Abstract
Most degenerative diseases are caused by free radicals. Antioxidin-RL peptide is a free radical scavenger found in the skin of plateau frog Odorrana livida, which is more stable than vitamin C as it resists light-induced degradation. However, whether and how antioxidin-RL protects cells from oxidative stress was not clear. Here we addressed this issue, and in addition, we designed a series of antioxidin cognates by adding tyrosine residues to enhance free radical-binding capability. We performed free radical-clearing assays in solution to screen the mutants, and found a mutant antioxidin-2 that was as stable as antioxidin-RL and cleared free radical faster. By using PC-12 cells as a model, we demonstrated that both antioxidin-2 and antioxidin-RL inhibited the accumulation of intracellular free radicals triggered by H2O2, reduced mitochondria membrane potential dissipation, maintained mitochondrial morphology, and decreased the expression of dynamin-related protein-1 in mitochondria, with antioxidin-RL more effective. Antioxidin-RL also attenuated the changes in SOD1 and GPx1 expression induced by H2O2. These findings provide insight into the anti-oxidative mechanisms of antioxidin-RL and its derivatives, which will provide rational basis for the development of more effective antioxidants to cure diseases.
Keywords: Antioxidin-RL, oxidative injury, PC12 cell, metabolic activity, mitochondria
Introduction
Most of degenerative diseases in human are caused by free radicals. For example, neuronal degenerative diseases such as Parkinson’s disease and Alzheimer’s diseases are closely associated with free radicals [1-3], mainly reactive oxygen species (ROS), cueing for the therapy by relieving neurons from ROS insult. However, most of antioxidants such as vitamin C are unstable. Therefore, it is important to develop antioxidants that are more stable and efficient than those commonly used.
It was recently found that the skin of a type of frogs, Odorranalivida, had extraordinarily low level of ROS despite high exposure to UV irradiation in the plateau environment, which was attributed to the peptides secreted in the skin, and one of the most efficient peptide was antioxidin-RL (AR) [4-6]. Intriguingly, as elucidated by the study on its ROS clearing mechanism, unlike ROS scavenging enzymes such as superoxide dismutase (SOD) [7], AR functions directly as a reactant instead of a catalyst to reduce ROS and not only works much faster than the ordinary antioxidant L-ascorbic acid (Vc) but also boasts a large free radical turnover because one molecule of AR is capable of reducing 6-7 molecules of 2,2’-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS)+ free radicals and binding additionally 4-6 molecules of unreduced ABTS+ free radicals.
To explore whether AR mutants could result in more potent antioxidant, we designed a series of peptides based on AR structure, and determined their effects, under ROS insult circumstance, on PC12 cells, a cell type often being used to study neuron functions and the underlying mechanism. First, a batch of antioxidin cognates was derived from rationally designed amino acid sequence in reference to the in vitro ROS clearing biochemical mechanism, providing a pool of peptide candidates for in vitro ROS clearing activity assay and thus identifying the promising mutants. Secondly, the effects of the peptides on cell metabolic activity and intracellular ROS level following H2O2 treatment were evaluated. Thirdly, since mitochondria is the most sensitive organelle in cells to oxidative stress and crucial for cell metabolic activity, mitochondria membrane potential and the expression of dynamin-related protein 1 (Drp1) were evaluated. Lastly, the expression of oxidative stress responsive genes was examined with and without the peptides protection against ROS insult to clarify the anti-oxidative mechanism.
Materials and methods
Peptide synthesis
AR, A2 and other mutant antioxidant peptides are chemically synthesized by GL Biochem (Shanghai) Ltd.
Cell culture
PC12 were cultured in high glucose DMEM (Thermofisher Scientific) supplemented with 10% fetal bovine serum (FBS) (Thermofisher Scientific) and 1% penicillin/streptomycin (Thermofisher Scientific) in a humidified incubator with 5% CO2 at 37°C.
