Abstract
Long noncoding RNA (lncRNA) small ubiquitin-like modifier 1 pseudogene 3 (SUMO1P3) acts a tumor promoter in several malignancies; however, its roles in colon cancer remain unclear. Herein, we demonstrated that SUMO1P3 expression was significantly higher in colon cancer tissues and cell lines than the corresponding non-tumor samples and normal colonic epithelial cells, respectively. The upregulation of SUMO1P3 was positively correlated with the advanced histological stages, metastases, angiogenesis and poor prognosis of colon cancer patients. SUMO1P3 knockdown repressed the proliferation, migration, invasion, and pro-angiogenesis of colon cancer cells in vitro. SUMO1P3 silencing reduced the growth, liver metastasis, and vascularization of colon cancer in vivo. Mechanistically, SUMO1P3 depletion decreased the levels of cyclin D1, Vimentin, and VEGFA while increased E-cadherin expression in xenograft tumor tissues. Overall, these results indicate that SUMO1P3 expedites the malignant behaviors of colon cancer and may be as a potential therapeutic target.
Keywords: SUMO1P3, colon cancer, growth, metastasis, angiogenesis
Introduction
Colon cancer is the third most common carcinoma and the second leading cause of cancer-related deaths worldwide [1]. The incidence and mortality rates of colon cancer are gradually increasing in China, with 376,300 new cases and 191,000 deaths in 2015 [2]. Despite improvements in colon cancer therapy, the 5-year survival rate remains at 30% due to recurrence and metastasis [3]. Reportedly, colon cancer is causally linked to a combination of genetic and environmental factors involving multistep progressions with genetic or epigenetic abnormalities [4,5]. Therefore, a clear understanding of the molecular mechanisms underlying the progression of colon cancer is essential in the development of new treatments and improvement of patient prognosis.
Long noncoding RNAs (lncRNAs) are a class of non-coding transcripts longer than 200 nucleotides. LncRNAs regulate gene expression at the epigenetic, transcriptional, and post-transcriptional levels and are involved in the physiological and pathological processes of human diseases [6]. Mounting evidences showed that lncRNAs participate in colon cancer pathogenesis by modulating several biological processes, such as differentiation, proliferation, migration, invasion, and angiogenesis [7,8]. For example, CCAT1 promotes the proliferation and invasion of colon cancer cells [9]. BLACAT1 indicates a poor prognosis of colorectal cancer and affects cell proliferation by regulating cell cycle progression [10]. HOTAIR is a predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition (EMT) in colon cancer [11]. ATB promotes colon cancer progression by promoting EMT [12]. Recently, it has been reported that lncRNA small ubiquitin-like modifier 1 pseudogene 3 (SUMO1P3) is upregulated in gastric cancer and is a potential prognostic and therapeutic target for gastric cancer [13]. Another study demonstrated that SUMO1P3 increases in bladder cancer and promotes tumor growth and metastasis [14]. However, the clinical significance and biological roles of SUMO1P3 in colon cancer remain unknown.
In this study, we found that SUMO1P3 was highly expressed in colon cancer tissues and cell lines. SUMO1P3 upregulation was positively associated with advanced tumor stage, lymphatic and distant metastases, and microvessel density (MVD) but negatively associated with the prognosis of colon cancer patients. Moreover, functional studies revealed that SUMO1P3 knockdown inhibited the proliferation, migration, invasion, and pro-angiogenesis of colon cancer cells in vitro and in vivo. Collectively, we dissected the oncogenetic functions of SUMO1P3 in colon cancer and found that SUMO1P3 is a promising prognostic marker and therapeutic target for colon cancer patients.
Materials and methods
Patients and clinical samples
Colon cancer tissues and matched adjacent non-tumor tissues were obtained from 120 colon cancer patients who underwent colectomy with a signed informed consent at the First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology (Luoyang, China) between October 2008 and December 2011. None of the patients received preoperative chemotherapy or radiotherapy. The samples were immediately immersed in 4% formaldehyde for immunohistochemistry (IHC) staining or frozen in tubes containing RNAlater preservation liquid and then stored in liquid nitrogen for quantitative real-time PCR (qPCR) assay. All samples were blindly confirmed by two experienced pathologists. Clinical stage was conducted in accordance with the protocols of the American Joint Committee on Cancer Staging System. A follow-up was carried out through outpatient examination or telephone (median follow-up time, 45.5 months; range, 10-60 months). This study was approved by the Ethics Committee of the First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology. The clinico-pathological parameters of the colon cancer patients are summarized in Table 1.
Table 1.
The correlation of SUMO1P3 expression with clinicopathological parameters of colon cancer
| Variables | Clinicopathological parameters | Case No. (n = 120) | USP22 expression | P value | |
|---|---|---|---|---|---|
|
| |||||
| High (n = 80) | Low (n = 40) | ||||
| Gender | Male | 50 | 34 | 16 | 0.382 |
| Female | 70 | 46 | 24 | ||
| Age (years) | < 65 | 45 | 28 | 17 | 0.424 |
| ≥ 65 | 75 | 52 | 23 | ||
| Size (cm) | < 5 | 62 | 42 | 20 | 0.121 |
| ≥ 5 | 58 | 38 | 20 | ||
| Differentiation | Well/Moderate | 66 | 30 | 36 | 0.001* |
| Poor | 54 | 50 | 4 | ||
| AJCC stage | I/II | 63 | 31 | 32 | 0.001* |
| III/IV | 57 | 49 | 8 | ||
| pT stage | T1/T2 | 42 | 24 | 18 | 0.026* |
| T3/T4 | 78 | 56 | 22 | ||
| pN stage | N0 | 60 | 32 | 28 | 0.002* |
| N1/N2 | 60 | 48 | 12 | ||
| pM stage | M0 | 96 | 60 | 36 | < 0.001* |
| M1 | 24 | 20 | 4 | ||
P < 0.05.
