Abstract
To investigate the role of dermal Vγ4 +γδ T cells in psoriasis-like skin inflammation induced by a re-challenge with imiquimod (IMQ), we compared the development of dermatitis induced by topical application of IMQ in primary challenged mice and re-challenged mice. We also compared the development of dermatitis induced by IMQ between re-challenged control mice and Vγ4 - depleted re-challenged mice that had been initially subjected to IMQ-induced dermatitis 30 prior. We found that the IMQ-induced dermatitis was exacerbated in the re-challenged group compared with the primary challenged group and the Vγ4 - depleted re-challenged group. In addition, the Vγ4 +γδ T cells increased in number and secreted more IL-17A, γ-IFN and IL-22 in the re-challenged control group compared with the primary challenged group. However, in the Vγ4 - depleted re-challenged group, the Vγ4 -γδ T cells increased in number and produced more IL-17A and IL-22 compared with re-challenged control mice. These findings suggest that dermal Vγ4 +γδ T cells enhance relapsing psoriasis-like skin inflammation induced by IMQ in C57BL/6 mice by secreting IL-17A and γ-IFN.
Keywords: Psoriasis, Vγ4+γδ T cells, cytokines, relapse
Introduction
Psoriasis is a relapsing inflammatory skin disease that affects 2-3% of the population worldwide and is characterized by erythema and scaly plaques over the skin surface. The main pathological changes that occur in psoriasis are the proliferation and differentiation of epidermal keratinocytes and the infiltration of inflammatory cells in skin tissues. The pathogenesis of psoriasis is still not fully understood. Previous studies have shown that psoriasis is an autoimmune disease associated with activated T cells. Moreover, recent studies have established that IL-17A-producing γδ T cells (γδ T17 cells) play a critical role in psoriasis [1-3].
There are six IL-17 family members, IL-17A through IL-17F. IL-17A (also commonly called IL-17) is involved in the development of autoimmune inflammatory diseases, including human psoriasis and models of psoriasis induced by imiquimod (IMQ) or IL-23 [4,5]. In addition, psoriasis-like skin inflammation is decreased in IL-17-/- mice, and an IL-17 neutralizing antibody has been tested in stage III clinical trials for the treatment of plaque-type psoriasis in human. These results indicate that IL-17 plays a central role in the development of psoriasis [6,7]. IL-17 cytokines, especially IL-17A, are produced by γδ T cells, which are a tiny subset of T cells and the main source of IL-17A in both the physiological state and some diseases [7-9]. In psoriasis lesions, γδ T cells are increased in number and produce a large mount of IL-17A [10]. In mouse skin, epidermal γδ T cells are called dendritic epidermal T cells, and 98% of these cells are Vγ5 + T cells. Dermal γδ T cells consist of Vγ4 +, Vγ5 + and Vγ4 -Vγ5 - subpopulations and are the mayor source of IL-17A following IL-23 stimulation [11]. To conveniently describe the functions of the Vγ5 + and Vγ4 -Vγ5 - subpopulations, they are collectively known as Vγ4 - cells in later sections. Recent studies have shown that Vγ4 + cells are a subset of γδ T cells and play a critical role in IMQ-induced psoriasis-like skin inflammation via production of IL-17A [12,13].
Previous studies have demonstrated that IFN-γ can initiate and enhance psoriasis by promoting the activation and proliferation of keratinocytes [14-16]. There are reports that γδ T cells in the draining lymph nodes and spleen produce IFN-γ in IMQ-induced psoriasis-like skin inflammation [17]. However, it remains unclear whether dermal Vγ4 +γδ T cells secrete the IFN-γ. The psoriasis-like skin inflammation induced by IMQ is reduced in IL-22-deficient mice and in mice treated with IL-22 neutralizing antibody, indicating that IL-22 is responsible for psoriasis-like lesions in the mouse IMQ model [18,19]. γδ T cells in the lymph nodes and spleen have been reported to secrete IL-22 [19]. Here, we detected the IFN-γ and IL-22-producing Vγ4 + cell in the dermis.
Previous report shave established that IL-23 is required for the differentiation and survival of IL-17-producing cells in the model of psoriasis-like dermatitis induced by IMQ, a Toll-like receptor ligand that is used as a model to elucidate developmental mechanism of psoriasis and to evaluate the efficacy of new treatments [20-22]. In the IMQ-induced psoriasis-like skin inflammation model, the number of IL-17A-producing Vγ4 + cells is increased, and these cells exhibit long-lived memory at least 3 months after initial stimulation with IMQ [23]. However, few studies have examined whether innate-memory Vγ4 + cells exacerbate IMQ-induced psoriasis-like dermatitis. Though recent studies have reported that memory Vγ4 + cells rapidly respond to repeated exposure to the same stimulus and enhance the dermatitis induced by IMQ re-challenge [23,24], the site of topical IMQ application in these studies was on the ear. Using the mouse ear, rather than the back skin, to study psoriasis-like dermatitis prevents full observation of the erythema and the scaly plaques that are the representative of psoriasis. Moreover, it has not previously been reported that dermal Vγ4 - cells produce IL-17A, IFN-γ and IL-22 in Vγ4 - depleted re-challenged mice or that dermal Vγ4 + cells secrete IFN-γ and IL-22 in primary challenged and re-challenged control mice.
Here, we compared the dermatitis induced by IMQ cream in a primary challenged group with that of a re-challenged control group and a Vγ4 - depleted re-challenged group. We found that dermal Vγ4 +T cells were increased in number and were the major source of IL-17A in the re-challenged group. In addition, the dermal Vγ4 +T cells secreted low amounts of IFN-γ and IL-22 in both the re-challenged control group and the Vγ4 - depleted re-challenged group. However, the IL-17A and IL-22-secreting Vγ4 - cells were increased in Vγ4 - depleted re-challenged group.
Materials and methods
Animals
C57BL/6J (B6) mice (female, 6 to 8 weeks old) were purchased from the Experimental Animal Department of the Third Military Medical University. All experiments were approved by the Institutional Animal Care and Use Committee of Third Military Medical University. The mice were kept under specific pathogen-free conditions before the experiments began, and the animals were housed in the Institute of Burn Research of South west Hospital, where the experiments were conducted.
