ABSTRACT
Commensal Streptococcus sanguinis and Streptococcus gordonii are pioneer oral biofilm colonizers. Characteristic for both is the SpxB-dependent production of H2O2, which is crucial for inhibiting competing biofilm members, especially the cariogenic species Streptococcus mutans. H2O2 production is strongly affected by environmental conditions, but few mechanisms are known. Dental plaque pH is one of the key parameters dictating dental plaque ecology and ultimately oral health status. Therefore, the objective of the current study was to characterize the effects of environmental pH on H2O2 production by S. sanguinis and S. gordonii. S. sanguinis H2O2 production was not found to be affected by moderate changes in environmental pH, whereas S. gordonii H2O2 production declined markedly in response to lower pH. Further investigation into the pyruvate node, the central metabolic switch modulating H2O2 or lactic acid production, revealed increased lactic acid levels for S. gordonii at pH 6. The bias for lactic acid production at pH 6 resulted in concomitant improvement in the survival of S. gordonii at low pH and seems to constitute part of the acid tolerance response of S. gordonii. Differential responses to pH similarly affect other oral streptococcal species, suggesting that the observed results are part of a larger phenomenon linking environmental pH, central metabolism, and the capacity to produce antagonistic amounts of H2O2.
IMPORTANCE Oral biofilms are subject to frequent and dramatic changes in pH. S. sanguinis and S. gordonii can compete with caries- and periodontitis-associated pathogens by generating H2O2. Therefore, it is crucial to understand how S. sanguinis and S. gordonii adapt to low pH and maintain their competitiveness under acid stress. The present study provides evidence that certain oral bacteria respond to environmental pH changes by tuning their metabolic output in favor of lactic acid production, to increase their acid survival, while others maintain their H2O2 production at a constant level. The differential control of H2O2 production provides important insights into the role of environmental conditions for growth competition of the oral flora.
KEYWORDS: Streptococcus, biofilms, microbial ecology
INTRODUCTION
Oral biofilm bacteria are exposed to constantly changing environmental conditions. Some of these environmental changes are triggered directly by host behaviors and promote the development of oral diseases (1, 2). One striking example is the development of caries as the result of increased carbohydrate exposure due to nutritional choices of the host (3). Oral bacteria can respond to environmental changes by modifying their gene expression. For example, exposure of Streptococcus mutans to low pH leads to an acid tolerance response (4). In the oral biofilm, frequent exposure to low pH selects for aciduric species, resulting in a shift toward a dysbiotic, caries-promoting microbial community (5). The selection process that favors aciduric species leads to a decline in the abundance of several commensal species that can be found in healthy oral biofilms. For example, recent clinical studies observed that Streptococcus sanguinis is strongly associated with oral health, but its prevalence declines in individuals with caries and periodontal diseases (6, 7). Similarly, Streptococcus gordonii seems to be an abundant species in healthy dental plaque (3, 8, 9).
It is largely unknown how exposure to lower pH influences commensal bacterial species in the oral biofilm. S. sanguinis and S. gordonii are less aciduric than S. mutans, thus providing a possible explanation for their decreased abundance in acidic dental plaques (10–12). The competitive abilities of commensal streptococci likely also play an integral role in oral biofilm ecology. Both S. sanguinis and S. gordonii have been demonstrated to successfully antagonize competitors in dual-species biofilms (13, 14), but it is unclear to what degree, if any, pH influences these abilities.
Our group and others have demonstrated the importance of the pyruvate oxidase SpxB in the competitive abilities of commensal streptococci (14–16). Under aerobic conditions, as found during early stages of oral biofilm formation, the catalytic activity of SpxB generates H2O2, CO2, and acetyl phosphate. Acetyl phosphate is further metabolized to acetate, generating ATP (14). Recent studies have demonstrated that the H2O2 produced by S. sanguinis and S. gordonii under aerobic conditions plays an important role in cell-cell aggregation and biofilm formation through the H2O2-mediated release of DNA (17, 18). Extracellular DNA (eDNA) not only has a structurally stabilizing role as part of the biofilm extracellular polymeric substances (EPS) but also serves as a source of DNA for horizontal gene transfer (17, 18). Moreover, H2O2 is the main inhibitory substance produced by S. sanguinis and S. gordonii to combat competitors such as S. mutans, one of the few oral streptococci that does not express SpxB (14). Thus, SpxB activity likely provides commensal colonizers with a competitive growth advantage during early oral biofilm formation.
Interestingly, spxB is encoded in the majority of oral streptococci with a high degree of sequence conservation (19) but little is known about its regulation. Initial investigations of spxB expression in S. sanguinis and S. gordonii revealed a striking difference between the species despite a largely conserved spxB promoter region. The presence of cre binding sites in the spxB promoter for the carbon catabolite regulator CcpA suggests carbohydrate-dependent regulation (20). As expected, deletion of CcpA in both species increased the expression of spxB (21, 22); however, carbohydrate-dependent repression of spxB was detected only in S. gordonii. Different carbohydrates had no influence on spxB expression in S. sanguinis (21, 22). The underlying mechanism for this differential expression regulation is currently unknown, which piqued our interest to further investigate how environmental factors influence spxB expression in the two commensal streptococci.
Here we demonstrate that S. gordonii and S. sanguinis exhibit vastly different H2O2 production phenotypes in response to pH. Although S. sanguinis produces less H2O2 overall, H2O2 production is similar at pH 6 and pH 7. In contrast, S. gordonii H2O2 production decreases significantly as the pH drops. Further investigations into the metabolic consequences of decreased SpxB activity in S. gordonii suggest a redirection of central metabolism toward lactic acid production at pH 6, contributing to the survival of S. gordonii at low pH. Additionally, we demonstrate that the differential responses to a change in pH are not confined to S. sanguinis and S. gordonii but also affect other oral streptococcal species.
RESULTS
Differential pH-dependent H2O2 production in S. gordonii and S. sanguinis.
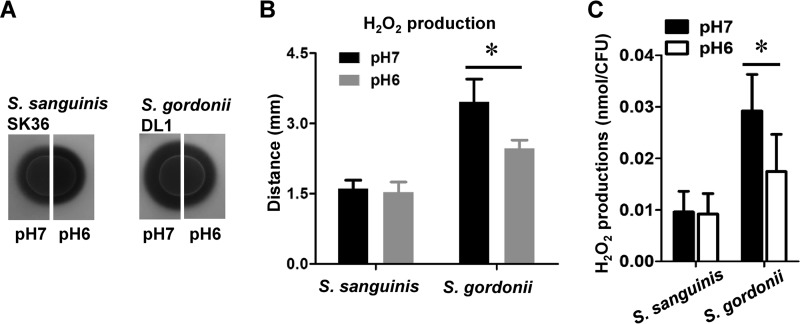
To determine whether H2O2 production in S. sanguinis and S. gordonii is influenced by changes in environmental pH, we employed a colorimetric plate assay for the detection of H2O2. The plate assay ensures maximum H2O2 production, due to naturally efficient aeration, as reported previously (23). The amount of H2O2 produced by S. gordonii at pH 6 was noticeably decreased, compared to that produced at pH 7, as evident from the smaller halo surrounding the respective inoculum (Fig. 1A). No difference was observed for S. sanguinis (Fig. 1A). Quantification of the halo size confirmed a statistically significant difference in H2O2 production for S. gordonii (Fig. 1B). The observation that S. sanguinis is not influenced by the environmental pH while S. gordonii responds with decreased H2O2 production was also determined with different initial cell densities. A positive correlation between the increase in cell density and H2O2 production was observed for both species, but the overall observation that S. sanguinis produces less H2O2 remained consistent (see Fig. S1 in the supplemental material). The H2O2 concentration was also determined with planktonic cells in liquid cultures grown aerobically and was normalized to CFU counts. The S. sanguinis H2O2 production levels were similar at pH 6 and pH 7, whereas S. gordonii H2O2 production decreased significantly at pH 6, consistent with the results determined by the colorimetric indicator plate assays (Fig. 1C).
