Abstract
Background: This study aimed to investigate the influence of IL-25 on the capacity of mesenchymal stem cells (MSCs) to induce intestinal epithelial cell regeneration. Methods: The CD4+IL-25R+ cells and LGR5+IL-25R+ cells in colonic mucosa of Crohn’s disease (CD) patients, ulcerative colitis (UC) patients and healthy controls were detected by immunofluorescence staining, and the CD4+IL-25R+ cells in peripheral blood were detected by flow cytometry. Rat MSCs were separated and stimulated with IL-25. Then, MSCs were further incubated in IL-25-free DMEM for 24 h, and this DMEM was collected as conditioned medium (CM). IEC-6 cells were divided into 3 groups: experimental group (CM and TNF-α), control group (DMEM and TNF-α) and negative control group (DMEM). Results: The CD4+IL-25R+ cells and LGR5+IL-25R+ cells significantly increased in the colonic mucosa of active CD patients and UC patients compared with IBD patients in remission and healthy controls. The CD4+IL-25R+ cells reduced in peripheral blood of IBD patients, which was inversely correlated with inflammatory markers (ESR and CRP). CM facilitated the migration and proliferation of IEC-6 cells in the presence of TNF-α. The protein expression of AKT, p38 and ERK increased in IEC-6 cells after treatment with CM and TNF-α. Conclusion: IL-25R is involved in Th-related mucosal inflammation and proliferation of intestinal stem cells in IBD. IL-25 enhances the capacity of MSC to induce intestinal epithelial cell regeneration, and MSC therapy with IL-25 may be a new direction for IBD treatment.
Keywords: Inflammatory bowel disease, mesenchymal stem cells, interleukin-25, intestinal stem cell, CD4
Introduction
Mesenchymal stem cells (MSCs) are primitive, supportive cells with the potential to differentiate into osteoblasts, adipocytes, chondrocytes, and other cell types. They possess immunomodulatory and wound-healing properties in vitro and in vivo [1]. Therefore, MSCs have the prospect as a therapeutic tool in transplantation and autoimmune diseases. Successful preclinical studies on MSCs in animal models of autoimmune diseases, inflammation, and tissue injury have paved the way for clinical trials. To date, more than 100 clinical trials regarding the MSCs treatment in autoimmune diseases have been registered in the Clinical Trials.gov database [2]. However, there remain many unanswered questions about how MSCs therapy works in autoimmune diseases. One of the possible mechanisms is that MSCs can replace the dysfunctional cells via their capacity to induce in situ cell differentiation and tissue growth [3].
Inflammatory bowel disease (IBD) comprises two types of chronic intestinal autoimmune diseases: Crohn’s disease (CD) and ulcerative colitis (UC). The etiology of IBD is associated with the dysregulation of mucosal immune reaction toward commensal bacterial flora, and excessive mucosal damage caused by specific intestinal antigens [4]. Accumulating evidence in animal and human studies has demonstrated that MSCs may be used for the IBD treatment, and clinical trials on the IBD therapy with MSCs have focused on complex fistula and intraperitoneal lesions [5-7]. The repair capability of MSCs seems to depend on many factors including culture condition (inflammatory environment), and thus modification of culture condition may enhance their therapeutic effects on IBD. Investigators have proven that IFN-γ-stimulated MSCs can significantly attenuate DSS-induced colitis and TNBS-induced colitis via inhibiting Th17 response [8]. In addition, MSCs conditioned medium (CM) under hypoxic state is effective for the recovery of DSS-induced colitis through inducing motility and viability of small intestine epithelial cells [9].
Interleukin (IL)-25, a member of the structurally related IL-17 cytokine family, has been shown to stimulate Th2 cell-mediated immune responses, resulting in epithelial cell hyperplasia and enhanced recruitment of inflammatory cells into injured tissues [10]. IL-25 also appears to attenuate the destructive inflammation in several autoimmune diseases via inhibiting Th1 or Th17 immune response [11]. Our previous study demonstrated that IL-25 was markedly decreased in inflamed mucosa of IBD and could inhibit IBD CD4+ T cell activation and differentiation into Th1/Th17 cells in an IL-10-dependent manner [12]. Recently, Wang et al found that MSCs could significantly suppress Th17 responses though increasing IL-25 expression, and knockdown of IL-25 expression in MSCs abrogated Th17 suppression in vitro and in vivo [13]. These results suggest that IL-25 is related to the pathogenesis of autoimmune diseases and immunomodulatory process of MSCs.
The receptor for IL-25 is IL-17RB, which is a 56-kDa single-transmembrane protein expressed abundantly in the kidney, intestine, and other peripheral organs [14]. In this study, the expression of IL-25R was detected in the CD4+ T cells of inflamed mucosa and peripheral blood of IBD patients, and the correlation of CD4+IL-25R+ cells with C reaction protein (CRP) and erythrocyte sedimentation rate (ESR) was further evaluated in IBD patients. Moreover, the expression of IL-25R in the intestinal stem cells of IBD patients was also detected, and the influence of IL-25 primed MSC medium on migration, viability and proliferation of intestinal epithelial cells was further explored. Our findings may provide a better understanding of the role of IL-25 in the pathogenesis of IBD and highlight that IL-25 pathway may serve as a potential target for the IBD treatment based on MSCs.