IMQ-induced psoriasis-like skin inflammation model
The induction of psoriasis-like skin inflammationby IMQ was performed as previously described [20]. To first induce dermatitis, mice at 6 to 8 weeks of age received a daily topical dosesof 60.0 mg of commercially available IMQ cream (5%) (Aldara; 3 M Pharmaceuticals) on the shaved back for 6 days. The animals were then housed separately in plastic cages until the experiments were repeated.
Invivo Vγ4 +T cell depletion
After 28 days, the animals that had been subjected to IMQ-induced psoriasis-like skin inflammation were randomly divided into two groups. In one group, the mice were depleted of Vγ4 +T cells by an intraperitoneal injectionof 200 μg of a hamster anti-Vγ4 UC3 monoclonalantibody (BioXCell) diluted in 200 ul of PBS on day 28 and day 30. The mice in the second group were injected with the same volume of PBS and regarded as the control group.
Scoring the severity of skin inflammation
The clinical Psoriasis Area and Severity Index (PASI) was used to directly measure the severity of inflammation on the back skin of mice, and the scores were determined as previously reported [20]. The erythema, scaling, and thickening scores were individually determined based on a standard from 0 to 4:0, none; 1, slight; 2, moderate; 3, marked; 4, very marked. The cumulative score, which included erythema, scaling, and thickening, served as the over all evaluation of the severity of inflammation (scale 0-12). To facilitate the scoring, the mice were photographed each day prior to the application of the topical cream.
Preparation of dermal single-cell suspensions
The dermal tissue was digested as previous described [25]. Skin samples were harvested from mice wit IMQ-induced psoriasis-like skin inflammation, and the fascial underneath the dermal tissue was removed. Next, the skin was cut into 5 mm × 5 mm pieces and flattened out on a cell culture dish. The pieces were soaked in 0.5% trypsin/GNK at 37°C for 1-2 hours, and the dermis and epidermis were then separated. The dermis was digested with DMEM containing 1000 U ml-1 collagenase type II (Worthington Biochemical) and 0.1% DNaseI (Sigma-Aldrich) for 60 minutes at 37°C. A70-μm or 100-um nylon sieve (BD Bioscience) was used to filter the cell suspensions.
Flow cytometry
Cell in suspensions were stained with the following monoclonal antibodies using a previously described staining method [13]: anti-TCR γδ (GL3, Tianjin Sungene Biotech, China), anti-Vγ2 (UC3-10A6, BD Biosciences), anti-IL-17A-BVL-421 (BD Biosciences), anti-IL-22-PE (Biolegend), and anti-IFN-γ-PE (BD Bioscience). For the cellular surface cytokine staining, the nonspecific binding sites were blocked with anti-CD16/32 (clone 2.4G2; Tianjin Sungene Biotech, China), and the cells were then incubated with the antibodies for 30 minutes at room temperature. To stain intracellular cytokines, the cells were stimulated with a cell stimulation cocktail (eBiosicence) for 4 h. The cells were then stained for surface antigens and then fixed with BD Cytofix Buffer, permeabilized with Perm/Wash reagent (BD Biosciences), and stained with anti-IL-22, anti-IL-17A, and anti-IFN-γ. The cells were analyzed with an Attune Acoustic Focusing Cytometer (Applied Biosystems, Life Technologies, CA, USA), and the data were analyzed by FlowJo software (Tree Star Incorporation, USA).
Detection of IL-23 expression in psoriasis-like skin inflammation by western blot
Dermal tissues from mice with psoriasis-like skin inflammation were obtained as mentioned in the section above. The dermal tissues were cut into pieces, weighed, frozen and minced in liquid nitrogen. Sequentially, lysis buffer (KeyGEN, China) containing 1% protease inhibitor cocktail, 5% pheny methyl sulphonyl fluoride and 5% phosphatase inhibitor cocktail was added. Proteins were extracted from the samples as previously described [26]. Equal amounts of protein (40 μg) from eachsample was loaded onto 12% SDS-PAGE gels. The proteins were first electrophoresed at 80 volts for 25 minutes and then at 100 volts for 70 minutes. The proteins werethen transferred to a nitrocellulose (NC) membrane (GE, USA) at 200 milliamps for 70 minutes. Subsequently, the membrane was incubated in Tris-buffered saline (TBS) containing 3% bovine serum albumin (BSA, Biosharp, China) for apporximately 2 hours at room temperature, and the membranes containing proteins with molecular weights between 15 kDa to 25 kDa and between 35 kDa to 40 kDa were incubated with primary antibodies (anti-IL-23 antibody, 1:1000 dilution, Abcam, UK; anti-GAPDH was used as an internal control, 1:5000 dilution, Sungene, China) at 4°C overnight. After shaking 30 minutes at room temperature, the membranes were washed 5 times with TBS containing 1% Tween-20 on ahorizontal rotator, followed by incubation with an HRP-labeled secondary antibody (1:5000) that corresponded to the primary antibody (Zhongshan Biology Company, China) for 60 minutes at room temperature. The membranes were then washed as described above. Using HRP-ECL chemiluminescent solution (Thermal Scientific, USA), the bound proteins were detected by the ChemiDoc TM XRS western blot detection system (Bio-Rad, USA).
Immunohistochemistry
To determine the key cytokines involved in psoriasis intiation and development in the dermal tissue, IL-23 was detected by immunohistochemical staining. Paraffin sections were dried at 67°C and rehydrated. The sections were washed with PBS, incubated in a 95°C-99°C water bath for 18 min, washed again with PBS and incubated in 3% H2O2 for 15 minutes. The sections were then washed 3 times with PBS and blocked with 10% normal goat serum (Zhongshan Biology Company, China) for 1 h at room temperature. The primary antibody (anti-IL-23 antibody ab189300, 1:1000 dilution, Abcam, UK) was then incubated with the tissue overnight at 4°C. The sections were incubated at room temperature for 30 minutes, washed 3 times with PBS, and incubated with biotinylated goat anti-rabbit IgG antibody and avidin peroxidase reagent (Zhongshan Biology Company, China) according to the manufacturer’s protocol.