FIG 1.
pH-dependent H2O2 production in S. sanguinis and S. gordonii. (A) Images of S. sanguinis and S. gordonii on H2O2 indicator plates adjusted to pH 7 and pH 6; representative images are shown. (B) Quantitative analysis of H2O2 production on indicator plates; production was determined by measuring the distance between the border of the colony and the border of the halo formed by the Prussian blue precipitate. (C) Quantitative analysis of H2O2 production in liquid cultures; production was determined enzymatically and normalized to CFU. All data were obtained in triplicate at separate times. *, significant difference (P < 0.05).
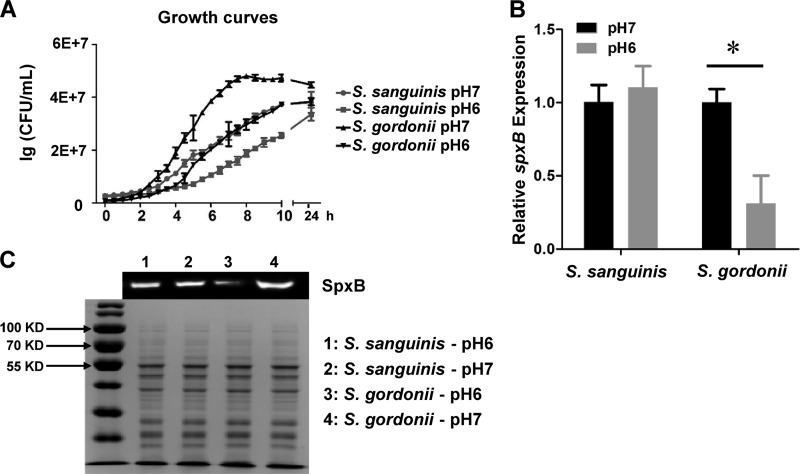
The growth of S. sanguinis and S. gordonii was slower at pH 6 than at pH 7, and both strains yielded fewer CFU than did cells grown at pH 7 after 24 h (Fig. 2A). Additionally, we found that the growth of both strains was largely inhibited at pH 5 (data not shown). To account for potential differences in cell densities at different pH values and to determine whether the reduced production of H2O2 in S. gordonii correlates with gene expression, the expression of spxB was determined with quantitative reverse transcription-PCR (qRT-PCR). S. sanguinis spxB gene expression was not affected by pH, in agreement with the phenotypic observations on H2O2 indicator plates. The expression of spxB in S. gordonii was decreased 3.5-fold at pH 6, compared to that at pH 7 (Fig. 2B). Furthermore, Western blot analysis was performed using isogenic strains of S. sanguinis and S. gordonii carrying engineered SpxB with FLAG tags. The FLAG epitope did not interfere with H2O2 production and was specific for SpxB (Fig. S2A). As predicted, SpxB in S. sanguinis exhibited the same abundance levels at pH 6 and pH 7. However, noticeably less SpxB was detected for S. gordonii at pH 6 versus pH 7 (Fig. 2C). The data indicate that S. sanguinis H2O2 production is largely insensitive to slightly acidic environmental pH, whereas the same pH reduces S. gordonii spxB gene expression, ultimately decreasing its H2O2 production.
FIG 2.
Growth, spxB expression, and SpxB abundance in S. sanguinis SK36 and S. gordonii DL1 at pH 7 and pH 6. (A) Growth curves for S. sanguinis SK36 and S. gordonii DL1 at pH 7 and pH 6. (B) Relative levels of expression of spxB genes in S. sanguinis SK36 and S. gordonii DL1 under different pH conditions; the relative cDNA abundance with SK36 and DL1 at pH 7 was set to 1. (C) Top, Western blot analysis of SpxB protein (72 kDa) abundance in S. sanguinis SK36 and S. gordonii DL1 at pH 7 and pH 6; bottom, SDS-PAGE gel with Coomassie blue staining, shown as a loading control. All data were obtained in triplicate at separate times. *, significant difference (P < 0.05).
Role of two-component signal transduction systems in the control of pH-dependent H2O2 adjustment.
The two-component systems (TCSs) ComDE, VicKR, and CiaHR have been associated with pH-dependent stress response regulation (24–27). Therefore, we were curious regarding whether expression of comC (the first gene in the comCDE operon), vicK, and ciaH responded to pH changes in S. sanguinis and S. gordonii, as a potential mechanism to explain the observed differential regulation of spxB expression during acidification. Expression of all three TCSs was indeed influenced by changes in pH; however, the differences were moderate (Fig. S3A and B). Thus, we did not observe compelling evidence to implicate TCS regulation of spxB gene expression at pH 6.
pH-dependent redirection of central metabolism toward lactic acid production in S. gordonii.
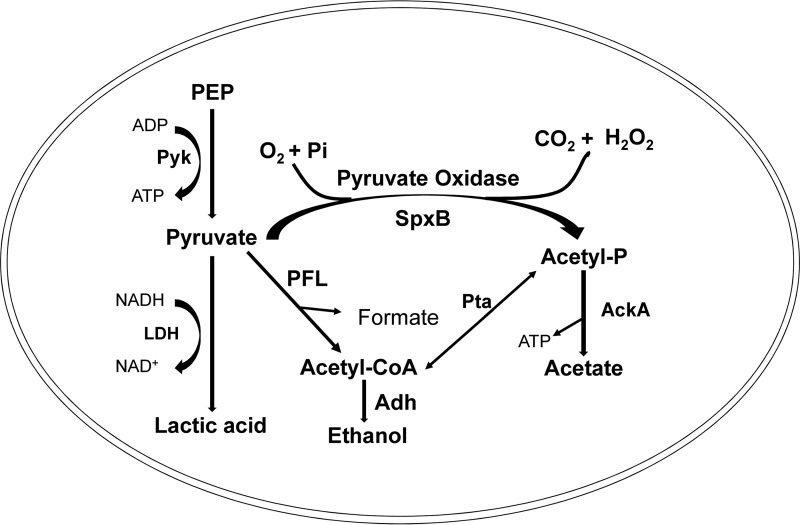
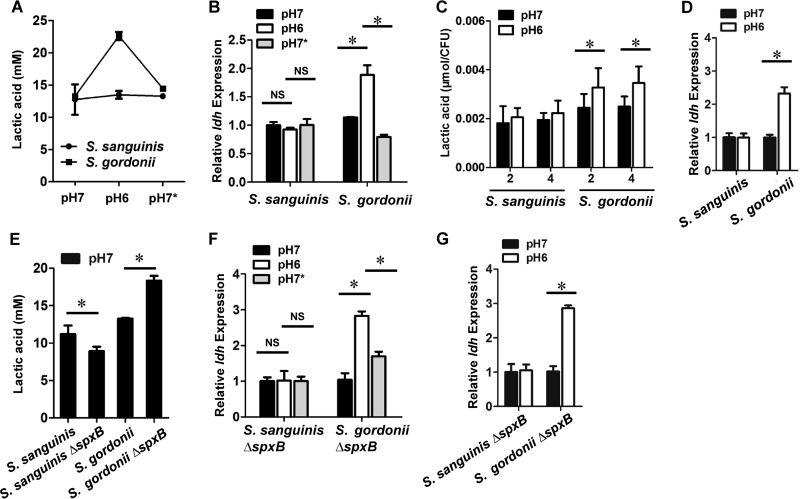
The pyruvate node of streptococcal central metabolism is responsible for the SpxB-dependent generation of acetyl-PO4, which is further converted to acetate via acetate kinase (AckA) and to lactic acid via lactate dehydrogenase (Ldh), thereby generating ATP and NAD+. Since these pathways are interconnected, we hypothesized that a decrease in SpxB activity, i.e., lower H2O2 production in S. gordonii, would potentially redirect pyruvate toward the Ldh pathway (see Fig. 7 for an overview of the pyruvate node). To test this, we compared the concentrations of lactic acid secreted from S. sanguinis and S. gordonii. As expected, a change from pH 7 to pH 6 or from pH 6 to pH 7 had no obvious impact on lactic acid production by S. sanguinis, whereas both conditions significantly altered lactic acid production by S. gordonii (Fig. 3A). Similarly, ldh gene expression in both strains correlated with their lactic acid production at pH 6 and pH 7 (Fig. 3B). Moreover, we measured lactic acid production and ldh gene expression when the pH was fixed to 7 or 6. The amount of lactic acid produced by S. gordonii at pH 6 was significantly increased, compared to that produced at pH 7, whereas no difference was observed for S. sanguinis 2 h or 4 h after the start of incubation (Fig. 3C). Consistently, ldh expression was noticeably upregulated in S. gordonii at pH 6, while no difference was observed for S. sanguinis (Fig. 3D). Interestingly, it was also possible to increase S. gordonii lactic acid production at pH 7 simply by deleting spxB (Fig. 3E). This is also reflected in increased ldh gene expression for the S. gordonii ΔspxB mutant at pH 6 (Fig. 3F). Interestingly, lactic acid production by the S. sanguinis spxB mutant was slightly decreased and spxB expression was unaffected during the pH shift (Fig. 3E and F). When the pH was fixed to 7 or 6, ldh gene expression in both strains was comparable to findings from the pH shift experiments, confirming the observation that S. gordonii adjusts its ldh expression to the lower pH condition (Fig. 3G).
