ABSTRACT
Heat-stable toxin (STa)-producing enterotoxigenic Escherichia coli (ETEC) strains are a top cause of moderate-to-severe diarrhea in children from developing countries and a common cause of travelers' diarrhea. Recent progress in using STa toxoids and toxoid fusions to induce neutralizing anti-STa antibodies has accelerated ETEC vaccine development. However, concern remains regarding whether the derived anti-STa antibodies cross-react with STa-like guanylin and uroguanylin, two guanylate cyclase C (GC-C) ligands regulating fluid and electrolyte transportation in human intestinal and renal epithelial cells. To further divert STa from guanylin and uroguanylin structurally and antigenically and to eliminate anti-STa antibody cross-reactivity with guanylin and uroguanylin, we mutated STa at the 9th (leucine), 12th (asparagine), and 14th (alanine) residues for the double and triple mutants STaL9A/N12S, STaL9A/A14H, STaN12S/A14T, and STaL9A/N12S/A14H. We then fused each STa mutant (three copies) to a monomeric heat-labile toxin (LT) mutant (mnLTR192G/L211A) for the toxoid fusions 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaL9A/A14H-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, and 3×STaL9A/N12S/A14H-mnLTR192G/L211A; examined each fusion for anti-STa immunogenicity; and assessed the derived antibodies for in vitro neutralization activity against STa toxicity and for cross-reactivity with guanylin and uroguanylin. Mice subcutaneously immunized with each fusion protein developed anti-STa antibodies, and the antibodies derived from 3×STaN12S-mnLTR192G/L211A, 3×STaL9A/N12S-mnLTR192G/L211A, or 3×STaN12S/A14T-mnLTR192G/L211A prevented STa from the stimulation of intracellular cGMP in T-84 cells. Competitive enzyme-linked immunosorbent assays (ELISAs) showed that guanylin and uroguanylin hardly blocked the binding of anti-STa antibodies to the coated STa-ovalbumin conjugate. These results indicated that antibodies derived from 3×STaN12S-mnLTR192G/L211A, 3×STaL9A/N12S-mnLTR192G/L211A, or 3×STaN12S/A14T-mnLTR192G/L211A neutralized STa and had little cross-reactivity with guanylin and uroguanylin, suggesting that these toxoid fusions are suitable antigens for ETEC vaccines.
IMPORTANCE Enterotoxigenic Escherichia coli (ETEC) strains are a leading cause of children's diarrhea and travelers' diarrhea. Currently, there is no licensed vaccine against ETEC diarrhea. One key challenge is to identify safe antigens to induce antibodies neutralizing the key STa without cross-reacting with guanylin and uroguanylin, two important ligands controlling homeostasis in human intestinal and renal epithelial cells. In this study, we generated nontoxic fusion antigens that induced antibodies that neutralize STa enterotoxicity in vitro and do not cross-react with guanylin or uroguanylin. These fusions have become the preferred antigens for the development of ETEC vaccines to potentially prevent the deaths of hundreds of thousands of young children and hundreds of millions of diarrheal cases each year.
KEYWORDS: ETEC, enterotoxigenic Escherichia coli, antibody cross-reactivity, diarrhea, guanylin, uroguanylin
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) bacteria that produce heat-stable toxin (STa) type Ib (hSTa [human-type STa] or STh), alone or together with heat-labile toxin (LT), are among the top five causes of moderate-to-severe diarrhea in children less than 5 years of age in developing countries (1) and the most common cause of diarrhea in children and adults traveling from developed countries to regions where ETEC is endemic (2–4). Currently, there is no licensed vaccine for ETEC-associated children's diarrhea or travelers' diarrhea. One key challenge in ETEC vaccine development is the inability to use safe STa antigens to induce neutralizing anti-STa antibodies (5, 6). However, recent studies demonstrated that a nontoxic STa mutant (toxoid; 1 to 3 copies) genetically fused to a monomeric LT toxoid (mnLT) (a peptide consisting of one LTA subunit mutant and one LTB subunit, to serve as the LT antigen and a carrier to facilitate STa antigenicity) induced antibodies that neutralized STa and LT enterotoxicity (7–10). Among those nontoxic STa-mnLT fusions, 3×STaN12S-mnLTR192G/L211A (previously named 3×STaN12S-dmLT [10]), which carried three copies of the STa toxoid STaN12S and one copy of the monomeric LT mutant mnLTR192G/L211A (with the LTA subunit mutated at residues 192 and the 211), was considered optimal in inducing neutralizing anti-STa antibodies (10, 11). Moreover, antibodies derived from the toxoid fusion 3×STaN12S-mnLTR192G/L211A prevented STa-positive (STa+) ETEC diarrhea in a pig challenge model (12), suggesting that 3×STaN12S-mnLTR192G/L211A is a leading ETEC vaccine antigen (13).
However, potential cross-reactivity between anti-STa antibodies and guanylin or uroguanylin remains a concern (14). Guanylin and uroguanylin are structurally and functionally similar to STa (15–17) (Fig. 1). Like STa, heat-stable guanylin and uroguanylin regulate water and electrolyte homeostasis in intestinal epithelial cells by activating the guanylate cyclase C (GC-C) pathway and controlling intracellular cGMP levels (15, 18–20). Guanylin- or uroguanylin-regulated Cl− and HCO3− secretion and Na+ absorption in host epithelial cells can be altered by cross-reactive anti-STa antibodies, potentially leading to adverse health consequences, including hypernatremia.
FIG 1.
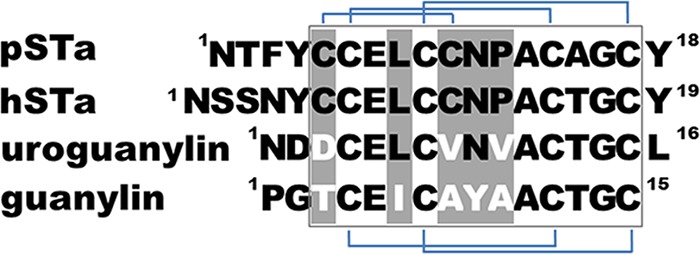
Amino acid sequences of mature porcine-type STa (pSTa) or human-type STa (hSTa), uroguanylin, and guanylin peptides. The boxed sequence represents the toxicity domain, and heterogeneous amino acid residues are in light gray.
