Abstract
Tumor-associated neutrophils (TANs) promote metastasis of multiple cancers, including oral squamous cell carcinoma (OSCC). Melatonin (Mel) reportedly exerts anti-metastatic effects on OSCC. However, little is known about the anti-OSCC effects of Mel involved in TANs. In this study, intensive infiltration of TANs was positively associated with advanced stage, lymphatic metastasis, and poor prognosis of OSCC. Moreover, Mel reduced the survival and migration of OSCC-associated neutrophils. Mechanistically, Mel suppressed the TAN release of C-X-C motif chemokine ligand 8, C-C motif chemokine ligand 2 (CCL2), CCL4, and matrix metalloproteinase-9 by blockage of p38 MAPK and Akt signaling. Mel-fostered TANs decreased the migration and invasion of OSCC cells and reduced tube formation in vitro. Additionally, Mel-hampered pro-motility and pro-angiogenesis effects of TANs were dependent on MMP-9 suppression in OSCC. Overall, The beneficial roles of melatonin in retarding OSCC metastasis were implicated with inhibition of TANs.
Keywords: Melatonin, neutrophils, oral squamous cell carcinoma, metastasis
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common head and neck cancers (HNCs) worldwide, accounting for 90% of oral cancers, with more than 49,000 newly diagnosed cases annually [1,2]. Despite considerable improvement in diagnoses and therapeutics of OSCC, the five-year survival rate is still less than 50% over the past two decades [3]. Local invasion and distant metastases are considered the major reasons for poor prognosis of OSCC patients [4]. Thus, understanding the mechanisms underlying metastasis and identifying an effective therapeutic agent for OSCC are urgently necessary.
Accumulating evidence indicates that an inflammatory tumor microenvironment contributes greatly to the development and metastasis of tumors [5]. Within this microenvironment, tumor cells can educate immune cells to restrict their immune functions and acquire tumor-promoting activities [6,7]. Tumor-associated neutrophils (TANs) play key tumor-supporting roles in multiple cancers, including OSCC [8,9]. TAN infiltration is a poor prognostic indicator of HNC [10]. Wang et al. [11] also found that neutrophil immigration is positively associated with lymph node metastasis, advanced clinical stage, and high recurrence of tongue squamous cell carcinoma (TSCC). HNC cells promote neutrophil motility and survival and increase their inflammatory activity by releasing pro-inflammatory factors, such as C-C motif chemokine ligand 4 (CCL4) and matrix metalloproteinase 9 (MMP-9) [10]. Neutrophil-derived inflammatory factors upon HNC cell stimulation have a feedback effect on tumor cells by enhancing their migratory properties [12]. Neutrophils increase the invasiveness of OSCC cells through the activation of invadopodia and extracellular matrix (ECM) degradation [13]. However, the molecular mechanisms underlying the migration-promoting effects of TANs on OSCC cells are poorly understood.
Melatonin (Mel; N-acetyl-5-methoxytryptamine) is an indoleamine mainly produced by the pineal gland in response to darkness [14]. Mel is a hormone and cell protector involved in immunomodulation, antioxidation, and hematopoiesis [15]. Numerous studies have proven that Mel possesses oncostatic properties in many types of cancers, including OSCC [16-20]. The antitumor mechanisms of Mel are associated with several hallmarks of cancer, such as anti-proliferation, apoptosis promotion, migration and invasion inhibition, and anti-angiogenesis [21]. Yang et al. [22] demonstrated that Mel reduces oral cancer cell proliferation in vivo and in vitro. Mel inhibits TPA-induced oral cancer cell migration by suppressing MMP-9 activation [20]. Moreover, Mel decreases cell viability of both OSCC cell lines (SCC-9 and SCC-25) and reduces the expression of pro-angiogenic genes (i.e., hypoxia inducible factor-1α and vascular endothelial growth factor [VEGF]) and the level of the pro-metastatic gene ROCK-1 in SCC-9 cells [23]. Nevertheless, whether the anti-metastatic properties of Mel on OSCC are related to the regulation of the tumor microenvironment involved in TANs remains unclear.
In this study, infiltration of TANs in OSCC tissues was enhanced compared with corresponding nontumor samples. More intratumoral TANs were observed in patients with OSCC in advanced stage (T4) or with lymphatic metastasis than in patients in low stages (T1, T2, and T3) or with non-lymphatic metastasis. High accumulation of TANs was associated with a poor survival rate among OSCC patients. In vitro studies showed that Mel reduced the migration and survival of OSCC-associated neutrophils. Mel inhibited the release of pro-inflammatory factors, including C-X-C motif chemokine ligand 8 (CXCL8), CCL2, CCL4, and MMP-9, from OSCC-associated neutrophils through inactivation of the p38 mitogen-activated protein kinase (MAPK) and Akt signaling pathways. In addition, Mel-educated TANs decreased the migration and invasion of OSCC cells, as well as the angiogenesis of endothelial cells in a MMP-9-dependent manner. Overall, these results demonstrated that Mel hindered migration, apoptosis resistance, and inflammatory responses of OSCC-associated neutrophils and suppressed the promotility and pro-angiogenesis effects of TANs, suggesting that Mel may be a potential therapeutic agent for OSCC.
Materials and methods
Patients and clinical samples
The experiments were approved by the committees of Zhongshan Hospital Affiliated to Dalian University (Dalian, China) and Fourth Military Medical University (Xi’an, China), and all the patients enrolled in this study provided written informed consent before sample collection. For neutrophil isolation, tumor tissues and peripheral blood were collected from 20 OSCC patients and peripheral blood was obtained from 20 healthy donors who had a medical check-up in Zhongshan Hospital. For immunohistochemical examination, paraffin-embedded OSCC samples and paired adjacent nontumor tissues were obtained from 112 patients (median age, 61 years; range, 40-76 years) who underwent curative resection between 2009 and 2011 in Zhongshan Hospital and School of Stomatology of Fourth Military Medical University. None of the patients had radiotherapy or chemotherapy before surgery. All the tissues were subjected to a blind histopathological examination by two pathologists. OSCC was staged according to the tumor-node-metastasis system. Follow up was conducted via outpatient examination or over the telephone (median time, 35 months; range, 10-60 months). Five-year overall survival was defined as the interval between surgery and death or between surgery and the last observation point. For surviving patients, the data were censored at the last follow-up evaluation.