FIG 7.
Schematic overview of the pyruvate node. Pyruvate is generated through the glycolytic pathway from phosphoenolpyruvate (PEP) via pyruvate kinase (Pyk). As relevant here, pyruvate can be channeled through pyruvate oxidase (SpxB), generating acetyl-phosphate (Acetyl-P), CO2, and H2O2, with subsequent generation of acetate by acetate kinase (AckA), or alternatively through lactate dehydrogenase (LDH), generating lactic acid and NAD+ from NADH, as well as through pyruvate-formate lyase (PFL), generating acetyl-CoA and formate, with subsequent generation of ethanol by alcohol dehydrogenase (Adh). Acetyl-CoA and acetyl-P can be converted into each other by phosphate acetyltransferase (Pta).
FIG 3.
pH-dependent influence of lactic acid production in S. sanguinis and S. gordonii. (A) Lactic acid levels in S. sanguinis SK36 and S. gordonii DL1 under different pH conditions. pH 7, pH 6, and pH 7* represent different time points illustrated in Fig. S4 in the supplemental material. Bacteria were adjusted to the same cell density and volume before lactic acid production measurements. (B) Relative ldh gene expression in S. sanguinis SK36 and S. gordonii DL1 under different conditions. pH 7, pH 6, and pH 7* represent different time points illustrated in Fig. S4. (C) Lactic acid levels in S. sanguinis SK36 and S. gordonii DL1 at different pH conditions and different time points. The mid-log-phase bacteria were cultured at a fixed pH (pH 7 or pH 6) for 2 h or 4 h. Lactic acid concentrations were normalized to CFU. (D) Relative ldh gene expression in S. sanguinis SK36 and S. gordonii DL1 at pH 7 and pH 6. The pH was fixed to pH 7 or 6, and the relative cDNA abundance with SK36 and DL1 at pH 7 was set to 1. (E) Lactic acid levels in wild-type S. sanguinis SK36 and S. gordonii DL1 and their respective ΔspxB mutants. Bacteria were adjusted to the same cell density and volume before lactic acid production measurements. (F) Relative ldh gene expression in the S. sanguinis SK36 ΔspxB mutant and the S. gordonii DL1 ΔspxB mutant under different conditions. pH 7, pH 6, and pH 7* represent different time points illustrated in Fig. S4. (G) Relative ldh gene expression in the S. sanguinis SK36 ΔspxB mutant and the S. gordonii DL1 ΔspxB mutant at pH 7 and pH 6. The pH was fixed to pH 7 or 6, and the relative cDNA abundance with the SK36 ΔspxB mutant and the DL1 ΔspxB mutant at pH 7 was set to 1. All data were obtained in triplicate at separate times. *, significant difference (P < 0.05). NS, not significant.
The pyruvate node involves several other gene products, including pyruvate kinase (pyk), acetate kinase (ackA), pyruvate formate lyase (pfl), alcohol dehydrogenase (adh), and phosphate acetyltransferase (pta) (see Fig. 7 for an overview). To initially assess whether a change in pH leads to alteration in expression of those genes, expression in S. sanguinis and S. gordonii at pH 6 and pH 7 was determined. Interestingly, pyk was upregulated at pH 6 in S. sanguinis, suggesting increased availability of pyruvate. Since S. sanguinis does not produce more H2O2 or lactic acid at pH 6, the increased expression of pfl and adh suggests that the pyruvate might be metabolized to ethanol. No difference of >2-fold was observed for the other tested genes of S. gordonii and S. sanguinis (Fig. S5).
Effects of pH-dependent redirection of central metabolism toward lactic acid production on acid survival of S. gordonii.
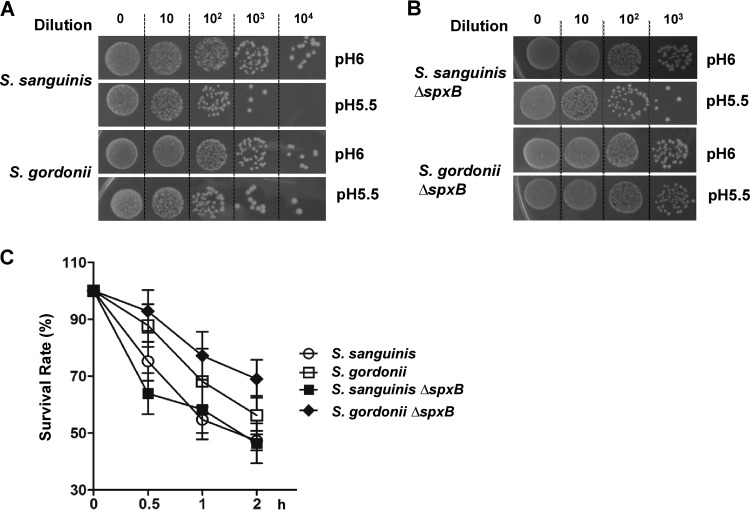
Acidogenic S. mutans can substantially lower the environmental pH via production of lactic acid, and it is able to survive this low pH for extended periods (4). Since S. gordonii seems to shift its metabolism toward lactic acid production at pH 6, we wondered whether this could be a strategy to activate its acid tolerance mechanisms. Cells of S. gordonii and S. sanguinis grown in brain heart infusion (BHI) broth at pH 7 were inoculated into BHI broth at pH 6 to trigger acid adaptation and were subsequently spotted onto a pH 5.5 plate. S. gordonii acid tolerance seems to be more robust than that of S. sanguinis, as evident by the growth of colonies at dilutions of 103 and 104 (Fig. 4A). The acid survival rate assay confirmed a higher survival rate for S. gordonii versus S. sanguinis at low pH (Fig. 4C). Interestingly, the S. gordonii ΔspxB mutation further increased the acid survival rate, while the same mutation demonstrated no impact on S. sanguinis survival (Fig. 4B and C). This observation confirms that, as the pH drops, the modification of S. gordonii metabolism toward lactic acid production increases its survival at low pH.
FIG 4.
Acid survival of S. sanguinis SK36 and S. gordonii DL1 wild-type strains and ΔspxB mutants at pH 5.5. (A) Representative images of the wild-type SK36 and DL1 strains. Cells grown in BHI broth at pH 7 were inoculated into BHI broth at pH 6 to adjust their metabolic activity. Subsequently, serial dilutions were spotted on pH 5.5 plates. Photographs of the plates were taken after 24 h of incubation. (B) Representative images of the SK36 and DL1 ΔspxB mutants. (C) Acid survival rates of planktonic cells exposed to pH 5.5 over time (0, 0.5, 1, and 2 h). All data were obtained in triplicate at separate times.
Effects of pH on pyruvate node plasticity of clinical isolates.