STa toxoids that induce antibodies that neutralize STa without cross-reacting with guanylin and uroguanylin are preferred for ETEC vaccine development. These STa toxoids would likely have mutations at certain residues to further divert them from guanylin and uroguanylin in structure and antigenicity. Toxoid fusion 3×STaN12S-mnLTR192G/L211A carrying the STa toxoid STaN12S induced strongly neutralizing anti-STa antibodies (10–12). However, anti-STa antibodies derived from this toxoid fusion have not been examined for cross-reactivity with guanylin or uroguanylin. Presumably, STaN12S would be separated further from uroguanylin or guanylin if this toxoid has an additional residue(s) mutated, although such STa mutants potentially have altered STa antigenic properties and may induce antibodies not specific to native STa and thus would not neutralize STa enterotoxicity.
In this study, we mutated STa at the 9th, 12th, and 14th residues for STa double or triple mutants; genetically fused each STa mutant (three copies) to the monomer LT mutant mnLTR192G/L211A (a single peptide of one LTA subunit mutated at residues 192 and 211 and one LTB subunit); and examined each toxoid fusion for anti-STa immunogenicity. Furthermore, the induced anti-STa antibodies were examined for neutralization activity against STa toxicity and for antibody cross-reactivity with guanylin and uroguanylin.
RESULTS
Production of four genetic fusions carrying three copies of an STa double or triple mutant and one copy of a monomeric LT mutant (mnLTR192G/L211A).
Guanylin, which has been reported to have no cross-reactivity with anti-STa antibodies (16), differs from uroguanylin (which showed some cross-reactivity with anti-STa antibodies) at the 3rd, 6th, 8th, 9th, and 10th residues (Fig. 1). The noncysteine residues at the 3rd and 8th positions separate both guanylin peptides (referred to as guanylin and uroguanylin) from STa by forming only two disulfide bridges. The 6th (leucine) and 9th (asparagine) residues (equivalent to the 9th and 12th residues of STa) are shared by STa and uroguanylin but not guanylin (Fig. 1). Differences at these two residues likely dictate anti-STa antibody cross-reactivity with uroguanylin but not guanylin. Therefore, mutations at the 9th and/or the 12th residue of STa should enhance antigenic heterogeneity and divert STa further from uroguanylin in structure and potential antigenicity. By adding a mutation at the 14th residue of STa, which was demonstrated to play a role in the enterotoxicity and immunogenicity of STa, we generated three STa double mutants, STaL9A/N12S, STaN12S/A14T, and STaL9A/A14H, and one triple mutant, STaL9A/N12S/A14H. Genetically fusing three copies of each STa mutant to mnLTR192G/L211A produced four fusions: 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, 3×STaL9A/A14H-mnLTR192G/L211A, and 3×STaL9A/N12S/A14H-mnLTR192G/L211A. Cloning each fusion gene (a single open reading frame [ORF]) at the NcoI and EagI sites of vector pET28α produced four recombinant strains to express fusion proteins without a 6×His tag.
Four tagless double or triple mutant fusion proteins and tagless 3×STaN12S-mnLTR192G/L211A (previously labeled 3×STaN12S-dmLT) were verified with anti-cholera toxin (anti-CT) (Fig. 2) and anti-STa antisera. Expressed fusion proteins were confirmed by the lack of STa enterotoxicity. Two hundred micrograms of the fusion protein or 25 μl of the supernatant filtrates from a bacterial culture of each recombinant strain grown overnight did not significantly elevate intracellular cGMP levels in T-84 cells. When administered subcutaneously (s.c.), all fusion proteins were well tolerated by mice, as none of the mice showed adverse effects except for redness and mild swelling at the injection sites.
FIG 2.
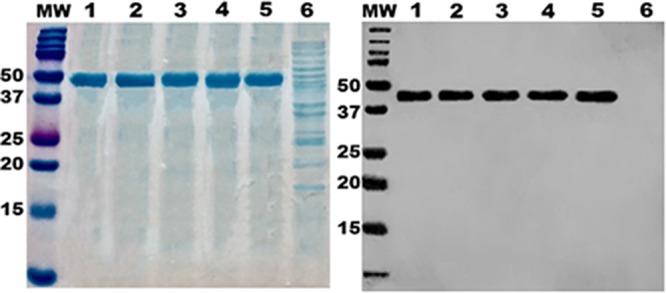
Expression and characterization of 3×STatoxoid-mnLTR192G/L211A fusion proteins. (Left) SDS-PAGE with Coomassie blue staining to show the purity of purified fusion proteins. (Right) Western blotting for detection of fusion proteins with anti-CT rabbit antiserum (Sigma). Lanes: 1, 3×STaL9A/N12S-mnLTR192G/L211A; 2, 3×STaN12S/A14T-mnLTR192G/L211A; 3, 3×STaL9A/N12S/A14H-mnLTR192G/L211A; 4, 3×STaL9A/A14H-mnLTR192G/L211A; 5, 3×STaN12S-mnLTR192G/L211A; 6, total protein of host E. coli strain BL21; MW, molecular weight marker (in thousands).
STatoxoid-mnLTR192G/L211A fusion proteins induce anti-STa and anti-LT antibodies in s.c. immunized mice.
All subcutaneously immunized mice developed anti-STa and anti-LT antibody responses (Fig. 3). Anti-STa IgG titers were 3.61 ± 0.46, 3.35 ± 0.65, 3.01 ± 0.61, and 3.53 ± 0.65 log10 units in serum samples of mice immunized with 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, 3×STaL9A/A14H-mnLTR192G/L211A, and 3×STaL9A/N12S/A14H-mnLTR192G/L211A, respectively. Anti-LT IgG titers in these groups were 3.63 ± 0.20, 3.52 ± 0.24, 3.64 ± 0.21, and 3.62 ± 0.24 log10 units, respectively. Anti-STa and anti-LT IgG titers in the serum samples of mice immunized with 3×STaN12S-mnLTR192G/L211A were 2.98 ± 0.46 and 3.85 ± 0.16 log10 units, respectively. No anti-STa or anti-LT IgG response was detected in serum samples of the control mice.
