooler filled with seawater at ambient temperature to the laboratory, and processed within 1 h of collection.
Quantification of cultivable Vibrio species.
Syringe contents were transferred into sterile 15-ml conical vials and vortexed for approximately 10 s. Then, 10 μl of SML from white pox lesions and 100 μl of all other sample types were spread in triplicate onto the Vibrionaceae selective medium, thiosulfate-citrate-bile salts-sucrose agar (TCBS; Oxoid), and incubated at ∼29°C. CFU were enumerated after approximately 24 h and expressed as mean CFU per milliliter. All counts were log-transformed to allow for a normally distributed data set (verified by the Shapiro-Wilk test of normality). Differences in the mean CFU per milliliter among sample type within each sampling period were examined using one-way ANOVA. An additional two-way ANOVA was performed on late-summer data to assess differences between years in addition to sample type. Significant groupings were determined by Tukey's pairwise tests, accounting for multiple comparisons.
Quantitative PCR.
We examined the relative abundance of Vibrio in relation to total bacteria during August 2014 among coral sample types (H, DH, and DPX mucus) by a SYBR green quantitative real-time PCR (qPCR) method targeting the 16S rRNA gene that has been previously described (71). DNA was obtained by pelleting duplicate 2-ml aliquots from each 12-ml syringe sample by centrifuging at 17,000 × g for 20 min. Supernatant fluids were decanted, and pellets were stored at −80°C until use. DNA was then extracted with the DNeasy blood and tissue kit (Qiagen, Valencia, CA), according to the manufacturer's protocol for Gram-positive bacteria. The duplicate pellets were thawed and resuspended in 180 μl of lysis buffer (20 mM Tris-HCl [pH 8], 2 mM EDTA, and 1.2% Triton X-100) containing lysozyme (20 mg · ml−1, final concentration) and incubated at 37°C for 30 min, followed by proteinase K digestion at 56°C for 1 h. Lysates of the duplicate 2-ml aliquots were combined onto a single DNeasy mini spin column for continuation of the DNeasy protocol. Purified DNA was eluted in 100 μl of Qiagen AE buffer. DNA was then diluted 1:10 in Qiagen AE buffer to reduce PCR inhibition.
The qPCR assay utilized Vibrio-specific primers targeting the V3-V4 region of the Vibrio 16S rRNA gene (Vib1 567f, 5′-GGCGTAAAGCGCATGCAGGT-3′; and Vib2 680r, 5′-GAAATTCTACCCCCCTCTACAG-3′ [72]) and general primers targeting the V6 region of the 16S rRNA gene for the domain Bacteria (967f, 5′-CAACGCGAAGAACCTTACC-3′; and 1046r, 5′-CGACAGCCATGCANCACCT-3′ [73]). Each reaction mixture contained 10 μl of PowerUp SYBR green master mix (Applied Biosystems, Thermo Fisher Scientific), 0.3 μM each primer, and 5 μl of DNA template, with molecular-grade water added, for a total reaction volume of 20 μl. All reactions were run in triplicate on a StepOne real-time PCR system (Applied Biosystems, Life Technologies, Grand Island, NY); they were performed under conditions according to the manufacturer's protocol for PowerUp SYBR green master mix and previously published methods (71): initial uracil-DNA glycosylase (UDG) activation at 50°C for 2 min, polymerase activation at 95°C for 2 min, and then 40 cycles of denaturation at 95°C for 15 s and annealing and elongation at 60°C for the Vibrio-specific qPCR. The qPCR using total bacteria was performed under the same cycling conditions, except the annealing and elongation were split into two steps: 61°C for 15 s for annealing and 72°C for 1 min for elongation. Each run was followed by a dissociation step (95°C for 30 s, 60°C for 30 s, and 95°C for 30 s) to determine a melt curve for the analysis of specificity and included three replicate negative (no-template) controls.