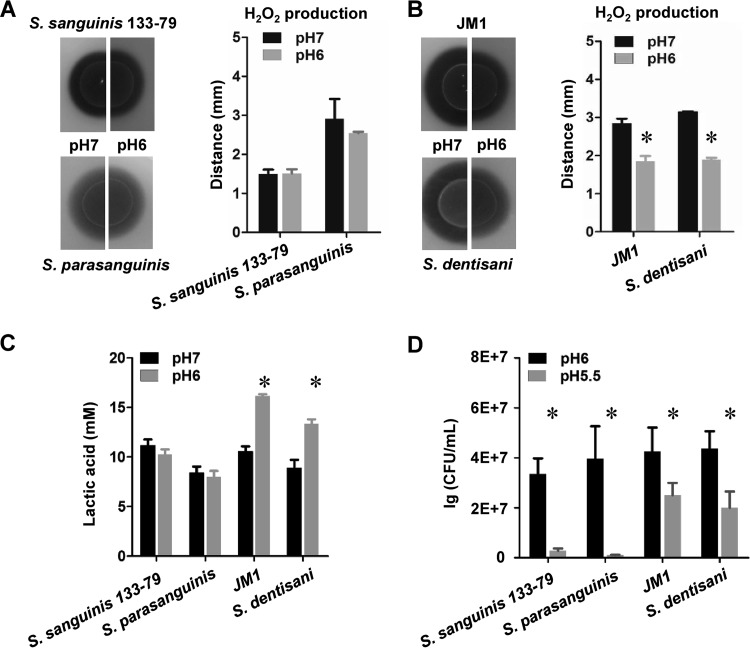
The spxB gene is highly conserved among H2O2-producing oral streptococci. Therefore, we were curious to compare pH-dependent H2O2 production using additional oral streptococci (19). Low-passage-number oral clinical isolates of Streptococcus parasanguinis, Streptococcus dentisani, and S. gordonii and an endocarditis S. sanguinis isolate were tested for H2O2 production using H2O2 indicator plates adjusted to pH 7 or pH 6. As shown in Fig. 5A, S. sanguinis 133-79 exhibited no significant differences in H2O2 production at pH 6 versus pH 7, consistent with our previous results with S. sanguinis SK36. The same observation was made with the S. parasanguinis isolate. In contrast, S. gordonii JM1 and S. dentisani both produced significantly less H2O2 at pH 6 than at pH 7 (Fig. 5B). The difference in H2O2 production also influenced the lactic acid production and the ability to survive an acid challenge. Compared to S. sanguinis 133-79 and S. parasanguinis, S. gordonii JM1 and S. dentisani increased lactic acid production at pH 6 (Fig. 5C) and survived the acid challenge from pH 6 to pH 5.5 to a greater extent (Fig. 5D).
FIG 5.
Effects of pH on H2O2 production in various oral streptococci. (A) H2O2 production of S. sanguinis 133-79 and S. parasanguinis at pH 7 and pH 6. (B) H2O2 production of S. gordonii JM1 and S. dentisani at pH 7 and pH 6. (C) Lactic acid levels in S. sanguinis 133-79, S. parasanguinis, S. gordonii JM1, and S. dentisani at pH 7 and pH 6. (D) CFU quantification of S. sanguinis 133-79, S. parasanguinis, S. gordonii JM1, and S. dentisani at pH 6 and pH 5.5 after overnight growth. All data were obtained at least three separate times. *, significant difference (P < 0.05).
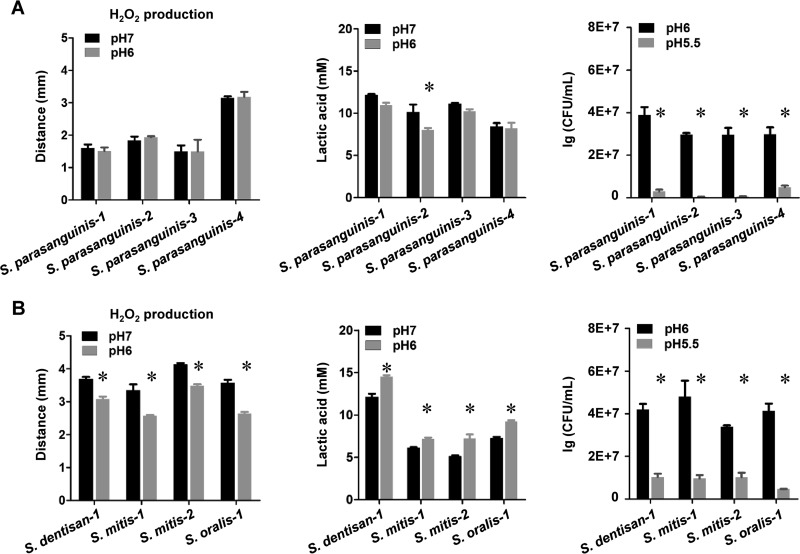
Furthermore, testing of several other low-passage-number S. parasanguinis isolates with unchanged or slightly lower production of H2O2 at pH 6 demonstrated lower survival rates at pH 6, compared to low-passage-number isolates of Streptococcus mitis, S. dentisani, and Streptococcus oralis (Fig. 6A). Accordingly, S. mitis, S. dentisani, and S. oralis all showed reduced production of H2O2 with increased lactic acid concentration at pH 6 (Fig. 6B). This suggests that the ability to adjust H2O2 production with changing environmental pH to increase survival at lower pH is likely characteristic of most or all S. gordonii strains and may be a feature shared by a variety of other oral streptococci as well.
FIG 6.
Effects of pH on H2O2 production in low-passage-number clinical isolates. (A) H2O2 production, lactic acid production, and acid stress survival of 4 S. parasanguinis isolates at pH 7 and pH 6. (B) H2O2 production, lactic acid production, and acid stress survival of additional S. dentisani, S. mitis, and S. oralis low-passage-number isolates at pH 7 and pH 6. *, significant difference (P < 0.05).
DISCUSSION
Polymicrobial biofilms have been implicated in the development of several chronic infections. Such infections are a problem of bacterial ecology, and efficacious treatment approaches require a holistic view of the microbial community and its specific interactions. In the case of oral diseases such as periodontitis and caries, recent next-generation sequencing studies have shown that the disruption of oral biofilm homeostasis leads to dysbiosis through a decline in commensal species (28, 29). However, we lack a fundamental mechanistic understanding of the early events that ultimately favor a dysbiotic ecology in the flora. In this study, we were interested to determine how changes in environmental pH influence the competitive capabilities of the commensal streptococci S. sanguinis and S. gordonii. Their survival at pH 5.5 is particularly relevant to caries development, since exposure to a pH below this value favors tooth enamel demineralization and also is associated with a steep decline in the population of commensal streptococcal species, most of which encode SpxB (3, 6, 8).
S. sanguinis exhibited no change in the production of H2O2 at pH 6; in sharp contrast, the amount of H2O2 produced by S. gordonii was significantly reduced at pH 6. These phenotypes were strongly correlated with both reduced spxB gene expression and lower SpxB protein abundance. The different responses to decreased environmental pH suggest that, compared with S. gordonii, S. sanguinis attempts to maintain its competitiveness via production of H2O2 as the pH becomes acidic. However, species such as S. gordonii adjust their metabolic output toward lactic acid production as the environment becomes more acidic. Part of this adjustment requires reduced activity from SpxB to favor pyruvate fermentation via lactate dehydrogenase (Fig. 7).
Since the response to low pH was a transcriptional adjustment in S. gordonii, our initial focus centered on gene expression control by TCSs. TCSs are known to integrate environmental information to modulate cellular behavior (30). TCSs ComDE, VicKR, and CiaHR of S. mutans (24, 26, 27) and Streptococcus pneumoniae (25) have been associated with stress response regulation as a result of pH changes. Therefore, we investigated whether comC, vicK, and ciaH expression responded to pH changes in S. sanguinis and S. gordonii (see Fig. S3 in the supplemental material). Overall, the expression responses to pH changes were moderate but more prominent in S. gordonii, suggesting that the involvement of the TCSs tested here is limited.