Materials and methods
Patients and sample collection
IBD patients were recruited from the Department of Gastroenterology, Affiliated Zhongshan Hospital of Xiamen University from May 2014 to March 2016. Inflamed ileal and/or colonic tissues were collected from 32 CD patients and 25 UC patients; whole venous blood was collected from 27 active CD patients and 22 active UC patients. Endoscopic biopsies were taken at the sites of active inflammation adjacent to ulcerations. Human peripheral blood mononuclear cells (hPBMCs) were separated from in all groups as previously described [15]. None received immunosuppressant therapy (i.e., cyclosporine A, azathioprine, 6-mercaptopurine) and biological therapy (i.e. infliximab) before the study. The diagnosis of IBD was based on the clinical manifestations, radiological and endoscopic features, and histological findings of the biopsies. Remission of CD was defined as the Crohn’s Disease Activity Index (CDAI) <150. Moreover, endoscopic biopsies from 35 healthy volunteers (20 males and 15 females; age: 22-50 years) were collected as controls, and venous blood was collected from 30 healthy controls (18 males and 12 females; age: 20-48 years). The study was approved by the Institutional Review Board for Clinical Research of Affiliated Zhongshan Hospital of Xiamen University. Written informed consent was obtained from all subjects before study. The clinical characteristics of these patients are shown in Supplementary Table 1.
Immunofluorescence staining
CD4+IL-25R+ and LGR5+IL-25R+ cells in the intestinal mucosa were examined by immunofluorescence staining. Briefly, 5-µm mucosal sections were obtained, and then fixed in cold acetone for 30 min and blocked with normal goat serum in PBS for 1 h at room temperature. The sections were incubated with primary antibodies (CD4 Ab: Santa Cruz, TX, USA; IL-25R Ab: R&D, MN, USA; LGR5 Ab: Novus, CO, USA) at 4°C overnight and then with fluorescent secondary antibodies (Invitrogen, Carlsbad, USA). After mounting with glycerol, sections were observed using a fluorescence microscope (Olympus BX43, Japan). The observers were blind to the study design. The double positive cells and total stromal cells were subsequently counted, and the percentage of positive cells was calculated as follow: [(positive cells)/(total cell number)] ×100%.
Flow cytometry
hPBMCs were washed with cold PBS and re-suspended in 100 µL of PBS and stained with FITC-conjugated anti-human CD4 (eBiosciences, CA, USA), and PE-conjugated anti-human IL-25R (R&D, MN, USA) antibodies at 2 µg/mL for 30 min at 4°C. Untreated cells were prepared as a control. Finally, the samples were fixed in 0.5 ml of 3% paraformaldehyde, and subjected to flow cytometry on the FACS Calibur instrument (BD Biosciences, CA, USA) and data were analyzed with CellQuest software.
Animals and cell culture
Sprague Dawley (SD) rats (age: 6-8 weeks, weight: 150-170 g) were purchased from Shanghai Laboratory Animal Research Center (Shanghai, China). The rat small intestine epithelial cell line (IEC-6 cells) was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). IEC-6 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco Invitrogen, Carlsbad, USA) supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified environment with 95% O2 and 5% CO2.
MSCs isolation
Isolation and culture of MSCs were performed as described previously [16]. Rats were sacrificed by cervical dislocation, and the tibiae were flushed to harvest bone marrow cells with low glucose DMEM (Gibco Invitrogen, Carlsbad, USA). Cells were then seeded into flasks and grown at 37°C in an environment with 5% CO2. After 3 days, non-adherent cells were removed and the medium was refreshed every 2-3 days. Once the cell confluence reached approximately 80%, cells were detached using 0.25% trypsin-EDTA (HyClone, Utah, USA) and then harvested for following experiments.
Preparation of conditioned medium and treatments of IEC-6 cells
Rat MSCs (2×105 cells/flask) were incubated with 50 ng/mL rat IL-25 (ProSpec, NJ, USA) for 24 h in vitro. Then, MSCs were washed with PBS and further incubated in serum-free DMEM for 24 h. The culture medium was collected, centrifuged at 1000 g for 5 min, filtered through a syringe filter and stored at -80°C as conditioned medium (CM). Control medium was prepared with untreated MSCs in serum-free DMEM following above procedures. In following experiments, IEC-6 cells were divided into 3 groups: negative control group (cells were grown in DMEM supplemented with 10% FBS), experimental group, and control group. In control group, cells were incubated with 200 ng/ml rat TNF-α (Novus, CO, USA) and control medium supplemented with 10% FBS; in experimental group, cells were incubated with 200 ng/ml rat TNF-α and CM supplemented with 10% FBS. Cells were harvested from each group 24 h later for further examinations.
