Abstract
Background: Resistance training (RT) has been associated with positive responses in patients with cardiovascular disease, and when it is combined with continuous aerobic training (CAT), favorable adaptations appear to be even more pronounced. However, the effects of high-intensity RT alone or in combined with CAT in the case of heart failure (HF) is not completely elucidated. Methods: 28 male Wistar rats with HF (90 days old) were allocated to 4 groups: high-intensity RT (RT, n=7), CAT (CAT, n=7), RT and CAT (RT+CAT, n=7) and sedentary (Sed, n=7). Trained animals were subjected to a RT protocol in an adapted squat apparatus for rats (4 bouts, 6-8 reps, 90 s interval, 3×/week, 75% to 85% of one maximum repetition (1RM) for 8 weeks). The animals subjected to CAT performed it 3×/week during 50 min/session at 16 m/min. The animals of the combined exercise regimen performed both the RT and CAT exercise protocols. Results: The left ventricular end-diastolic pressure (LVEDP), collagen volume fraction and right ventricular hypertrophy were lower in RT, CAT and RT+CAT groups when compared to Sed group (P<0.05) for all outcomes. Regarding the inflammatory profile, only the CAT group showed greater IL-10 concentrations. Conclusion: We concluded that RT combined with CAT was able to improve the strength in animals with HF, which was associated to improvement in ventricular structure and function.
Keywords: Muscle strength, exercise, hemodynamic function, collagen, acute myocardial infarction
Introduction
Heart failure (HF) is related to a cardiac muscle modifies that lead to a systemic disorders, consequently yielding symptoms such as dyspnea, fatigue and exercise intolerance [1]. Currently, muscle weakness has become a major target of research because the development of skeletal muscle strength is associated with survival of patients with HF [2,3].
Changes in the extracellular matrix of cardiac collagen are connected with impairments in cardiac compliance, systolic and diastolic function and in the electrical behavior of the heart [4,5]. Interestingly, physical exercise has produced favorable responses on interstitial collagen fractions in animal models [6,7] and physical exercise has been described as an effective tool to implement cardiac reverse remodeling in the pathophysiology of HF [8].
Various exercise protocols have been employed as a strong non-pharmacological strategy in animal models of acute myocardial infarction (AMI) [6,7,9]. The use of resistance training (RT) in the experimental model with healthy rats has exhibited value as a tool for positive adaptations with respect to cardiac function, cardiovascular remodeling [10] and skeletal muscle [11].
The effects of aerobic and anaerobic physical training and its variables as type, frequency and intensity need to be elucidated [12]. Several studies using animal models of HF have employed the effects of continuous aerobic training (CAT) [7,13]. However, the effects of high-intensity RT combined with aerobic training of moderate intensity are scarce. Therefore the aim of present study was to evaluate whether such responses may be associated with an improvement in hemodynamic function, inflammatory profiles and attenuated collagen deposition in animals with HF.
Methods
This experimental study was based on ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines [14].
Sample size, animals and ethical approval
Male Wistar rats (n=52, 220 to 270 g, 90 days of age) were obtained from the breeding animal unit of the Universidade Federal de Ciências da Saúde de Porto Alegre and were housed under standard conditions. This investigation followed the rules established by the guide for care and use of animals in experiments published by National Institute of Health (NIH publication no. 41-87, revised in 2011). All procedures were approved by the Ethics Committee in Research of Universidade Federal de Ciências da Saúde de Porto Alegre (protocol 199-13).
Acute myocardial infarction induction
The animals were weighed and anesthetized by inhalation with 2% isofluorane (Isofluorine, 100 ml-Cristália) in 100% oxygen [15] and were immediately artificially ventilated (Samway VR 15, 60 breaths/min). The ligature of coronary artery was performed to induce AMI as described previously [7].
Experimental design
Five weeks after AMI, 28 animals (with HF) were allocated into 4 groups: Sedentary (Sed), Resistance Training (RT), Continuous Aerobic Training (CAT) and Resistance Training combined with Continuous Aerobic Training (RT+CAT) (Figure 1).
Figure 1.