The chromogenic agent was diaminobenzidine solution. Mayer’s hematoxylin (Bosterbio,China) was used to counterstain the cell nucleus, and the sections were photographed using an optical microscope (CTR6000, Leica, Germany).
Statistical analysis
The PASI scores were evaluated by two-way repeated measures ANOUA. Differences between two sets of data were evaluated by 2-tailed Student’s t-test. Data were presented as the mean ± SD or SEM. P values <0.05 were considered significant.
Results
IMQ-induced psoriasis-like skin inflammation was more serious in re-challenged mice than in primary challenged mice
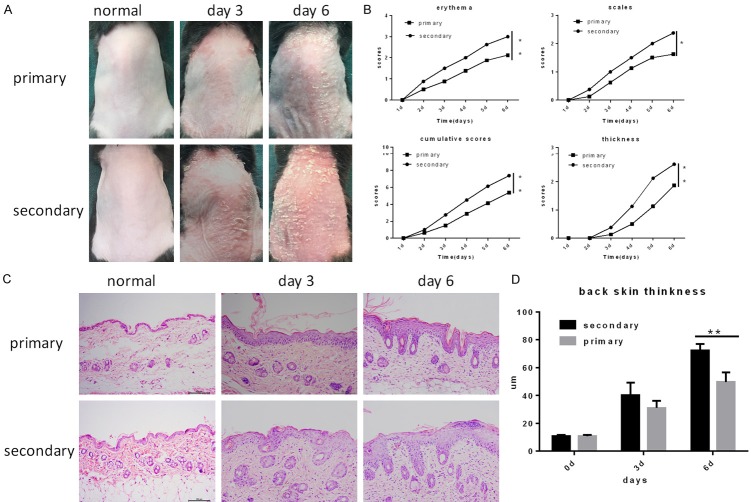
To investigate whether psoriasis-like skin inflammation is enhanced in mice re-challenged with IMQ 30 days after the initial induction of inflammation. We compared the severity of psoriasis-like skin inflammation between primary challenged and re-challenged mice that were the same weight. As expected, in this study, we found that the phenotypic inflammation of the back skin of the re-challenged mice was more serious than that of the primary challenged mice (Figure 1A). We evaluated the PSAI scores of individual mice in the two groups and observed that erythema, scaling, and thickness were more serious in the re-challenged group after day 3 (Figure 1B). Moreover, acanthosis, parakertosis and the dermal infiltration of inflammatory cells were more obvious in the re-challenged mice than in the primary challenged mice, as determined by hematoxylin and eosin (H&E-stained )staining of dermal skin sections from the two groups (Figure 1C). The epidermal tissue thickness was also increased in relapsed mice, as indicated by Image J software measurements. These results thus confirmed that IMQ-induced psoriasis-like skin inflammation was obviously enhanced in the re-challenged group compared with the primary challenged group.
Figure 1.
Psoriasis-like skin inflammation is exacerbated in mice upon re-challenge with IMQ. Psoriasis-like skin inflammation was induced in mice by IMQ cream, and the mice were re-challenged with IMQ 30 days later. Meanwhile, mice of the same weight were also subjected to induced dermatitis under the same conditions. A. Presentation of mouse back skin before treatment and phenotypical presentation after treatment on day 3 and 6. B. Erythema, scaling, and thickness of the back skin were assessed according to PASI, and the cumulative score (erythema plus scaling plus thickness) is depicted. Symbols indicate the mean score of eight mice per group. C. H&E staining of the back skin before treatment and after treatment on day 3 and 6. D. The back skin thickness was determined in H&E-stained tissue by Image J software; the bars represent the mean ± SEM for at least three mice per group.
Dermal Vγ4 +γδ T cells rapidly respond to a secondary challenge with the same IMQ stimulus
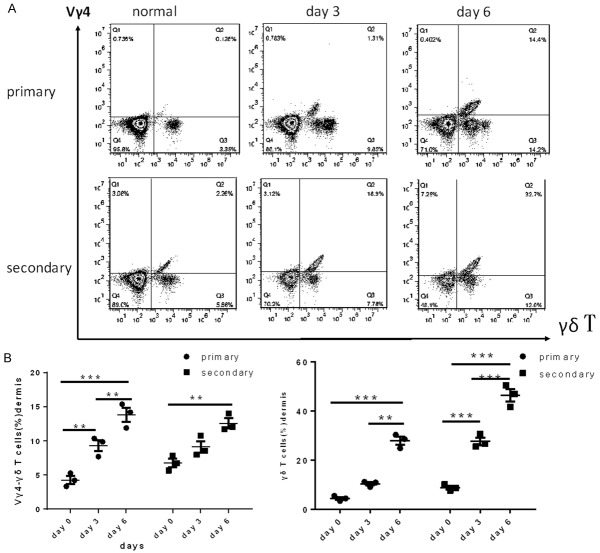
Vγ4 +γδ T cells have been demonstrated to play a pivotal role in the initiation of IMQ-induced dermatitis [7]; however, the function of dorsal dermal Vγ4 +γδ T cells in dermatitis upon rechallenged remained unknown. To count the dermal Vγ4 +γδ T cells in both the primary challenged and the re-challenged mice, cell suspensions were obtained from mice after the first and second IMQ treatment periods and measured by FACS. The Vγ4 +γδ T cell numbers were low in normal skin and increased after the first induction of psoriasis-like skin inflammation by IMQ beginning at day 3. Furthermore, we found that the number of Vγ4 +γδ T cells increased in dermis tissue from mice treated with a second round of IMQ compared with mice after the first IMQ treatment (Figure 2A). By contrast, the increase in Vγ4 -γδ T cells was slower in re-challenged mice than in primary challenged mice (Figure 2B), but the total number of γδ T cells obviously increased in the re-challenged group (Figure 2B). Interestingly, we detected that the number Vγ4 +γδ T cells was significantly reduced in the mice that had only been initially challenged with IMQ30 days prior (Figure 2A). These results thus suggested that Vγ4 +γδ T cells rapidly respond to a secondary challenge with the same IMQ stimulus.