PCR standards were prepared from genomic DNA of V. alginolyticus (American Type Culture Collection strain 33839). The DNeasy blood and tissue kit (Qiagen, Valencia, CA) was used to extract DNA from an overnight culture, according to the manufacturer's protocol for Gram-negative bacteria. Standards for the general bacterial assay were prepared from six serial dilutions of purified genomic DNA, factoring 11 copies of the 16S rRNA gene for V. alginolyticus ATCC strain 33839 (74). The Vibrio-specific qPCR assay utilized linearized plasmid standards prepared from amplicon products of the Vib1 and Vib2 primers (described above). Briefly, amplicons were inserted into a PCR-4 vector and cloned into E. coli using a TA-TOPO kit (Life Technologies Grand Island, NY), and the plasmid was extracted (QIAquick Spin miniprep kit; Qiagen). The cloned region was sequenced to verify the correct insert and linearized with NotI (Roche, Indianapolis, IN) after cleanup (QIAquick PCR purification kit; Qiagen). Linearized standards for the Vibrio-specific qPCR assay were quantified using a Qubit fluorometer, serially diluted in Qiagen AE buffer, and run with each Vibrio-specific qPCR assay.
Calculation of Vibrio relative abundance.
The Vibrio relative abundance index (VAI) was calculated as the ratio of Vibrio species genome equivalents (GE) to total bacterial GE. Vibrio species GE were determined by dividing the sample copy number in Vibrio-specific qPCR assays by the average per-genome copy of the 16S rRNA gene in Vibrio (n = 9.1), which is published in the rRNA operon copy number database (rrnDB) version 5.0 (74). To determine total bacterial GE, we acquired publically available 16S rRNA gene community data from apparently healthy A. palmata in the Florida Keys (70). The mean per-genome copy number for the entire community (n = 2.65) was calculated by weighting the published rrnDB 16S rRNA gene copy number for all bacterial taxa present in the A. palmata samples by their mean relative abundance (see Data Set S1 in the supplemental material). Total bacterial GE were then determined by dividing the sample copy number in the general bacterial qPCR assays by 2.65. Vibrio species GE were divided by the total bacterial GE for each sample individually to obtain the sample VAI (Table S3). The arcsine square root transformation for proportional data was used on all VAI data preceding one-way ANOVA and Tukey's multiple-comparison tests.
Multilocus sequence analysis.
To investigate the diversity of Vibrio-associated diseased and healthy A. palmata, Vibrio colonies were selected in a haphazard fashion from TCBS agar plates in August 2012 and July 2013. Colonies were transferred and stored in deep-agar stabs of Zobell marine agar 2216 (Difco) and were later purified by streak isolation on TCBS medium a minimum of three times. Sixty-nine isolates were chosen in a haphazard fashion for full-genome sequencing and were distributed across sample types, including H mucus (n = 15), DH mucus (n = 19), DPX mucus (n = 18), surface H2O (n = 6), and reef H2O (n = 11) (Table 2). To obtain genomic DNA, the purified bacterial isolates were grown overnight in Zobell marine broth 2216 (Difco) at 29°C. Then, 750 μl of overnight growth was pelleted (1,300 × g for 5 min), washed twice with phosphate-buffered saline, and resuspended in 180 μl of Qiagen ATL buffer. We proceeded using the DNeasy blood and tissue kit (Qiagen, Valencia, CA) extractions according to the manufacturer's protocol for Gram-negative bacteria.
Whole-genome sequences (WGS) were obtained using the Illumina NextSeq platform in the mid-output mode (150 cycles with 150-bp paired-end reads). The sequence reads were subsampled for Bayesian error correction and de novo assembled using SPAdes version 3.6.2, with the careful mode turned on (75). The resulting contigs were further scaffolded using with SSPACE version 2.0 (76), and then gaps in regions with ambiguous nucleotides were closed with GapFiller version 1.11 (77).
MLSA was performed using regions of the eight housekeeping genes gapA, gyrB, ftsZ, mreB, pryH, recA, rpoA, and topA, as suggested by Sawabe and colleagues (41, 42) for inferring the evolutionary history of Vibrio. The nucleotide sequences of the housekeeping genes were retrieved from the draft genomes following autoannotation by the online server for Rapid Annotation of Microbial Genomes using Subsystems Technology (RAST) (78). For all 69 assembled genomes, each of the eight housekeeping genes was present as a continuous sequence (with no ambiguous nucleotides) on a single contig. Reference housekeeping gene sequences were obtained for Vibrio strains used in previous phylogenetic studies (41, 42) from the NCBI GenBank nucleotide sequence database and the Taxonomy of the Vibrios online database (http://www.taxvibrio.lncc.br/project). Additional reference sequences were retrieved from annotated Vibrio genomes available on the Pathosystems Resource Integration Center (PATRIC) online database version 3.2.76 (79). All references sequences are listed with the accession numbers in Table S1.