Cell cycle and CCK-8 assays
For cell cycle assay, IEC-6 cells at 1×106 cells/ml were fixed in ice-cold 70% ethanol for 12 h, washed and incubated in dark with 500 ml PI/RNase Staining Buffer (BD Pharmingen, USA) for 15 min at room temperature. Cells were subjected to flow cytometry (BD Biosciences, CA, USA) and data were analyzed with Cell Quest Software (Becton Dickinson). In addition, cell viability was detected by Cell Counting Kit-8 assay (Dojindo Laboratories, Japan). In brief, cells were plated at 3×103 cells/well in 96-well plates with four replicates in each group. Different culture media were added. Cells in experimental group and control group were simultaneously treated with 200 ng/ml rat TNF-α. Then, WST-8 reagent was added 24 h later, and cells were incubated for 2 h. Optical density (absorbance) of each well was measured at 450 nm with a microplate reader (Bio-Rad Laboratories, USA). Three independent experiments were performed and means were calculated.
Scratch wound healing assay
The migration of IEC-6 cells in vitro was measured by scratch wound healing assay. The IEC-6 monolayer (5×105 cells/well) in a six-well plate was scraped in crossed straight lines with a 200-μl pipette tip. Then, cells were washed with PBS twice, and 1 ml of control medium, CM or DMEM supplemented with 10% FBS was added. Cells in experimental group and control group were simultaneously treated with 200 ng/ml rat TNF-α. 24 h later, cells were observed under a light microscope and photographed. Each experiment was repeated at least three times.
Western blotting
Total protein was extracted from IEC-6 cells of each group using RIPA buffer with a cocktail of protease and phosphatase inhibitors. After quantification of protein concentration using BCA assay by microplate spectrophotometry (Thermo, Massachusetts, USA), proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes (Millipore, Massachusetts, USA). Membranes were treated with primary antibodies: anti-AKT1 (Novus, CO, USA), p38 (Novus, CO, USA), ERK (Proteintech IL, USA) and GAPDH (Bioworld, MN, USA) at 4°C overnight. After incubation with secondary antibody, immunoblot detection was performed by Odyssey Imaging System (Li-COR Biosciences, Nebraska, USA).
Statistical analysis
Statistical analysis was performed with SPSS statistical software 17.0 (Chicago, IL, USA). Data are expressed as mean ± standard error (SEM). Differences between groups were compared with t test for 2 independent samples. Correlation was analyzed by Spearman’s correlation analysis. A value of P<0.05 was considered statistically significant.
Results
CD4+IL-25R+ cells and LGR5+IL-25R+ cells in inflamed mucosa
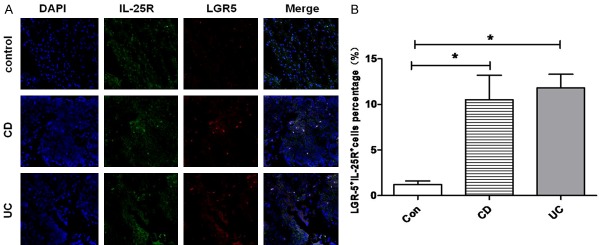
Double immunofluorescence staining was performed to determine in situ expression of IL-25R in mucosal CD4+ T cells of IBD patients and healthy controls. As shown in Figure 1, IL-25R was expressed in CD4+ cells in the lamina propria (LP) of colonic mucosa. Compared with healthy controls (3.7% ± 1.6%), the proportion of CD4+IL-25R+ cells in the LP of inflamed mucosa in CD patients (23.2% ± 6.7%) and UC patients (20.8% ± 7.5%) significantly increased (P<0.05), and no significant difference was observed between CD and UC patients (P>0.05). Moreover, the coexpression of IL-25R and LGR-5 (a marker of intestinal stem cells) was further investigated in the mucosa. As shown in Figure 2, a small amount of LGR5+IL-25R+ cells with cytoplasmic staining was present in the crypts of intestinal mucosa. The proportion of LGR5+IL-25R+ cells in the inflamed ileum/colon was 12.3% ± 2.4% in CD patients and 11.6% ± 1.9% in UC patients, which were significantly higher than in healthy controls (1.5% ± 0.6%; P<0.05). No significant difference was observed between CD and UC patients (P>0.05). Interestingly, the CD4+IL-25R+ cells and LGR5+IL-25R+ cells markedly decreased in the intestinal mucosa of IBD patients in remission or after successful treatment (P<0.05, Figure 3). Thus, the elevated LGR5 expression in the intestinal mucosa may be a feedback response to the insufficient expression of IL-25 and the extensive injury of intestinal epithelium.
Figure 1.
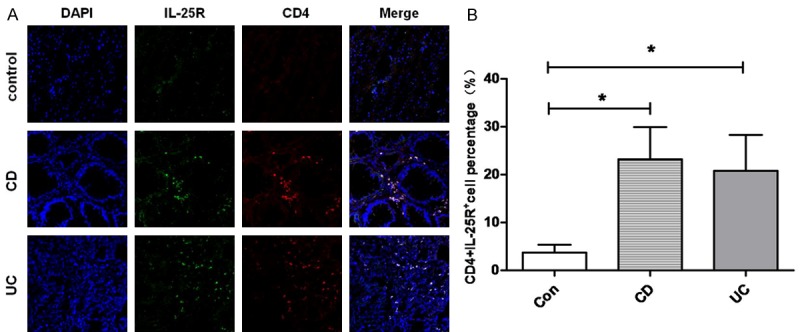
Colocalization between IL-25R and CD4 in the intestinal mucosa. A: Representative images from inflamed mucosa of a CD patient, a UC patient, and a healthy control (×200). IL-25R+CD4+ cells were detected by double immunofluorescence staining. B: Quantification of IL-25R+CD4+ cells in the intestinal mucosa of healthy control, CD patient, and UC patient (*P<0.05 vs control group). Data are expressed as mean number of positive cells per high power field ± SEM from 3 independent experiments.