Cell culture
The human colon cancer cell lines (HT29, HCT116, SW480, SW620, and LoVo), and the human normal colonic epithelial cell line (NCM460) and human umbilical vein endothelial cells (HUVECs) were purchased from the American Type Culture Collection (Manassas, VA, USA). The authenticity of each cell line was verified by short tandem repeat analysis. All cells were cultured in Roswell Park Memorial Institute 1640 medium (RPMI-1640; Gibco, BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified incubator containing 5% CO2.
Plasmid construction, transfection, and lentivirus infection
Two short hairpin RNA (shRNA)-targeted SUMO1P3 were designed by GenePharma (Shanghai, China) and cloned into pRNAT-U6.1/Neo plasmid (Biovector, Beijing, China) to generate two pRNAT-U6.1/Neo-shSUMO1P3 plasmids (shSUMO1P3-1 and shSUMO1P3-2). The empty pRNAT-U6.1/Neo plasmid was used as the negative control (shNC). All constructs were confirmed by DNA sequencing. To knock down SUMO1P3 expression, SW620 and LoVo cells were transfected with shNC, shSUMO1P3-1, or shSUMO1P3-2 by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. For establishing a cell line with stable silence of SUMO1P3, the plasmids carrying shSUMO1P3-1 or shNC were co-transfected with packaging vectors, namely, pMDLg/pRRE, pRSV-REV, and pCMV-VSVG, to produce pseudotyped lentiviruses designated as Lv-shSUMO1P3-1 and Lv-shNC. The lentiviruses were concentrated by ultracentrifugation and then infected SW620 and LoVo cells. After 2 weeks of screening using 200 μg/mL neomycin (Sigma) for SW620 and LoVo cells transfected with shSUMO1P3-1 or shNC plasmids (in vitro assays) or using 300 μg/mL neomycin for SW620 cells infected with Lv-shSUMO1P3-1 and Lv-shNC (in vivo assays), single clones were collected.
qPCR analysis
Total RNAs were extracted from tissues and shSUMO1P3-1- or shNC-transfected SW620 and LoVo cells using TRIzol reagent (Invitrogen) in accordance with the manufacturer’s protocol. RNA was reverse transcribed into cDNA using the PrimeScript RT reagent Kit (Takara, Dalian, China). qPCR assay was performed on an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, USA) with SYBR Green Real-time PCR Master Mix (Takara). The quantitative value was expressed using the 2-ΔΔCt method. SUMO1P3 expression was normalized against that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primers were as follows: for SUMO1P3, 5’-ACTGGGAATGGAGGAAGA-3’ (forward) and 5’-TGAGAAAG GATTGAGGGAAAAG-3’ (reverse); for GAPDH, 5’-GGGAGCCAAAAGGGTCA T-3’ (forward) and 5’-GAGTCCTTCCACGATACCAA-3’ (reverse).
IHC staining
The human and mouse tissues were soaked in 4% formaldehyde, dehydrated with alcohol, and then embedded in low-melting paraffin. Paraffin blocks were subsequently cut into 4 μm sections and mounted on the polylysine-covered slides (Maixin Biotech., Fuzhou, China). The sections were boiled in citrate buffer (pH 6.0) to retrieve the antigenicity following deparaffinization and dehydration. Next, sections were incubated with anti-human or anti-mouse CD34 antibodies (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C followed by treatment with a ChemMate EnVision Kit containing biotinylated secondary antibody (DAKO, Hamburg, Germany) at 37°C for 20 min. The sections were stained with diaminobenzidine (Maixin Biotech.), counterstained with hematoxylin, and observed and photographed under a light microscope (Leica, Wetzlar, Germany).
Evaluation of tumor microvascular density (MVD)
MVD was assessed by CD34 expression (IHC staining) as previously described [15,16]. In brief, the sections stained with CD34 were scanned at a low magnification (100 ×), and all areas with a high density of highlighted microvessels (“hot spots”) were identified. Five hot spots were randomly selected and observed, and the number of MVD values was calculated by scanning at a total magnification of 200 ×. Any positively stained endothelial cells or clusters that were clearly separated from adjacent microvessels, tumor cells, or other connective tissues were considered as separate countable vessels. The mean score of the five areas was calculated as the level of MVD for each sample.
Cell viability assay
Cell viability was determined by MTT assay. In brief, SW620 and LoVo cells untransfected or transfected with shSUMO1P3-1 or shNC plasmids were seeded into 96-well plates (2 × 103/well) in triplicate. At 24, 48, 72, and 96 h after transfection, the cells were incubated with MTT (5 mg/mL; Sigma) for 4 h at 37°C. MTT solution was then carefully removed, and dimethyl sulfoxide (Sigma) was added to each well. The optical density (OD) of each well was measured at 490 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Cell cycle analysis by flow cytometry
The untransfected and shNC- or shSUMO1P3-1-transfected SW620 and LoVo cells were trypsinized and washed with cold phosphate-buffered saline (PBS). The cells were then fixed with ice-cold 70% ethanol at 4°C overnight. After washing with PBS, the cells were treated with RNAase (Takara) for 30 min at 37°C. Intracellular DNA was labeled with propidium iodide (50 μg/mL; Sigma) at 4°C for 30 min and then analyzed using BD FACSCalibur flow cytometry (BD Bioscience, San Jose, CA, USA). The proportions of cells in the G1, S, and G2/M phases were calculated using the ModFit software (Verity Software House Inc., Topsham, ME, USA).