Sequences for each housekeeping gene were aligned separately using MUSCLE version 3.8.31 (80) and trimmed, allowing no gaps, with trimAl version 1.3 (81). The trimmed sequences of the eight housekeeping genes were concatenated, resulting in a multilocus sequence 4,203 bp long for each Vibrio strain. The 8-gene concatenations were aligned with MUSCLE version 3.8.31, and the alignment was used for maximum likelihood (ML) phylogenetic analysis with RAxML version 8.2.4 (82), with the general time-reversible (GTR) plus gamma model and 20 randomized starting trees. Concatenated loci were partitioned, and the shapes of the gamma distribution, nucleotide frequencies, and nucleotide substitution rates were estimated individually for each of the eight loci. Tree topology was checked by 500 bootstrap replicates. The average nucleotide identity (ANI) for the 8-gene locus concatenation was calculated for the Vibrio species isolates and the reference strains they clustered with using JSpecies version 2.7.0 (83, 84); this is reported in Table 3.
Accession number(s).
The accession numbers for the 69 Vibrio species isolates sequenced in this study are listed in Table 2 and in the NCBI database under BioProject accession number PRJNA385961.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NSF grants EF-1015342 and OCE-1357423 (to E.K.L).
Jeff Turner provided helpful suggestions and feedback for the phylogenetic analyses. We thank Dustin Kemp, Kathryn Sutherland, and James Porter, who assisted in field surveys and sample collection. We also acknowledge the staff at the Mote Tropical Research Lab on Summerland Key for their logistical support. Erich Bartels provided HOBO logger data. Mucus and other specimens were obtained under permit FKNMS-2010-131-A.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01035-17.
REFERENCES
- 1.Ainsworth TD, Thurber RV, Gates RD. 2010. The future of coral reefs: a microbial perspective. Trends Ecol Evol 25:233–240. doi: 10.1016/j.tree.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Bourne DG, Morrow KM, Webster NS. 2016. Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu Rev Microbiol 70:317–340. doi: 10.1146/annurev-micro-102215-095440. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler M, Seneca FO, Yum LK, Palumbi SR, Voolstra CR. 2017. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat Commun 8:14213. doi: 10.1038/ncomms14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tout J, Siboni N, Messer LF, Garren M, Stocker R, Webster NS, Ralph PJ, Seymour JR. 2015. Increased seawater temperature increases the abundance and alters the structure of natural Vibrio populations associated with the coral Pocillopora damicornis. Front Microbiol 6:403–412. doi: 10.3389/fmicb.2015.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee STM, Davy SK, Tang S-L, Fan T-Y, Kench PS. 2015. Successive shifts in the microbial community of the surface mucus layer and tissues of the coral Acropora muricata under thermal stress. FEMS Microbiol Ecol 91:fiv142. doi: 10.1093/femsec/fiv142. [DOI] [PubMed] [Google Scholar]
- 6.Vega Thurber R, Willner-Hall D, Rodriguez-Mueller B, Desnues C, Edwards RA, Angly F, Dinsdale E, Kelly L, Rohwer F. 2009. Metagenomic analysis of stressed coral holobionts. Environ Microbiol 11:2148–2163. doi: 10.1111/j.1462-2920.2009.01935.x. [DOI] [PubMed] [Google Scholar]
- 7.Zaneveld JR, Burkepile DE, Shantz AA, Pritchard CE, McMinds R, Payet JP, Welsh R, Correa AMS, Lemoine NP, Rosales S, Fuchs C, Maynard JA, Thurber RV. 2016. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat Commun 7:11833. doi: 10.1038/ncomms11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourne D, Iida Y, Uthicke S, Smith-Keune C. 2007. Changes in coral-associated microbial communities during a bleaching event. ISME J 2:350–363. doi: 10.1038/ismej.2007.112. [DOI] [PubMed] [Google Scholar]