FIG 3.
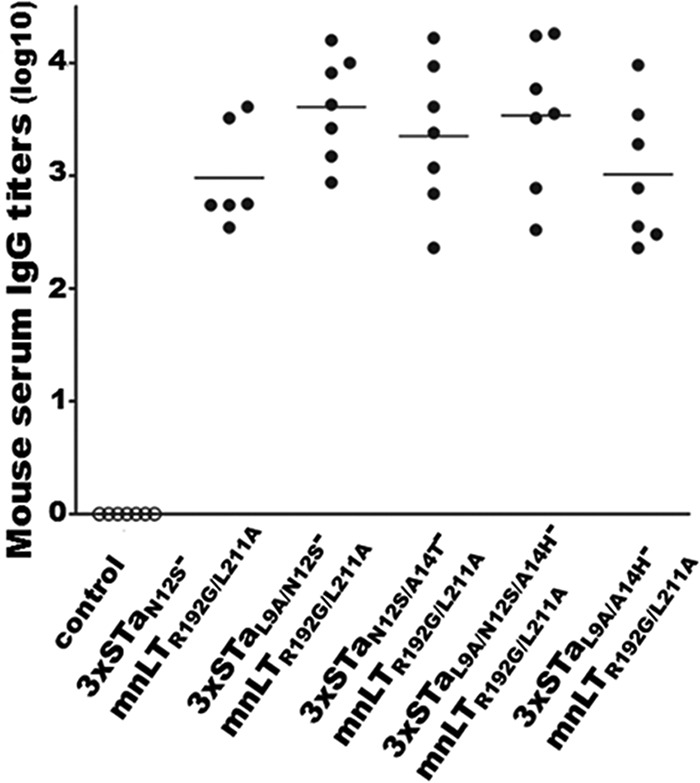
Anti-STa IgG titers (in log10 units) from serum samples of mice immunized s.c. with the 3×STaN12S-mnLTR192G/L211A, 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, 3×STaL9A/N12S/A14H-mnLTR192G/L211A, or 3×STaL9A/A14H-mnLTR192G/L211A fusion protein. Each dot represents the anti-STa IgG titer from a mouse in the immunized or control group. Bars indicate the mean IgG titers in each immunized group.
Serum samples of mice immunized with 3×STaN12S-mnLTR192G/L211A, 3×STaL9A/N12S-mnLTR192G/L211A, or 3×STaN12S/A14T-mnLTR192G/L211A neutralize STa in vitro.
Intracellular cGMP levels in T-84 cells incubated with STa and serum samples from mice immunized with 3×STaN12S-mnLTR192G/L211A, 3×STaL9A/N12S-mnLTR192G/L211A, or 3×STaN12S/A14T-mnLTR192G/L211A were 1.87 ± 0.28, 1.82 ± 0.51, and 1.81 ± 0.41 pmol/ml, respectively. These levels were significantly lower than the cGMP levels in T-84 cells incubated with STa and serum samples of mice immunized with 3×STaL9A/A14H-mnLTR192G/L211A (8.51 ± 0.58 pmol/ml; P < 0.001) or 3×STaL9A/N12S/A14H-mnLTR192G/L211A (36.1 ± 2.35 pmol/ml; P < 0.001). The cGMP level in T-84 cells incubated with STa and control mouse serum samples was 46 ± 1.77 pmol/ml, and the cGMP level in cells incubated with STa alone was 50 ± 1.23 pmol/ml (Fig. 4).
FIG 4.
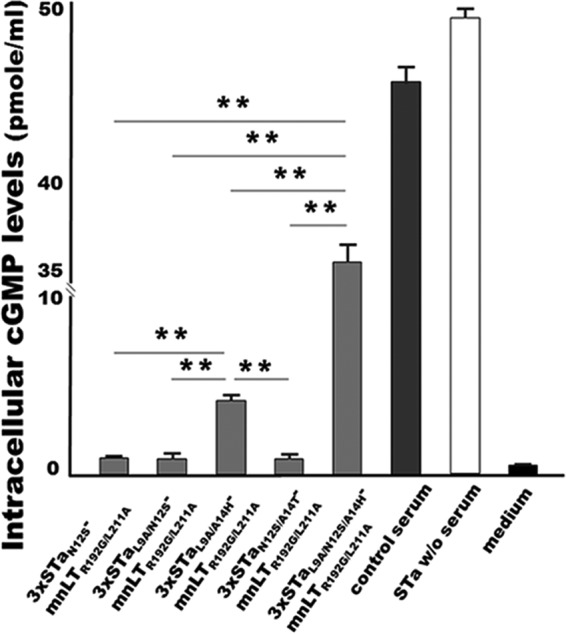
In vitro neutralization activity of mouse serum antibodies against STa. Intracellular cyclic GMP levels (picomoles per milliliter) in T-84 cells incubated with 2 ng STa and serum samples of mice immunized s.c. with the 3×STaN12S-mnLTR192G/L211A, 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, 3×STaL9A/N12S/A14H-mnLTR192G/L211A, or 3×STaL9A/A14H-mnLTR192G/L211A fusion protein were measured by using a cGMP EIA kit (Enzo Life). STa elevates intracellular cGMP levels in T-84 cells; neutralizing anti-STa antibodies prevent STa from stimulating cGMP production in T-84 cells. T-84 cells incubated with STa alone or STa with a control mouse serum sample were also included as controls. T-84 cells incubated with cell culture medium were used to show baseline cGMP levels in T-84 cells. ** indicates a P value of <0.01.