Figure 2.
The number of Vγ4 +γδ T cells in dermal tissue. A. Vγ4 +γδ T cells were measured in a suspension of digested dermal cells from mice with psoriasis-like skin inflammation on day 0, 3 and 6, gated on total T cells. B. The percent of Vγ4 -γδ T cells and γδ T cells on different days. The values were calculated as the mean ± SEM (n=3), **p<0.01, ***p<0.001.
Dermal Vγ4 + cells were the major source of IL-17A and secreted γ-IFN and IL-22 upon secondary challenge
Recently, Vγ4 + cells, a subset of γδ T cells, have been demonstrated to play a role in the pathogenesis of IMQ-induced psoriasis-like skin inflammation [7], but their role in dermatitis induced by a secondary IMQ challenge was still not understood. To characterize the role of Vγ4 + cells upon a secondary challenge, the expression of cytokines was measured by FACS. IMQ cream was applied on both initial challenged mice and re-challenged mice. On day 3 and day 6, the mice were killed and single-cell suspensions were prepared for intracellular staining. In the mice treated with IMQ twice, both the expression of IL-17A and the proportion of IL-17A-producing Vγ4 + cells were significantly increased on day 3 and day 6 compared with the mice treated with IMQ once (Figure 3A, 3B). These results indicated that Vγ4 + cells were the major source of IL-17A upon a secondary challenge.
Figure 3.
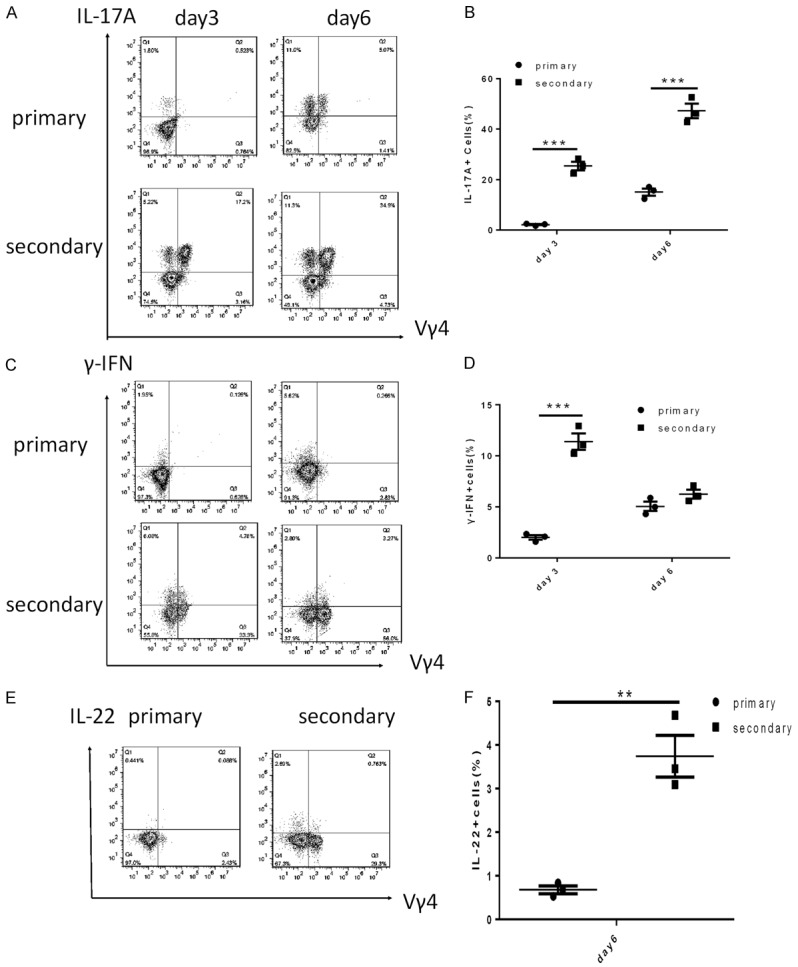
The dermal Vγ4 + cells produced IL-17A, γ-IFN and IL-22 after application of IMQ cream. (A) Representation of IL-17A+ Vγ4 + cells from mice with psoriasis-like skin inflammation on day 3 and 6. (B) The percent of IL-17+ cells on different days. (C) Representation of γ-IFN+ Vγ4 + cells and (D) the percent of IFN-γ+ cells from mice with psoriasis-like skin inflammation on day 3 and 6. (E) The numbers of IL-22+ Vγ4 + cells and (F) the percent of IL-22+ cells in mice treated with IMQ for 6 days. The values were calculated as the mean ± SEM (n=3), **p<0.01, ***p<0.001.
In addition, previous studies have shown that IFN-γ-producing splenic or thymus γδ T cells play a role in the development of psoriasis [17], but there was little research regarding IFN-γ-producing dermal Vγ4 +T cells. We next examined the dermal Vγ4 + cells on day 3 and day 6 of IMQ treatment by FACS analysis. Compared to primary challenge mice, we found that dermal Vγ4 + cells from re-challenged mice could secrete IFN-γ, and the proportion of IFN-γ-producing Vγ4 + cells was higher in re-challenged mice than in primary challenged mice (Figure 3C, 3D). Unexpectedly, the expression of IFN-γ by cells on day 6 was reduced in the secondary challenge group compared with that observed on day 3. This result was inconsistent with the increases IFN-γ expression observed on the same day of the first IMQ treatment. In addition, we further examined the IL-22-producing Vγ4 + cells in the dermis. Vγ4 + cells secreted low amounts of IL-22 after the second IMQ treatment; however, almost no IL-22 secretion was observed after the first IMQ treatment (Figure 3E, 3F). Meanwhile, the expression of IL-17A, IL-22 and IFN-γ was not detected by FACS under non-inflammatory conditions (data not shown).
The above results suggested that dermal Vγ4 + cells were the major source of IL-17A and secreted IFN-γ and IL-22 in the secondary challenge group compared with the primary challenge group.