Neutrophil isolation and culture
Normal peripheral blood neutrophils (nPBNs) and TANs were isolated as previously described [24]. Peripheral blood samples were collected from healthy volunteers and used for nPBN isolation. For TAN isolation, the fresh OSCC tissues were sliced into small pieces and digested in Roswell Park Memorial Institute 1640 (RPMI 1640; Gibco, BRL, Carlsbad, CA, USA) medium supplemented with 0.05% collagenase IV (Sigma-Aldrich, St. Louis, MO, USA), 0.002% DNase I (Roche, Indianapolis, IN, USA), and 20% fetal bovine serum (FBS; Gibco) at 37°C for 30 min. The dissociated cells were filtered through a 150 mm mesh. Subsequently, 1 mL of cell suspension and 10 mL of Ficoll-Hypaque (Stemcell Technologies, Vancouver, Canada) were centrifuged at 2500 rpm for 20 min in a 15 mL tube. The leukocytes were harvested, and CD66b+ cells were isolated using the EasySep PE Selection Kit (STEMCELL Technologies) according to the manufacturer’s protocol. The purification of neutrophils was confirmed by fluorescence-activated cell sorter analysis with phycoerythrin (PE)-conjugated anti-CD66b antibody (BD Bioscience, San Jose, CA, USA), showing a purity of greater than 95% and 85% for nPBNs and TANs, respectively.
Cell culture
Two human OSCC cell lines (SCC-9 and SCC-25) and human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). OSCC cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% FBS. HUVECs were cultured in RPMI 1640 medium. All cells were grown under a humidified condition at 37°C with 95% CO2. Quality and identity of the cell lines were validated consistently according to ATCC Technical Bulletin No. 8, including regular microscopic controls of morphology, growth curve recordings, and PCR-based testing for mycoplasma infection. Conditioned medium (CM) from SCC-9 and SCC-25 cells were prepared by culturing SCC-9 and SCC-25 cells (1 × 106 cells/mL) for 24 h at 37°C.
Neutrophil treatment
For nPBN activation, nPBNs (1 × 106 cells/mL) were cultured with SCC-9-CM and SCC-25-CM or control (Ctrl)-CM for 24 h. For Mel treatment, TANs and nPBNs were seeded into dishes and cultured in RPMI 1640 medium until 80% confluence. The medium was replaced to minimize any stimulated effects on neutrophils. The neutrophils were exposed to 1 mM Mel (Sigma) for the indicated time points. To evaluate the signaling pathways involved in TANs and OSCC-educated TAN-like neutrophils, the cells were pretreated with SB203580 (10 μM, p38 inhibitor; Merck Millipore, Billerica, MA, USA) or LY294002 (50 μM, PI3K inhibitor; Merck Millipore) at 37°C and 5% CO2 for 1 h, washed with PBS three times, and exposed to the indicated stimuli. To explore the molecules by which TAN mediated OSCC cell migration and angiogenesis, the cells were pretreated with MMP-9 inhibitor I (1 μM; Merck Millipore) and Scramble siRNA (siScrb) or MMP-9 siRNA (siMMP-9; GenePharma, Shanghai, China) followed by the indicated stimulus. SiScrb or siMMP-9 was used to knock down MMP-9 expression in TANs by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions.
Immunohistochemistry
Paraffin-embedded OSCC and the paired nontumor samples were sectioned to visualize CD66b-positive cells. In brief, after deparaffinization, rehydration, and microwave antigen retrieval, the sections were incubated with mouse anti-human CD66b monoclonal antibody (Beckman Coulter, Fullerton, CA, USA) at 4°C overnight, followed by incubation with secondary antibody (Maixin Biotech., Fuzhou, China) at 37°C for 20 min. Staining was performed with 3,3’-diaminobenzidine (Maixin Biotech.) and counterstained with hematoxylin (Sigma). The omitted primary antibody was used as the negative control. The sections were observed and photographed under a light microscope (Carl-Zeiss, Berlin, Germany). CD66b-positive cells were rated with the score “-” for no, and “+,” “++,” and “+++” for weak, medium, and strong staining, respectively. According to the score system, the cells were divided into two groups: CD66b low-level group (- and +) and CD66b high-level group (++ and +++).
Neutrophil chemotaxis assay
Neutrophil chemotaxis was measured by a Transwell system (Corning Costar, NY, USA) with 3 mm polycarbonate membranes as described previously [10]. Ctrl-CM, SCC-9-CM, or SCC-25-CM was plated into the bottom chambers. nPBNs and TANs (1 × 105 cells/100 mL) with or without Mel treatment for 1 h were suspended in RPMI 1640 containing 2% FBS, added to the upper wells and then incubated for 3 h at 37°C. The neutrophils that migrated to the lower chamber were collected and counted by CASY®Model TT (Roche Innovatis, Bielefeld, Germany). The negative control comprised nPBNs that migrated to RPMI 1640 alone. The chemotactic index was calculated according to the following formula: chemotactic index = neutrophils that migrated to the membranes induced by the various attractants/nPBNs that migrated toward RPMI 1640 alone. By definition, nPBNs that migrated toward RPMI 1640 alone have a chemotactic index of 1 [10].
Measurement of neutrophil apoptosis by flow cytometry
Neutrophil apoptosis was measured using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BD Bioscience) according to the manufacturer’s instructions. In brief, nPBNs and TANs with different treatments were harvested, centrifuged, and resuspended in binding buffer. After the addition of Annexin V-FITC (10 μL) in the mixture, the cells were incubated at 37°C for 15 min and counterstained with 5 μL of propidium iodide (PI) in the dark for 30 min. Cell apoptosis was determined by BD FACSCalibur flow cytometry (BD Bioscience). The results were analyzed by CellQuest software (BD Bioscience).
Western blot analysis
Neutrophils with the indicated treatments were lysed with RIPA lysis buffer (Beyotime Biotechnology, Jiangsu, China). Protein samples were boiled and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane (Millipore). The membranes were blocked with 5% skimmed milk at room temperature for 1 h, followed by incubation with specific primary antibodies, including phosphorylated (p)-p38T180/Y182, p38, p-AktS473, Akt, and GAPDH (all from Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight. Afterwards, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Sigma) at 37°C for 1 h. Bands were detected by enhanced chemiluminescence detection reagents (Santa Cruz, Dallas, TX, USA).
Enzyme-linked immunosorbent assay (ELISA)
The levels of cytokines/chemokines in the supernatants of neutrophils with indicated treatments were measured using commercial human ELISA kits (R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturer’s instructions. In brief, 100 μL of sample was placed into each well. The plates were incubated for 3 h at room temperature, followed by conjugate incubation for 2 h. After washing, substrate solution was added to determine immunoreactivity, and the absorbance was read at 490 nm on a microplate reader (Bio-Rad, Hercules, CA, USA).
Migration and invasion assays
Migration and invasion of OSCC cells were measured using Transwell inserts (8 μm; Corning Costar) precoated with or without Matrigel. For migration assay, SCC-9 and SCC-25 cells (5 × 104) were suspended in 500 μL of 10% FBS/DMEM and seeded in the upper chamber of the inserts uncoated with Matrigel. The bottom chamber was filled with 1 mL of 10% FBS/DMEM with nPBNs-, TANs-, (TANs + Mel)-, (TAN + MMP-9 inhibitor)-, (TAN + siScrb)-, or (TAN + MMP-9)-CM for chemotaxis and allowed to migrate for 24 h. The bottom chamber with only 10% FBS/DMEM was used as control. For the invasion assay, except for the upper chamber of the inserts precoated with 5 mg/mL Matrigel (Sigma), the other procedures were the same as those for the migration assay. The cells that migrated or invaded to the membranes were fixed with methanol for 30 min, stained with crystal violet (Sigma) for another 30 min, and counted under a microscope (Carl-Zeiss). The migration or invasion index was calculated as follows: migration or invasion index = OSCC cells that migrated or invaded to the membranes induced by the various attractants/OSCC cells that migrated or invaded toward DMEM alone. By definition, OSCC cells that migrated or invaded toward DMEM alone had a migration or invasion index of 1.