Colony formation assay
For colony formation assay, the untransfected and shSUMO1P3-1- or shNC-transfected SW620 and LoVo cells were placed in 6-well plates (1 × 103/well) and then maintained in RPMI-1640 medium containing 10% FBS, with each medium replaced every 3 days. After 14 days, the cells were fixed with methanol and stained with 0.5% crystal violet (Sigma). Visible colonies were counted under a fluorescent microscope (Olympus, Tokyo, Japan).
Wound healing assay
The untransfected and shSUMO1P3-1- or shNC-transfected SW620 and LoVo cells were seeded into 6-well plates (5 × 105/well) precoated with 10 μg/mL type I collagen (Sigma) and then cultured until 100% confluence. The head of a 200 μL tip was used to scratch the cells, creating a scraped line (wound). After washing with ice-cold PBS, the cells were cultured in RPMI-1640 medium containing 3% FBS and then incubated for 24 h. Wound closure was viewed under a phase-contrast microscope (Carl-Zeiss) at 0 and 24 h after scratching. The migrated extent of the cells into the wound area was determined using Image Pro Plus software (Media Cybernetics Inc., Bethesda, MD, USA).
Migration and invasion assays
Cell migration and invasion assays were performed using Transwell chambers (8-μm pore size; BD Biosciences). For the migration assay, 3 × 104 SW620 and LoVo cells transfected with shSUMO1P3-1 or shNC plasmids for 48 h were seeded into the upper chamber of the inserts in serum-free medium. For the invasion assay, after transfection with shSUMO1P3-1 or shNC plasmids for 48 h, SW620 and LoVo cells (1 × 105) in a serum-free medium were seeded into the upper chamber of the inserts precoated with Matrigel (BD Biosciences). A medium containing 10% FBS was added to the lower chamber for chemotaxis. After 24 h incubation, the cells remaining on the upper membrane were removed. The cells that migrated or invaded through the membrane were stained with 0.1% crystal violet, imaged, and counted using a fluorescent microscope (Olympus).
Tube formation assay
Tube formation assay was carried out to evaluate angiogenesis. In brief, 48-well plates were coated with Matrigel (BD Biosciences; 200 µL/well) and then allowed to polymerize at 37°C for 30 min. HUVECs (1 × 105) were cultured in conditioned media from untransfected and shSUMO1P3-1- or shNC-transfected SW620 and LoVo cells. After 24 h of incubation, tube formation was assessed with a phase-contrast microscope (Carl-Zeiss). The relative tubule length, number of tubule, and number of branch points were measured using Image Pro Plus software (Media Cybernetics Inc.).
Enzyme-linked immunosorbent assay (ELISA)
The secretion of vascular endothelial growth factor A (VEGFA) by SW620 and LoVo cells was evaluated using an ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA) in accordance with the manufacturer’s protocol. In brief, the untransfected and shSUMO1P3-1- or NC-transfected SW620 and LoVo cells were seeded into 12-well plates and cultured for 24 h. The supernatants were collected for VEGFA measurement. Final results were expressed as ng/μL.
Western blot analysis
Total protein was obtained from fresh tissues with RIPA buffer (Beyotime, Shanghai, China) and then centrifuged at 14,000 g and 4°C for 10 min. Proteins were separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). After blocking in 5% nonfat milk for 2 h at room temperature, the membranes were incubated with primary antibodies, including cyclin D1, E-cadherin, Vimentin, VEGFA, and β-actin (all from Cell Signaling Technology), at 4°C overnight. Next, the membranes were incubated with horseradish-peroxidase-conjugated secondary antibodies (Sigma) at 37°C for 1 h. Protein bands were visualized and detected using the enhanced chemiluminescence Detection System (Thermo Fisher Scientific, Waltham, MA, USA).
In vivo tumor growth and metastasis assay
Five-week-old male severe combined immune deficiency (SCID) mice (weighing 22-24 g) were obtained from the Institute of Zoology, Chinese Academy of Sciences (Beijing, China) and maintained under specific pathogen-free conditions at 22-24°C with a regular 12 h day/night cycle. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of the First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology.
For subcutaneous xenograft assay, 5 × 105 SW620 cells infected with Lv-shNC or Lv-shSUMO1P3-1 were subcutaneously inoculated in the flanks of the mice (n = 10 mice/group). A caliper was used to examine the tumor volumes once a week and the volumes were calculated as follows: tumor volume = width2 × length/2. At 6 weeks post-inoculation, the mice were sacrificed by euthanasia. The tumors were then removed, photographed, and weighed. Half of the tumor tissues were stored at -80°C for qPCR and Western blot analyses, and the remaining tissues were immediately soaked in 4% formaldehyde and embedded in paraffin for IHC staining.
Liver metastasis of colon cancer was performed as previously described [17]. In brief, SCID mice (n = 10 mice/group) were anesthetized with 4% chloral hydrate (10 mL/kg), and a small left abdominal incision was made under sterile conditions. After the spleen was exteriorized, 1 × 106 SW620 cells expressing shNC or shSUMO1P3 were injected into the spleen using a sterile tuberculin syringe with a 27-gauge needle. At 10 min after the injection, splenectomy was performed. The abdominal incision was closed with nylon sutures. After 3 weeks, the mice were euthanized and liver metastases were examined. Livers with visible tumor colonies were collected and sectioned for hematoxylin/eosin staining. The total number of metastases per liver section was calculated.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard deviation (SD). The difference between groups was analyzed using Student’s t-test or one-way ANOVA and the Bonferroni post-hoc test. The frequencies were evaluated with the Chi-squared test. Survival curves were plotted using the Kaplan-Meier method and measured using the log rank test. Statistical significance was considered at P < 0.05.