Vγ4 - depletion ameliorated IMQ-induced psoriasis-like skin inflammation in mice
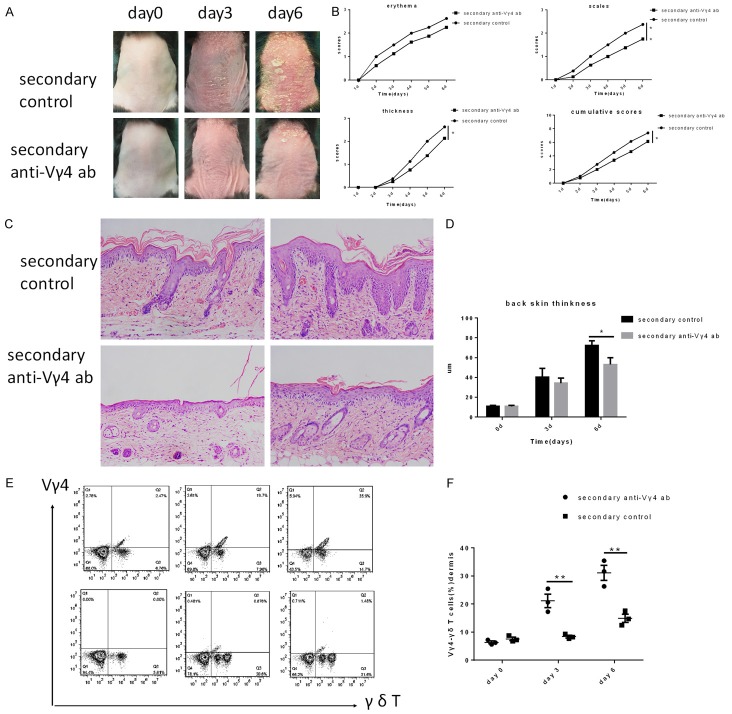
To evaluate the function of Vγ4 + cells in the pathogenesis of recurring psoriasis, Vγ4 + cells were depleted using an anti-Vγ4 antibody in wild-type mice that had been subjected to IMQ-induced psoriasis-like skin inflammation. Consistent with a previous report [25], Vγ4 + cells were completely ablated from the dermis within 72 h after intraperitoneal injection of the antibody. IMQ cream was again applied on the shaved back skin of Vγ4 - depleted mice and control mice, which had previously been treated wit IMQ to induce dermatitis. The severity of dermatitis was evaluated as mentioned in the section above. As expected, Vγ4 - depleted mice showed significantly lower levels of dermatitis compared with control mice after the secondary challenge, as judged by the PASI score of the shaved back skin (Figure 4A, 4B) and the epidermis thickness determined by H&E staining of the dermatitis tissue (Figure 4C, 4D).Meanwhile, on days 0, 3 and 6 of IMQ treatment, we measured the numbers of Vγ4 +γδ T cells and analyzed the percent of Vγ4 -γδ T cells in both Vγ4 - depleted mice and control mice.Vγ4 +γδ T cells increased with the day of IMQ topical treatment in re-challenged control mice; however, the Vγ4 +γδ T cells were not fully depleted in the Vγ4 - depleted mice. Interestingly, when psoriasis-like dermatitis was induced for the second time, we found that the Vγ4 -γδ T subset significantly increased in Vγ4 - depleted mice. These results thus suggested that IMQ-induced psoriasis-like skin inflammation was reduced in Vγ4 - depleted mice.
Figure 4.
Vγ4 -depleted mice show decreased IMQ-induced psoriasis-like skin inflammation. C57BJ/6 mice that were subjected to induced psoriasis-like dermatitis 28 days prior were randomly divided into two groups, the re-challenged anti-Vγ4 antibody group and the re-challenged control group. A. Phenotypical presentation of mouse back skin on day 0, 3, and 6 of IMQ treatment. B. The PASI score was determined for the back skin (n=8 per group). C. H&E staining of the back skin (n=3 per group). D. The thickness of the epidermis skin on day 0, 3, and 6 of IMQ treatment (n=3 per group). E. The number of Vγ4 +γδ T cells in the dermis was evaluated by FACS analysis on day 0, 3, and 6. F. The percent of Vγ4 +γδ T cells on different days (n=3 per group). The values represent the mean ± SEM. *p<0.05.
Dermal cell from Vγ4 - depleted mice showed decreased the expression of IL-17A and γ-IFN
To demonstrate the role of Vγ4 + cells upon a secondary challenged, the expression of cytokines was measured by FACS. IL-17A, one of the most important cytokines in the development of psoriasis, was first detected by FACS, gated on γδ T cells. The number of IL-17A-producing Vγ4 +γδ T cells significantly increased in the control group compared with the Vγ4 - depleted groups (Figure 5A). Unexpectedly, the subsets of Vγ4 -γδ T cells capable of producing IL-17A increased in the Vγ4 - depleted mice compared with the control mice (Figure 5B). This result was consistent with the increased numbers of Vγ4 -γδ T cells described in the section above.
Figure 5.