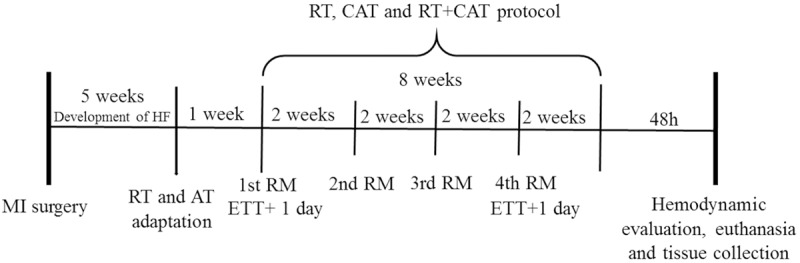
Timeline of experimental procedures. Acute myocardial infarction, AMI; resistance training, RT; continuous aerobic training, CAT; exercise tolerance test, ETT; 1st test of a maximum repetition, 1st 1RM; 2nd test of a maximum repetition, 2nd 1RM; 3rd test of a maximum repetition, 3rd 1RM; and 4th test of a maximum repetition, 4th 1RM.
Resistance training (RT)
Adaptation
The trained groups were subjected to a familiarization period with the adapted squat apparatus [16]. The animals were encouraged to perform 5 to 10 repetitions with 40% to 60% of body weight for one week [6].
1RM test
The training loads were determined based in percentage of 1RM test and adjusted every two weeks.
RT protocol
A neoprene vest attached to apparatus was used by animals to remain in the standard position on their lower limbs. An electric stimulus (4-5 mA, 1 s duration, with a 3 s interval between each repetition) was applied to the rats’ tails. The training protocol has been described elsewhere [6].
Continuous aerobic training (CAT)
Adaptation to treadmill
Five weeks after AMI induction surgery, the animals were encouraged to run (10 m/min; 0% inclination) for five minutes on the first and second day and ten minutes on the third, fourth and fifth days.
Exercise test tolerance/incremental load test
The animals were subjected to an exercise tolerance test (ETT) five weeks after AMI surgery and after eight weeks of training. The protocol employed consisted of a run on the treadmill with an inclination of 15° until exhaustion. The protocol began with a speed of 5 m/min and there was an increment of 5 m/min every 3 minutes until exhaustion of the animal [17].
CAT
The run training protocol lasted for eight weeks, with 50 min/d; 3 times per week at 10 m/min, which was progressively increased up to 15 m/min (0% inclination).
Protocol of combined physical training (RT+CAT)
The Animals of the RT+CAT initially performed the RT and immediately at the end of RT, the animals were subjected to CAT.
Evaluation of hemodynamic function
The recordings were performed 48 hours after the end of the training protocol. The animals were anesthetized and a polyethylene catheter model P50 was introduced into the left ventricle (LV) via the right carotid artery. Blood pressure was recorded for 5 minutes immediately following the catheter being positioned within the LV to perform the registration of the ventricular pressure. This method has been described elsewhere [6].
Blood sample and collection of tissue
Blood collection was conducted with the tube already positioned in the left carotid artery and samples were taken in two 1.5 ml tubes containing 3.2% sodium citrate (1:9 v/v) and next centrifuged at 500×g for 10 min at 4°C. The plasma was stored at -22°C. Immediately after the hemodynamic assessment and blood collection, the animals were sacrificed by decapitation and the heart, lung and liver tissues were harvested [6].
Cardiac hypertrophy, pulmonary and hepatic congestion
Cardiac hypertrophy was determined by ratio to body weight [6,7,18]. To evaluate lung and liver congestion was utilized:
Congestion (%H2O) = (Wet weight Dry weight)/(Wet weight × 100)
Determining the size of the infarcted area
Left ventricles were filled with a latex pad and submerged in buffered formaldehyde for 10% over 24 h. The size of the infarcted area was determined by histological evaluation adapted from the method previously described by Martinez et al. [19].
Analysis of interstitial collagen deposition in the heart muscle
Cross cuts were performed on the LV and these fragments were fixed in 10% buffered formaldehyde for subsequent inclusion in paraffin [20,21]. Histological sections of 6 microns were stained with picrosirius Red (PSR) and were employed for measurements of collagen volume fraction (CVF) present in LV. The fields selected were located far from the infarcted area and distant from the pericardial region as previously described [6]. The CVF was defined as the sum of all areas of interstitial collagen tissue (highlighted by polarization) divided by total tissue area [20].
Determination of plasmatic levels of interleukins
TNF-α and IL-10 plasma level were assessed through a Milliplex Map kit (RCYTO-80K, Millipore-Billerica, MA, USA) following the manufacturer’s recommendations.