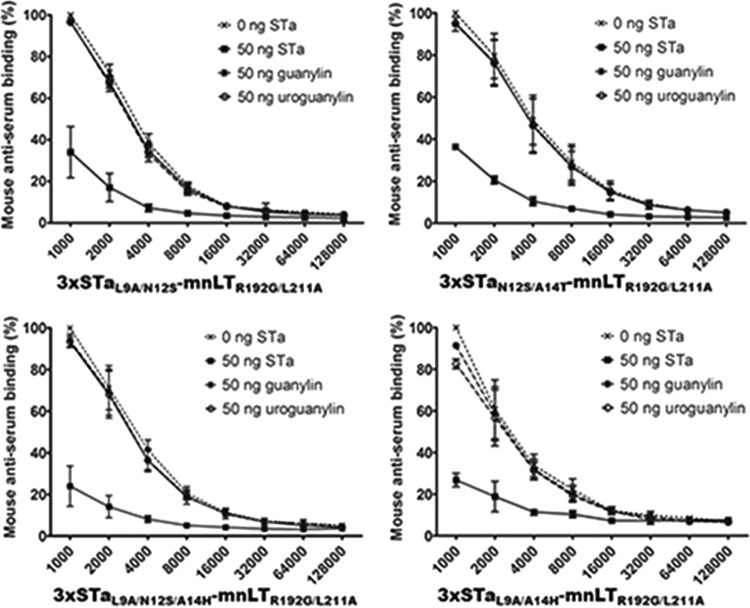
Serum antibodies from mice immunized with the toxoid fusion 3×STaN12S-mnLTR192G/L211A show little cross-reactivity with guanylin or uroguanylin in a competitive STa ELISA.
Competitive STa enzyme-linked immunosorbent assays (ELISAs) showed that guanylin or uroguanylin (50 ng) exhibited little competition against the coated STa-ovalbumin conjugates for reacting with anti-STa antibodies in the sera of mice immunized with the toxoid fusion 3×STaN12S-mnLTR192G/L211A (Fig. 5). Compared to the level of reactivity (99.7% ± 0.4%) of anti-STa serum antibodies (at a 1:1,000 dilution) with the coated STa-ovalbumin conjugates (without competition from STa or guanylin peptides), the level of mouse serum anti-STa antibody reactivity with the STa-ovalbumin conjugates was determined to be 96.7% ± 2.2% or 96.8% ± 2.5% when 50 ng guanylin or 50 ng uroguanylin was added, respectively. This showed that only about 3% of the reactivity of anti-STa antibodies with STa-ovalbumin was reduced by guanylin or uroguanylin, while the background reactivity reading in wells without the STa-ovalbumin coating conjugate was 2.2% ± 0.4%. In contrast, when STa (50 ng) was included, the reactivity of anti-STa antibodies with the coated STa-ovalbumin conjugate was measured at 30.9% ± 2.3%, indicating that STa reacted with anti-STa antibodies in mouse serum samples and blocked about 69% of the reactivity of anti-STa antibodies with coated STa-ovalbumin (Fig. 5).
FIG 5.
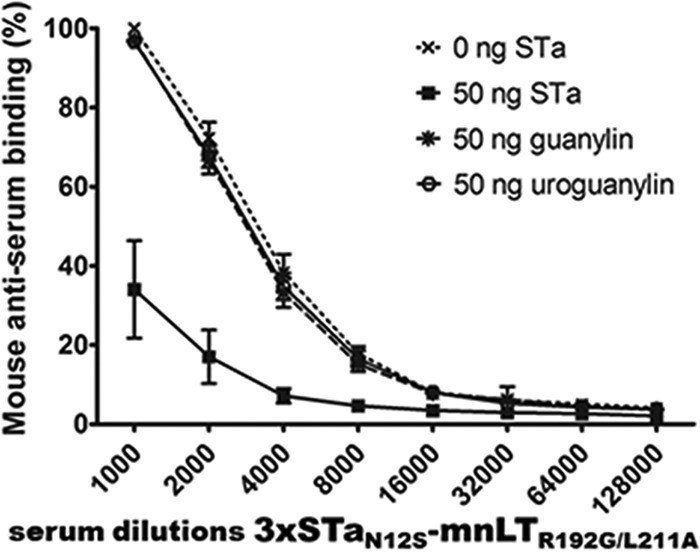
Competitive STa ELISAs to measure the cross-reactivity of anti-STa antibodies with guanylin and uroguanylin in serum samples of mice immunized s.c. with 3×STaN12S-mnLTR192G/L211A. ELISA plates coated with STa-ovalbumin conjugates (50 ng per well) were incubated with mouse serum samples (diluted 2-fold from 1:1,000 to 1:128,000) mixed with PBS, 50 ng STa, 50 ng guanylin, or 50 ng uroguanylin. The secondary antibody HRP-conjugated goat anti-mouse IgG (1:3,300; Sigma) and the TMB substrate (2C; KPL) were used to measure the optical absorbance at 405 nm. Reactivity is presented as a percentage, with the level of reactivity of mouse serum antibodies (at a 1:1,000 serum dilution) with the coated STa-ovalbumin conjugates being 100%.
Serum antibodies from mice immunized with the fusion 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, 3×STaL9A/A14H-mnLTR192G/L211A, or 3×STaL9A/N12S/A14H-mnLTR192G/L211A show little or limited cross-reactivity with guanylin or uroguanylin.
Serum samples from mice immunized with 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, or 3×STaL9A/N12S/A14H-mnLTR192G/L211A showed little cross-reactivity with guanylin or uroguanylin, whereas serum samples of mice immunized with 3×STaL9A/A14H-mnLTR192G/L211A showed a low level of reactivity with guanylin peptides (Fig. 6). The levels of reactivity of coated STa-ovalbumin with serum samples (1:1,000 dilution) of mice immunized with 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, and 3×STaL9A/N12S/A14H-mnLTR192G/L211A were 96.3% ± 1.5%, 94.6% ± 3.0%, and 96.3% ± 4.2%, respectively, when guanylin was added. When uroguanylin was added, the levels of reactivity were 95.8% ± 1.4%, 95.8% ± 1.4%, and 95.8% ± 4.3%, respectively. The background reactivity readings ranged from 2.2% ± 0.2% to 3.6% ± 0.2%.
FIG 6.
Competitive STa ELISAs to measure guanylin and uroguanylin cross-reactivity with anti-STa antibodies in serum samples of mice immunized s.c. with the 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, 3×STaL9A/N12S/A14H-mnLTR192G/L211A, or 3×STaL9A/A14H-mnLTR192G/L211A fusion protein. PBS, 50 ng STa toxin, 50 ng guanylin, or 50 ng uroguanylin mixed with serum dilutions from each immunization group was added to wells of competitive STa ELISA plates. The OD405 was measured and converted to a percentage to indicate antibody reactivity, with the OD in the wells incubated with a 1:1,000 dilution of mouse serum and PBS having a value of 100% (0% antibody cross-reactivity).