In vitro angiogenesis assay
In vitro tube formation assay was conducted to determine the pro-angiogenic capacity of neutrophils. HUVECs (1 × 105) were seeded on the 24-well plates precoated with Matrigel (Sigma; 400 μL/well) and cultured in RPMI 1640 medium with nPBNs-, TANs-, (TANs + Mel)-, (TAN + MMP-9 inhibitor)-, (TAN + siScrb)-, or (TAN + MMP-9)-CM. The tube formation capacity was observed under a phase-contrast microscope (Carl-Zeiss) at 24 h after incubation, and the number of tubes and branch points were measured using Image Pro Plus software (Media Cybernetics Inc., Bethesda, MD, USA).
Statistical analysis
Data were presented as the mean ± standard deviation (SD). Differences between the two groups or multiple groups were compared using Student’s t-test or one-way ANOVA followed by Tukey’s post-hoc test. Survival curves were plotted by the Kaplan-Meier method and analyzed using the log rank test. Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as P < 0.05.
Results
Increased TAN infiltration in OSCC was correlated with tumor progression and poor prognosis
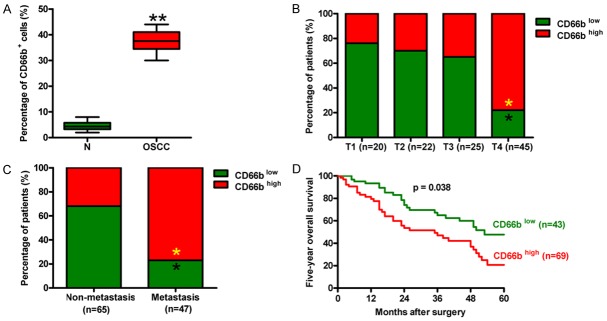
To assess the relevance between OSCC-associated neutrophils and their clinical significance, we first analyzed the extent of TAN infiltration in OSCC tissues and the paired nontumor samples. As shown in Figure 1A, the percentage of CD66b-positive cells was much higher in OSCC tissues than that of nontumor samples. According to the scoring system of TAN infiltration by using anti-CD66b staining, 95% of OSCC tissues exhibited TAN infiltration, among which weak, medium, and strong infiltrations were found in 42%, 31%, and 22%, respectively, of the tissues (data not shown). Most of the T4 tumors displayed high CD66b expression, whereas T1, T2, and T3 tumors exhibited low TAN infiltration (Figure 1B). The patients with lymphatic metastasis had more CD66b high-expression cells than the non-metastatic patients (Figure 1C). The survival rate of the patients with CD66b low-expression cells was much higher than that of the patients with CD66b high-expression cells (Figure 1D). These results indicate that increased TAN infiltration was positively associated with the advanced stage, lymphatic metastasis, and poor prognosis of OSCC.
Figure 1.
Enhancement of TAN infiltration was positively associated with the advanced stage, lymphatic metastasis, and poor prognosis of OSCC. (A) The percentage of CD66b-positive neutrophils in OSCC tissues and the corresponding nontumor tissues. CD66b-positive neutrophil was rated with the score “-” for no and “+,” “++,” and “+++” for weak, medium, and strong staining, respectively. (B and C) Tumor infiltration by CD66b-positive neutrophils was analyzed in 112 patients with OSCC. The percentages of the infiltration scores are indicated for each T stage (B) and the tissues with or without lymphatic metastasis (C, D) The five-year survival rate of OSCC patients with CD66b high- or low-expression cells. All data represent the mean ± SD of three replicates. *P < 0.05 compared with the T1, T2, or T3 group in (B) or compared with the non-metastasis group in (C). **P < 0.01 compared with N tissues. N = Nontumor.
Mel reduced migration and increased apoptosis of OSCC-associated neutrophils
To investigate the effects of Mel on the migration and apoptosis of OSCC-associated neutrophils, we performed neutrophil chemotaxis and flow cytometry assays. Compared with nPBNs, the migration of TANs markedly increased, which was significantly reduced by Mel treatment (Figure 2A). Flow cytometry assay demonstrated lower levels of apoptotic TANs than nPBNs. However, Mel significantly increased TAN apoptosis (Figure 2B). To explore whether the increased migration and decreased apoptosis of TANs are induced by OSCC cells, we detected the migration and apoptosis of nPBNs with OSCC-CM treatment. As expected, SCC-9-CM or SCC-25-CM notably increased migration and decreased apoptosis of nPBNs compared with the nPBNs alone or Ctrl-CM treatment, whereas Mel reversed these effects caused by OSCC cells (Figure 2C and 2D). These data demonstrate that Mel inhibited migration and enhanced apoptosis of OSCC-associated neutrophils.
Figure 2.
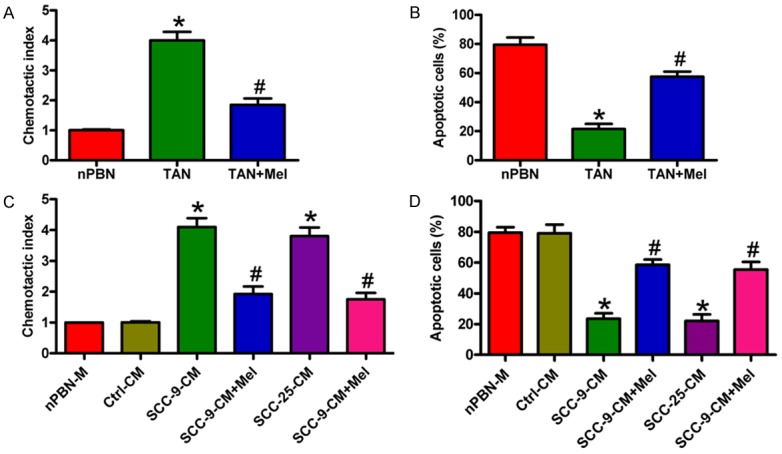
Mel inhibited migration and increased apoptosis of OSCC-associated neutrophils. (A and B) TANs were treated with or without 1 mM Mel for 1 h. nPBNs without any treatment was used as control. Cell migration was counted 3 h after Mel treatment (A), and cell apoptosis was measured at 24 h after Mel exposure (B, C) nPBNs with or without 1 h of Mel (1 mM) pretreatment were allowed to migrate toward Ctrl-, SCC-9-, or SCC-25-CM for 3 h. (D) nPBNs with or without 1 h of Mel (1 mM) pretreatment were added Ctrl-, SCC-9-, or SCC-25-CM and cultured for 24 h. Cell apoptosis was measured by flow cytometry. All data represent the mean ± SD of three replicates. *P < 0.05 compared with the nPBN group in (A and B) or compared with the nPBN-M or Ctrl-CM group in (C and D). #P < 0.05 compared with the TAN group in (A and B) or compared with the SCC-9- or SCC-25-CM group in (C and D). Ctrl: control; CM: conditioned medium; M: medium; Mel: melatonin.