Figure 2.
Colocalization between IL-25R and LGR5 in the intestinal mucosa. A: Representative images from inflamed mucosa of a CD patient, a UC patient, and a healthy control (×200). IL-25R+LGR5+ cells were detected by double immunofluorescence staining. B: Quantification of IL-25R+LGR5+ cells in the intestinal mucosa of healthy control, CD patient, and UC patient (*P<0.05 vs control group). Data are expressed as mean number of positive cells per high power field ± SEM from 3 independent experiments.
Figure 3.
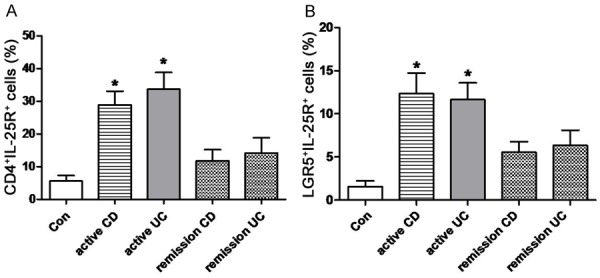
CD4+IL-25R+ cells (A) and LGR5+IL-25R+ cells (B) in the intestinal mucosa of IBD patients in remission or active stage. Cells were detected by double immunofluorescence staining. *P<0.05 vs healthy controls.
CD4+IL-25R+ cells in the peripheral blood
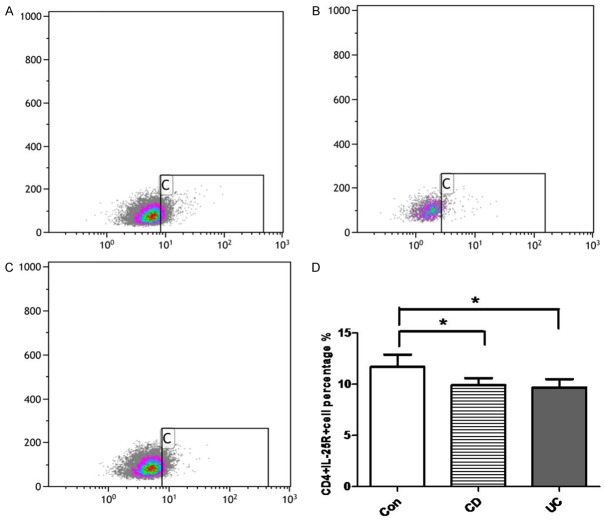
The CD4+IL-25R+ cells were further detected in the peripheral blood of IBD patients and healthy controls by flow cytometry. As shown in Figure 4, the CD4+IL-25R+ cells significantly decreased in the peripheral blood of CD patients (9.9% ± 0.6%) and UC patients (9.6% ± 0.8%) when compared with healthy controls (11.7% ± 1.21%) (P<0.05), but no significant difference was observed between CD and UC patients (P>0.05). The decrease in CD4+IL-25R+ cells of peripheral blood was different from their increase in the inflamed mucosa of IBD patients. Thus, we speculate that the feedback response to the insufficient expression of IL-25 might only exist in local inflamed intestinal mucosa rather than in peripheral circulation of IBD patients.
Figure 4.
Proportion of CD4+IL-25R+ cells in the peripheral blood. CD4+IL-25R+ cells in the peripheral blood of healthy controls (A), CD patients (B) and UC patients (C) were detected by flow cytometry. (D) Quantification of CD4+IL-25R+ cells in the intestinal mucosa of healthy control, CD patient, and UC patient. *P<0.05 vs healthy controls.
Correlation of CD4+IL-25R+ cells with CRP and ESR
Clinically, CRP and ESR are two main inflammatory markers, and may reflect the severity of IBD. The correlations of CD4+IL-25R+ cells with CRP and ESR were further evaluated in IBD patients. Results showed the proportion of CD4+IL-25R+ cells in the peripheral blood was inversely related to the CRP and ESR in both CD patients and UC patients (CRP: CD, r = -0.79; UC, r = -0.72; ESR: CD, r = -0.64; UC, r = -0.69; P<0.05, Figure 5). These findings indicate that the CD4+IL-25R+ cells are closely related to the clinical severity of inflammation in IBD.
Figure 5.