Statistical analysis
Variables were presented as mean ± SD. After employing the normality test, ANOVA followed by a Tukey’s post hoc test to compare symmetrical variables between groups was used. A Two-way repeated measurement ANOVA followed by Tukey’s post hoc test was employed to compare the strength gain between groups at different experimental times and also to compare speed, distance and time in the ETT. Pearson’s correlation test was applied to examine the relationship among gain maximum strength and other variables. P values <0.05 were considered significant. GraphPad Prism version 6.01 (GraphPad Software, San Diego, CA, USA) for Windows. Sigmaplot version 12.0 (Systat Software Inc., San Jose, CA, USA) were utilized to statistical analysis.
Results
Mortality, exclusion and adverse effects
The mortality in AMI induction surgery was ~39% (20/52). The only animals with an infarcted area (≥40%) were included. Experimental groups were Sed (n=7), RT (n=7), CAT (n=7), and RT+CAT (n=7). No deaths occurred during the exercise protocols.
Morphological variables and pulmonary and hepatic congestion
The initial and final animal body weights as well other morphological variables were similar between groups in the pre- and post-training states. Sed-IC group showed greater cardiac hypertrophy and pulmonary congestion when compared to all training groups (Table 1).
Table 1.
Body weight, infarcted area size, cardiac hypertrophy, lung and liver congestion
| Sed | RT | CAT | RT+CAT | P One way ANOVA | |
|---|---|---|---|---|---|
| Initial weight, g | 264.3±9.1 | 262.6±20.4 | 270.4±9.5 | 274.3±7.8 | 0.401 |
| Final weight, g | 359.7±41.6 | 344.6±34.0 | 350.0±26.8 | 370.8±21.5 | 0.523 |
| Infarcted area% | 42.52±2.5 | 41.51±1.7 | 42.82±2.2 | 44.63±3.3 | 0.169 |
| MW/BW mg/g | 3.87±0.5 | 3.72±0.4 | 3.60±0.1 | 3.39±0.3 | 0.827 |
| LVW/BW mg/g | 2.71±0.4 | 2.96±0.3 | 2.73±0.3 | 3.11±0.5 | 0.274 |
| RVW/BW mg/g | 1.16±0.3* | 0.76±0.1 | 0.86±0.2 | 0.61±0.4 | 0.030 |
| Pulmonary congestion (PC)% | 78.19±2.7† | 70.17±4.0 | 72.52±2.4 | 73.00±1.4 | <0.001 |
| Hepatic congestion (HC)% | 69.87±0.5 | 70.28±0.8 | 71.09±2.2 | 70.10±0.7 | 0.743 |
Values are means ± SD. Sedentary group, Sed; resistance training group, RT; continuous aerobic training group, CAT; resistance training combined with continuous aerobic training group, RT+CAT. Ratio of the total myocardial Weight to body weight, MW/BW; ratio of left ventricular weight to body weight, LVW/BW e ratio of right ventricle weight to body weight, RVW/BW.
compared to RT+CAT.
compared to all groups.
Hemodynamic evaluation
RT group demonstrated lower LVEDP compared to Sed group. Higher value of -dP/dtmax was observed in RT+CAT compared to Sed group. The combined training protocol was also able to enhance cardiac relaxation as reflected by an increase of -dP/dtmax, mmHg/s (Table 2).
Table 2.