Results
Upregulation of SUMO1P3 is positively associated with advanced histological grade, metastasis, angiogenesis, and poor prognosis of colon cancer patients
qPCR analysis was performed to evaluate SUMO1P3 expression in 120 colon cancer tissues and the corresponding non-tumor counterparts. Results showed that SUMO1P3 expression was significantly increased in colon cancer tissues compared with the non-tumor samples (Figure 1A). The correlation of SUMO1P3 enhancement with clinicopathological features of colon cancer patients was then explored. Results revealed a positive association between SUMO1P3 upregulation and advanced histological grade, poor differentiation, and lymphatic and distant metastases (Figure 1B and 1C; Table 1). However, no correlation was found between SUMO1P3 elevation and patient gender, age, and tumor size (Table 1). To investigate the association of SUMO1P3 expression with angiogenesis and prognosis of colon cancer patients, the colon cancer samples were divided into two groups: SUMO1P3 high-expression group and SUMO1P3 low-expression group. As shown in Figure 1D, intensive MVD was observed in the SUMO1P3 high-expression samples. In addition, the patients with high levels of SUMO1P3 had shorter survival rates than those with low levels of SUMO1P3 (Figure 1E). These findings indicate that SUMO1P3 augment plays a key role in the development and progression of colon cancer and could serve as a useful prognosis marker for colon cancer.
Figure 1.
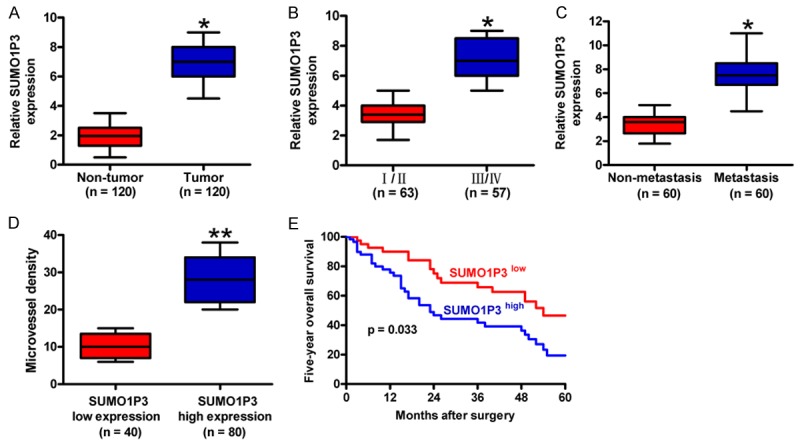
The expression and clinical significance of SUMO1P3 in colon cancer. (A) SUMO1P3 expression was measured by qPCR assay and normalized to GAPDH in 120 pairs of colon cancer tissues and corresponding non-tumor tissues. (B and C) Comparisons of SUMO1P3 levels in colon cancer patients at different stages (I/II stage, n = 63; III/IV stage, n = 57) (B) and with (n = 60) or without (n = 60) lymphatic metastasis (C). (D) Association between SUMO1P3 expression and MVD determined by CD34 expression. CD34 expression was evaluated by IHC assay in colon cancer tissues from SUMO1P3 high-expression (n = 80) and low-expression (n = 40) groups. Magnification: 100 ×. (E) Five-year survival rate of colon cancer patients with high expression (n = 80) and low expression (n = 40) of SUMO1P3. All data are represented as the mean ± SD of three replicates. *P < 0.05, **P < 0.01.
Knockdown of SUMO1P3 reduces colon cancer cell viability and proliferation in vitro
Next, we measured SUMO1P3 expression in five colon cancer cell lines (HT29, HCT116, SW480, SW620, and LoVo) and normal colonic epithelial cells (NCM460) by using qPCR assay. SUMO1P3 was highly expressed in the five colon cancer cell lines compared with NCM460 cells (Figure 2A). SW620 and LoVo were selected for the subsequent studies because they had the highest SUMO1P3 levels among the cell lines. To address the functional role of SUMO1P3 in colon cell proliferation, shSUMO1P3-1, shSUMO1P3-2, and shNC plasmids were transfected into SW620 and LoVo cells. As shown in Figure 2B, shSUMO1P3 transfection significantly reduced the SUMO1P3 levels in SW620 and LoVo cells. ShSUMO1P3-1 treatment showed more effective inhibition of SUMO1P3 expression, which was selected for the following investigations. MTT assay showed a notable reduction of cell viability in the SUMO1P3-depleted SW620 and LoVo cells (Figure 2C). Flow cytometric analysis revealed that SUMO1P3 knockdown induced cell cycle arrest of SW620 and LoVo cells, reflecting a significant increase in the G1 stage and a marked decrease in the S phase (Figure 2D). Moreover, fewer and smaller colonies were observed in the shSUMO1P3-1-transfected SW620 and LoVo cells than in those transfected with shNC (Figure 2E and 2F). These data suggest that SUMO1P3 silencing reduces the viability and proliferation of colon cancer cells in vitro.
Figure 2.