In vitro free radical scavenging evaluation
The ability of AR and its mutants to reduce free radical was tested with ABTS+ free radical, the reductive product of which presented a maximum absorbance at 340 nm whereby free radical scavenging ability could be measured by calculating the speed of OD340 increase. ABTS+ radical stock solution was made by addition of potassium persulfate to 7 mM ABTS (Sigma) water solution to the final concentration of 3 mM and incubation at room temperature for at least 5 hours. Shortly before the test, the stock solution was diluted by 75 folds with phosphate buffered saline (PBS). The in vitro reaction was started by the addition of 3 μM AR, its mutants or Vc. OD340 change was monitored by a microplate reader (BioTek). Each point represents the mean of triplicates ± SD.
Cell metabolic activity evaluation
Cell metabolic activity was measured by using CCK-8 kit (Beyotime Biotechnology). The assay uses sodium-4-(3-(2-Methoxy-4-Nitrophenyl)-2-(4-Nitrophenyl)-2H-Tetrazol-3-ium-5-yl) Benzene-1,3-disulfonate (WST-8) that works in the same principle as 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide (MTT) [8]. Briefly, cells were incubated in 96 well plate at the density of 5000/well until 80% confluent, cells were treated with 10-50 μM AR, A2 and Vc for 12 hours, then 200 μM H2O2 (Sigma) was used to treat cells. After 12 hours of H2O2 treatment, cells were washed with PBS (Thermofisher Scientific) thrice before the addition of 10% (v/v) CCK-8 solution in 100 μL DMEM to cells in each well. Cells were incubated for another 1 hour, and the samples were analyzed by using a microplate reader at 450 nm. All experiments were carried out in triplicates and the data were represented as mean ± SD.
Intracellular ROS evaluation
ROS accumulated in PC12 cells were tested quantitatively with 2’,7’-Dichlorofluorescin diacetate (DCFH-DA) probe (Sigma). DCFH-DA molecule is not fluorescent; it is cell permeable and can be hydrolyzed to DCFH by intracellular esterase. Once oxidized by ROS in cells, DCFH is transformed to DCF, emitting fluorescence at 525 nm when excited under 488 nm so that intracellular ROS level is reflected indirectly by fluorescence intensity [9]. Briefly, cells were seeded in 96 well plate to 80% confluent and pretreated at 37°C with 10-50 μM AR, A2 or Vc for 12 hours, and 200 μM H2O2 was added for another 12 hours of culture. Cells were then washed with serum-free DMEM twice and incubated with 10 μM DCFH-DA at 37°C for 30 min. After DCFH-DA probe loading, cells were washed with serum free DMEM twice, and the fluorescent emission at 525 nm was examined by a microplate reader with the excitation at 488 nm. All experiments were carried out in triplicates and the data were represented as mean ± SD. The ROS level was shown as the percentage with respect to the control.
Mitochondrial membrane potential (MMP) evaluation
MMP evaluation was conducted with JC-10 Kit (Yeasen). JC-10 is a soluble fluorescent probe and is able to permeate through inner mitochondria membrane and gather in mitochondrial matrix as either monomer at low concentration or dimer at high concentration. The concentration of JC-10 molecules in mitochondria is positively correlated to MMP, consequently the MMP in uninjured cells tends to amass high concentration of JC-10, mostly resulting in dimers, while with MMP declining, JC-10 concentration in mitochondria decreases so that a part of the dimers convert to monomers reversibly. The dimer emits red fluorescence at 590 nm and the monomer emits green fluorescence at 525 nm. Taken together, the ratio between the intensity of 590 nm to 525 nm (Emission at 590 nm/Emission at 525 nm) represents MMP. The final result was the relative JC-10 ratio calculated as the percentage of the JC-10 ratio of the untreated cells [10]. 80% confluent PC12 cells were incubated in 96 well plate with or without 10-50 μM AR, A2 or Vc for 12 hours, followed by the treatment of 200 μM H2O2 for 12 more hours. After these treatments, culture media was aspirated from the plate and 100 μL of 10 μM JC-10 work solution was applied to each well of cells and incubated for 25 min. The samples were tested with a microplate reader (BioTek) at 490/525 and 540/590 nm (Excitation/Emission). All experiments were carried out in triplicates and the data were represented as mean ± SD. The ratio of Emi590 to Emi525 reflected the MMP and the final results were shown as the percentage of the control.