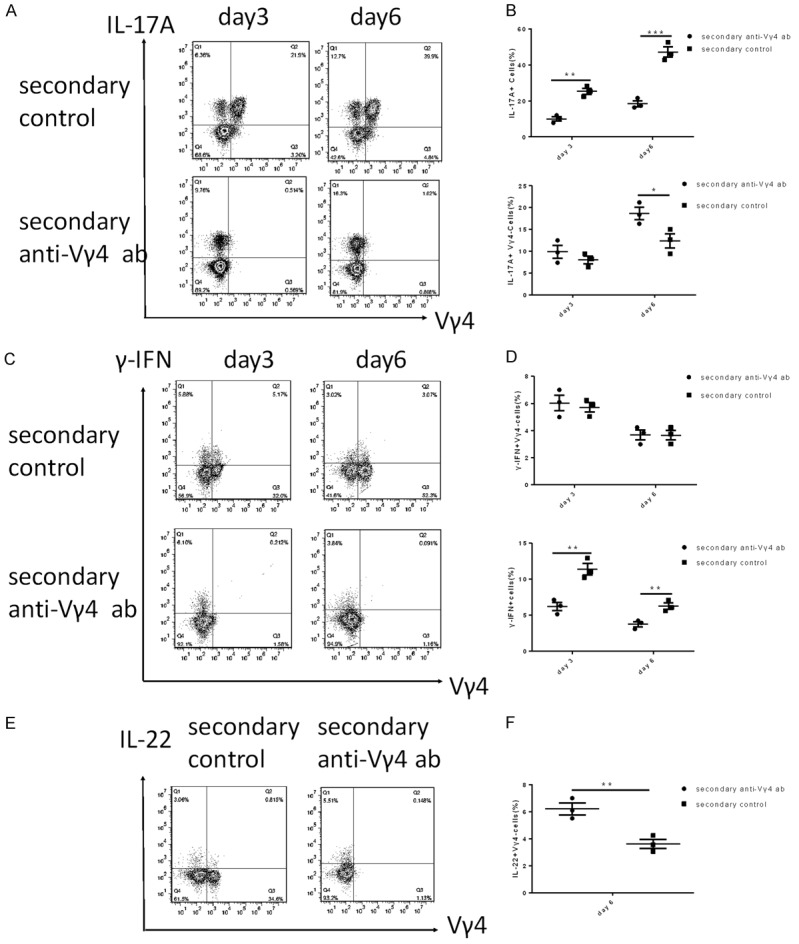
The expression of cytokines in the re-challenged control group and the re-challenged anti-Vγ4 antibody group. (A) IMQ application increased the IL-17A-producing Vγ4 +γδ T cells in the dermis and (B) the percent of IL-17A+ Vγ4 - cells and IL-17A+ cells. (C) The IFN-γ-producing Vγ4 +γδ T cells were detected by FACS. (D) The percent of γ-IFN-producing Vγ4 -γδ T cells and γ-IFN+ cells on different days. (E) The IL-22-producing Vγ4 +γδ T cells on day 6 and (F) the IL-22-producing Vγ4 -γδ T cells were detected by FACS. The values represent the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
The IFN-γ results indicated that Vγ4 +γδ T cells and Vγ4 -γδ T cells produced IFN-γ in both control mice and Vγ4 - depleted mice (Figure 5C). The level of IFN-γ-producing Vγ4 -γδ T cells was higher on day 3 than on day 6 in both control mice and Vγ4 - depleted mice (Figure 5D). Furthermore, after 6 consecutive days of IMQ treatment, we also observed that the number of IL-22-producing γδ T cells was enhanced in the Vγ4 - depleted group compared with the control group (Figure 5E, 5F). These results suggested that Vγ4 depletion reduced the expression of IL-17A and IFN-γ; however, other pro-inflammatory cytokine-producing γδ T cells may be increased in the Vγ4 - depleted group compared with the control group.
The expression of IL-23 was higher in re-challenged control group than in both the re-challenged Vγ4 - depleted group and the primary challenged group
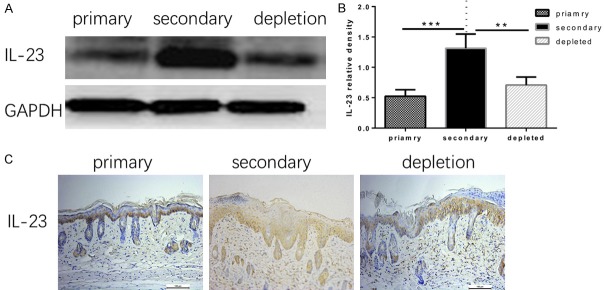
Previous studies confirmed that IL-23 activates γδ T cells and promotes Vγ4 +T cells to proliferate to produce IL-17A [12,27]. However, how IL-23 is altered in Vγ4 - depleted mice remained unknown. Here, we examined IL-23 expression in skin tissue by western blot and immunohistochemical staining. IL-23 expression was significantly enhanced in the re-challenged group compared with the re-challenged Vγ4 - depleted group and the primary challenged group (Figure 6).
Figure 6.
(A) The expression of IL-23 in dermal tissues with psoriasis-like dermatitis induced by IMQ treatment for 6 days was determined by western blot, and (B) the relative densities of the IL-23 protein level in each group are shown. (C) IL-23 immunohistochemical staining in skin with psoriasis-like inflammation on day 6 of IMQ treatment. The symbols represent the mean ± SD (n=3), **p<0.01, ***p<0.001.
Discussion
In this study, we analyzed the development of psoriasis-like skin inflammation induced by IMQ in re-challenged mice and primary challenged mice. The dermatitis induced by IMQ treatment resembles human plaques-type psoriasis with respect to erythema, scaly plaques and epidermal proliferation, which were observed in re-challenged mice. We found that the severity of psoriasis-like skin inflammation was enhanced in re-challenged mice. In addition, the dermal tissue of the re-challenged mice showed increased expression of IL-23, which stimulates dermal γδ T cells to differentiate and proliferate [23,28]. Moreover, the number of IL-17A-producing Vγ4 + cells was increased in the re-challenged group. These findings suggest that Vγ4 + cells are critical in the development of psoriasis-like skin inflammation induced by IMQ.
The importance of γδ T cells in the progression of psoriasis is supported by the observation that γδ T cells secrete IL-17A, IFN-γ and IL-22, which are elevated in psoriasis, and by the fact that dermatitis is alleviated in the TCR δ-/- mice [7]. It has recently been reported that the Vγ4 + subset of γδ T cells increase psoriasis-like skin inflammation by secreting IL-17A. IL-17A is closely associated with the development of psoriasis, and clinical trials of an IL-17A antibody in the treatment of human psoriasis are currently underway and have reached phase III [29]. Vγ4 depletion alleviated the dermatitis induced by IMQ in mice, indicating that Vγ4 + cells play an important role in the development of psoriasis [13,25].