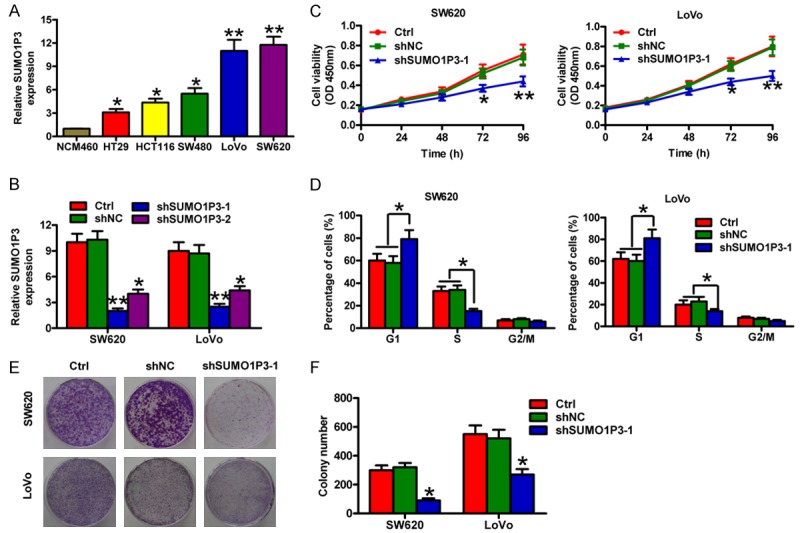
SUMO1P3 silencing suppressed the viability and proliferation of colon cancer cells in vitro. (A) SUMO1P3 expression was measured by qPCR assay and normalized to GAPDH expression in five colon cancer cell lines (HT29, HCT116, SW480, SW620, and LoVo) and the normal colonic epithelial cells NCM460. (B) Inhibitory efficiency of shSUMO1P3-1 and shSUMO1P3-2 on SUMO1P3 expression in SW620 and LoVo cells was evaluated by qPCR assay. (C-F) SW620 and LoVo cells were untransfected (control) and transfected with shSUMO1P3-1 or shNC plasmids. (C) MTT assay was performed to determine cell viability at 24, 48, 72, and 96 h after transfection. (D) Flow cytometry analysis for cell cycle of SW620 and LoVo cells. The cell percentages in the G1, S, and G2/M phases were calculated. (E) Representative colony formation images of the cells. (F) Colonies were counted in (E). Data are represented as the mean ± SD of three replicates. *P < 0.05, **P < 0.01 compared with Ctrl or shNC group. Ctrl: control; shNC: negative control.
SUMO1P3 depletion inhibits the migration and invasion of colon cancer cells in vitro
To determine the roles of SUMO1P3 in the motility of colon cancer cells, wound healing and Transwell assays were performed in SW620 and LoVo cells. Wound healing assays showed that the migration of the shSUMO1P3-1-transfected SW620 and LoVo cells was obviously inhibited compared with that of the shNC-transfected or control cells (Figure 3A and 3B). Similarly, Transwell assays demonstrated that the number of migrated SW620 and LoVo cells was significantly reduced in the shSUMO1P3-1-transfected group (Figure 3C and 3D). Also, the invasion of SW620 and LoVo cells was markedly suppressed by SUMO1P3 silencing (Figure 3E and 3F). These data demonstrate that SUMO1P3 knockdown inhibits the motility of colon cancer cells in vitro.
Figure 3.
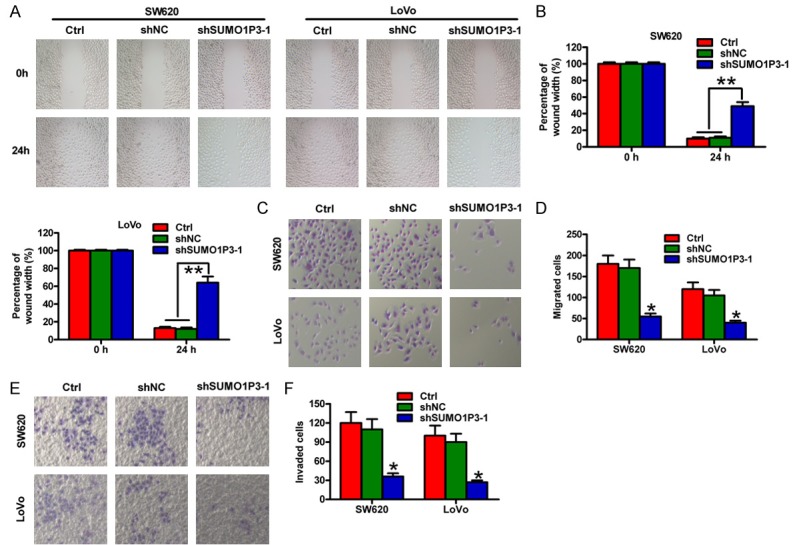
SUMO1P3 knockdown reduced the migratory and invasive abilities of colon cancer cells in vitro. SW620 and LoVo cells were untransfected (control) and transfected with shSUMO1P3-1 or shNC plasmids. (A) Wound healing assay of SW620 and LoVo cells. Scale bar: 10 μm. (B) Quantification of the wound width in (A) at 0 and 24 h after transfection. (C and E) Transwell assays of the migration (C) and invasion (E) of SW620 and LoVo cells. (D and F) The numbers of migrated (D) and invaded (F) cells were calculated in (C and E) at 24 h after transfection. Data are represented as the mean ± SD of three replicates. *P < 0.05, **P < 0.01 compared with the Ctrl or shNC group. Ctrl: control; shNC: negative control.