The levels of reactivity of the coated STa-ovalbumin conjugates with serum samples (1:1,000 dilution) of mice immunized with 3×STaL9A/A14H-mnLTR192G/L211A were 90.1% ± 3.3% and 81.3% ± 2.6% when guanylin and uroguanylin were included, respectively. This showed that guanylin and uroguanylin cross-reacted with 10% and 19% of anti-STa antibodies or blocked 10% and 19% of the anti-STa antibody reactivity with the coated STa-ovalbumin conjugates, respectively.
Comparatively, when 50 ng STa was included, the levels of reactivity of the coated STa-ovalbumin conjugates with serum antibodies of mice immunized with 3×STaL9A/N12S-mnLTR192G/L211A, 3×STaN12S/A14T-mnLTR192G/L211A, 3×STaL9A/N12S/A14H-mnLTR192G/L211A, and 3×STaL9A/A14H-mnLTR192G/L211A were 33.8% ± 11.3%, 36% ± 2.2%, 33.8% ± 8.8%, and 26.4% ± 2.5%, respectively (Fig. 6).
DISCUSSION
The cross-reactivity of anti-STa antibody with guanylin peptides (guanylin and uroguanylin) has not been well investigated. It was reported previously that STa did not cross-react with an antiguanylin monoclonal antibody (MAb) (21). A recent study examined two polyclonal antibodies, one against STa type Ib (hSTa [human-type STa]) and the other against STa type Ia (pSTa [porcine-type STa]), as well as four monoclonal antibodies (one against hSTa and three against pSTa) and found that rabbit anti-STa antiserum did not cross-react with guanylin but cross-reacted with uroguanylin at a low level (14). Those findings suggested a possible solution for the design of ETEC vaccines for anti-STa antibodies that do not cross-react with guanylin and uroguanylin. Data from the present study showed that the constructed fusion proteins carrying an STa double or triple mutant induced anti-STa and anti-LT antibody responses as effectively as did the STa single mutant fusion 3×STaN12S-mnLTR192G/L211A in subcutaneously immunized mice, and 3×STaL9A/N12S-mnLTR192G/L211A and 3×STaN12S/A14T-mnLTR192G/L211A induced antibodies that neutralized STa enterotoxicity without cross-reacting with guanylin and uroguanylin.
The introduction of double or triple mutations into STa significantly reduced the homology of STa with guanylin and uroguanylin. These STa mutants are preferred as ETEC vaccine antigens to induce antibodies that neutralize STa enterotoxicity but do not cross-react with guanylin peptides. Primary sequence comparisons indicated that while both guanylin peptides differ from STa at the 3rd, 8th, and 10th residues, guanylin further differs from STa (and uroguanylin) at the 6th and 9th residues (equal to the 9th and 12th residues of STa) (Fig. 1). The heterogeneity at these two residues (the 6th and 9th residues) separates guanylin further from STa and may directly result in a lack of anti-STa antibody cross-reactivity with guanylin (14). In contrast, sharing of the 6th and 9th residues by uroguanylin and STa likely causes anti-STa antibody cross-reactivity with uroguanylin. Data from this study showed that antibodies from fusions carrying STa mutated at these two residues had little cross-reactivity with guanylin or uroguanylin. The data also suggested that the heterogeneity at the 9th residue of uroguanylin (equal to the 12th residue of STa) likely plays a more important role in diverting STa mutants from guanylin peptides antigenically, since anti-STa antibodies derived from fusions carrying STa peptides mutated at the 12th asparagine residue (alone or combined with other mutations) showed no or little cross-reactivity with guanylin and uroguanylin. In contrast, serum antibodies from mice immunized with 3×STaL9A/A14H-mnLTR192G/L211A, which had the native 12th residue of STa, had 10% and 19% cross-reactivities with guanylin and uroguanylin, respectively. Future studies to compare the structural or antigenic divergence of STaL9A/A14H with those of the other four mutants may help us to understand differences in anti-STa antibody cross-reactivity with guanylin and uroguanylin.
Five STa mutants with mutations at the 9th, 12th, and 14th residues were targeted for the construction of toxoid fusions and for the examination of antibody cross-reactivity with guanylin peptides in this study. Selecting the 9th and 12th residues is obvious because STa shares the 9th residue (leucine) and the 12th residue (asparagine) with uroguanylin but not with guanylin. Additionally, the 9th residue (leucine) was thought to be associated with STa antigenic properties together with enterotoxicity. STa lost its reactivity with anti-STa antibodies and also enterotoxicity when the leucine residue at the 9th position was replaced with glutamine, arginine, glycine, or serine. On the other hand, STa mutants retained some anti-STa antibody reactivity and also some enterotoxicity when the leucine residue at the 9th position was replaced with isoleucine or lysine (22). Later antigenicity and enterotoxicity studies confirmed that a majority of STa mutants with mutations at the 9th residue had 90% of the antigenicity and enterotoxicity of STa reduced, and only a few mutants, including STaL9M, STaL9V, and STaL9F, retained a portion of the antigenicity and also the enterotoxicity of STa, although a few exceptional mutants, including STaL9G, STaL9S, and STaL9A, retained some STa antigenicity but not STa enterotoxicity (16). The STaL9A mutant was selected because the toxoid fusion 3×STaL9A-mnLTR192G/L211A (3xSTaL9A-dmLT) was previously demonstrated to induce neutralizing anti-STa IgG and IgA antibodies (10). The results from the present study also confirmed that a single mutation of the asparagine residue at the 12th position to serine or a double mutation of the 12th and 9th residues (leucine to alanine) did not significantly alter the propensity of STa for antigenicity, which was demonstrated by the induction of neutralizing anti-STa antibodies by 3×STaN12S-mnLTR192G/L211A and 3×STaL9A/N12S-mnLTR192G/L211A. These results indicated that mutations at the 12th residue or at both the 9th and 12th residues separated STa further from guanylin and uroguanylin, which was proven by the lack of cross-reactivity with guanylin or uroguanylin from antibodies derived from 3×STaN12S-mnLTR192G/L211A, 3×STaL9A/N12S-mnLTR192G/L211A, or 3×STaL9A/N12S/A14H-mnLTR192G/L211A. Mutation of the 14th residue of STa was also included in this study even though this alanine residue is identical among STa and the guanylin peptides. The alanine residue at the 14th position was previously suggested to play an important role in the enterotoxicity and antigenicity of STa (10, 16, 23). Additionally, the STaN12S single mutant was used as a reference mutant. The STa single mutant fusion 3×STaN12S-mnLTR192G/L211A induced antibodies that neutralized STa in vitro, protected piglets against STa+ ETEC diarrhea in vivo (10–12), and is currently considered the leading toxin antigen for ETEC vaccines. Data from this study suggested that the single mutant fusion 3×STaN12S-mnLTR192G/L211A was equally as effective as the double mutant fusions at inducing neutralizing and non-cross-reacting anti-STa antibodies.