- 9.Bourne DG, Munn CB. 2005. Diversity of bacteria associated with the coral Pocillopora damicornis from the Great Barrier Reef. Environ Microbiol 7:1162–1174. doi: 10.1111/j.1462-2920.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee STM, Davy SK, Tang S-L, Kench PS. 2017. Water flow buffers shifts in bacterial community structure in heat-stressed Acropora muricata. Sci Rep 7:43600. doi: 10.1038/srep43600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutherland KP, Berry B, Park A, Kemp DW, Kemp KM, Lipp EK, Porter JW. 2016. Shifting white pox aetiologies affecting Acropora palmata in the Florida Keys, 1994–2014. Philos Trans R Soc Lond B Biol Sci. 371:20150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweet MJ, Bulling MT. 2017. On the importance of the microbiome and pathobiome in coral health and disease. Front Mar Sci 4:2261–2211. doi: 10.3389/fmars.2017.00009. [DOI] [Google Scholar]
- 13.Nelson A, De Soyza A, Perry JD, Sutcliffe IC, Cummings SP. 2012. Polymicrobial challenges to Koch's postulates: ecological lessons from the bacterial vaginosis and cystic fibrosis microbiomes. 18:774–783. [DOI] [PubMed] [Google Scholar]
- 14.Vayssier-Taussat M, Albina E, Citti C, Cosson JF, Jacques MA, Lebrun MH, Le Loir Y, Ogliastro M, Petit MA, Roumagnac P, Candresse T. 2014. Shifting the paradigm from pathogens to pathobiome: new concepts in the light of meta-omics. Front Cell Infect Microbiol 4:29. doi: 10.3389/fcimb.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamont RJ, Hajishengallis G. 2015. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med 21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Precht WF, Gintert BE, Robbart ML, Fura R, van Woesik R. 2016. Unprecedented disease-related coral mortality in southeastern Florida. Sci Rep 6:31374. doi: 10.1038/srep31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Moreno D, Willis BL, Page AC, Weil E, Croquer A, Vargas-Angel B, Jordan-Garza AG, Jordán-Dahlgren E, Raymundo L, Harvell CD. 2012. Global coral disease prevalence associated with sea temperature anomalies and local factors. Dis Aquat Org 100:249–261. doi: 10.3354/dao02488. [DOI] [PubMed] [Google Scholar]
- 18.Maynard J, van Hooidonk R, Eakin CM, Puotinen M, Garren M, Williams G, Heron SF, Lamb J, Weil E, Willis B, Harvell CD. 2015. Projections of climate conditions that increase coral disease susceptibility and pathogen abundance and virulence. Nat Clim Chang 5:688–694. doi: 10.1038/nclimate2625. [DOI] [Google Scholar]
- 19.Randall CJ, van Woesik R. 2015. Contemporary white-band disease in Caribbean corals driven by climate change. Nat Clim Chang 5:375–379. doi: 10.1038/nclimate2530. [DOI] [Google Scholar]
- 20.Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, Peters EC, Santavy DL, Smith GW. 2002. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc Natl Acad Sci U S A 99:8725–8730. doi: 10.1073/pnas.092260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aronson RB, Precht WF. 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38. doi: 10.1023/A:1013103928980. [DOI] [Google Scholar]
- 22.Alvarez-Filip L, Dulvy NK, Gill JA, Cote IM, Watkinson AR. 2009. Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc R Soc B Biol Sci 276:3019–3025. doi: 10.1098/rspb.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams DE, Miller MW. 2011. Attributing mortality among drivers of population decline in Acropora palmata in the Florida Keys (USA). Coral Reefs 31:369–382. doi: 10.1007/s00338-011-0847-y. [DOI] [Google Scholar]
- 24.Joyner JL, Sutherland KP, Kemp D, Berry B, Griffin A, Porter J, Amador MHB, Noren HKG, Lipp EK. 2015. Systematic analysis of white pox disease in Acropora palmata of the Florida Keys and the role of Serratia marcescens. Appl Environ Microbiol 81:4451–4457. doi: 10.1128/AEM.00116-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May LA, Avadanei AR, Rogers CS, Miller J, Woodley CM. 2011. Microbial community analysis of Acropora palmata mucus swabs, water and sediment samples from Hawksnest Bay, St. John, U.S. Virgin Islands. NOAA technical memorandum NOS NCCOS 123 and CRCP 14. National Oceanic and Atmospheric Administration, Silver Spring, MD. https://www.coris.noaa.gov/activities/aplm_usvi/aplm_usvi_tm.pdf.