Based on our previous findings (14, 20), H2O2 production in S. gordonii seems to be strongly influenced by environmental cues. For example, the presence of carbohydrates can elicit a carbon catabolite repression of spxB expression (22). Considering that, in general, S. gordonii produces more H2O2 than does S. sanguinis, adaptation to diverse environmental conditions could be a strategy to ensure overall fitness. Interestingly, S. gordonii is also lower in abundance than S. sanguinis, which is one of the most abundant streptococci in the oral biofilm (31). We speculate that high H2O2 production is important for initial competitiveness but adaptation is more important than antagonism for S. gordonii to counter the environmental stress. Hence, S. gordonii is willing to sacrifice H2O2 production for acid protection at low pH. Alternatively, it could be that the acidification of the environment is a result of increased population growth and therefore is indicative of an established biofilm population, where H2O2 is unlikely to be produced due to an ever-increasing anoxic environment. Thus, S. gordonii may respond accordingly by decreasing spxB gene expression to further bias its metabolism toward pyruvate fermentation. In either scenario, the net result is increased acid production and acid tolerance for S. gordonii and various other oral streptococci. For S. sanguinis, no change was observed at pH 6, compared with pH 7, regarding spxB expression, H2O2 production, and vicK and ciaH expression. We did observe increased expression of pyk, pfl, and adh, suggesting that the change in pH has an effect on the pyruvate node but potentially directs the metabolic flow toward ethanol production. This is somewhat surprising, since in general the pyruvate formate lyase is considered an oxygen-sensitive enzyme (32) and we performed our experiments aerobically. However, gene expression does not necessarily reflect protein production. In addition, the pyruvate-formate lyase of S. sanguinis was shown to be less oxygen sensitive than other streptococci (33). Overall, the observation that S. sanguinis does not change spxB expression in response to changes in pH is analogous to our previous finding that, unlike S. gordonii, S. sanguinis does not adjust spxB expression in response to changes in carbohydrate concentrations (20–22). The finding that the major carbon catabolite regulatory protein CcpA represses spxB expression in a carbohydrate-independent manner in S. sanguinis suggests that H2O2 production has a slightly different ecological purpose in this species. S. sanguinis is an abundant early colonizer and this potentially affects how S. sanguinis responds to environmental changes and stresses. If its overall abundance is high and the cells reside in a biofilm, then perhaps biofilm-mediated protection ensures S. sanguinis survival when it is temporarily exposed to lower pH. Alternatively, it is possible that S. sanguinis never developed a significant acid tolerance response, since high sugar consumption is, from an evolutionary perspective, a recent development (34).
Another notable finding in the current study is that the lactic acid levels in S. gordonii increased with reduced pH, whereas the levels in S. sanguinis showed no difference. Lactate dehydrogenase converts pyruvate to lactic acid with concomitant oxidation of NADH to NAD+, which is critical for metabolic fitness (35). The amount of lactic acid production by the S. gordonii spxB mutant was significantly increased, compared to the wild type. A similar result was demonstrated with an spxB mutant of S. pneumoniae, compared to the wild type (36). At pH 6, the increase in lactic acid production from S. gordonii and its isogenic spxB mutant improves acid stress survival. This may partially explain why, in subjects with caries, S. gordonii levels diminish much less dramatically than those of S. sanguinis (6). Interestingly, we demonstrated that other SpxB-encoding oral streptococci behave like S. sanguinis and S. gordonii. In particular, our low-passage-number clinical isolates of S. mitis, S. oralis, and S. dentisani, like S. gordonii, reduce the production of H2O2 when exposed to pH 6 and exhibit enhanced survival at pH 5.5. Consistent with our results, S. dentisani was recently reported to grow better at pH 6 than at pH 6.5. The authors speculated that S. dentisani is able to activate the arginolytic pathway at pH 6, which aides in its acid survival (37). Overall, our results suggest that plasticity in the pyruvate node might give certain streptococci an advantage at lower pH. The mechanisms governing the physiological changes that occur due to changes in the pyruvate node await further investigation.
Finally, it should be noted that, although H2O2 production in S. gordonii was compromised at pH 6, the amount produced was still comparable to that of S. sanguinis. It is known that the abundance of S. gordonii in the oral cavity is typically much lower than that of S. sanguinis (38), but the two species are commonly isolated from the same intraoral sites. From an ecological perspective, it is conceivable that S. sanguinis and S. gordonii might coordinate H2O2 production against their competitors in the biofilm. Therefore, when the biofilm community encounters a temporal change in pH, both species might still produce constant levels of H2O2 that are capable of inhibiting their main competitors. This ability, however, is severely affected when the acid challenge changes from temporary fluctuations to more permanent changes in pH (for example, through frequent carbohydrate consumption), leading to a more consistently acidic environment favoring dysbiosis and cariogenesis.
MATERIALS AND METHODS
Bacterial species and culture conditions.
Bacterial strains used in this study are listed in Table 1. S. sanguinis SK36, S. gordonii DL1, and their derivatives were routinely grown aerobically as static cultures (in 5% CO2 at 37°C) in BHI broth (Difco, Sparks, MD) or on BHI agar plates. The pH of the plates was adjusted to pH 7 or pH 6 with HCl prior to autoclaving; the pH of the liquid medium was adjusted to pH 7 or pH 6 after autoclaving, and the medium was filtered with 0.45-μm filters (VWR). Escherichia coli cells were grown aerobically at 37°C in LB medium (Difco). For antibiotic selection, 500 μg ml−1 spectinomycin (Sigma-Aldrich, St. Louis, MO) or 10 μg ml−1 erythromycin (Fisher Scientific, Pittsburgh, PA) was used for S. sanguinis and S. gordonii, and 100 μg ml−1 spectinomycin was used for E. coli. To measure cell growth at pH 7 and pH 6, overnight cultures were diluted 1:30 into fresh medium, and then CFU were determined every 30 min for 10 h and again after 24 h of growth.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| SK36 | Wild-type S. sanguinis | 45 |
| DL1 | Wild-type S. gordonii | 46 |
| SK36 ΔspxB | SK36, ΔspxB; Ermr | This study |
| DL1 ΔspxB | DL1, ΔspxB; Ermr | This study |
| SK36 spxB-FLAG | SK36 with spxB-FLAG tag (markerless); Ermr | This study |
| DL1 spxB-FLAG | DL1 with spxB-FLAG tag (markerless); Ermr | This study |
| 133-79 | Wild-type S. sanguinis, endocarditis isolate | 47 |
| JM1 | S. gordonii, low-passage-number oral isolate | Unpublished data |
| S. parasanguinis | Five low-passage-number oral isolates | 19 |
| S. dentisani | Two low-passage-number oral isolates | 19 |
| S. mitis | Two low-passage-number oral isolates | 19 |
| S. oralis | Low-passage-number oral isolate | 19 |
| pDL278 | Shuttle vector; Spcr | 48 |
Ermr, erythromycin resistant; Spcr, spectinomycin resistant.
DNA manipulations.
PCR was performed using a Bio-Rad thermocycler (Bio-Rad, Hercules, CA). DNA polymerases were purchased from New England BioLabs (Ipswich, MA) and used according to the manufacturer's protocol. Oligonucleotides (Table 2) were designed using S. sanguinis SK36 and S. gordonii DL1 sequence data obtained from the NCBI database (http://www.ncbi.nlm.nih.gov/genome) and were synthesized by Integrated DNA Technologies (Coralville, IA).
TABLE 2.
Primers used in this study
a Chromosomal locus tags can be found in Table S1 in the supplemental material.
Design and synthesis of in-frame deletion cassette.
To create in-frame markerless deletions and insertions, an in-frame deletion cassette (IFDC) for use in S. sanguinis (NCBI reference sequence NC_009009.1) and S. gordonii (NCBI reference sequence NC_009785.1) was created on the basis of an IFDC created previously for S. mutans (39). The IFDC was designed with the 278-bp S. sanguinis ldh promoter region. Downstream of this region, the pheS gene of S. sanguinis was engineered with an A316G point mutation (pheS*); pheS* was controlled by its native ribosome binding site. Silent mutations were created immediately following the point mutation within pheS*, to prevent homologous recombination with the chromosomal pheS. An erythromycin resistance cassette (ermAM) was placed directly following pheS*. The IFDC was flanked by BamHI and HindIII restriction sites at the 5′ and 3′ ends, respectively, to enable ligation and cloning for storage in E. coli DH10b cells (Lucigen). The IFDC was synthesized by Integrated DNA Technologies in three gBLocks and pieced together via PCR with the IFDC primers listed in Table 2.
Deletion of spxB in S. gordonii DL1 and S. sanguinis SK36.