Mel inhibited the release of inflammatory factors from OSCC-associated neutrophils through p38 MAPK and PI3K/Akt signaling
Zhou et al. [24] reported that hepatocellular carcinoma (HCC) cells educate nPBNs to become TAN-like and express CCL2 and CCL17 through the induction of p38 MAPK and PI3K/Akt signaling. A previous study showed that head and neck cells can induce p38 MAPK activation to stimulate the release of CCL4, CXCL8, and MMP-9 by neutrophils [25]. Mel reportedly reduces the levels of p38 MAPK and p-Akt in ovarian carcinoma [18]. Western blot analysis was performed to determine the signaling pathways involved in OSCC-induced activation of neutrophils and by which Mel inhibited neutrophil activation. As shown in Figure 3A, the levels of p-p38 MAPK and p-Akt were much higher in TANs and in SCC-9-CM- or SCC-25-CM-stimulated nPBNs than in nPBNs alone or Ctrl-CM-treated nPBNs. However, Mel significantly reduced p-p38 MAPK and p-Akt levels in TANs or OSCC-CM-exposed nPBNs. We then investigated whether the reduced release of inflammatory mediators from TANs by Mel is dependent on p38 MAPK and Akt activation. Secretion of CXCL8 (Figure 3B), CCL2 (Figure 3C), CCL4 (Figure 3D), and MMP-9 (Figure 3E) was markedly increased in TANs and SCC-9-CM- or SCC-25-CM-treated nPBNs, whereas those were decreased in the Mel treatment groups. Blocking the p38 MAPK and Akt pathways with SB203580 and LY294002 pretreatments, respectively, reduced the increased release of CXCL8, CCL2, CCL4, and MMP-9, which was further inhibited by Mel (Figure 3B-E). These results suggest that Mel suppressed the release of inflammatory mediators from OSCC-activated neutrophils, which require p38 MAPK and PI3K/Akt signaling inactivation.
Figure 3.
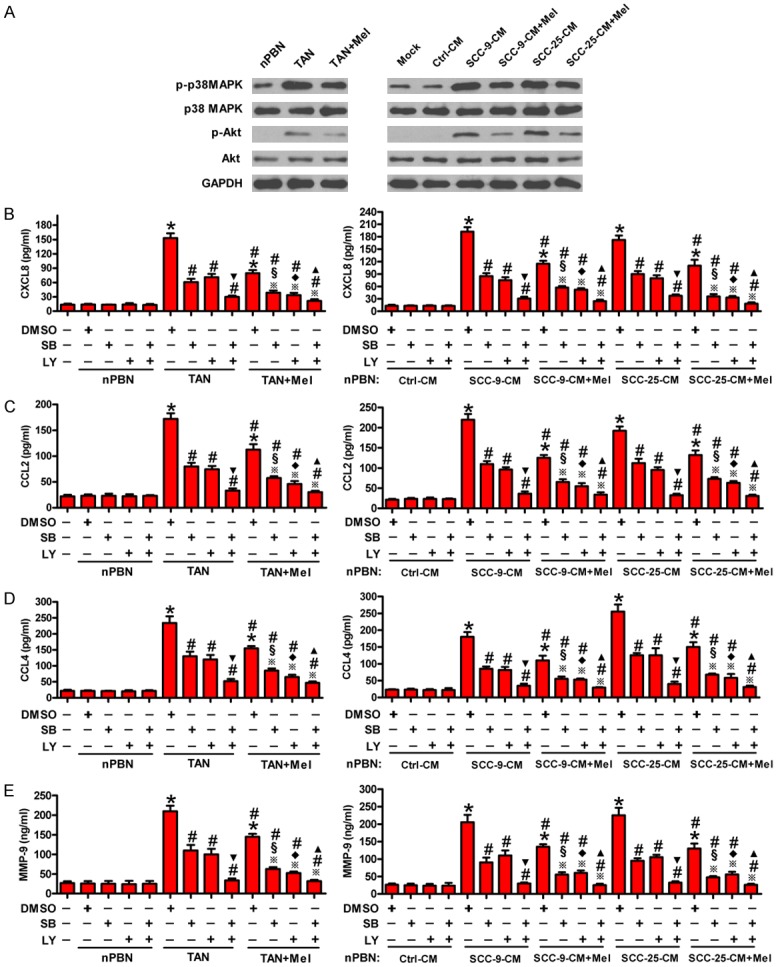
Mel reduced the release of inflammatory mediators from OSCC-associated neutrophils involving inactivation of p38 MAPK and PI3K/Akt signaling. (A) TANs were treated with or without 1 mM Mel for 1 h. nPBNs with or without 1 h of Mel (1 mM) pretreatment were cultured with Ctrl-, SCC-9-, and SCC-25-CM. Western blot analyses of the expression of phosphorylated (p)-p38T180/Y182, p38, p-AktS473, and Akt in nPBNs and TANs at 4 h after Mel treatment. GAPDH was used as the endogenous control. (B-E) Left panel: TANs were treated with or without 1 mM Mel treatment in the presence or absence of SB203580 (10 μM) or LY294002 (50 μM) for 1 h. Right panel: nPBNs pretreated with or without 1 h of Mel (1 mM) in the presence or absence of SB203580 (10 μM) or LY294002 (50 μM) and were cultured with SCC-9- or SCC-25-CM. The release of CXCL8 (B), CCL2 (C), CCL4 (D), and MMP-9 (E) in the cell media was assessed by ELISA at 24 h after Mel treatment. All data represent the mean ± SD of three replicates. *P < 0.05 compared with the nPBN groups in (B-E left panel) or compared with Ctrl-CM groups in (B-E right panel); #P < 0.05 compared with TAN + DMSO group in (B-E left panel) or compared with SCC-9-CM + DMSO or SCC-25-CM + DMSO group in (B-E right panel); ▼P < 0.05 compared with TAN + SB or TAN + LY group in (B-E left panel) or compared with SCC-9-CM + SB or SCC-9-CM + LY group or SCC-25-CM + SB or SCC-25-CM + LY group in (B-E right panel); ※P < 0.05 compared with (TAN + Mel) + DMSO group in (B-E left panel) or compared with (SCC-9-CM + Mel) + DMSO or (SCC-25-CM + Mel) + DMSO group in (B-E right panel); §P < 0.05 compared with TAN + SB group in (B-E left panel) or compared with SCC-9-CM + SB or SCC-25-CM + SB group in (B-E right panel); ♦P < 0.05 compared with TAN + LY group in (B-E left panel) or compared with SCC-9-CM + SB or SCC-25-CM + LY group in (B-E right panel); ▲P < 0.05 compared with (TAN + Mel) + SB or (TAN + Mel) + LY group in (B-E left panel) or compared with (SCC-9-CM + Mel) + SB or (SCC-9-CM + Mel) + LY group or (SCC-25-CM + Mel) + SB or (SCC-25-CM + Mel) + LY group in (B-E right panel). Ctrl: control; CM: conditioned medium; Mel: melatonin; DMSO: dimethyl sulfoxide; SB: SB203580; LY: LY294002.