Hemodynamic variables of the experimental groups
| Sed | RT | CAT | RT+CAT | P One way ANOVA | |
|---|---|---|---|---|---|
| HR, bpm | 249.4±40.6 | 269.3±52.6 | 253.4±71.0 | 244.7±57.7 | 0.790 |
| SBP, mmHg | 92.8±15.5 | 107.4±16.6 | 110.6±27.6 | 114.1±15.3 | 0.237 |
| DBP, mmHg | 76.0±12.5 | 83.05±10.2 | 82.1±11.7 | 92.9±12.5 | 0.186 |
| MAP, mmHg | 84.6±13.3 | 97.1±16.2 | 98.4±22.1 | 103.6±12.8 | 0.203 |
| LVEDP, mmHg | 25.6±4.1* | 8.12±3.0 | 13.1±2.3† | 7.4±2.8 | <0.001 |
| LVSP, mmHg | 88.5±21.1 | 102.6±10.2 | 113.0±29.8 | 106.6±3.9 | 0.117 |
| VHR, bpm | 246.9±39.1 | 236.3±33.6 | 210.2±9.9‡ | 226.4±13.1 | 0.039 |
| +dP/dtmax, mmHg/s | 3553±1064 | 4921±782 | 5463±1938‡ | 4929±449 | 0.035 |
| -dP/dtmax, mmHg/s | -2260±597 | -3279±560 | -3759±1625‡ | -3293±247‡ | 0.012 |
Values are means ± SD. Sedentary group, Sed; resistance training group, RT; continuous aerobic training group, CAT; resistance training combined with continuous aerobic training group, RT+CAT. Heart rate, HR; Systolic blood pressure; SBP; diastolic blood pressure, DBP; Mean arterial pressure, MAP; end diastolic pressure of the left ventricle, LVEDP; left ventricular systolic pressure, LVSP; ventricular heart rate, VHR; maximum positive left ventricular change in pressure over time, +dP/dtmax; Maximum negative left ventricular change in pressure over time, -dP/dtmax.
compared to all groups.
compared to RT and RT+CAT.
compared to Sed.
Maximum strength gains: responses intra- and extra-groups
Effects within groups
Figure 2 details the gain of absolute (A) and relative (B) force over time (1st to 4th 1RM). In terms of the 1st 1RM, there were no differences between groups in absolute and relative tests, suggesting that all groups started the protocol under similar conditions. The RT and RT+CAT groups presented gain maximum strength, in both, absolute and relative load in all tests compared to the initial test.
Figure 2.
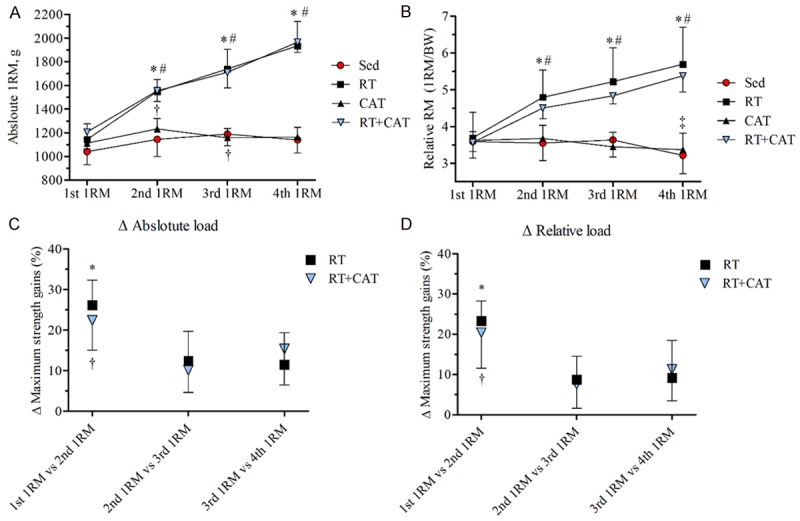
Maximum strength gain over time. A: Absolute load; B: Relative load. Magnitude of strength gain between the segments of the tests (1st 1RM vs 2nd 1RM; 2nd 1RM vs 3rd 1RM; and 3rd 1RM vs 4th 1RM). C: Absolute; D: Relative. Values are mean ± SD. Sedentary group, Sed; resistance training group, RT; continuous aerobic training group, CAT; resistance training combined with continuous aerobic training group, RT+CAT. Graph A and B: *P<0.01 RT and RT+CAT groups comparing the 1st 1RM test with absolute and relative load. †P<0.05 Sed and CAT groups compared to the 1st 1RM test with absolute load. ‡P<0.05 CAT group compared to the 4th RM test with 1st 1RM test with relative load. #P<0.01 RT and RT+CAT groups compared to the 2nd, 3rd and 4th 1RM test of the Sed and CAT with absolute and relative load. Graph C and D: *P<0.05 compared the percentage differences of the 2nd 1RM vs 3rd 1RM tests and the 3rd 1RM vs 4th 1RM tests for the RT group. †P<0.05 compared the percentage differences of the 2nd 1RM vs 3rd 1RM tests for the RT+CAT group.