SUMO1P3 silencing suppresses colon cancer cell-induced angiogenesis in vitro
Matrigel tube formation assay was conducted to evaluate the effects of colon cancer cells on angiogenesis. The suppressive effects of tube formation were observed on HUVECs treated with the conditioned medium from SW620 and LoVo cells transfected with the shSUMO1P3-1 plasmid (Figure 4A). The tube length (Figure 4B), number of tubules and branch points (Figure 4C and 4D) were significantly reduced in the conditioned medium-cultured HUVECs. Furthermore, the secretion of pro-angiogenic growth factor VEGFA was repressed by SUMO1P3 knockdown in SW620 and LoVo cells (Figure 4E). These results show that SUMO1P3 silencing attenuates the pro-angiogenesis effects of colon cancer cells in vitro.
Figure 4.
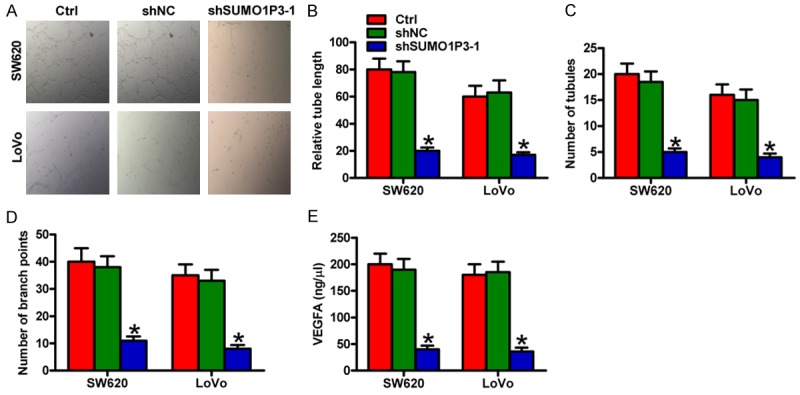
SUMO1P3 silencing inhibited the pro-angiogenesis ability of colon cancer cells in vitro. SW620 and LoVo cells were untransfected (control) and transfected with shSUMO1P3-1 or shNC plasmids. (A) Tube formation assay was performed to evaluate the effect of conditioned media from SW620 and LoVo cells with different treatments on the angiogenesis of HUVECs. (B-D) Relative tube length (B) and number of tubules and branch points (C and D) were counted and calculated. (E) VEGFA levels were determined by using ELISA assays. Data are represented as the mean ± SD of three replicates. *P < 0.05 compared with the Ctrl or shNC group. Ctrl: control; shNC: negative control.
SUMO1P3 knockdown suppresses the growth, metastasis and pro-angiogenesis of colon cancer in vivo
The roles of SUMO1P3 in colon cancer cells in vitro were further verified using in vivo mouse models. SUMO1P3-silenced SW620 cells were subcutaneously injected into the flank of SCID mice. Compared with control or Lv-shNC group, the SUMO1P3-depleted group revealed significant reductions in tumor volume (Figure 5A), tumor size and weight (Figure 5B-D). IHC analysis of CD34 expression was performed to detect the MVD in xenograft tumors. Results showed that SUMO1P3 knockdown significantly reduced MVD (Figure 5E). To explore the effects of SUMO1P3 on liver metastasis of colon cancer cell, SUMO1P3-silenced SW620 cells were injected into the spleen of SCID mice. As shown in Figure 5F, the rate of liver metastasis was lower in SUMO1P3 knockdown group than in Lv-shNC and control groups. Molecular analysis of tumor tissues showed that the levels of SUMO1P3, cyclin D1, Vimentin, and VEGFA were markedly decreased but E-cadherin was increased in the SUMO1P3-silenced group (Figure 5G, 5H and Supplemental Figures 1, 2). These data indicate that SUMO1P3 depletion represses the growth, metastasis and pro-angiogenesis of colon cancer cells in vivo.
Figure 5.
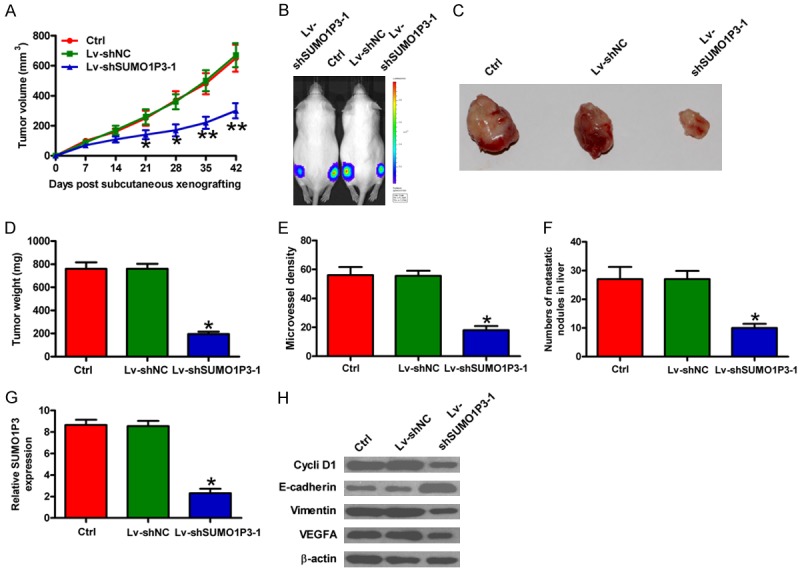
SUMO1P3 depletion hindered the tumorigenesis, metastasis, and pro-angiogenesis of colon cancer cells in vivo. Male 5-week-old SCID mice received uninfected (control) and Lv-shSUMO1P3-1- or Lv-shNC-infected SW620 cells through a subcutaneous injection into the hind flanks or through spleen injection. (A) The volumes in subcutaneous xenografts were measured and calculated once a week for 6 weeks. (B and C) Representative tumor photos of each group. (D) Tumor weights were measured at 6 weeks post-implantation. (E) IHC analysis of CD34 expression in tumor tissues. MVD was assessed by CD34 expression. (F) Quantification of microscopic nodules in the livers of each group at 3 weeks after spleen injection. (F and G) SUMO1P3 levels (G) and protein expression (H) of cyclin D1, E-cadherin, Vimentin, and VEGFA in xenografted tumor tissues of each group were detected by qPCR and Western blot assays, respectively. Data are represented as the mean ± SD of three replicates. *P < 0.05, **P < 0.01 compared with the Ctrl or Lv-shNC group. Ctrl: control; Lv-shNC: negative control.