Mel-fostered OSCC-associated neutrophils decreased migration and invasion of OSCC cells
A recent study demonstrated that neutrophils increase the invasiveness of OSCC [13]. Mel inhibits oral cancer cell migration by suppressing MMP-9 activation [20], which is mainly produced by TANs in the tumor microenvironment [26]. To address whether the inhibition of Mel on OSCC cell migration and invasion is dependent on neutrophil activation, Transwell assays were performed. Compared with Ctrl or nPBN-CM-stimulated OSCC cells, the migration of TAN-CM-treated SCC-9 and SCC-25 cells substantially increased, whereas CM from TANs with Mel treatment attenuated the enhancement of the migrated cells (Figure 4A and 4B). Similarly, (TANs + Mel)-CM reduced the invasion of SCC-9 and SCC-25 cells increased by TANs (Figure 4C and 4D). These results imply that Mel-educated OSCC-associated neutrophils reduced the migration and invasion of OSCC cells.
Figure 4.
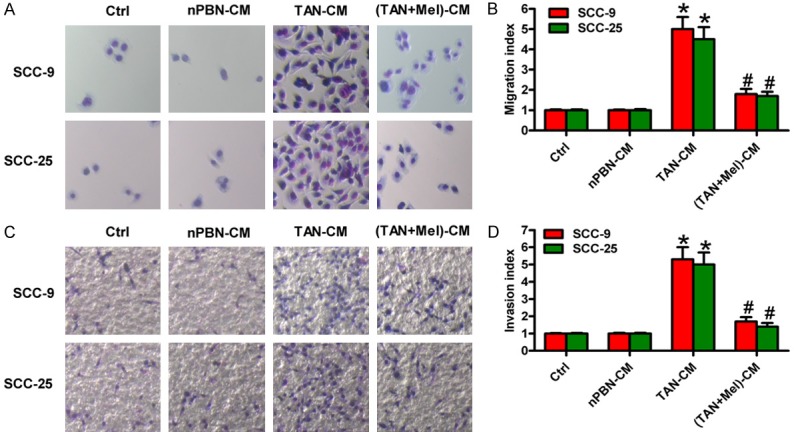
Mel-educated TANs decreased the migration and invasion of OSCC cells. SCC-9 and SCC-25 cells were cultured in Ctrl, nPBN-, TAN-, or (TAN + Mel)-CM for 24 h. (A and C) The (A) migration and (C) invasion of SCC-9 and SCC-25 cells were measured by Transwell assays. (Magnification: 200×). (B and D) The (B) migrated cells in (A) and (D) invaded cells (C) were calculated. (Magnification: 100×). All data represent the mean ± SD of three replicates. *P < 0.05 compared with the Ctrl or nPBN-CM group; #P < 0.05 compared with the TAN-CM group. CM: conditioned medium; Ctrl: control; Mel: melatonin.
Mel-educated OSCC-associated neutrophils hampered angiogenesis in vitro
Angiogenesis is essential for tumor growth and metastasis. Neutrophils promote tumor angiogenesis [27], whereas Mel inhibits angiogenesis in endothelial cell cultures [28]. To explore whether the anti-angiogenic effect of Mel is through inhibiting TAN activation, in vitro tube formation assay was performed. As shown in Figure 5A, TAN-CM treatment led to a significant increase in tube-like structures of HUVECs compared with the Ctrl or nPBN-CM-cultured cells. However, CM from Mel-treated TANs attenuated the capacity of tube formation induced by TANs. Quantitatively, (TANs + Mel)-CM incubation decreased the number of tubes (Figure 5B) and branch points (Figure 5C) of HUVECs. The data suggest that Mel inhibited the pro-angiogenic effect of TANs in OSCC.
Figure 5.
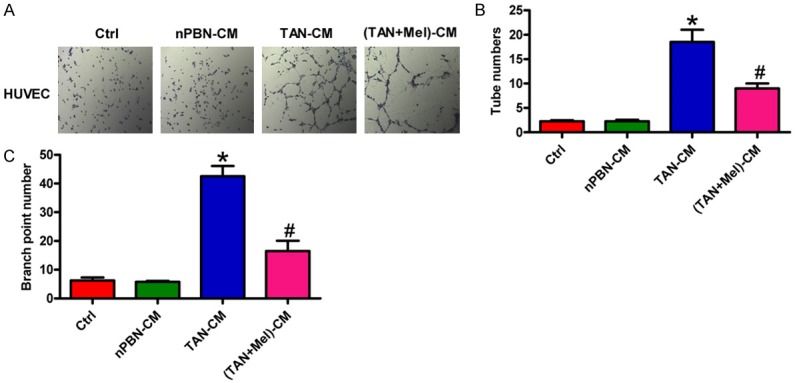
Mel-fostered OSCC-associated neutrophils inhibited angiogenesis in vitro. SCC-9 and SCC-25 cells were cultured in Ctrl, nPBN-, TAN-, or (TAN + Mel)-CM for 24 h. (A) Tube formation assay was performed to evaluate the effect of TANs on the angiogenesis of HUVECs. (Magnification: 50×). (B and C) The number of tubes (B) and branch points (C) were counted and calculated. All data represent the mean ± SD of three replicates. *P < 0.05 compared with the Ctrl or nPBN-CM group; #P < 0.05 compared with the TAN-CM group. CM: conditioned medium; Ctrl: control; Mel: melatonin.
Inhibition of the pro-motility and pro-angiogenic effects of TANs by Mel was in a MMP-9-dependent manner in OSCC
MMP-9 promotes tumor metastasis by degrading the ECM and contributing to neovascularization [29]. To verify whether Mel inhibits TAN-induced migration and invasion of OSCC cells and angiogenesis of HUVECs through MMP-9 suppression, MMP-9 inhibitor or siMMP-9 was used for MMP-9 inhibition assays. As shown in Figure 6A and 6B, TAN-CM-enhanced migration and invasion of SCC-9 and SCC-25 cells were markedly reduced by (TANs + Mel)-, MMP-9 inhibitor-, or siMMP-9-CM treatment. Compared with the MMP-9 inhibitor or siMMP-9 alone group, (MMP-9 inhibitor + Mel)- or (siMMP-9 + Mel)-CM-treated group exhibited less migrated and invaded cells. Similar results were observed in the number of tubes (Figure 6C) and branch points (Figure 6D). These data indicate that the pro-motility and pro-angiogenic effects of TANs suppressed by Mel were through MMP-9 suppression in OSCC.