- 26.Lesser MP, Jarett JK. 2014. Culture-dependent and culture-independent analyses reveal no prokaryotic community shifts or recovery of Serratia marcescens in Acropora palmata with white pox disease. FEMS Microbiol Ecol 88:457–467. doi: 10.1111/1574-6941.12311. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Gil B, Thompson CC, Matsumura Y, Sawabe T, Iida T, Christen R, Thompson F, Sawabe T. 2014. The family Vibrionaceae, p 659–747. In Rosenberg E, Delong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes. Springer Berlin Heidelberg, Heidelberg, Germany. [Google Scholar]
- 28.Rubio-Portillo E, Yarza P, Penalver C, Ramos-Espla AA, Anton J. 2014. New insights into Oculina patagonica coral diseases and their associated Vibrio spp. communities. ISME J 8:1794–1807. doi: 10.1038/ismej.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kushmaro A, Banin E, Loya Y, Stackebrandt E, Rosenberg E. 2001. Vibrio shiloi sp. nov., the causative agent of bleaching of the coral Oculina patagonica. Int J Syst Evol Microbiol 51:1383–1388. doi: 10.1099/00207713-51-4-1383. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Haim Y, Zicherman-Keren M, Rosenberg E. 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl Environ Microbiol 69:4236–4242. doi: 10.1128/AEM.69.7.4236-4242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil-Agudelo DL, Smith GW, Weil E. 2006. The white band disease type II pathogen in Puerto Rico. Rev Biol Trop 54:59–67.18457175 [Google Scholar]
- 32.Ritchie KB, Smith GW. 1998. Type II white-band disease. Rev Biol Trop 46:199–203. [Google Scholar]
- 33.Cervino JM, Thompson FL, Gomez-Gil B, Lorence EA, Goreau TJ, Hayes RL, Winiarski-Cervino KB, Smith GW, Hughen K, Bartels E. 2008. The Vibrio core group induces yellow band disease in Caribbean and Indo-Pacific reef-building corals. J Appl Microbiol 105:1658–1671. doi: 10.1111/j.1365-2672.2008.03871.x. [DOI] [PubMed] [Google Scholar]
- 34.Sussman M, Willis BL, Victor S, Bourne DG. 2008. Coral pathogens identified for White Syndrome (WS) epizootics in the Indo-Pacific. PLoS One 3:e2393. doi: 10.1371/journal.pone.0002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ushijima B, Videau P, Burger AH, Shore-Maggio A, Runyon CM, Sudek M, Aeby GS, Callahan SM. 2014. Vibrio coralliilyticus strain OCN008 is an etiological agent of acute Montipora white syndrome. Appl Environ Microbiol 80:2102–2109. doi: 10.1128/AEM.03463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ushijima B, Smith A, Aeby GS, Callahan SM. 2012. Vibrio owensii induces the tissue loss disease Montipora white syndrome in the Hawaiian reef coral Montipora capitata. PLoS One 7:e46717-10. doi: 10.1371/journal.pone.0046717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweet M, Bythell J. 2015. White syndrome in Acropora muricata: nonspecific bacterial infection and ciliate histophagy. Mol Ecol 24:1150–1159. doi: 10.1111/mec.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luna GM, Bongiorni L, Gili C, Biavasco F, Danovaro R. 2009. Vibrio harveyi as a causative agent of the White syndrome in tropical stony corals. Environ Microbiol Rep 2:120–127. doi: 10.1111/j.1758-2229.2009.00114.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhenyu X, Shaowen K, Chaoqun H, Zhixiong Z, Shifeng W, Yongcan Z. 2013. First characterization of bacterial pathogen, Vibrio alginolyticus, for Porites andrewsi white syndrome in the South China Sea. PLoS One 8:e75425-8. doi: 10.1371/journal.pone.0075425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller EM, van Woesik R. 2014. Genetic susceptibility, colony size, and water temperature drive white-pox disease on the coral Acropora palmata. PLoS One 9:e110759-. doi: 10.1371/journal.pone.0110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawabe T, Kita-Tsukamoto K, Thompson FL. 2007. Inferring the evolutionary history of vibrios by means of multilocus sequence analysis. J Bacteriol 189:7932–7936. doi: 10.1128/JB.00693-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawabe T, Ogura Y, Matsumura Y, Gao F, Amin AR, Mino S, Nakagawa S, Sawabe T, Kumar R, Fukui Y, Satomi M, Matsushima R, Thompson FL, Gomez-Gil B, Christen R, Maruyama F, Kurokawa K, Hayashi T. 2013. Updating the Vibrio clades defined by multilocus sequence phylogeny: proposal of eight new clades, and the description of Vibrio tritonius sp. nov. Front Microbiol 4:414. doi: 10.3389/fmicb.2013.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie KB, Smith GW. 1995. Carbon-source utilization patterns of coral-associated marine heterotrophs. J Mar Biotechnol 3:105–107. [Google Scholar]