The competitive STa ELISA was optimized by using 50 ng STa-ovalbumin coating antigen, 50 ng competitive antigen (guanylin, uroguanylin, or STa), and serum dilutions from 1:1,000 to 1:128,000 to examine anti-STa antibody cross-reactivity. Checkerboard tests showed that excessive anti-STa antibodies or insufficient competing agents (guanylin or uroguanylin) skewed anti-STa antibody reactivity with the coating antigen STa-ovalbumin, masking the detection of any cross-reactivity with guanylin or uroguanylin. On the other hand, a lower dose of coating antigen or more-diluted mouse serum samples resulted in low antibody reactivity to the coating antigen, as indicated by lower optical density at 405 nm (OD405) readings. It needs to be pointed out that the cross-reactivity of guanylin and uroguanylin currently measured in competitive ELISAs is reported relative to the reactivity of anti-STa antibodies with the coating antigen without any competitive molecules. Additionally, future studies using a pig challenge model or a controlled human challenge model (CHIM) to examine anti-STa and anti-LT antibodies induced by these toxoid fusions against ETEC diarrhea will help us to better assess their candidacy for ETEC vaccine development.
In conclusion, this study showed that the STa single, double, and triple mutants STaN12S, STaL9A/N12S, STaN12S/A14T, STaL9A/A14H, and STaL9A/N12S/A14H, after being genetically fused to a monomeric LT mutant (mnLTR192G/L211A), induced antibody responses to STa and LT. Antibodies derived from the fusions 3×STaN12S-mnLTR192G/L211A, 3×STaL9A/N12S-mnLTR192G/L211A, and 3×STaN12S/A14T-mnLTR192G/L211A neutralized STa in vitro and also did not cross-react with guanylin or uroguanylin. These results indicate that the toxoid fusions 3×STaN12S-mnLTR192G/L211A, 3×STaL9A/N12S-mnLTR192G/L211A, and 3×STaN12S/A14T-mnLTR192G/L211A are potentially desirable antigens for developing safe ETEC vaccines.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli bacteria and plasmids used in this study are included in Table 1. E. coli recombinant strain 9471, which was modified from 9331 (10) by removing the 6-histidine tag to express the tagless 3×STaN12S-mnLTR192G/L211A toxoid fusion, was used as the DNA template for toxoid fusion construction. Vector pET28α (Novagen, Madison, WI) was used to clone toxoid fusion genes, and E. coli strain BL21 was used to express toxoid fusion proteins.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| BL21 | F− ompT hsdS(rB− mB−) gal dcm | GE Healthcare |
| 9471 | 3×STaN12S-mnLTR192G/L211A recombinant strain | This study |
| 9600 | 3×STaL9A/N12S-mnLTR192G/L211A recombinant strain | This study |
| 9601 | 3×STaN12S/A14T-mnLTR192G/L211A recombinant strain | This study |
| 9602 | 3×STaL9A/N12S/A14H-mnLTR192G/L211A recombinant strain | This study |
| 9603 | 3×STaL9A/A14H-mnLTR192G/L211A recombinant strain | This study |
| Plasmids | ||
| pET28α | Novagen | |
| p9463 | Tagless 3×STaN12S-mnLTR192G/L211A gene in pET28α | This study |
| p9596 | Tagless 3×STaL9A/N12S-mnLTR192G/L211A gene in pET28α | This study |
| p9597 | Tagless 3×STaN12S/A14T-mnLTR192G/L211A gene in pET28α | This study |
| p9598 | Tagless 3×STaL9A/N12S/A14H-mnLTR192G/L211A gene in pET28α | This study |
| p9599 | Tagless 3×STaL9A/A14H-mnLTR192G/L211A gene in pET28α | This study |
Construction of the 3×STatoxoid-mnLTR192G/L211A toxoid fusion.
To avoid confusion with the holotoxin-structured double mutant LTR192G/L211A (dmLT), we designated the monomeric LT mutant mnLTR192G/L211A. This mutant consists of one LTA subunit and one LTB subunit as a monomer peptide (from a single ORF), with the A subunit mutated at positions 192 and 211. Splicing by overlap extension (SOE) PCR was used to mutate the STa gene (astA) for double or triple STa mutants and to fuse three copies of each STa mutant to the mnLTR192G/L211A gene for the construction of 3×STatoxoid-mnLTR192G/L211A fusion genes by using the PCR primers listed in Table 2. As described previously (9, 10), six nucleotide fragments were initially PCR amplified with Pfu DNA polymerase (Agilent Technologies, Santa Clara, CA) and primer pairs T7-F/STatoxoid-R, STatoxoid-F/LTA310-R, LTA310-F/STatoxoid-R, STatoxoid-F/LTB44-R, LTB44--F/STatoxoid-R, and STatoxoid-F/T7-R. Primers STatoxoid-F and STatoxoid-R were specific for each STa double or triple mutant. Overlapping the first two fragments, the third and the fourth fragments, and the last two amplicons generated three fragments carrying the first, second, and third copies of an STa toxoid and a part of mnLTR192G/L211A, respectively. Overlapping these three fragments together in a single SOE PCR yielded a 3×STatoxoid-mnLTR192G/L211A toxoid fusion gene. Each toxoid fusion gene was digested with NcoI and EagI and ligated into the pET28a vector.