Effects between groups
Figure 2A and 2B depict the absolute and relative strength gains between the groups. RT protocol alone or combined with CAT had an increase in their average values in the 2nd, 3rd and 4th 1RM compared to Sed and CAT groups.
Magnitude strength gain between the percentage differences of maximum load tests
Figure 2C and 2D details strength gains in the absolute and relative load in different moments of RM test for the RT and RT+CAT groups. These results suggest that during the protocol, there would be maximum strength gains, though on a smaller scale when compared to the effects of the initial two weeks of the RT protocol.
ETT
Figure 3A-C exhibit the variables analyzed in the ETT of the pre- and post-intervention. In Pre-intervention no differences were observed between the groups, suggesting that all groups presented similar conditions at begin of study. CAT group showed an increase in speed, time and distance in post compared to pre-ETT. There were no differences between endurance-trained animals when compared to Sed and RT groups as well as CAT pre to post protocol.
Figure 3.
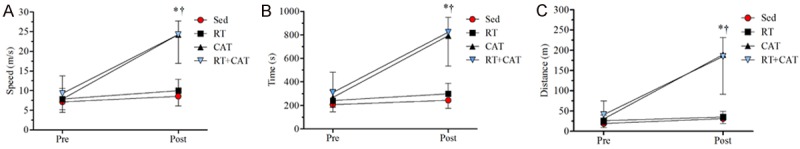
Exercise tolerance test (ETT). A: Speed (m/s); B: Time (s) and C: Distance (m). Values are means ± SD. The sedentary group, Sed; resistance training group, RT; continuous aerobic training group, CAT; resistance training combined with continuous aerobic training group, RT+CAT. *P<0.001; CAT and RT+CAT groups compared to the preintervention time. †P<0.001 compared to groups Sed and RT.
Inflammatory profile
CAT group showed higher IL-10 levels compared to Sed group and there was no difference compared to RT and RT+CAT (Figure 4A and 4B). No differences were observed in both, TNF-α plasma level and TNF-α/IL-10 ratio between groups.
Figure 4.
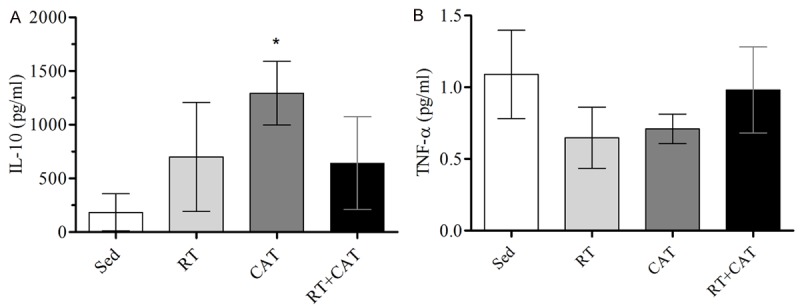
Analysis of inflammatory markers in plasma. A: IL-10 e; B: TNF-α. Values are mean ± SD. Sedentary group, Sed; resistance training group, RT; continuous aerobic training group, CAT; resistance training combined with continuous aerobic training group, RT+CAT. *P<0.05 compared to the Sed group.
Interstitial collagen volume fraction
The RT isolated or combined with CAT groups demonstrates a lower collagen volume compared to Sed group (Figure 5).
Figure 5.

Collagen deposition analysis. Values are mean ± SD. A: Sedentary group with HF, Sed; B: Resistance training group, RT; C: Continuous aerobic training group, CAT; D: Resistance training combined with continuous aerobic training group, RT+CAT. E: Representative graph of the quantification of collagen volume fraction. *P<0.05 compared to Sed group.
Correlations between central responses and maximum strength gain
Figure 6A-E, portray correlations between gain strength and the hemodynamic parameters and interstitial collagen fraction. There were significant correlation between LVEDP and the 4th 1RM (Figure 6A), LVEDP and the change in maximum strength gain (Figure 6B) and LVEDP and CVF (Figure 6C). In addition, there were significant correlations between CVF and the maximum strength gain as well as the change in maximum power gain (Figure 6D and 6E, respectively).
Figure 6.

Correlations between the left ventricle end-diastolic pressure (LVEDP) with maximum gain in strength and volume fraction of collagen. red circles (Mark 1), Sed; dark square (■), RT; dark triangle (▲), CAT; and blue triangle (Mark 2), RT+CAT. A: LVEDP with 4th 1RM/BW; B: LVEDP with Δ maximum strength gains (%); C: LVEDP with fraction of collagen volume (%); D: Fraction of collagen volume with 4th 1RM/BW; E: Fraction of collagen volume with Δ maximum strength gains (%).