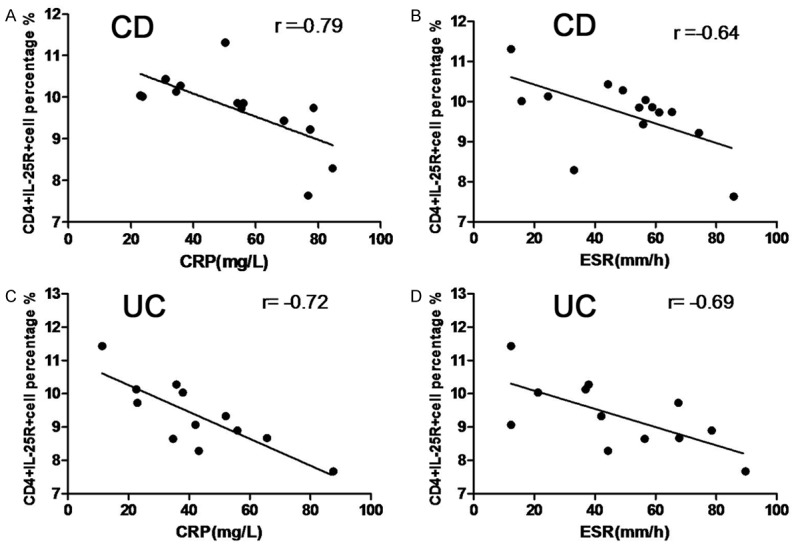
Correlation of CD4+IL-25R+ cells with CRP and ESR in IBD patients. A negative correlation of CD4+IL-25R+ cells percentage with CRP and ESR was observed in both CD (A, B) and UC patients (C, D). Data were analyzed by Spearman’s correlation analysis.
Effects of IL-25 primed MSC medium on migration, viability and cell cycle
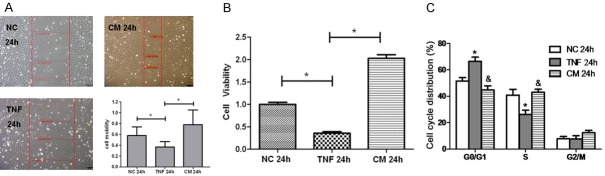
Studies have reported that the proliferative effect of MSCs on the intestinal epithelium seems to depend on many factors such as culture condition (inflammatory environment) [17,18]. In the present study, the effects of IL-25 primed MSC medium on cell migration, viability and cell cycle were further explored in IEC-6 cells. As shown in Figure 6A, the migration of IEC-6 cells in vitro was measured by a monolayer wound healing assay. The scratch wound of IEC-6 monolayer became wider after TNF-α treatment, and IL-25 primed MSC medium significantly facilitated the wound closure as compared to control medium and DMEM treatments (P<0.05). CCK8 assay indicated that treatment with IL-25 primed MSC medium was better than other treatments (control medium and DMEM) to increase IEC-6 cell viability in the presence of TNF-α, (Figure 6B, P<0.05). In addition, cells in G1 phase increased and cells in S phase reduced in IEC-6 cells after 24-h treatment with TNF-α, which was significantly inhibited after treatment with IL-25 primed MSC medium (Figure 6C). Taken together, these findings confirm that IL-25 effectively facilitates the proliferative effect of MSCs on intestinal epithelium.
Figure 6.
Effect of IL-25 primed MSCs medium on cell migration, viability and cell cycle. A: The migration of IEC-6 cells in vitro was measured by wound healing assay. Red line: cell wound. *P<0.05. B: CCK8 assay of IEC-6 cells treated with different media for 24 h. *P<0.05. C: Cell cycle distribution of IEC-6 cells after 24-h exposure to different media was examined by flow cytometry. *P<0.05 vs NC group, &P<0.05 vs TNF-α group. NC: negative control group; TNF: control medium group; CM: IL-25 primed MSCs medium group.
Activation of Akt and mitogen-activated protein kinase signal pathway in IEC-6 cells after IL-25 primed MSC medium stimulation
There is evidence showing that the PI3K-Akt pathway is involved in the epithelial cell survival in the inflammatory microenvironment in vivo [19]. To investigate the underlying mechanism of MSC proliferative effect on intestinal epithelium after IL-25 treatment, the expression of PI3K-Akt pathway related proteins was measured by western blotting. As shown in Figure 7, the protein expression of Akt1, P38 and ERK markedly decreased in IEC-6 cells after 24-h exposure to 200 ng/ml TNF-α (P<0.05), but the incubation in IL-25 primed MSC medium resulted in significant up-regulation of Akt1, P38 and ERK expression in the presence of TNF-α (P<0.05). Interestingly, the protein expression of phosphorylated Akt1, P38 and ERK was not significantly affected by IL-25 primed MSC medium in vitro (P>0.05, data not shown). These findings suggest that PI3K-Akt pathway plays a critical role in the MSC proliferative effect on intestinal epithelium after IL-25 treatment.
Figure 7.