The deletion of spxB in S. gordonii DL1 and S. sanguinis SK36 was performed via allelic replacement. An upstream fragment and a downstream fragment of spxB were amplified by PCR using Accuprime Pfx DNA polymerase (Invitrogen). Subsequently, the upstream and downstream fragments were combined in an overlapping PCR with an erm resistance cassette, and the resulting amplicons were transformed into DL1 and SK36, generating DL1 ΔspxB and SK36 ΔspxB, respectively. In detail, the primer pairs spxB_up_del_S_F and spxB_up_del_GS_R (SK36) and spxB_up_seq_GPa_F and spxB_up_del_GS_R (DL1) were used for the upstream fragments. The downstream fragments were amplified using the primer pairs spxB_dn_del_S_F and spxB_dn_seq_R (SK36) and spxB_dn_del_G_F and spxB_dn_seq_R (DL1). The erm resistance cassette was amplified by using the primer pair ermR_spxB_SG_del_F and ermR_spxB_S_del_R (deletion of spxB in SK36) or the primer pair ermR_spxB_SG_del_F and ermR_spxB_G_del_R (deletion of spxB in DL1). For overlap PCR, 0.5 μl of the unpurified PCR products was added directly to 22.5 μl of Accuprime Pfx DNA polymerase reaction mixture. After 10 initial cycles with 80 s of elongation, the primer pairs spxB_up_del_S_F and spxB_dn_seq_R (SK36) and spxB_up_del_GPa_F and spxB_dn_seq_R (DL1) were added, followed by 30 additional amplification cycles (150 s of elongation). The resulting amplicon was transformed into wild-type SK36 and DL1 strains induced for competence with their respective competence-stimulating peptides (CSPs). The correct deletion of spxB was verified by PCR and sequencing. Prussian blue agar plates (23) were used to detect H2O2 production in in S. gordonii ΔspxB mutant and the S. sanguinis ΔspxB mutant. The representative images demonstrated that the spxB mutants of both strains did not produce H2O2 (see Fig. S2A in the supplemental material).
Construction of S. gordonii DL1 and S. sanguinis SK36 with spxB-FLAG tags.
To measure the abundance of SpxB protein, a FLAG tag epitope (DYKDDDDK) was attached to the SpxB C terminus. In the first step, spxB was replaced with an IFDC cassette using allelic replacement. This cassette contained ermAM for positive selection and a site-directed mutant of pheS (SK36) for negative selection (39). In the second step, the IFDC cassette was replaced with spxB-FLAG. To introduce the IFDC cassette into SK36 and DL1, an upstream fragment was amplified using Accuprime Pfx DNA polymerase (Invitrogen) and the following primer pairs: Ss_spxB_Pex_U_F and spxB_ml_del_SG_U_R (SK36) and spxB_up_seq_GPa_F and spxB_ml_del_SG_U_R (DL1). The respective downstream fragment was amplified with spxB_ml_del_SG_D_F and spxB_dn_seq_R. The IFDC cassette was amplified with spxB_IFDC_SG_F and spxB_IFDC_SG_R. Overlap PCR was performed as described previously, utilizing the primer pairs Ss_spxB_Pex_U_F and spxB_dn_seq_R (SK36) and spxB_up_seq_GPa_F and spxB_dn_seq_R (DL1). After transformation, IFDC-positive clones (SK36 ΔspxB-IFDC and DL1 ΔspxB-IFDC) were selected with erythromycin. The correct integration of IFDC was verified by PCR and sequencing. For IFDC replacement, spxB was amplified with the primer pairs spxB_up_del_S_F and SG_flag_spxB_R (SK36) and spxB_up_seq_GPa_F and SG_flag_spxB_R (DL1). The downstream fragment was amplified with the primer pairs S_flag_spxB_dn_F and spxB_checker_R (SK36) and G_flag_spxB_dn_F and spxB_checker_R (DL1). Overlap PCR was performed using the primer pairs spxB_up_del_S_F and spxB_checker_R (SK36) and spxB_up_seq_GPa_F and spxB_checker_R (DL1). The resulting amplicons were transformed into SK36 ΔspxB-IFDC and DL1 ΔspxB-IFDC generating SK36 spxB-FLAG and DL1 spxB-FLAG, respectively. Transformants were selected with dl-4-chlorophenylalanine (39) and verified by PCR and sequencing.
Measurement of H2O2 production.
For the quantification of H2O2 production at different pH values, overnight cultures of S. sanguinis and S. gordonii were diluted 1:30 in fresh BHI medium and grown to an A600 of ∼0.5. Cells were collected by centrifugation and resuspended in an equal volume of fresh BHI broth to remove residual H2O2 that might have accumulated in the supernatant. Fifteen microliters of cells was then spotted onto Prussian blue agar plates (adjusted to pH 7 or pH 6 as described above) essentially as described previously (23). After 16 h of aerobic growth at 37°C, H2O2 production was quantified (in millimeters) by measuring the blue halos on the indicator plates with a digital caliper (Digimatic Caliper, Mitutoyo, Japan). The means ± standard deviations (SDs) of three replicates are presented. The same method was used to determine H2O2 production of low-passage-number oral clinical isolates collected in a previous study (19). The concentrations of H2O2 in liquid cultures were determined using a modification of the protocol described by Gilliland (40). Bacteria grown to mid-logarithmic phase were centrifuged, and 40 μl of cell-free supernatant was mixed with 160 μl of freshly prepared 0.1 M sodium acetate (pH 4.5) containing 0.1 μg horseradish peroxidase (Thermo Scientific) and 10 μl of 1 mg/ml o-dianisidine (Alfa Aesar) in methanol. The reaction mixture was protected from light and incubated at room temperature for 10 min. The absorbance at 570 nm was determined using a microplate reader (model 680; Bio-Rad). A standard curve was determined using serial dilutions of a commercial 30% H2O2 solution in Milli-Q water; the detectable range was 0.1 to 4.0 mM H2O2. To determine cell numbers for normalization, cells were serially diluted and 100-μl aliquots were spotted onto BHI agar plates. CFU were counted after 24 h of incubation at 37°C. The H2O2 concentrations were calculated according to the standard curves and were normalized to the CFU counts. All experiments were performed in triplicate.
Western blotting.
Western blotting was performed to investigate the protein abundance of SpxB-FLAG in S. sanguinis and S. gordonii (21). SK36 SpxB-FLAG and DL1 SpxB-FLAG strains were grown in BHI medium as planktonic cultures until mid-logarithmic phase (A600 values of ∼0.5), collected by centrifugation at 11,000 × g for 15 min at 4°C, and resuspended in 1 ml of phosphate-buffered saline (PBS). Cytoplasmic extracts were generated by mechanical disruption using a Precellys homogenizer (Bertin Corp.), with 0.1-mm silica beads (Thermo Fisher Scientific). Cell debris was separated by centrifugation at 11,000 × g for 15 min at 4°C. Aliquots of the supernatants were adjusted to approximately the same protein concentration using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific), and appropriate volumes were separated by 10% SDS-PAGE. For Western immunoblotting, the proteins were transferred to nitrocellulose membranes (Thermo Fisher Scientific), and the membranes were blocked for 1 h with a solution of 5% skim milk dissolved in Tris-buffered saline containing 0.1% Tween 20 (TBST). The membranes were incubated overnight at 4°C with primary antibody solution (anti-FLAG M2 antibody; Stratagene), followed by three washes with TBST, and then were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (Thermo Fisher Scientific). After being washed with TBST, the blots were developed using the ECL chemiluminescence detection system (Thermo Fisher Scientific).
Lactic acid measurement after pH challenge.
Lactic acid levels were measured essentially as described previously (41). Lactic acid assays were performed following a specific incubation protocol, as outlined in Fig. S4. Briefly, 10 ml of mid-log-phase cells of S. sanguinis SK36 and S. gordonii DL1 were harvested by centrifugation at 4,000 × g for 15 min at 4°C, suspended in fresh BHI broth (pH 7), and incubated at 37°C for 2 h (Fig. S4, 1, pH 7). The bacteria were adjusted to the same cell density (A600 values of ∼0.5), and 10 ml of each culture was used for centrifugation. Aliquots were collected for lactic acid measurements, and the cells were resuspended in fresh BHI broth (pH 6) to a final A600 value of ∼0.5. After 2 h of cultivation at 37°C (Fig. S4, 2, pH 6), the cultures were adjusted to A600 values of ∼0.5, and 10 ml of each culture was centrifuged. Aliquots were collected, the cells were transferred to fresh BHI broth (pH 7) (A600 values of ∼0.5) and incubated at 37°C for 2 h, the cultures were adjusted again to A600 values of ∼0.5, and 10 ml was centrifuged. The supernatants were collected for lactic acid measurements after centrifugation (Fig. S4, 3, pH 7*). The cells from each time point were collected and stored at −80°C for RNA extraction, to determine ldh gene expression. All supernatants were filter-sterilized through a 0.22-μm-pore-size filter (EMD Millipore, Billerica, MA). Lactic acid concentrations were determined using an enzymatic (lactic dehydrogenase) method (41). Standard curves were prepared fresh each time, using a standard lactic acid solution (Supelco Analytical, Bellefonte, PA). All samples and standards were prepared in triplicate.