- 44.Pantos O, Bythell JC. 2006. Bacterial community structure associated with white band disease in the elkhorn coral Acropora palmata determined using culture-independent 16S rRNA techniques. Dis Aquat Org 69:79–88. doi: 10.3354/dao069079. [DOI] [PubMed] [Google Scholar]
- 45.Alves N Jr, Neto OSM, Silva BSO, De Moura RL, Francini-Filho RB, Barreira e Castro C, Paranhos R, Bitner-Mathé BC, Kruger RH, Vicente ACP, Thompson CC, Thompson FL. 2010. Diversity and pathogenic potential of vibrios isolated from Abrolhos Bank corals. Environ Microbiol Rep 2:90–95. doi: 10.1111/j.1758-2229.2009.00101.x. [DOI] [PubMed] [Google Scholar]
- 46.Bythell JC, Wild C. 2011. Biology and ecology of coral mucus release. J Exp Mar Biol Ecol 408:88–93. doi: 10.1016/j.jembe.2011.07.028. [DOI] [Google Scholar]
- 47.Wegley L, Edwards R, Rodriguez-Brito B, Liu H, Rohwer F. 2007. Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environ Microbiol 9:2707–2719. doi: 10.1111/j.1462-2920.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- 48.Dang H, Lovell CR. 2016. Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev 80:91–138. doi: 10.1128/MMBR.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee STM, Davy SK, Tang S-L, Kench PS. 2016. Mucus sugar content shapes the bacterial community structure in thermally stressed Acropora muricata. Front Microbiol 7:3274–3212. doi: 10.3389/fmicb.2016.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glas MS, Sato Y, Ulstrup KE, Bourne DG. 2012. Biogeochemical conditions determine virulence of black band disease in corals. ISME J 6:1526–1534. doi: 10.1038/ismej.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweet MJ, Croquer A, Bythell JC. 2010. Bacterial assemblages differ between compartments within the coral holobiont. Coral Reefs 30:39–52. doi: 10.1007/s00338-010-0695-1. [DOI] [Google Scholar]
- 52.Santos Ede O, Alves N Jr, Dias GM, Mazotto AM, Vermelho A, Vora GJ, Wilson B, Beltran VH, Bourne DG, Le Roux F, Thompson FL. 2011. Genomic and proteomic analyses of the coral pathogen Vibrio coralliilyticus reveal a diverse virulence repertoire. ISME J 5:1471–1483. doi: 10.1038/ismej.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munn CB, Marchant HK, Moody AJ. 2008. Defences against oxidative stress in vibrios associated with corals. FEMS Microbiol Lett 281:58–63. doi: 10.1111/j.1574-6968.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 54.Westrich JR, Ebling AM, Landing WM, Joyner JL, Kemp KM, Griffin DW, Lipp EK. 2016. Saharan dust nutrients promote Vibrio bloom formation in marine surface waters. Proc Natl Acad Sci U S A 113:5964–5969. doi: 10.1073/pnas.1518080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shade A, Jones SE, Caporaso JG, Handelsman J, Knight R, Fierer N, Gilbert JA. 2014. Conditionally rare taxa disproportionately contribute to temporal changes in microbial diversity. mBio 5:e01371-. doi: 10.1128/mBio.01371-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu ND, Vollmer SV. 2016. Caribbean corals house shared and host-specific microbial symbionts over time and space. Environ Microbiol Rep 8:493–500. doi: 10.1111/1758-2229.12412. [DOI] [PubMed] [Google Scholar]
- 57.Pootakham W, Mhuantong W, Yoocha T, Putchim L, Sonthirod C, Naktang C, Thongtham N, Tangphatsornruang S. 2017. High resolution profiling of coral-associated bacterial communities using full-length 16S rRNA sequence data from PacBio SMRT sequencing system. Sci Rep 7:215–214. doi: 10.1038/s41598-017-00308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chimetto LA, Brocchi M, Thompson CC, Martins RCR, Ramos HR, Thompson FL. 2008. Vibrios dominate as culturable nitrogen-fixing bacteria of the Brazilian coral Mussismilia hispida. Syst Appl Microbiol 31:312–319. doi: 10.1016/j.syapm.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Lema KA, Willis BL, Bourne DG. 2014. Amplicon pyrosequencing reveals spatial and temporal consistency in diazotroph assemblages of the Acropora millepora microbiome. Environ Microbiol. 16:3345–3359. doi: 10.1111/1462-2920.12366. [DOI] [PubMed] [Google Scholar]