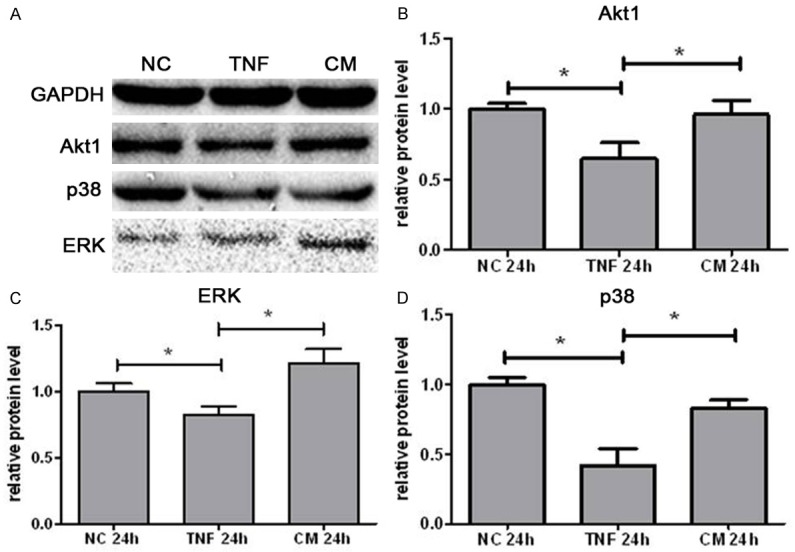
Protein expression of AKT1 (B), ERK(C) and P38 (D) in IEC-6 cells after 24-h exposure to different media. Protein expression was detected by Western blotting. (A) Representative WB images were shown for NC groups, TNF group and CM group. Data are represented as means ± SEM from three independent experiments (*P<0.05). NC: negative control group; TNF: control medium group; CM: IL-25 primed MSCs medium group.
Discussion
In our previous study, results showed IL-25 expression markedly decreased in the inflamed mucosa of IBD, which was related to the intestinal mucosal inflammation and disease activity and could inhibit IBD CD4+ T cell activation and their differentiation into Th1/Th17 cells. In this study, we further investigated the role of IL-25R in the Th-mediated immunity of IBD patients. Our results showed an increase in CD4+IL-25R+ cells in the inflamed mucosa, nut they decreased in peripheral blood of IBD patients. Moreover, the proportion of CD4+IL-25R+ cells was inversely related to ESR and CRP, two inflammatory markers. Furthermore, we investigated whether IL-25 was able to enhance the capacity of MSCs to induce intestinal epithelial cell regeneration. Results showed that IL-25R expression was up-regulated in the intestinal stem cells of IBD patients as compared to healthy controls. In vitro experiments indicated that IL-25 primed MSC medium could promote the migration, viability and proliferation of intestinal epithelial cells (IEC-6 cells), which was, at least partially, mediated through PI3K-Akt pathway.
Although the specific causes of IBD are still poorly understood, it is now widely accepted that, atypical infection, genetic susceptibility, and autoimmune responses to luminal flora play major roles in the pathogenesis of human IBD [20-22]. Most current theories concentrate on the disordered intestinal mucosal lymphocyte activation and the imbalance between pro-inflammatory and anti-inflammatory signals. Theoretically, CD seems to be driven by the Th1 and Th17 activated CD4+ lymphocyte response, with an increase in TNF-α, IL-17 and IL-21, while UC is associated with a cytokine profile similar to the Th2-mediated response, producing IL-5 and IL-13 but not IL-4 [23,24]. Cytokines play a key role in this process and determine the differentiation of Th1, Th2 and Th17 cells. Cytokines temporally and spacially orchestrate the development, recurrence and exacerbation of inflammatory response in IBD [25,26].
IL-25 has been found to suppress the development of Th1/Th17 immune response. Studies have shown that IL-25 is able to inhibit the production of Th1 or Th17-associated cytokines, and prevents and treats experimental murine colitis [27]. In addition, a systematic defect of IL-25 has been found in patients with several autoimmune diseases, including IBD, indicating that IL-25 deficiency may predispose to magnified inflammatory responses [28,29]. IL-25 acts through binding to IL-25R, which is expressed in Th2 central memory cells, eosinophils, monocytes, and non-immune cells, such as epithelial and endothelial cells [14]. The binding between IL-25 and IL-25R leads to the activation of some transcription factors, such as NF-κB, STAT6, 212GATA3, NF-ATC1, JUNNB, MAPK, and JNK [30]. In this study, the IL-25R protein expression was found to be up-regulated in CD4+ cells of inflammed mucosa in IBD patients, which was inconsistent with the systematic reduction in IL-25 expression in these patients. The elevated IL-25R production in the intestinal mucosa may be a feedback response of CD4+ cells to the reduced expression of IL-25. However, the IL-25R expression decreased in CD4+ cells of peripheral blood from IBD patients, suggesting that this feedback response might only exist in local inflamed intestinal mucosa rather than in peripheral circulation of IBD patients. In addition, the CD4+IL-25R+ cells markedly decreased in the intestinal mucosa of IBD patients in remission or after successful treatment, and the proportion of CD4+IL-25R+ cells in the peripheral blood of IBD patients were inversely related to serum CRP and ESR, two main markers of IBD disease activity. These results indicate that the change in IL-25R of CD4+ cells in IBD patients is closely associated with the inflammatory responses.