Additionally, overnight cultures of S. sanguinis SK36 and S. gordonii DL1 were inoculated in fresh BHI broth. Mid-log-phase cells were harvested by centrifugation at 4,000 × g for 15 min at 4°C, suspended in fresh BHI broth (pH 7 or pH 6), and incubated at 37°C for 2 h or 4 h. The CFU of each culture were determined by serial dilution; the cultures were subsequently centrifuged, and aliquots were collected for lactic acid measurements. CFU were counted after 24 h of incubation at 37°C. All supernatants were filter-sterilized and used for determination of lactic acid concentrations. The lactic acid concentrations were calculated according to the standard curves and normalized by CFU. All experiments were performed in triplicate.
RNA isolation, cDNA synthesis, and quantitative PCR.
Overnight cultures of S. sanguinis SK36 and S. gordonii DL1 were inoculated into fresh BHI medium. Cells at mid-log phase were divided into two groups. For condition 1, cells were collected by centrifugation and resuspended in 20 ml fresh BHI broth adjusted to pH 7 or pH 6. After an additional 2 h of aerobic incubation, cells were collected by centrifugation (Fig. S4, a). For condition 2, 100 μl of log-phase cells was inoculated onto BHI agar plates (6 plates/group, pH 7 or pH 6). All plates were further incubated for 2 h at 37°C, and cells were collected by addition of 5 ml BHI broth to the plates and scraping with sterile cotton swabs, followed by collection of the liquid into 50-ml conical tubes and centrifugation at 11,000 × g for 15 min at 4°C (Fig. S4, b). Cell pellets were stored at −80°C until further use. Total RNA was extracted by following the TRIzol method, according to the manufacturer's protocol. Isolated RNA was treated with DNase I (Invitrogen) to remove traces of chromosomal DNA. RNA samples were cleaned with the Qiagen RNeasy kit. The concentrations and integrity of RNA samples were confirmed using NanoDrop spectrophotometer measurements and gel electrophoresis. cDNA was synthesized from 2 μg of RNA using SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was performed using SYBR green master mix (Bio-Rad), according to the manufacturer's instructions. Threshold cycle (CT) values were determined, and data were analyzed with Bio-Rad CFX MANAGER software (version 2.0), using the 2−ΔΔCT method (42). The 16S rRNA gene was used as the housekeeping reference control.
Acid survival assay.
Acid survival of S. sanguinis SK36 and S. gordonii DL1 and their ΔspxB mutants at pH 6 or pH 5.5 was determined by modification of a previously published method (43, 44). Briefly, mid-log-phase cells were inoculated into fresh BHI broth (pH 7) for 24 h. Subsequently, cells were collected by centrifugation and resuspended in fresh BHI broth (pH 6). After 24 h of incubation at 37°C, cells were washed twice with PBS (pH 6), adjusted to the same A600 values, and divided into two groups. For condition 1, cells were serially diluted and 15 μl was spotted onto BHI agar plates adjusted to pH 6 or pH 5.5. After 24 h of incubation at 37°C, plates were photographed for documentation. For condition 2, cells were resuspended in 0.1 M glycine buffer (pH 6 or pH 5.5). CFU of cells incubated for 0, 0.5, 1, or 2 h were determined. Survival rates were defined as the ratio of CFU per milliliter at pH 5.5 to CFU per milliliter at pH 6.
Statistical analysis.
All data were obtained in triplicate at separate times and statistically analyzed with SPSS (version 18.0 for Windows; SPSS Inc., Chicago, IL). Student's t test was used to compare the data for two groups, followed by the Student-Newman-Keuls test. Data were considered significantly different if the two-tailed P value was <0.05.
Supplementary Material
ACKNOWLEDGMENTS
J.K. acknowledges support through NIH-NIDCR grant DE021726, and J.M. is supported by NIH-NIDCR grants DE022083 and DE018893.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01697-17.
REFERENCES
- 1.Liu C, Niu Y, Zhou X, Zhang K, Cheng L, Li M, Li Y, Wang R, Yang Y, Xu X. 2013. Hyperosmotic response of Streptococcus mutans: from microscopic physiology to transcriptomic profile. BMC Microbiol 13:275. doi: 10.1186/1471-2180-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu C, Niu Y, Zhou X, Zheng X, Wang S, Guo Q, Li Y, Li M, Li J, Yang Y, Ding Y, Lamont RJ, Xu X. 2015. Streptococcus mutans copes with heat stress by multiple transcriptional regulons modulating virulence and energy metabolism. Sci Rep 5:12929. doi: 10.1038/srep12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh PD, Head DA, Devine DA. 2015. Ecological approaches to oral biofilms: control without killing. Caries Res 49(Suppl 1):S46–S54. doi: 10.1159/000377732. [DOI] [PubMed] [Google Scholar]
- 4.Lemos JA, Burne RA. 2008. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154:3247–3255. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen WH. 2016. Dental caries — not just holes in teeth! A perspective. Mol Oral Microbiol 31:228–233. doi: 10.1111/omi.12132. [DOI] [PubMed] [Google Scholar]
- 6.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL. 2002. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mira A, Simon-Soro A, Curtis MA. 2017. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J Clin Periodontol 44(Suppl 18):S23–S38. doi: 10.1111/jcpe.12671. [DOI] [PubMed] [Google Scholar]
- 8.Zeng L, Martino NC, Burne RA. 2012. Two gene clusters coordinate galactose and lactose metabolism in Streptococcus gordonii. Appl Environ Microbiol 78:5597–5605. doi: 10.1128/AEM.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuramitsu HK, Wang BY. 2006. Virulence properties of cariogenic bacteria. BMC Oral Health 6(Suppl 1):S11. doi: 10.1186/1472-6831-6-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender GR, Sutton SV, Marquis RE. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun 53:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton IR, Svensater G. 1998. Acid-regulated proteins induced by Streptococcus mutans and other oral bacteria during acid shock. Oral Microbiol Immunol 13:292–300. doi: 10.1111/j.1399-302X.1998.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi N, Yamada T. 1999. Acid-induced acid tolerance and acidogenicity of non-mutans streptococci. Oral Microbiol Immunol 14:43–48. doi: 10.1034/j.1399-302X.1999.140105.x. [DOI] [PubMed] [Google Scholar]
- 13.Kreth J, Giacaman RA, Raghavan R, Merritt J. 2017. The road less traveled — defining molecular commensalism with Streptococcus sanguinis. Mol Oral Microbiol 32:181–196. doi: 10.1111/omi.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreth J, Zhang Y, Herzberg MC. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol 190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrero ER, Slomka V, Boon N, Bernaerts K, Hernandez-Sanabria E, Quirynen M, Teughels W. 2016. Dysbiosis by neutralizing commensal mediated inhibition of pathobionts. Sci Rep 6:38179. doi: 10.1038/srep38179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Palmer SR, Ahn SJ, Richards VP, Williams ML, Nascimento MM, Burne RA. 2016. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol 82:2187–2201. doi: 10.1128/AEM.03887-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itzek A, Zheng L, Chen Z, Merritt J, Kreth J. 2011. Hydrogen peroxide-dependent DNA release and transfer of antibiotic resistance genes in Streptococcus gordonii. J Bacteriol 193:6912–6922. doi: 10.1128/JB.05791-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreth J, Vu H, Zhang Y, Herzberg MC. 2009. Characterization of hydrogen peroxide-induced DNA release by Streptococcus sanguinis and Streptococcus gordonii. J Bacteriol 191:6281–6291. doi: 10.1128/JB.00906-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L, Xu Y, Ferretti JJ, Kreth J. 2014. Probing oral microbial functionality: expression of spxB in plaque samples. PLoS One 9:e86685. doi: 10.1371/journal.pone.0086685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng L, Itzek A, Chen Z, Kreth J. 2011. Environmental influences on competitive hydrogen peroxide production in Streptococcus gordonii. Appl Environ Microbiol 77:4318–4328. doi: 10.1128/AEM.00309-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng L, Chen Z, Itzek A, Ashby M, Kreth J. 2011. Catabolite control protein A controls hydrogen peroxide production and cell death in Streptococcus sanguinis. J Bacteriol 193:516–526. doi: 10.1128/JB.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng L, Chen Z, Itzek A, Herzberg MC, Kreth J. 2012. CcpA regulates biofilm formation and competence in Streptococcus gordonii. Mol Oral Microbiol 27:83–94. doi: 10.1111/j.2041-1014.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giacaman RA, Torres S, Gomez Y, Munoz-Sandoval C, Kreth J. 2015. Correlation of Streptococcus mutans and Streptococcus sanguinis colonization and ex vivo hydrogen peroxide production in carious lesion-free and high caries adults. Arch Oral Biol 60:154–159. doi: 10.1016/j.archoralbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Biswas I, Drake L, Erkina D, Biswas S. 2008. Involvement of sensor kinases in the stress tolerance response of Streptococcus mutans. J Bacteriol 190:68–77. doi: 10.1128/JB.00990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Echenique JR, Chapuy-Regaud S, Trombe MC. 2000. Competence regulation by oxygen in Streptococcus pneumoniae: involvement of ciaRH and comCDE. Mol Microbiol 36:688–696. doi: 10.1046/j.1365-2958.2000.01891.x. [DOI] [PubMed] [Google Scholar]
- 26.Kreth J, Hung DC, Merritt J, Perry J, Zhu L, Goodman SD, Cvitkovitch DG, Shi W, Qi F. 2007. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology 153:1799–1807. doi: 10.1099/mic.0.2007/005975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senadheera MD, Guggenheim B, Spatafora GA, Huang YC, Choi J, Hung DC, Treglown JS, Goodman SD, Ellen RP, Cvitkovitch DG. 2005. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J Bacteriol 187:4064–4076. doi: 10.1128/JB.187.12.4064-4076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duran-Pinedo AE, Frias-Lopez J. 2015. Beyond microbial community composition: functional activities of the oral microbiome in health and disease. Microbes Infect 17:505–516. doi: 10.1016/j.micinf.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nascimento MM, Zaura E, Mira A, Takahashi N, Ten Cate JM. 2017. Second era of OMICS in caries research: moving past the phase of disillusionment. J Dent Res 96:733–740. doi: 10.1177/0022034517701902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu Rev Biochem 69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 31.Nobbs AH, Zhang Y, Khammanivong A, Herzberg MC. 2007. Streptococcus gordonii Hsa environmentally constrains competitive binding by Streptococcus sanguinis to saliva-coated hydroxyapatite. J Bacteriol 189:3106–3114. doi: 10.1128/JB.01535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yesilkaya H, Spissu F, Carvalho SM, Terra VS, Homer KA, Benisty R, Porat N, Neves AR, Andrew PW. 2009. Pyruvate formate lyase is required for pneumococcal fermentative metabolism and virulence. Infect Immun 77:5418–5427. doi: 10.1128/IAI.00178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi N, Abbe K, Takahashi-Abbe S, Yamada T. 1987. Oxygen sensitivity of sugar metabolism and interconversion of pyruvate formate-lyase in intact cells of Streptococcus mutans and Streptococcus sanguis. Infect Immun 55:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornejo OE, Lefebure T, Bitar PD, Lang P, Richards VP, Eilertson K, Do T, Beighton D, Zeng L, Ahn SJ, Burne RA, Siepel A, Bustamante CD, Stanhope MJ. 2013. Evolutionary and population genomics of the cavity causing bacteria Streptococcus mutans. Mol Biol Evol 30:881–893. doi: 10.1093/molbev/mss278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson KP, Hillman JD. 1982. Competitive properties of lactate dehydrogenase mutants of the oral bacterium Streptococcus mutans in the rat. Arch Oral Biol 27:513–516. doi: 10.1016/0003-9969(82)90093-0. [DOI] [PubMed] [Google Scholar]
- 36.Taniai H, Iida K, Seki M, Saito M, Shiota S, Nakayama H, Yoshida S. 2008. Concerted action of lactate oxidase and pyruvate oxidase in aerobic growth of Streptococcus pneumoniae: role of lactate as an energy source. J Bacteriol 190:3572–3579. doi: 10.1128/JB.01882-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Lopez A, Camelo-Castillo A, Ferrer MD, Simon-Soro A, Mira A. 2017. Health-associated niche inhabitants as oral probiotics: the case of Streptococcus dentisani. Front Microbiol 8:379. doi: 10.3389/fmicb.2017.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tappuni AR, Challacombe SJ. 1993. Distribution and isolation frequency of eight streptococcal species in saliva from predentate and dentate children and adults. J Dent Res 72:31–36. doi: 10.1177/00220345930720010401. [DOI] [PubMed] [Google Scholar]
- 39.Xie Z, Okinaga T, Qi F, Zhang Z, Merritt J. 2011. Cloning-independent and counterselectable markerless mutagenesis system in Streptococcus mutans. Appl Environ Microbiol 77:8025–8033. doi: 10.1128/AEM.06362-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilliland S. 1968. Enzymatic determination of residual hydrogen peroxide in milk. J Dairy Sci 52:321–324. doi: 10.3168/jds.S0022-0302(69)86554-9. [DOI] [Google Scholar]
- 41.Wang SP, Ge Y, Zhou XD, Xu HH, Weir MD, Zhang KK, Wang HH, Hannig M, Rupf S, Li Q, Cheng L. 2016. Effect of anti-biofilm glass-ionomer cement on Streptococcus mutans biofilms. Int J Oral Sci 8:76–83. doi: 10.1038/ijos.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng X, Zheng X, Zhou X, Zeng J, Ren Z, Xu X, Cheng L, Li M, Li J, Li Y. 2016. Regulation of oxidative response and extracellular polysaccharide synthesis by a diadenylate cyclase in Streptococcus mutans. Environ Microbiol 18:904–922. doi: 10.1111/1462-2920.13123. [DOI] [PubMed] [Google Scholar]
- 43.Cheng X, Xu X, Chen J, Zhou X, Cheng L, Li M, Li J, Wang R, Jia W, Li YQ. 2014. Effects of simulated microgravity on Streptococcus mutans physiology and biofilm structure. FEMS Microbiol Lett 359:94–101. doi: 10.1111/1574-6968.12573. [DOI] [PubMed] [Google Scholar]
- 44.Guo Q, Ahn SJ, Kaspar J, Zhou X, Burne RA. 2014. Growth phase and pH influence peptide signaling for competence development in Streptococcus mutans. J Bacteriol 196:227–236. doi: 10.1128/JB.00995-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu P, Alves JM, Kitten T, Brown A, Chen Z, Ozaki LS, Manque P, Ge X, Serrano MG, Puiu D, Hendricks S, Wang Y, Chaplin MD, Akan D, Paik S, Peterson DL, Macrina FL, Buck GA. 2007. Genome of the opportunistic pathogen Streptococcus sanguinis. J Bacteriol 189:3166–3175. doi: 10.1128/JB.01808-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pakula R, Walczak W. 1963. On the nature of competence of transformable streptococci. J Gen Microbiol 31:125–133. doi: 10.1099/00221287-31-1-125. [DOI] [PubMed] [Google Scholar]
- 47.Herzberg MC, Gong K, MacFarlane GD, Erickson PR, Soberay AH, Krebsbach PH, Manjula G, Schilling K, Bowen WH. 1990. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect Immun 58:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunny GM, Lee LN, LeBlanc DJ. 1991. Improved electroporation and cloning vector system for Gram-positive bacteria. Appl Environ Microbiol 57:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.