TABLE 2.
PCR primers used to mutate the STa gene (estA) and to construct 3×STa-toxoid-mnLTR192G/L211A toxoid fusion genes in this study
Expression and characterization of the 3×STatoxoid-mnLTR192G/L211A fusion protein.
Toxoid fusion proteins without a 6×His tag expressed in E. coli strain BL21 were extracted and refolded as described previously (10, 24). Briefly, recombinant bacteria of each toxoid fusion (from a single colony) grown overnight in 5 ml lysogeny broth (LB) were added to 200 ml 2×YT medium (2× yeast extract, Tryptone) and continuously cultured until the OD reached 0.6. Bacteria were then induced with 0.5 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG; Sigma, St. Louis, MO). Bacteria were collected and processed for the extraction of inclusion body proteins with bacterial protein extraction reagent (B-PER; Pierce, Rockford, IL) according to the manufacturer's protocol. Extracted inclusion body proteins were solubilized and refolded by using a protein refolding kit (Novagen) according to the manufacturer's protocol.
Refolded toxoid fusion proteins were electrophoresed in a 12% sodium dodecyl sulfate-polyacrylamide gel, visualized by Coomassie blue staining, and characterized by Western blotting with rabbit anti-CT antiserum (Sigma) and rabbit anti-STa antiserum (provided by D. C. Robertson at Kansas State University), as previously described (10).
Subcutaneous immunization of mice with the 3×STatoxoid-mnLTR192G/L211A fusion protein.
Groups of six or seven adult female BALB/c mice were subcutaneously immunized with 40 μg of each refolded toxoid fusion protein. Two micrograms of holotoxin-structured dmLT (provided by the Walter Reed Army Institute of Research, Silver Spring, MD) was included in each immunization as an adjuvant. A group of seven mice immunized with protein buffer was used as the control. Each mouse received two boosters at intervals of 2 weeks. All mice were sacrificed 2 weeks after the second booster. The mouse study was performed according to 1996 National Research Council guidance (25) and was approved and supervised by the Kansas State University IACUC.
Mouse serum anti-STa and anti-LT IgG antibody titration.
Serum samples from each immunized or control mouse were titrated for anti-STa and anti-LT IgG antibodies as previously described (7, 8, 10, 11). Briefly, 10 ng STa-ovalbumin conjugates (provided by D. C. Robertson at Kansas State University) was used to coat each well of Costar plates (Corning Inc., Corning, NY) to titrate anti-STa antibodies, and each well of 2HB plates (Thermo Scientific, Rochester, NY) was coated with 100 ng LT (List Biological Laboratories Inc., Campbell, CA) to titrate anti-LT antibodies. Mouse serum samples were diluted 2-fold from 1:200 to 1:256,000. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:3,300; Sigma) was used as the secondary antibody. The tetramethylbenzidine (TMB) microwell peroxidase substrate kit (KPL, Gaithersburg, MD) was used to measure the OD405.
Mouse serum antibody neutralization against STa.
Mouse serum samples from each immunization group and the control group were examined for neutralization activity against STa by using T-84 cells and a cGMP enzyme immunoassay (EIA) kit (Enzo Life, Farmingdale, NY) as previously described (7, 8, 10). Briefly, a 30-μl serum sample mixed with 2 ng STa (in 150 μl Dulbecco's modified Eagle's medium [DMEM]) was incubated at room temperature for 1 h. The mixture was added to T-84 cells (in Eagle's minimum essential medium, with a final volume of 1 ml). After 1 h of incubation in a CO2 incubator, T-84 cells were lysed, and intracellular cGMP concentrations (picomoles per milliliter) in T-84 cells were measured with the cGMP EIA kit according to the manufacturer's protocol (Enzo Life).
Mouse serum antibody cross-reactivity with guanylin and uroguanylin.
A competitive STa ELISA (26, 27) was used to measure guanylin or uroguanylin cross-reactivity with anti-STa antibodies derived from each toxoid fusion. Briefly, Costar plates (Corning) coated with STa-ovalbumin conjugates (50 ng per well) were incubated with 2-fold dilutions of mouse serum (from 1:1,000 to 1:128,000) from each immunization group and phosphate-buffered saline (PBS), 50 ng STa toxin, 50 ng guanylin, or 50 ng uroguanylin. Wells without the STa-ovalbumin conjugate coating antigen were included to measure background readings. After incubation for 1 h at 37°C, plates were washed and then incubated with HRP-conjugated goat anti-mouse IgG (1:3,300; Sigma), followed by TMB peroxidase substrates (KPL). The OD405 was then measured.
Data analyses.
Mouse serum samples were examined in duplicates. Antibody titration and neutralization assays were replicated two times, and antibody cross-reactivity assays were repeated three times. Differences between treatment groups were analyzed with two-sided nonparametric Mood's median test by using Sigma XL (Kitchener, ON, Canada) with 95% confidence.
ACKNOWLEDGMENTS
We thank D. C. Robertson (Kansas State University) for providing STa-ovalbumin conjugates, the Walter Reed Army Institute of Research for providing dmLT, and the STa Toxoid Vaccine Consortium Group (Eileen Barry, James P. Nataro, John C. Clements, Halvor Sommerfelt, and Pal Puntervoll) for discussion of study design.
Financial support for this study was provided by NIH grant R01AI121067, PATH, and Kansas State University.