Traditional therapy for IBD employs corticosteroids, 5-aminosalicylates, antimicrobiotics, immunosuppressive agents and/or monoclonal antibodies, but not all the patients are responsive to these medications and some of drugs have multiple adverse effects. In addition, intestinal resection is still needed for IBD patients after treatment failure [31,32]. Several reports have demonstrated that MSC transplantation is a promising treatment for IBD in both clinical trials and animal models [7,33]. MSCs possess the capabilities of differentiation and self-renewal, and they can migrate to the site of injury and repair the damaged tissues [34]. The migration and differentiation of MSCs to specific tissues are largely dependent on chemotactic signals at the injured or inflamed sites [35]. TNFα-primed cells are more sensitive to most chemokines, suggesting that the mobilization of MSCs and their subsequent differentiation into injured tissues depend on the systemic and local inflammatory states [36]. LGR5 expression is mainly found in the crypt base columnar cells of the intestine, and thus LGR5 has been used as a marker of stem cells in the small intestine, colon, stomach pylorus, and other organs such as hair follicle [37]. In our study, results showed a small amount of LGR5+IL-25R+ cells in the crypts of intestinal mucosa, indicating that IL-25 may act on intestinal stem cells through binding to IL-25R. Furthermore, the proportion of LGR5+IL-25R+ cells increased in the inflamed mucosa in active IBD patients, while its expression markedly decreased in remission of IBD or after successful treatment. We speculate that the inflammatory milieu of IBD might up-regulate the IL-25R expression in the intestinal stem cells, finally leading to the augmented response of intestinal stem cells to IL-25. Our results are in concordance with the findings of Saenz et al that IL-25 could promote the accumulation of multipotent progenitor cell population in the gut-associated lymphoid tissues through inducing Th2 cytokine response [38].
We further investigated whether IL-25 influenced the effect of MSCs on the intestinal epithelium repair. Recent studies reported that the MSCs related repair is independent of their direct engraftment, but relies on their capacity to release trophic factors favoring tissue regeneration in a special immune microenvironment [39]. For instance, hypoxia may profoundly affect the compositions of MSC-conditioned medium, which has a lot of pleiotropic gut trophic factors involved in wound healing, proliferation, and tissue remodeling [9]. Our results showed that IL-25 primed MSCs medium could facilitate the migration, viability and proliferation of IEC-6 cells in the presence of TNF-α, strongly implicating the IL-25 induced paracrine mechanism in the proliferative effect of MSCs on the intestinal epithelium. Moreover, IL-25 primed MSC medium could up-regulate the expression of PI3K-Akt pathway related proteins in IEC-6 cells after 24-h exposure to TNF-α. Of note, PI3K-Akt pathway is involved in the epithelial cell survival to resist the inflammatory damage [19]. Therefore, we hypothesized that IL-25 might promote the anti-inflammatory and proliferative effects of MSCs on the intestinal epithelium via the downstream signaling pathways of PI3K-Akt. However, the factors in the IL-25 primed MSCs medium that promote the proliferation of IEC-6 cells remain to be further identified.
Conclusions
In summary, our results show that IL-25R is involved in Th-related mucosal inflammation and proliferation of intestinal stem cells in IBD patients. IL-25 enhances the capability of MSCs to induce intestinal epithelial cell regeneration. IL-25 mediated MSC-based therapy may be a new treatment for IBD.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81400601) and the Young Innovative Research Foundation of Fujian Province (No. 2014-2-71) to Jingling Su.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munir H, McGettrick HM. Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells Dev. 2015;24:2091–2100. doi: 10.1089/scd.2015.0008. [DOI] [PubMed] [Google Scholar]
- 3.Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 5.de Witte SF, Franquesa M, Baan CC, Hoogduijn MJ. Toward development of imesenchymal stem cells for immunomodulatory therapy. Front Immunol. 2015;6:648. doi: 10.3389/fimmu.2015.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 7.Panes J, Garcia-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 8.Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549–1558. doi: 10.1002/stem.698. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe S, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nasuno M, Yamashita K, Idogawa M, Naishiro Y, Murata M, Adachi Y, Fujimiya M, Imai K, Shinomura Y. Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. J Gastroenterol. 2014;49:270–282. doi: 10.1007/s00535-013-0901-3. [DOI] [PubMed] [Google Scholar]
- 10.Kempuraj D, Frydas S, Conti P, Kandere-Grzybowska K, Boucher W, Letourneau R, Madhappan B, Huang SH, Sugimoto K, Papadopoulou NG, Christodoulou S, Theoharides TC. Interleukin-25 (or IL-17E): a new IL-17 family member with growth factor/inflammatory actions. Int J Immunopathol Pharmacol. 2003;16:185–188. doi: 10.1177/039463200301600301. [DOI] [PubMed] [Google Scholar]