IL-17A-producing Vγ4 + cells from the dermis and draining lymph nodes are increased and persist for months in mice with IMQ-induced psoriasis-like skin inflammation [23,24]. Jason G. Cyster reported that Vγ4 + cells could rapidly increase in number and produce more IL-17A when dermatitis was re-induced with IMQ. In agreement with this study, we confirmed that the Vγ4 + cells rapidly responded to the same stimulus and became the major source of IL-17A in the re-challenged mice. BurkhardBecher reported that Vγ4 + cells persisted in the skin for months; however, our results indicated that the number of dermal Vγ4 + cells was significantly reduced 30 days after the initial inflammatory stimulus. This finding was seemingly contrary to their conclusion. The different experimental conclusions may be due to the different proportions and properties of Vγ4 + cells on the ear skin and the back skin. In addition, we also examined the IFN-γ and IL-22-producing dermal Vγ4 + cells in the primary challenged mice and re-challenged mice. Similar to lymph nodes γδ T cells, dermal γδ T cells also secreted IFN-γ. However, we found that dermal Vγ4 + cells were not the major source of IFN-γ, and the production of IFN-γ was reduced in the re-challenged mice on day 6 of IMQ treatment compared with day 3.This may be the because that IFN-γ-secreting γδ T cells are involved in the early stage of psoriasis, unlike IL-17A, which is associated with the severity of inflammation. Laure Dumontier reported that T lymphocytes and γδ T cells were a major source of IL-22 in draining lymph nodes. We found that the dermal γδ T cells also produce IL-22, but in much lower amounts, especially in the primary challenged mice, indicating that IL-22-producing dermal γδ T cells may not have an effect on the development of IMQ-induced psoriasis-like skin inflammation.
Vγ4 - depleted re-challenged mice exhibited reduced epidermal thickness when treated on the ear skin. In our study, we observed that the erythema, scaly plaques and the thinckness of the epidermis were reduced in Vγ4 - depleted re-challenged mice compared with re-challenged control mice. We also verified that the accumulation of IL-17A-producing dermal γδ T cells was significantly decreased in Vγ4 - depleted mice. This finding supported the conclusion that dermal Vγ4 + cells are the main source of IL-17A in the IMQ-induced dermatitis model. Importantly, a larger increase in Vγ4 - cells was observed in Vγ4 - depleted re-challenged mice than re-challenged control mice. In addition, the Vγ4 -γδ T cells in Vγ4 - depleted re-challenged mice produced more IL-17A than those in re-challenged control mice, indicating that Vγ4 -γδ T cells may also have a role in the development of psoriasis-like skin inflammation. Cai et al reported that dermal Vγ4 +T cells and Vγ6 +T cells both secreted IL-17A and that Vγ4 +T cells were more competitive than Vγ6 + cells in dermal γδ T cells reconstitution after the induction of psoriasis-like skin inflammation and in mice lacking Vγ4 +T cells, Vγ6 + cells are able to induce psoriasis-like dermatitis upon IMQ treatment [12,30-32]. The different experimental conclusions may be explained by the fact that the Vγ6 +T cell experiment included concluded Vγ4 -γδ T cells. Furthermore, these reports may explain why the Vγ4 -γδ T cells increased in number and produced IL-17A in the Vγ4 - depleted re-challenged mice in our study. In addition, IFN-γ production was consistent between Vγ4 - depleted re-challenged mice and re-challenged control mice, and the frequencies of IFN-γ-producing Vγ4 -γδ T cells were similar in Vγ4 - depleted re-challenged mice and re-challenged control mice. These results may indicate that the altered constitution of dermal γδ T cells did not have an effect on IFN-γ production in psoriasis-like dermatitis.
Previous reports have suggested that IL-22 is responsible for the dermatitis induced by IMQ and is produced by both T cells and innate immune cells. However, whether the dermal γδ T cells produce IL-22 remained unclear. Here, we demonstrated that dermal γδ T cells produce IL-22, which is secreted at low amounts by Vγ4 + cells. Interestingly, we found that the secretion of IL-22 from the remaining γδ T cells increased in Vγ4 - depleted re-challenged mice. Given the critical role of IL-22 in the development of psoriasis, this may be another reason why psoriasis-like skin inflammation was still able to be induced in the Vγ4 - depleted mice.
IL-23 is required for the proliferation and survival of IL-17A-producing cells, which augment the expression of IL-17A in skin with psoriasis [33]. Here, we demonstrated that the expression of IL-23 is obviously enhanced in control mice re-challenged with IMQ for 6 successive days compared with both primary challenged mice and Vγ4 - depleted re-challenged mice. These results indicated that our findings were consistent with previous reports.
Although a Vγ4 depletion antibody could neutralize the Vγ4 +γδ T cells in wild-type mice [25], it did not fully deplete the Vγ4 +γδ T cells in the mice with IMQ-induced dermatitis in our study. However, our results showed that the number of remaining Vγ4 +γδ T cells in the Vγ4 - depleted group was limited and did not have an effect on our experiments.
In conclusion, our studies showed that the dermal Vγ4 +γδ T cells are the main source of IL-17A in re-challenged mice and that dermal Vγ4 + cells produce IFN-γ. IL-22 secretion from the remaining γδ T cells increased when the Vγ4 + cells were depleted in mice that were subjected to induced dermatitis 28 days prior to depletion. These findings suggest that Vγ4 + cells enhance the relapsed symptoms of psoriasis-like skin inflammation. However, the remaining γδ T cells can still promote the development of psoriasis in Vγ4 - depleted re-challenged mice.
Acknowledgements
This work was supported by grants from the National Naturnal Science Foundation of China (81271767). All experiments were performed in the Institute of Burn Research Southwest Hospital.
Disclosure of conflict of interest
None.