Figure 6.
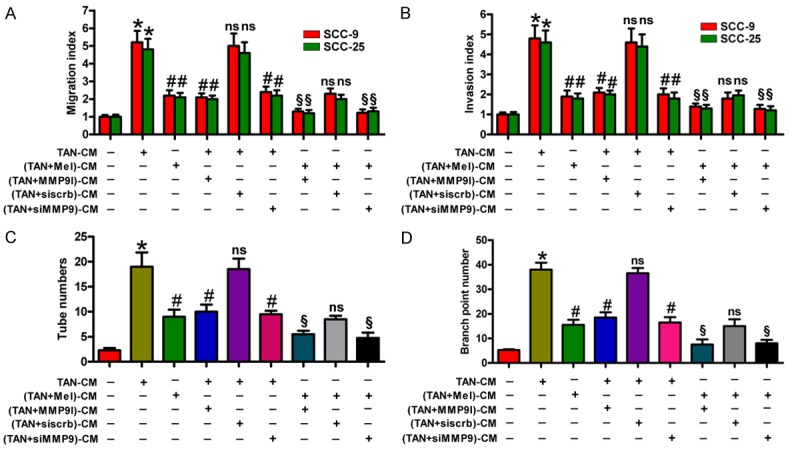
Mel repressed the pro-motility and pro-angiogenesis effects of OSCC-associated neutrophils through MMP-9 suppression. (A and B) SCC-9 and SCC-25 cells were cultured in TAN-, (TAN + Mel)-, (TAN + MMP-9 inhibitor)-, (TAN + siscrb)-, or (TAN + siMMP-9)-CM for 24 h. The migration (A) and invasion (B) of SCC-9 and SCC-25 cells were evaluated by Transwell assays. The migration or invasion index of TAN-CM treatment was defined as 1. (C and D) HUVECs were cultured in TAN-, (TAN + Mel)-, (TAN + MMP-9 inhibitor)-, (TAN + siscrb)-, or (TAN + siMMP-9)-CM for 24 h. Tube formation assay was performed to measure the number of tubes (C) and branch points (D). All data represent the mean ± SD of three replicates. *P < 0.05 compared with the Ctrl group; #P < 0.05 compared with the TAN-CM group; ns: no significance compared with the TAN-CM group or (TAN + Mel)-CM group; §P < 0.05 compared with the (TAN + Mel)-CM group. CM: conditioned medium; Ctrl: control; Mel: melatonin; MMP-9 I: MMP-9 inhibitor.
Discussion
Several key findings were observed in this study. First, TAN accumulation increased in OSCC tissues compared with the corresponding nontumor samples. Second, levels of intratumoral TANs in OSCC patients in advanced stage (T4) or with lymphatic metastasis were higher than those in low stages (T1, T2, and T3) or with non-lymphatic metastasis. Third, the OSCC patients with CD66b high-expression cells had a shorter survival time than those with CD66b low-expression cells. Fourth, the migratory and viable abilities of TANs were reduced by Mel treatment. Fifth, the reduced release of CXCL8, CCL2, CCL4, and MMP-9 from OSCC-associated neutrophils by Mel was dependent on the inactivation of p38 MAPK and Akt signaling. Sixth, (TANs + Mel)-CM incubation decreased the migration and invasion of OSCC cells. Seventh, Mel-educated OSCC-associated neutrophils suppressed angiogenesis in vitro. Finally, the inhibition of pro-motility and pro-angiogenesis effects of TANs by Mel was in a MMP-9-dependent manner in OSCC. Overall, these data indicate that Mel retarded OSCC metastasis by inhibiting TAN activation and Mel may be as a potential therapeutic agent for OSCC.
Neutrophils are the most abundant immune cell in humans, representing 50%-70% of all leukocytes. The circulating neutrophils have a half-life of approximately 7 h in healthy humans [30] and 8-10 h in mice [31]. However, animal experiments showed that a small pool of noncirculating neutrophils can survive for several days in tissues [32,33]. Neutrophils are also retained for a long time in tumors [34], suggesting that the tumor microenvironment encourages their survival both locally and systemically. Emerging evidence demonstrates that neutrophils are an essential component of the tumor microenvironment, and neutrophil infiltration plays a key role in tumor progression [35]. Human hypopharyngeal carcinoma cell stimulates the migration and prolong the survival time of neutrophils [10]. In line with this finding, our results show that OSCC cell stimulation enhanced the migration and survival of OSCC-associated neutrophils. However, Mel significantly reduced the migration and survival of TANs. Aberrant accumulation of neutrophils has been found in multiple cancers and is often related to poor prognosis [36-38]. Trellakis et al. [10] reported that increased neutrophil infiltration, as detected by CD66b-positive cells, is associated with poor outcomes among HNC patients. TSCCs with neutrophil infiltration exhibit increased lymphatic metastasis, advanced clinical stage, and enhanced tumor recurrence [11]. Consistently, this study demonstrate that high levels of CD66b-positive neutrophils were found in OSCC tissues, and increased neutrophil accumulation was positively associated with advanced stage, lymphatic metastasis, and poor outcomes of OSCC patients.
Solid tumors are often accompanied with a state of so-called cancer-related inflammation. Inflammation is recognized as an important contributor to cancer development, which can fuel both primary tumor growth and metastasis [39]. As a key type of inflammatory cell, neutrophils can be mobilized and recruited to tumors. Two differing polarized populations of TANs (N1 and N2) were characterized in a murine model. Transforming growth factor-β (TGF-β) within the tumor microenvironment induces a population (N2) of TANs with a protumor phenotype, whereas blockade of TGF-β results in the recruitment and activation of TANs with an antitumor phenotype (N1) [40]. N2 neutrophils are particularly relevant for OSCC patients, as evidenced by increased expression of IL-1β, IL-6, and TGF-β [41]. Neutrophils can release many pro-inflammatory, immunoregulatory, and angiogenic factors, including TNF-α, IL-1β, IL-8, MMP-9, and VEGF, in a tumor microenvironment [10,13,25]. FaDu cells can increase the production of CCL4, CXCL8, and MMP-9 in neutrophils [10,25]. The co-culture of OSCC cells and neutrophils leads to enhanced secretion of TNF-α and IL-8 [13]. CCL2 and CCL17 are secreted exclusively by TANs as tumor-promoting prognostic factors in HCC [24]. CXCL8 promotes tumor progression by enhancing neutrophil chemotaxis and degranulation [42], and serum CXCL8 has been suggested as a possible survival biomarker for HNC patients [43]. CCL2 expressed by TANs is associated with lymphatic metastasis of OSCC [44]. CCL4 released by neutrophils at the tumor site may further recruit mononuclear immune cells to intensify the inflammatory tumor-host interaction [45]. MMP-9 is one of the most important contributors of tumor progression, and it is a key player in ECM degradation and angiogenesis in OSCC [46]. In the present study, we found that pro-inflammatory factors, including CXCL8, CCL2, CCL4, and MMP-9, were produced largely by OSCC-associated neutrophils, which were significantly reduced by Mel treatment. Previous studies demonstrated the expression of CCL2 and CCL17 in HCC-educated TAN-like cells via induction of p38 MAPK and PI3K/Akt signaling [24] and the release of CCL4, CXCL8, and MMP-9 from FaDu cell-stimulated neutrophils by induction of p38 MAPK activation [25]. In this study, we also found that Mel inhibited the release of inflammatory factors from OSCC-associated neutrophils through p38 MAPK and PI3K/Akt signaling.