- 60.Lema KA, Clode PL, Kilburn MR, Thornton R, Willis BL, Bourne DG. 2015. Imaging the uptake of nitrogen-fixing bacteria into larvae of the coral Acropora millepora. ISME J 10:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raina J-B, Dinsdale EA, Willis BL, Bourne DG. 2010. Do the organic sulfur compounds DMSP and DMS drive coral microbial associations? Trends Microbiol 18:101–108. doi: 10.1016/j.tim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Raina J-B, Tapiolas D, Willis BL, Bourne DG. 2009. Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl Environ Microbiol 75:3492–3501. doi: 10.1128/AEM.02567-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benavides M, Bednarz VN, Ferrier-Pagès C. 2017. Diazotrophs: overlooked key players within the coral symbiosis and tropical reef ecosystems? Front Mar Sci 4:2261–2217. doi: 10.3389/fmars.2017.00010. [DOI] [Google Scholar]
- 64.Kimes NE, Grim CJ, Johnson WR, Hasan NA, Tall BD, Kothary MH, Kiss H, Munk AC, Tapia R, Green L, Detter C, Bruce DC, Brettin TS, Colwell RR, Morris PJ. 2012. Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus. ISME J 6:835–846. doi: 10.1038/ismej.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chow J, Tang H, Mazmanian SK. 2011. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol 23:473–480. doi: 10.1016/j.coi.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Round JL, Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moyat M, Velin D. 2014. Immune responses to Helicobacter pylori infection. World J Gastroenterol 20:5583–5593. doi: 10.3748/wjg.v20.i19.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamada N, Chen GY, Inohara N, Núñez G. 2013. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aiyar SE, Gaal T, Gourse RL. 2002. rRNA promoter activity in the fast-growing bacterium Vibrio natriegens. J Bacteriol 184:1349–1358. doi: 10.1128/JB.184.5.1349-1358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kemp DW, Rivers AR, Kemp KM, Lipp EK, Porter JW, Wares JP. 2015. Spatial homogeneity of bacterial communities associated with the surface mucus layer of the reef-building coral Acropora palmata. PLoS One 10:e0143790. doi: 10.1371/journal.pone.0143790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vezzulli L, Brettar I, Pezzati E, Reid PC, Colwell RR, Höfle MG, Pruzzo C. 2012. Long-term effects of ocean warming on the prokaryotic community: evidence from the vibrios. ISME J 6:21–30. doi: 10.1038/ismej.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson JR, Randa MA, Marcelino LA, Tomita-Mitchell A, Lim E, Polz MF. 2004. Diversity and dynamics of a North Atlantic coastal Vibrio community. Appl Environ Microbiol 70:4103–4110. doi: 10.1128/AEM.70.7.4103-4110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sogin ML, Morrison HG, Huber JA, Mark Welch D, Huse SM, Neal PR, Arrieta JM, Herndl GJ. 2006. Microbial diversity in the deep sea and the underexplored “rare biosphere.” Proc Natl Acad Sci U S A 103:12115–12120. doi: 10.1073/pnas.0605127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stoddard SF, Smith BJ, Hein R, Roller BRK, Schmidt TM. 2015. rrnDB: improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res 43:D593–D598. doi: 10.1093/nar/gku1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. 2011. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27:578–579. doi: 10.1093/bioinformatics/btq683. [DOI] [PubMed] [Google Scholar]
- 77.Boetzer M, Pirovano W. 2012. Toward almost closed genomes with GapFiller. Genome Biol 13:R56. doi: 10.1186/gb-2012-13-6-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2013. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, Machi D, Mao C, Nordberg EK, Olson R, Overbeek R, Pusch GD, Shukla M, Schulman J, Stevens RL, Sullivan DE, Vonstein V, Warren A, Will R, Wilson MJC, Yoo HS, Zhang C, Zhang Y, Sobral BW. 2013. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res 42:D581–D591. doi: 10.1093/nar/gkt1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richter M, Rosselló-Móra R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A 106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richter M, Rosselló-Móra R, Oliver Glöckner F, Peplies J. 2016. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 32:929–931. doi: 10.1093/bioinformatics/btv681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arboleda M, Reichardt WT. 2010. Vibrio sp. causing Porites ulcerative white spot disease. Dis Aquat Org 90:93–104. doi: 10.3354/dao02222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.