- 11.Saadoun D, Terrier B, Cacoub P. Interleukin-25: key regulator of inflammatory and autoimmune diseases. Curr Pharm Des. 2011;17:3781–3785. doi: 10.2174/138161211798357872. [DOI] [PubMed] [Google Scholar]
- 12.Su J, Chen T, Ji XY, Liu C, Yadav PK, Wu R, Yang P, Liu Z. IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:720–728. doi: 10.1097/MIB.0b013e3182802a76. [DOI] [PubMed] [Google Scholar]
- 13.Wang WB, Yen ML, Liu KJ, Hsu PJ, Lin MH, Chen PM, Sudhir PR, Chen CH, Chen CH, Sytwu HK, Yen BL. Interleukin-25 mediates transcriptional control of PD-L1 via STAT3 in multipotent human mesenchymal stromal cells (hMSCs) to suppress th17 responses. Stem Cell Reports. 2015;5:392–404. doi: 10.1016/j.stemcr.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredo G, Storie J, Shrestha Palikhe N, Davidson C, Adams A, Vliagoftis H, Cameron L. Interleukin-25 initiates Th2 differentiation of human CD4(+) T cells and influences expression of its own receptor. Immun Inflamm Dis. 2015;3:455–468. doi: 10.1002/iid3.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Feng BS, Yang SB, Chen X, Su J, Yang PC. Interleukin (IL)-23 suppresses IL-10 in inflammatory bowel disease. J Biol Chem. 2012;287:3591–3597. doi: 10.1074/jbc.M111.304949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155–162. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 17.Park JS, Yi TG, Park JM, Han YM, Kim JH, Shin DH, Tak SJ, Lee K, Lee YS, Jeon MS, Hahm KB, Song SU, Park SH. Therapeutic effects of mouse bone marrow-derived clonal mesenchymal stem cells in a mouse model of inflammatory bowel disease. J Clin Biochem Nutr. 2015;57:192–203. doi: 10.3164/jcbn.15-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang WQ, Dong K, Zhou L, Jiao GH, Zhu CZ, Li WW, Yu G, Wu WT, Chen S, Sun ZN, Wang YM, Liu WT, Zhang J, Wang BM, Feng XM. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin. 2015;36:1377–1387. doi: 10.1038/aps.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 20.Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113–120. doi: 10.2147/JIR.S65979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hisamatsu T, Kanai T, Mikami Y, Yoneno K, Matsuoka K, Hibi T. Immune aspects of the pathogenesis of inflammatory bowel disease. Pharmacol Ther. 2013;137:283–297. doi: 10.1016/j.pharmthera.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca-Camarillo G, Yamamoto-Furusho JK. Immunoregulatory pathways involved in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2188–2193. doi: 10.1097/MIB.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 24.Kmiec Z, Cyman M, Slebioda TJ. Cells of the innate and adaptive immunity and their interactions in inflammatory bowel disease. Adv Med Sci. 2017;62:1–16. doi: 10.1016/j.advms.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:374–388. e374. doi: 10.1053/j.gastro.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen ML, Sundrud MS. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:1157–1167. doi: 10.1097/MIB.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franze E, Rizzo A, Caruso R, Pallone F, Monteleone G. Interleukin-25 negatively controls pathogenic responses in the gut. Inflamm Allergy Drug Targets. 2011;10:187–191. doi: 10.2174/187152811795564028. [DOI] [PubMed] [Google Scholar]
- 28.Caruso R, Stolfi C, De Nitto D, Pallone F, Monteleone G. The dual role of interleukin-25 in the control of immune-mediated pathologies. Curr Mol Med. 2011;11:26–30. doi: 10.2174/156652411794474365. [DOI] [PubMed] [Google Scholar]
- 29.Javan MR, Seyfizadeh N, Aslani S, Farhoodi M, Babaloo Z. Molecular analysis of interleukin-25 exons 1 and 2 and its serum levels in Iranian patients with multiple sclerosis. Am J Clin Exp Immunol. 2014;3:91–96. [PMC free article] [PubMed] [Google Scholar]
- 30.Monteleone G, Pallone F, Macdonald TT. Interleukin-25: a two-edged sword in the control of immune-inflammatory responses. Cytokine Growth Factor Rev. 2010;21:471–475. doi: 10.1016/j.cytogfr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Grevenitis P, Thomas A, Lodhia N. Medical therapy for inflammatory bowel disease. Surg Clin North Am. 2015;95:1159–1182. vi. doi: 10.1016/j.suc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–278. doi: 10.1038/nrgastro.2016.208. [DOI] [PubMed] [Google Scholar]
- 33.Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH, Du HT, Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013;19:4702–4717. doi: 10.3748/wjg.v19.i29.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 36.Jin P, Zhao Y, Liu H, Chen J, Ren J, Jin J, Bedognetti D, Liu S, Wang E, Marincola F, Stroncek D. Interferon-gamma and tumor necrosis factor-alpha polarize bone marrow stromal cells uniformly to a Th1 phenotype. Sci Rep. 2016;6:26345. doi: 10.1038/srep26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 38.Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, Peterson LW, Wherry EJ, Goldrath AW, Bhandoola A, Artis D. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med. 2013;210:1823–1837. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao Y, Lei J, Liu M, Lin W, Hong D, Tuo Y, Jiang MH, Xia H, Wang M, Huang W, Xiang AP. Mesenchymal stromal cells mitigate experimental colitis via insulin-like growth factor binding protein 7-mediated immunosuppression. Mol Ther. 2016;24:1860–1872. doi: 10.1038/mt.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.