Local or distant metastases are aggressive hallmarks of OSCC. The progression of cancer metastases is a complex multi-step process involving dynamic interactions between cancer cells and their microenvironment [47]. Migration of cells from the primary tumor requires the breakdown of the basement membrane and remodeling of the ECM. Proteolytic cleavage and degradation of ECM proteins during tumor invasion are coordinated by a number of enzymes, particularly by MMPs [48]. MMPs regulate tumor cell invasion through autocrine or paracrine pathways [49]. In the tumor microenvironment, neutrophils are recruited to the niches of distant cancer metastasis and may be a key player in the establishment of metastasis [5]. Neutrophils secrete MMP-8, MMP-9, elastase, and other matrix proteinases that degrade the ECM directly to facilitate cancer cell invasion [50] or activate membrane type 1-MMP in tumor cells to promote cancer progression indirectly [51]. Previous studies demonstrated increased expression of MMPs involved in the invasion and angiogenesis of HNC [52]. MMP-9 induced by collagen XVI is reported to facilitate OSCC cell invasion [53]. A significant relationship was found between microvessel density count and MMP-9 expression in OSCC [54]. Intriguingly, MMP-9 is highly expressed in HNC tissue-infiltrating neutrophils [25], and MMP-9 inhibition reduces neutrophil-enhanced OSCC cell invasion and ECM degradation [13]. In line with these results, we found that OSCC-associated neutrophils promoted the migration and invasion of OSCC cells and angiogenesis of HUVECs in vitro. Moreover, knockdown of MMP-9 markedly eliminated the pro-aggressive behaviors of TANs in OSCC. Mel-educated TANs led to less migrated and invaded OSCC cells and tube formation ability, and they presented additive effects with MMP-9 inhibition. These results suggest that Mel inhibited the pro-motility and pro-angiogenesis effects of OSCC-associated neutrophils by repressing MMP-9.
In summary, our findings describe a novel mechanism linking TANs to OSCC metastasis involved in the antitumor effects of Mel. We demonstrated that increased CD66b-positive neutrophils were correlated with advanced stage, lymphatic metastasis, and poor prognosis of OSCC. Mel inhibited migration and apoptosis resistance of OSCC-associated neutrophils and reduced CXCL8, CCL2, CCL4, and MMP-9 release from OSCC-associated neutrophils by inactivating p38 MAPK and PI3K/Akt pathways. The pro-motility and pro-angiogenesis effects of OSCC-associated neutrophils decreased by Mel were dependent on MMP-9 suppression in OSCC. Overall, Mel may be used as an anti-OSCC therapeutic agent by reducing migration, apoptosis resistance, inflammatory response, and pro-motility and pro-angiogenesis effects of TANs.
Acknowledgements
This study was supported by Shaanxi Province Social Development Scientific and Technological Project Fund (No. 2015SF168).
Disclosure of conflict of interest
None.
References
- 1.Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Reboiras-Lopez MD, Gandara Rey JM, Garcia-Garcia A. Genetic and molecular alterations associated with oral squamous cell cancer (Review) Oncol Rep. 2009;22:1277–1282. doi: 10.3892/or_00000565. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Global oral cancer incidence. Br Dent J. 2016;221:288. doi: 10.1038/sj.bdj.2016.672. [DOI] [PubMed] [Google Scholar]
- 4.Mishra R. Biomarkers of oral premalignant epithelial lesions for clinical application. Oral Oncol. 2012;48:578–584. doi: 10.1016/j.oraloncology.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL, Ruvolo PP. Introduction to tumor microenvironment regulation of cancer cell survival, metastasis, inflammation, and immune surveillance. Biochim Biophys Acta. 2016;1863:379–381. doi: 10.1016/j.bbamcr.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiteside TL. Tricks tumors use to escape from immune control. Oral Oncol. 2009;45:e119–123. doi: 10.1016/j.oraloncology.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 9.Magalhaes MA, Glogauer JE, Glogauer M. Neutrophils and oral squamous cell carcinoma: lessons learned and future directions. J Leukoc Biol. 2014;96:695–702. doi: 10.1189/jlb.4RU0614-294R. [DOI] [PubMed] [Google Scholar]
- 10.Trellakis S, Bruderek K, Dumitru CA, Gholaman H, Gu X, Bankfalvi A, Scherag A, Hutte J, Dominas N, Lehnerdt GF, Hoffmann TK, Lang S, Brandau S. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer. 2011;129:2183–2193. doi: 10.1002/ijc.25892. [DOI] [PubMed] [Google Scholar]
- 11.Wang N, Feng Y, Wang Q, Liu S, Xiang L, Sun M, Zhang X, Liu G, Qu X, Wei F. Neutrophils infiltration in the tongue squamous cell carcinoma and its correlation with CEACAM1 expression on tumor cells. PLoS One. 2014;9:e89991. doi: 10.1371/journal.pone.0089991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumitru CA, Gholaman H, Trellakis S, Bruderek K, Dominas N, Gu X, Bankfalvi A, Whiteside TL, Lang S, Brandau S. Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. Int J Cancer. 2011;129:859–869. doi: 10.1002/ijc.25991. [DOI] [PubMed] [Google Scholar]
- 13.Glogauer JE, Sun CX, Bradley G, Magalhaes MA. Neutrophils increase oral squamous cell carcinoma invasion through an invadopodiadependent pathway. Cancer Immunol Res. 2015;3:1218–1226. doi: 10.1158/2326-6066.CIR-15-0017. [DOI] [PubMed] [Google Scholar]
- 14.Grant SG, Melan MA, Latimer JJ, Witt-Enderby PA. Melatonin and breast cancer: cellular mechanisms, clinical studies and future perspectives. Expert Rev Mol Med. 2009;11:e5. doi: 10.1017/S1462399409000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luchetti F, Canonico B, Betti M, Arcangeletti M, Pilolli F, Piroddi M, Canesi L, Papa S, Galli F. Melatonin signaling and cell protection function. Faseb J. 2010;24:3603–3624. doi: 10.1096/fj.10-154450. [DOI] [PubMed] [Google Scholar]
- 16.Joo SS, Yoo YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009;47:8–14. doi: 10.1111/j.1600-079X.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 17.Borin TF, Arbab AS, Gelaleti GB, Ferreira LC, Moschetta MG, Jardim-Perassi BV, Iskander AS, Varma NR, Shankar A, Coimbra VB, Fabri VA, de Oliveira JG, Zuccari DA. Melatonin decreases breast cancer metastasis by modulating Rho-associated kinase protein-1 expression. J Pineal Res. 2016;60:3–15. doi: 10.1111/jpi.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira GM, Martinez M, Camargo IC, Domeniconi RF, Martinez FE, Chuffa LG. Melatonin Attenuates Her-2, p38 MAPK, p-AKT, and mTOR levels in ovarian carcinoma of ethanolpreferring rats. J Cancer. 2014;5:728–735. doi: 10.7150/jca.10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ordonez R, Carbajo-Pescador S, Prieto-Dominguez N, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL. Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J Pineal Res. 2014;56:20–30. doi: 10.1111/jpi.12092. [DOI] [PubMed] [Google Scholar]