Discussion
Colon cancer is a common malignancy worldwide. Substantial researches are needed to elucidate the molecular basis of colon cancer and improve clinical outputs. However, only a few reliable prognostic markers and therapeutic approaches of colon cancer are available [18]. Thus, identifying novel useful prognostic biomarkers and treatment methods of colon cancer is essential and urgent.
Aberrant genetic or epigenetic alterations of oncogenes and tumor suppressors have occurred in colon cancer [19]. A large number of lncRNAs contribute to the development and progression of colon cancer [20]. Among those are CCAT1 [9], BLACAT1 [10], HOTAIR [11], and ATB [12]. LncRNA SUMO1P3 is upregulated in gastric cancer and used as a diagnostic biomarker of gastric cancer [13]. Moreover, SUMO1P3 enhancement predicts poor prognosis and promotes the growth and metastasis of bladder cancer [14]. However, the expression and biological roles of SUMO1P3 in colon cancer are not elucidated. In this study, the SUMO1P3 level in colon cancer tissues was significantly higher than that in non-tumor tissues. SUMO1P3 elevation in colon cancer patients positively correlated with tumor stage, metastasis, and angiogenesis, but negatively associated with poor prognosis. These results suggest that SUMO1P3 plays an important role in colon cancer progression.
Uncontrolled cell proliferation and sustained cell cycle progression are the significant hallmarks of human cancers [21]. Previous studies demonstrated that several lncRNAs play crucial roles in colon cancer by regulating cell proliferation and cell cycle progression [9,10,22]. Su et al. [10] showed that G1/G0 phase was increased in both HCT116 and SW480 cells when BLACAT1 was repressed. Cyclin D1 is a crucial mediator of G1 to S progression, and its upregulation results in the rapid growth of colon cancer cells [23]. In the present study, we found that lncRNA SUMO1P3 knocked down reduced colon cancer cell proliferation and G1 phase arrest in vitro and significantly attenuated tumor growth in vivo. Mechanisticly, the expression of cyclin D1 was decreased by SUMO1P3 depletion in the tumor tissues of xenograft mouse models. These findings indicate that SUMO1P3 regulates the proliferation of colon cancer cells.
Tumor spreading and metastasis are the aggressive behaviors of colon cancers. Several lncRNAs promote the migratory and invasive abilities of colon cancer cells by promoting EMT [11,12]. EMT is a biologic process that allows a polarized epithelial cell, which normally interacts with basement membrane via its basal surface, to undergo multiple biochemical changes that enable it to assume a mesenchymal cell phenotype, which includes enhanced migratory and invasive capacities, as well as elevated resistance to apoptosis [24]. When EMT occurs, the epithelial markers, such as E-cadherin, and α-catenin are downregulated, while the mesenchymal markers, such as N-cadherin, Vimentin, slug, snail, and β-catenin are upregulated [25]. Wu et al. [11] demonstrated that HOTAIR silencing increased E-cadherin and decreased Vimentin level in SW480 and HT29 cells. Knockdown of ATB enhanced expression of E-cadherin and ZO-1, and reduced levels of ZEB1 and N-cadherin in SW620 cells [12]. In this study, lncRNA SUMO1P3 knockdown inhibited the migration and invasion of SW620 and LoVo cells. Low-frequency and small liver nodules were found in SUMO1P3-silenced tumor tissues. Moreover, SUMO1P3 depletion led to the downregulation of Vimentin and upregulation of E-cadherin in vivo. These results suggest that SUMO1P3 facilitates colon cancer metastasis by facilitating EMT.
Angiogenesis is crucial for tumor progression because tumor growth, invasion, and metastasis are angiogenesis dependent [26]. Angiogenesis is a complex procedure by which capillaries sprout from vessels and allow tumor cells to metastasize to distant sites from primary lesions [27]. The angiogenic phenotype is characterized by an increase in the production of pro-angiogenic molecules, such as VEGF, which is the key regulator of tumor angiogenesis [28]. Among the six members of VEGF, VEGFA is the predominant factor that promotes the formation and growth of new vessels [29]. The present study investigated the role of SUMO1P3 in the regulation of the pro-angiogenetic ability of colon cancer cells. SUMO1P3 knockdown repressed colon cancer cell-induced VEGFA release and angiogenesis in vitro. SUMO1P3 silencing reduced VEGFA expression and MVD in xenograft tumors. These findings indicate that SUMO1P3 promotes colon cancer-induced angiogenesis.
In summary, SUMO1P3 was highly expressed in colon cancer tissues and cell lines. Upregulation of SUMO1P3 positively correlated with the advanced tumor stage, lymphatic and distant metastases, angiogenesis, and poor prognosis of colon cancer patients. SUMO1P3 knockdown reduced the proliferative, migratory, invasive, and pro-angiogenic abilities of colon cancer cells in vitro and hindered tumor growth, liver metastasis, and vascularization in vivo. Overall, these findings illuminate that SUMO1P3 contributes to growth and metastasis of colon cancer and could be used as a potential prognostic marker and therapeutic target for colon cancer patients.