Discussion
To our knowledge, this is the first study to investigate the effects of 8 weeks of high-intensity RT (75% to 85% of 1RM) and CAT of moderate intensity (60% of the speed of ETT), alone or in combination, in HF rats. Based on our results, the trained groups showed improvement in the following variables: Strength gain, LVEDP, +dP/dtmax, -dP/dtmax, PC (% water), right ventricular hypertrophy, IL-10 and CVF. The maximum strength gain was an important outcome found in our study, because many studies have shown that muscle weakness is an independent risk factor for mortality in populations with HF [2,3]. In this way, our study was able to show an increase in strength gain, which can be directly related to peripheral muscle preservation as a positive prognosis in HF syndrome.
Coronary artery ligation produces a marked ventricular dysfunction that is closely related to the infarct area size [6,7] and therefore may be observed as a major impairment in hemodynamic function. In the present study, the infarcted areas were close to ~43%, which reflects directly a high LVEDP. Among the hemodynamic responses, we are able to highlight LVEDP, an important marker of ventricular dysfunction in HF [22].
In our study, LVEDP [22] was lower in the RT (67.8%), CAT (48.7%) and RT+CAT (71.1%) groups compared to Sed group. We also saw that those animals that had an increase in muscular strength showed lower LVEDP values as demonstrated by the negative correlations in Figure 6A and 6B (r=-0.62 and r=-0.73, respectively). Previous studies in our laboratory with HF animals subjected to respiratory muscle training [23], swimming training [13] and CAT [7] showed similar responses in LVEDP. These improvements in cardiac function demonstrate a positive effect of exercise training.
The +dP/dtmax and -dP/dtmax exhibited favorable changes in the CAT group, while the group with combined exercise showed improvement solely in -dP/dtmax. Similar results have been reported in experimental studies of HF with different forms of interventions [7,13]. In fact, the different exercise models like RT and CAT performed in our study leads to specific physiological adaptations on cardiac function. On the other hand, from a translational perspective, clinical studies have suggested there are controversial effects from the impact of exercise on systolic and diastolic function after acute myocardial infarction [12,24,25].
The exercise protocols used in the present study, were able to lower pulmonary congestion corroborating with other studies that employed RT or CAT as the primary intervention in rats with HF [6,26]. Additionally, we observed lower values in the RVW/BW ratio in the RT+CAT group, which can be related to a cardiac remodeling associated to lower pulmonary congestion. These outcomes were also found in a previous study that utilized only the RT in rats with HF [6].
In our study, we observed an increase in plasma levels of IL-10 only in the CAT group. In fact, the moderate intensity of aerobic exercise is able to improve inflammatory profile [27]. Interestingly, the combined exercise group did not show improvement in this variable, possibly because the total volume and specific energy metabolism required in high intensity RT. These different characteristics of training may have interfered negatively to increased plasma levels of IL-10.
The modification of extracellular matrix collagen is part in a natural process of an adaptive response to increased mechanical load on the heart during exercise. However, the chronic overload arising from cardiac injury develops a negative response with respect to distensibility of the heart, valvular function, systolic and diastolic dysfunction and electrical transmission, ultimately leading to myocardial arrhythmia in HF [4,5]. In the present study, the interstitial collagen accumulation of animals in the Sed group was higher when compared to groups subjected to physical training, demonstrating a possible reverse remodeling of the extracellular matrix, corroborating with the results of previous studies [26,28]. These findings show that a sedentary behavior is directly related to a poor prognosis in HF condition. Here, a positive correlation was also observed between increased collagen accumulation and LVEDP (r=0.64; P=0.0002, Figure 6C). These results contributed to understand the relationship between hemodynamic injury and structural changes of the heart. Yet, it was observed in this study that an increase in muscle strength can influence the reduction of the accumulation of collagen as demonstrated by the correlation of Figure 6D and 6E (r=-0.49 and r=-0.57; P=0.007 for both, respectively).