- 20.Yeh CM, Lin CW, Yang JS, Yang WE, Su SC, Yang SF. Melatonin inhibits TPA-induced oral cancer cell migration by suppressing matrix metalloproteinase-9 activation through the histone acetylation. Oncotarget. 2016;7:21952–21967. doi: 10.18632/oncotarget.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiter RJ. Mechanisms of cancer inhibition by melatonin. J Pineal Res. 2004;37:213–214. doi: 10.1111/j.1600-079X.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang CY, Lin CK, Tsao CH, Hsieh CC, Lin GJ, Ma KH, Shieh YS, Sytwu HK, Chen YW. Melatonin exerts anti-oral cancer effect via suppressing LSD1 in patient-derived tumor xenograft models. Oncotarget. 2017;8:33756–33769. doi: 10.18632/oncotarget.16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncalves Ndo N, Rodrigues RV, Jardim-Perassi BV, Moschetta MG, Lopes JR, Colombo J, Zuccari DA. Molecular markers of angiogenesis and metastasis in lines of oral carcinoma after treatment with melatonin. Anticancer Agents Med Chem. 2014;14:1302–1311. doi: 10.2174/1871520614666140812110246. [DOI] [PubMed] [Google Scholar]
- 24.Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumorassociated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150:1646–1658. e1617. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 25.Dumitru CA, Fechner MK, Hoffmann TK, Lang S, Brandau S. A novel p38-MAPK signaling axis modulates neutrophil biology in head and neck cancer. J Leukoc Biol. 2012;91:591–598. doi: 10.1189/jlb.0411193. [DOI] [PubMed] [Google Scholar]
- 26.Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissueinfiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16:771–788. doi: 10.1016/j.neo.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez-Garcia V, Gonzalez A, Alonso-Gonzalez C, Martinez-Campa C, Cos S. Antiangiogenic effects of melatonin in endothelial cell cultures. Microvasc Res. 2013;87:25–33. doi: 10.1016/j.mvr.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179:1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saverymuttu SH, Peters AM, Keshavarzian A, Reavy HJ, Lavender JP. The kinetics of 111indium distribution following injection of 111indium labelled autologous granulocytes in man. Br J Haematol. 1985;61:675–685. doi: 10.1111/j.1365-2141.1985.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 31.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- 32.Vincent PC, Chanana AD, Cronkite EP, Joel DD. The intravascular survival of neutrophils labeled in vivo. Blood. 1974;43:371–377. [PubMed] [Google Scholar]
- 33.Cheretakis C, Leung R, Sun CX, Dror Y, Glogauer M. Timing of neutrophil tissue repopulation predicts restoration of innate immune protection in a murine bone marrow transplantation model. Blood. 2006;108:2821–2826. doi: 10.1182/blood-2006-04-018184. [DOI] [PubMed] [Google Scholar]
- 34.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 35.Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4:83–91. doi: 10.1158/2326-6066.CIR-15-0313. [DOI] [PubMed] [Google Scholar]
- 36.Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98:349–354. doi: 10.1007/s004010051093. [DOI] [PubMed] [Google Scholar]
- 37.Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. 2013;108:2116–2122. doi: 10.1038/bjc.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue TC, Zhang L, Xie XY, Ge NL, Li LX, Zhang BH, Ye SL, Ren ZG. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: a meta-analysis. PLoS One. 2014;9:e96072. doi: 10.1371/journal.pone.0096072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 40.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JJ, Chang YL, Lai WL, Ko JY, Kuo MY, Chiang CP, Azuma M, Chen CW, Chia JS. Increased prevalence of interleukin-17-producing CD4(+) tumor infiltrating lymphocytes in human oral squamous cell carcinoma. Head Neck. 2011;33:1301–1308. doi: 10.1002/hed.21607. [DOI] [PubMed] [Google Scholar]
- 42.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 43.Hathaway B, Landsittel DP, Gooding W, Whiteside TL, Grandis JR, Siegfried JM, Bigbee WL, Ferris RL. Multiplexed analysis of serum cytokines as biomarkers in squamous cell carcinoma of the head and neck patients. Laryngoscope. 2005;115:522–527. doi: 10.1097/01.mlg.0000157850.16649.b8. [DOI] [PubMed] [Google Scholar]
- 44.Fujita S, Ikeda T. The CCL2-CCR2 axis in lymph node metastasis from oral squamous cell carcinoma: an immunohistochemical study. J Oral Maxillofac Surg. 2017;75:742–749. doi: 10.1016/j.joms.2016.09.052. [DOI] [PubMed] [Google Scholar]
- 45.Blum DL, Koyama T, M’Koma AE, Iturregui JM, Martinez-Ferrer M, Uwamariya C, Smith JA Jr, Clark PE, Bhowmick NA. Chemokine markers predict biochemical recurrence of prostate cancer following prostatectomy. Clin Cancer Res. 2008;14:7790–7797. doi: 10.1158/1078-0432.CCR-08-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikebe T, Takeuchi H, Jimi E, Beppu M, Shinohara M, Shirasuna K. Involvement of proteasomes in migration and matrix metalloproteinase-9 production of oral squamous cell carcinoma. Int J Cancer. 1998;77:578–585. doi: 10.1002/(sici)1097-0215(19980812)77:4<578::aid-ijc18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 47.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 50.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, Mignatti P. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 52.Iizuka S, Ishimaru N, Kudo Y. Matrix metalloproteinases: the gene expression signatures of head and neck cancer progression. Cancers (Basel) 2014;6:396–415. doi: 10.3390/cancers6010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bedal KB, Grassel S, Oefner PJ, Reinders J, Reichert TE, Bauer R. Collagen XVI induces expression of MMP9 via modulation of AP-1 transcription factors and facilitates invasion of oral squamous cell carcinoma. PLoS One. 2014;9:e86777. doi: 10.1371/journal.pone.0086777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andisheh-Tadbir A, Mardani M, Pourshahidi S, Nezarati K, Bahadori P. Prognostic value of matrix metalloproteinase-9 expression in oral squamous cell carcinoma and its association with angiogenesis. J Clin Exp Dent. 2016;8:e130–135. doi: 10.4317/jced.52712. [DOI] [PMC free article] [PubMed] [Google Scholar]