References
- 1.Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol. 2012;9:302–309. doi: 10.1038/cmi.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Y, Fleming C, Yan J. Dermal γδ T cells-A new player in the pathogenesis of psoriasis. Int Immunopharmacol. 2013;16:388–391. doi: 10.1016/j.intimp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, Russell CB. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol. 2013;133:17–26. doi: 10.1038/jid.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Malakouti M, Brown GE, Wang E, Koo J, Levin EC. The role of IL-17 in psoriasis. J Dermatolog Treat. 2015;26:41–44. doi: 10.3109/09546634.2013.879093. [DOI] [PubMed] [Google Scholar]
- 6.Frleta M, Siebert S, McInnes IB. The interleukin-17 pathway in psoriasis and psoriatic arthritis: disease pathogenesis and possibilities of treatment. Curr Rheumatol Rep. 2014;16:414. doi: 10.1007/s11926-014-0414-y. [DOI] [PubMed] [Google Scholar]
- 7.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, Yan J. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becher B, Pantelyushin S. Hiding under the skin: Interleukin-17-producing γδ T cells go under the skin? Nat Med. 2012;18:1748–1750. doi: 10.1038/nm.3016. [DOI] [PubMed] [Google Scholar]
- 9.Bird L. γδ T cells: innate source of IL-17. Nat Rev Immunol. 2009;9:671. [Google Scholar]
- 10.Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, Smith CH, Hayday AC, Nickoloff BJ, Nestle FO. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2T cell subset with a potential role in psoriasis. J Immunol. 2011;187:2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, Shklovskaya E, Fazekas de St Groth B, Triccas JA, Weninger W. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Y, Xue F, Fleming C, Yang J, Ding C, Ma Y, Liu M, Zhang HG, Zheng J, Xiong N, Yan J. Differential developmental requirement and peripheral regulation for dermal Vγ4 and Vγ6T17 cells in health and inflammation. Nat Commun. 2014;5:3986. doi: 10.1038/ncomms4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray EE, Ramírez-Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE, Cyster JG. Deficiency in IL-17-committed Vγ4(+) γδ T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis. Nat Immunol. 2013;14:584–592. doi: 10.1038/ni.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and ‘Type 1’ inflammatory gene expression. Trends Immunol. 2004;25:295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Fierlbeck G, Rassner G. Treatment of psoriasis and psoriatic arthritis with interferon gamma. J Invest Dermatol. 1990;95:138S–141S. doi: 10.1111/1523-1747.ep12875040. [DOI] [PubMed] [Google Scholar]
- 16.Chen SH, Arany I, Apisarnthanarax N, Rajaraman S, Tyring SK, Horikoshi T, Brysk H, Brysk MM. Response of keratinocytes from normal and psoriatic epidermis to interferon-gamma differs in the expression of zinc-alpha(2)-glycoprotein and cathepsin D. FASEB J. 2000;14:565–571. doi: 10.1096/fasebj.14.3.565. [DOI] [PubMed] [Google Scholar]
- 17.Corpuz TM, Stolp J, Kim HO, Pinget GV, Gray DH, Cho JH, Sprent J, Webster KE. Differential responsiveness of innate-like IL-17- and IFN-γ-producing γδ T cells to homeostatic cytokines. J Immunol. 2016;196:645–654. doi: 10.4049/jimmunol.1502082. [DOI] [PubMed] [Google Scholar]
- 18.Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 19.Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, Fouser LA, Renauld JC, Dumoutier L. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2011;188:462–469. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- 20.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 21.Yoshiki R, Kabashima K, Honda T, Nakamizo S, Sawada Y, Sugita K, Yoshioka H, Ohmori S, Malissen B, Tokura Y, Nakamura M. IL-23 from langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing γδ T cells. J Invest Dermatol. 2014;134:1912–1921. doi: 10.1038/jid.2014.98. [DOI] [PubMed] [Google Scholar]
- 22.Takaishi M, Nakajima K, Ouyang W, Sano S. Psoriasis-like skin lesions are dependent on IL-23 but develop in the absence of IL-22 in a model mouse. J Dermatol Sci. 2014;73:261–264. doi: 10.1016/j.jdermsci.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Ramírez-Vallea F, Gray EE, Cyster JG. Inflammation induces dermal Vγ4+γδ T17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci U S A. 2015;26:8046–8051. doi: 10.1073/pnas.1508990112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B. Dermal IL-17-producing γδ T cells establish long-lived memory in the skin. Eur J Immunol. 2015;45:3022–3033. doi: 10.1002/eji.201545883. [DOI] [PubMed] [Google Scholar]
- 25.Nakamizo S, Egawa G, Tomura M, Sakai S, Tsuchiya S, Kitoh A, Honda T, Otsuka A, Nakajima S, Dainichi T, Tanizaki H, Mitsuyama M, Sugimoto Y, Kawai K, Yoshikai Y, Miyachi Y, Kabashima K. Dermal Vgamma4(+) gammadelta T cells possess a migratory potency to the draining lymph nodes and modulate CD8(+) T-cell activity through TNF-alpha production. J Invest Dermatol. 2015;135:1007–1015. doi: 10.1038/jid.2014.516. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Xu Y, Zhang X, Liang G, Chen L, Xie J, Tang J, Zhao J, Shu B, Qi S, Chen J, Luo G, Wu J, He W, Liu X. Defects in dermal Vγ4 γ δ T cells result in delayed wound healing in diabetic mice. Am J Transl Res. 2016;8:2667–2680. [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garber K. Anti-IL-17 mAbs herald new options in psoriasis. Nat Biotechnol. 2012;30:475–477. doi: 10.1038/nbt0612-475. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien RL, Born WK. gammadelta T cell subsets: a link between TCR and function? Semin Immunol. 2010;22:193–198. doi: 10.1016/j.smim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akitsu A, Ishigame H, Kakuta S, Chung SH, Ikeda S, Shimizu K, Kubo S, Liu Y, Umemura M, Matsuzaki G, Yoshikai Y, Saijo S, Iwakura Y. IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2(+)V6(+)γδ T cells. Nat Commun. 2015;6:7464. doi: 10.1038/ncomms8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rei M, Goncalves SN, Lanca T, Thompson RG, Mensurado S, Balkwill FR, Kulbe H, Pennington DJ, Silva SB. Murine CD27(-) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A. 2014;34:3562–3570. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diveu C, McGeachy MJ, Cua DJ. Cytokines that regulate autoimmunity. Curr Opin Immunol. 2008;20:663–668. doi: 10.1016/j.coi.2008.09.003. [DOI] [PubMed] [Google Scholar]