There were some limitations in this study. First, the most robust biochemical analyzes in cardiac and peripheral muscle tissue could help to a better understand of possible balance between the molecular pathways of anabolism and catabolism induced by disease and exercise. Second, the absence of a load cell coupled to the squat apparatus for rats could help to increase the precision of maximum strength tests and control of the loads during the training protocol. This information could contribute to adjustments of intensity of training in the HF rats, especially in the RT training.
Conclusions
The RT protocol of high-intensity, performed alone or combined with CAT of moderate intensity, was able to increase the strength in animals with HF syndrome. This response was closely related to the improvement of ventricular structure and function. Nevertheless, the high-intensity RT protocol does not appear to offer benefits in terms of inflammatory markers (IL-10 and TNF-α). However, only the CAT protocol of moderate intensity was capable of exhibiting a positive influence on IL-10 plasma level. We emphasize that the exercise protocols need to be better balanced in relation to the volume, intensity and frequency, since these variables can directly to influence the functional and structural adaptations induced by physical training programs in HF.
Acknowledgements
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. We are grateful to Prof. Luís Fernando Deresz, Terezinha Stein, Rosalva Theresa Meurer, Ignês Cristiane de Souza Paiva and Mário Pereira (in memoriam) for support during this study.
Disclosure of conflict of interest
None.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 2.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 3.Hulsmann M, Quittan M, Berger R, Crevenna R, Springer C, Nuhr M, Mortl D, Moser P, Pacher R. Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail. 2004;6:101–107. doi: 10.1016/j.ejheart.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 4.van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol. 2016;93:133–142. doi: 10.1016/j.yjmcc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Whittaker P. Unravelling the mysteries of collagen and cicatrix after myocardial infarction. Cardiovasc Res. 1995;29:758–762. [PubMed] [Google Scholar]
- 6.Alves JP, Nunes RB, Stefani GP, Dal Lago P. Resistance training improves hemodynamic function, collagen deposition and inflammatory profiles: experimental model of heart failure. PLoS One. 2014;9:e110317. doi: 10.1371/journal.pone.0110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunes RB, Alves JP, Kessler LP, Dornelles AZ, Stefani GP, Lago PD. Interval and continuous exercise enhances aerobic capacity and hemodynamic function in CHF rats. Braz J Phys Ther. 2015;19:257–263. doi: 10.1590/bjpt-rbf.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams V, Niebauer J. Reversing heart failure-associated pathophysiology with exercise: what actually improves and by how much? Heart Fail Clin. 2015;11:17–28. doi: 10.1016/j.hfc.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Batista ML Jr, Rosa JC, Lopes RD, Lira FS, Martins E Jr, Yamashita AS, Brum PC, Lancha AH Jr, Lopes AC, Seelaender M. Exercise training changes IL-10/TNF-alpha ratio in the skeletal muscle of post-MI rats. Cytokine. 2010;49:102–108. doi: 10.1016/j.cyto.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Barauna VG, Rosa KT, Irigoyen MC, de Oliveira EM. Effects of resistance training on ventricular function and hypertrophy in a rat model. Clin Med Res. 2007;5:114–120. doi: 10.3121/cmr.2007.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamaki T, Uchiyama S, Nakano S. A weightlifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med Sci Sports Exerc. 1992;24:881–886. [PubMed] [Google Scholar]
- 12.Fontes-Carvalho R, Azevedo AI, Sampaio F, Teixeira M, Bettencourt N, Campos L, Goncalves FR, Ribeiro VG, Azevedo A, Leite-Moreira A. The effect of exercise training on diastolic and systolic function after acute myocardial infarction: a randomized study. Medicine (Baltimore) 2015;94:e1450. doi: 10.1097/MD.0000000000001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunes RB, Tonetto M, Machado N, Chazan M, Heck TG, Veiga AB, Dall’Ago P. Physical exercise improves plasmatic levels of IL-10, left ventricular end-diastolic pressure, and muscle lipid peroxidation in chronic heart failure rats. J Appl Physiol (1985) 2008;104:1641–1647. doi: 10.1152/japplphysiol.00062.2008. [DOI] [PubMed] [Google Scholar]