REFERENCES
- 1.Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, Adegbola RA, Alonso PL, Breiman RF, Faruque AS, Saha D, Sow SO, Sur D, Zaidi AK, Biswas K, Panchalingam S, Clemens JD, Cohen D, Glass RI, Mintz ED, Sommerfelt H, Levine MM. 2012. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis 55(Suppl 4):S232–S245. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang ZD, DuPont HL. 2017. Etiology of travellers' diarrhea. J Travel Med 24(Suppl 1):S13–S16. doi: 10.1093/jtm/tax003. [DOI] [PubMed] [Google Scholar]
- 3.Hill DR, Beeching NJ. 2010. Travelers' diarrhea. Curr Opin Infect Dis 23:481–487. doi: 10.1097/QCO.0b013e32833dfca5. [DOI] [PubMed] [Google Scholar]
- 4.Sack DA, Shimko J, Torres O, Bourgeois AL, Francia DS, Gustafsson B, Karnell A, Nyquist I, Svernnerholm AM. 2007. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. coli diarrhoea of travellers to Guatemala and Mexico. Vaccine 25:4392–4400. doi: 10.1016/j.vaccine.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Sack DA. 2012. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev Vaccines 11:677–694. doi: 10.1586/erv.12.37. [DOI] [PubMed] [Google Scholar]
- 6.Walker RI. 2015. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine 33:954–965. doi: 10.1016/j.vaccine.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC. 2010. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect Immun 78:316–325. doi: 10.1128/IAI.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M, Ruan X, Zhang C, Lawson SR, Knudsen DE, Nataro JP, Roberston DC, Zhang W. 2011. Heat-labile- and heat-stable-toxoid fusions (LTR192G-STaP13F) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect Immun 79:4002–4009. doi: 10.1128/IAI.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, Knudsen DE, Liu M, Robertson DC, Zhang W, STa Toxoid Vaccine Consortium Group. 2013. Toxicity and immunogenicity of enterotoxigenic Escherichia coli heat-labile and heat-stable toxoid fusion 3xSTaA14Q-LTS63K/R192G/L211A in a murine model. PLoS One 8:e77386. doi: 10.1371/journal.pone.0077386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan X, Robertson DC, Nataro JP, Clements JD, Zhang W, STa Toxoid Vaccine Consortium Group. 2014. Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infect Immun 82:1823–1832. doi: 10.1128/IAI.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nandre RM, Ruan X, Duan Q, Zhang W. 2016. Enterotoxigenic Escherichia coli heat-stable toxin and heat-labile toxin toxoid fusion 3xSTaN12S-dmLT induces neutralizing anti-STa antibodies in subcutaneously immunized mice. FEMS Microbiol Lett 363:fnw246. doi: 10.1093/femsle/fnw246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandre RM, Duan Q, Wang Y, Zhang W. 2016. Passive antibodies derived from intramuscularly immunized toxoid fusion 3xSTaN12S-dmLT protect against STa+ enterotoxigenic Escherichia coli (ETEC) diarrhea in a pig model. Vaccine 35:552–556. doi: 10.1016/j.vaccine.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Sack DA. 2015. Current progress in developing subunit vaccines against enterotoxigenic Escherichia coli (ETEC) associated diarrhea. Clin Vaccine Immunol 22:983–991. doi: 10.1128/CVI.00224-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taxt AM, Diaz Y, Bacle A, Grauffel C, Reuter N, Aasland R, Sommerfelt H, Puntervoll P. 2014. Characterization of immunological cross-reactivity between enterotoxigenic Escherichia coli heat-stable toxin and human guanylin and uroguanylin. Infect Immun 82:2913–2922. doi: 10.1128/IAI.01749-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forte LR., Jr 2004. Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther 104:137–162. doi: 10.1016/j.pharmthera.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Taxt AM, Diaz Y, Aasland R, Clements JD, Nataro JP, Sommerfelt H, Puntervoll P. 2016. Towards rational design of a toxoid vaccine against the heat-stable toxin of Escherichia coli. Infect Immun 84:1239–1249. doi: 10.1128/IAI.01225-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan Q, Zhang W. 2016. Structure, enterotoxicity, and immunogenicity of enterotoxigenic Escherichia coli heat-stable type I toxin (STa) and derivatives, p 1–22. In Gopalakrishnakone PSB, Alape-Giron A, Dubreuil JD, Mandale M (ed), Microbial toxins. Springer, Dordrecht, Netherlands. [Google Scholar]
- 18.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. 1992. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A 89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chin DT, Tompkins JA, Fok KF, Smith CE, Duffin KI, Siegel NR, Currie MG. 1993. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci U S A 190:10464–10468. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sindic A, Schlatter E. 2006. Cellular effects of guanylin and uroguanylin. J Am Soc Nephrol 17:607–616. doi: 10.1681/ASN.2005080818. [DOI] [PubMed] [Google Scholar]
- 21.Martin S, Adermann K, Forssmann WG, Kuhn M. 1999. Regulated, side-directed secretion of proguanylin from isolated rat colonic mucosa. Endocrinology 140:5022–5029. doi: 10.1210/endo.140.11.7103. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Zhang C, Mateo K, Nataro JP, Robertson DC, Zhang W. 2011. Modified heat-stable toxins (hSTa) of enterotoxigenic Escherichia coli lose toxicity but display antigenicity after being genetically fused to heat-labile toxoid LTR192G. Toxins (Basel) 3:1146–1162. doi: 10.3390/toxins3091146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirayama T. 1995. Heat-stable enterotoxin of Escherichia coli, p 281–296. In Moss J, Iglewski B, Vaughan M, Tu AT (ed), Bacterial toxins and virulence factors in disease. Marcel Dekker, Inc, New York, NY. [Google Scholar]
- 24.Nandre RM, Ruan X, Duan Q, Sack DA, Zhang W. 2016. Antibodies derived from an enterotoxigenic Escherichia coli (ETEC) adhesin tip MEFA (multiepitope fusion antigen) against adherence of nine ETEC adhesins: CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, CS21 and EtpA. Vaccine 34:3620–3625. doi: 10.1016/j.vaccine.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
- 26.Lockwood DE, Robertson DC. 1984. Development of a competitive enzyme-linked immunosorbent assay (ELISA) for Escherichia coli heat-stable enterotoxin (STa). J Immunol Methods 75:295–307. doi: 10.1016/0022-1759(84)90113-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Robertson DC, Zhang C, Bai W, Zhao M, Francis DH. 2008. Escherichia coli constructs expressing human or porcine enterotoxins induce identical diarrheal diseases in a piglet infection model. Appl Environ Microbiol 74:5832–5837. doi: 10.1128/AEM.00893-08. [DOI] [PMC free article] [PubMed] [Google Scholar]