Acknowledgements
This study was partly supported by Scientific Research Fund of Henan Provincial Education Department (No.142300410329).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Lee YC, Lee YL, Chuang JP, Lee JC. Differences in survival between colon and rectal cancer from SEER data. PLoS One. 2013;8:e78709. doi: 10.1371/journal.pone.0078709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Kita Y, Yonemori K, Osako Y, Baba K, Mori S, Maemura K, Natsugoe S. Noncoding RNA and colorectal cancer: its epigenetic role. J Hum Genet. 2017;62:41–47. doi: 10.1038/jhg.2016.66. [DOI] [PubMed] [Google Scholar]
- 8.Saus E, Brunet-Vega A, Iraola-Guzman S, Pegueroles C, Gabaldon T, Pericay C. Long Non-Coding RNAs as potential novel prognostic biomarkers in colorectal cancer. Front Genet. 2016;7:54. doi: 10.3389/fgene.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He X, Tan X, Wang X, Jin H, Liu L, Ma L, Yu H, Fan Z. C-Myc-activated long noncoding RNA CCAT1 promotes colon cancer cell proliferation and invasion. Tumour Biol. 2014;35:12181–12188. doi: 10.1007/s13277-014-2526-4. [DOI] [PubMed] [Google Scholar]
- 10.Su J, Zhang E, Han L, Yin D, Liu Z, He X, Zhang Y, Lin F, Lin Q, Mao P, Mao W, Shen D. Long noncoding RNA BLACAT1 indicates a poor prognosis of colorectal cancer and affects cell proliferation by epigenetically silencing of p15. Cell Death Dis. 2017;8:e2665. doi: 10.1038/cddis.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu ZH, Wang XL, Tang HM, Jiang T, Chen J, Lu S, Qiu GQ, Peng ZH, Yan DW. Long noncoding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol Rep. 2014;32:395–402. doi: 10.3892/or.2014.3186. [DOI] [PubMed] [Google Scholar]
- 12.Yue B, Qiu S, Zhao S, Liu C, Zhang D, Yu F, Peng Z, Yan D. LncRNA-ATB mediated E-cadherin repression promotes the progression of colon cancer and predicts poor prognosis. J Gastroenterol Hepatol. 2016;31:595–603. doi: 10.1111/jgh.13206. [DOI] [PubMed] [Google Scholar]
- 13.Mei D, Song H, Wang K, Lou Y, Sun W, Liu Z, Ding X, Guo J. Up-regulation of SUMO1 pseudogene 3 (SUMO1P3) in gastric cancer and its clinical association. Med Oncol. 2013;30:709. doi: 10.1007/s12032-013-0709-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhan Y, Liu Y, Wang C, Lin J, Chen M, Chen X, Zhuang C, Liu L, Xu W, Zhou Q, Sun X, Zhang Q, Zhao G, Huang W. Increased expression of SUMO1P3 predicts poor prognosis and promotes tumor growth and metastasis in bladder cancer. Oncotarget. 2016;7:16038–16048. doi: 10.18632/oncotarget.6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preusser M, Heinzl H, Gelpi E, Schonegger K, Haberler C, Birner P, Marosi C, Hegi M, Gorlia T, Hainfellner JA. Histopathologic assessment of hot-spot microvessel density and vascular patterns in glioblastoma: poor observer agreement limits clinical utility as prognostic factors: a translational research project of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Cancer. 2006;107:162–170. doi: 10.1002/cncr.21973. [DOI] [PubMed] [Google Scholar]
- 16.Lv J, Xia Q, Wang J, Shen Q, Zhang J, Zhou X. EphB4 promotes the proliferation, invasion, and angiogenesis of human colorectal cancer. Exp Mol Pathol. 2016;100:402–408. doi: 10.1016/j.yexmp.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Hirakawa E, Mori S, Hamada Y, Kawaguchi N, Matsuura N. Cleavage of carcinoembryonic antigen induces metastatic potential in colorectal carcinoma. Biochem Biophys Res Commun. 2005;333:223–229. doi: 10.1016/j.bbrc.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 18.Das V, Kalita J, Pal M. Predictive and prognostic biomarkers in colorectal cancer: a systematic review of recent advances and challenges. Biomed Pharmacother. 2017;87:8–19. doi: 10.1016/j.biopha.2016.12.064. [DOI] [PubMed] [Google Scholar]
- 19.Grady WM, Markowitz SD. Genetic and epigenetic alterations in colon cancer. Annu Rev Genomics Hum Genet. 2002;3:101–128. doi: 10.1146/annurev.genom.3.022502.103043. [DOI] [PubMed] [Google Scholar]
- 20.Okugawa Y, Grady WM, Goel A. Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology. 2015;149:1204–1225. e1212. doi: 10.1053/j.gastro.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Thorenoor N, Faltejskova-Vychytilova P, Hombach S, Mlcochova J, Kretz M, Svoboda M, Slaby O. Long non-coding RNA ZFAS1 interacts with CDK1 and is involved in p53-dependent cell cycle control and apoptosis in colorectal cancer. Oncotarget. 2016;7:622–637. doi: 10.18632/oncotarget.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 24.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 27.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Hofstaetter JG, Saad FA, Samuel RE, Wunderlich L, Choi YH, Glimcher MJ. Differential expression of VEGF isoforms and receptors in knee joint menisci under systemic hypoxia. Biochem Biophys Res Commun. 2004;324:667–672. doi: 10.1016/j.bbrc.2004.09.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