- 14.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munkvik M, Lunde PK, Aronsen JM, Birkeland JA, Sjaastad I, Sejersted OM. Attenuated fatigue in slow twitch skeletal muscle during isotonic exercise in rats with chronic heart failure. PLoS One. 2011;6:e22695. doi: 10.1371/journal.pone.0022695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krisan AD, Collins DE, Crain AM, Kwong CC, Singh MK, Bernard JR, Yaspelkis BB 3rd. Resistance training enhances components of the insulin signaling cascade in normal and high-fat-fed rodent skeletal muscle. J Appl Physiol (1985) 2004;96:1691–1700. doi: 10.1152/japplphysiol.01054.2003. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira JC, Rolim NP, Bartholomeu JB, Gobatto CA, Kokubun E, Brum PC. Maximal lactate steady state in running mice: effect of exercise training. Clin Exp Pharmacol Physiol. 2007;34:760–765. doi: 10.1111/j.1440-1681.2007.04635.x. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes T, Hashimoto NY, Magalhaes FC, Fernandes FB, Casarini DE, Carmona AK, Krieger JE, Phillips MI, Oliveira EM. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin ii, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1-7) Hypertension. 2011;58:182–189. doi: 10.1161/HYPERTENSIONAHA.110.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez PF, Okoshi K, Zornoff LA, Oliveira SA Jr, Campos DH, Lima AR, Damatto RL, Cezar MD, Bonomo C, Guizoni DM, Padovani CR, Cicogna AC, Okoshi MP. Echocardiographic detection of congestive heart failure in postinfarction rats. J Appl Physiol (1985) 2011;111:543–551. doi: 10.1152/japplphysiol.01154.2010. [DOI] [PubMed] [Google Scholar]
- 20.Matsubara LS, Matsubara BB, Okoshi MP, Cicogna AC, Janicki JS. Alterations in myocardial collagen content affect rat papillary muscle function. Am J Physiol Heart Circ Physiol. 2000;279:H1534–1539. doi: 10.1152/ajpheart.2000.279.4.H1534. [DOI] [PubMed] [Google Scholar]
- 21.Whittaker P, Boughner DR, Kloner RA. Analysis of healing after myocardial infarction using polarized light microscopy. Am J Pathol. 1989;134:879–893. [PMC free article] [PubMed] [Google Scholar]
- 22.Musch TI, Wolfram S, Hageman KS, Pickar JG. Skeletal muscle ouabain binding sites are reduced in rats with chronic heart failure. J Appl Physiol (1985) 2002;92:2326–2334. doi: 10.1152/japplphysiol.00686.2001. [DOI] [PubMed] [Google Scholar]
- 23.Jaenisch RB, Quagliotto E, Chechi C, Calegari L, Dos Santos F, Borghi-Silva A, Dal Lago P. Respiratory muscle training improves chemoreflex response, heart rate variability, and respiratory mechanics in rats with heart failure. Can J Cardiol. 2017;33:508–514. doi: 10.1016/j.cjca.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Alves AJ, Ribeiro F, Goldhammer E, Rivlin Y, Rosenschein U, Viana JL, Duarte JA, Sagiv M, Oliveira J. Exercise training improves diastolic function in heart failure patients. Med Sci Sports Exerc. 2012;44:776–785. doi: 10.1249/MSS.0b013e31823cd16a. [DOI] [PubMed] [Google Scholar]
- 25.Smart N, Haluska B, Jeffriess L, Marwick TH. Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. Am Heart J. 2007;153:530–536. doi: 10.1016/j.ahj.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 26.de Moraes WM, Melara TP, de Souza PR, Guimaraes Fde S, Bozi LH, Brum PC, Medeiros A. Impact of leucine supplementation on exercise training induced anti-cardiac remodeling effect in heart failure mice. Nutrients. 2015;7:3751–3766. doi: 10.3390/nu7053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunes RB, Alves JP, Kessler LP, Dal Lago P. Aerobic exercise improves the inflammatory profile correlated with cardiac remodeling and function in chronic heart failure rats. Clinics (Sao Paulo) 2013;68:876–882. doi: 10.6061/clinics/2013(06)24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanzelli AS, Medeiros A, Rolim N, Bartholomeu JB, Cunha TF, Bechara LR, Gomes ER, Mattos KC, Sirvente R, Salemi VM, Mady C, Negrao CE, Guatimosim S, Brum PC. Integrative effect of carvedilol and aerobic exercise training therapies on improving cardiac contractility and remodeling in heart failure mice. PLoS One. 2013;8:e62452. doi: 10.1371/journal.pone.0062452. [DOI] [PMC free article] [PubMed] [Google Scholar]