Mitochondrial morphology imaging
Cells were cultured to 80% confluence and then treated with or without 50 μM AR, A2 or L-ascorbic acid (Vc) respectively for 12 hours. After being treated with 200 μM H2O2 for 12 hours, cells were washed with HBSS and incubated with 0.2 μM MitoTracker Green (the excitation and emission wavelength of MitoTracker Green are 490 and 516 nm) for 25 min. Then cells were washed with HBSS twice and observed with confocal microscope (Leica) at 40 x objective.
Drp1 expression test
PC12 cells were treated with or without 10 μM of AR, A2 and Vc for 12 hours, and 200 μM H2O2 was added for another 12 hours. Proteins were isolated from the cells with Whole Cell Extraction Kit (Chemicon). The extracted proteins were quantified by BCA Protein Assay Kit (Novagen). For Western blotting analysis, 12% SDS-polyacrylamide gel was used for electrophoresis, and then protein samples were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). The membrane was blocked with 5% fat free milk in 1 X TBST buffer (10 x TBS contains 88 g NaCl, 24 g Tris Base and 13 ml HCl in 1 L dH2O, 1 X TBST was made up of 1 L 1 x TBS and 1 mL Tween-20) and incubated with rabbit monoclonal antibody Drp1 (Novus Biological Inc) and β-actin (Sigma) (1:1000 dilution) at 4°C overnight. After incubation with the primary antibodies, the membrane was incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (R&D) for 1 hour at room temperature. Chemiluminescent reaction was performed with Chemiluminescent HRP Substrate Kit (Millipore). The image was presented and analyzed by Chemi Doc XRS imaging system (BIO-RAD). β-actin was used as the internal reference for normalization.
Real-time quantitative PCR
PC12 cells were treated with or without 10 μM of AR, A2 and Vc for 12 hours, and 200 μM H2O2 was added to the culture for 12 hours. Total RNA from cells was isolated with TRIzol® Reagent kit (Invitrogen) following the manufacturer’s protocol. First strand cDNA was synthesized with the template of the extracted total RNA through TIANScript cDNA kit (Tiangen). About 100 ng of cDNA for each type of treatment was used for quantification with SuperReal Premix Plus SYBR Green kit (Tiangen) and ABI 7900HT Real Time PCR system. All the samples were run in triplicates in a 384 well plate. qPCR primers are listed below: GAPDH (forward)-5’ GTGCCAGCC TCGTCTCATAG 3’, GAPDH (reverse)-5’ GAACTTGCCGTGGGTAGAGT 3’, SOD1 (forward)-5’ CCACGAGAAACAAGATGACT 3’, SOD1 (reverse)-5’ GACTCAGACCACATAGGGAA 3’, GPx1 (forward)-5’ ATAGAAGCCCTGCTGTCCAA 3’, GPx1 (reverse)-5’ ATCACCAAGCCCAGATACCA 3’. Real-Time PCR analyses were carried out using the 2(-Delta Delta C(T)) method (2-ΔΔCt). The data were analyzed with the 2(-Delta Delta C(T)) method (2-ΔΔCt). All experiments were carried out in triplicates and the data were represented as mean ± SD.
Statistical analysis
The results were displayed as means ± standard deviation. SPSS 17.0 was used to analyze the data in one-way analysis of variance (ANOVA) and LSD post hoc test. P<0.05 was defined statistically significant. Besides, two-factor analyses of variance followed by LSD post hoc test were used for comparison among efficacy of Vc, AR and A2 on H2O2 injured PC12 cells.
Results and discussion
A2 scavenges ABTS+ free radical faster than the native counterpart AR
We designed a series of mutants based on the conserved and variant domains of AR peptide. The amino acid sequence alignment among AR and its homologs antioxidin-RP1 and antioxidin-RP2 highlighted a group of conserved amino acid residues (Figure 1A), among which tyrosine 6 and 12 (Y6 and Y12) and cysteine 10 (C10) were reported to play a key role in free radical reduction with Y6 and Y12 binding free radicals and C10 forming anion first and then donating an electron to reduce the bound free radicals. The formation of cysteine anion depends on the basic residue at the position 8 separated from C10 by proline 9 (P9). The deprotonation of the thiol group in C10 is mediated by the positive charge on the position 8 basic amino acid which was arginine (R) in AR and lysine (K) in both antioxidin-RP1 and antioxidin-RP2 [4].
Figure 1.
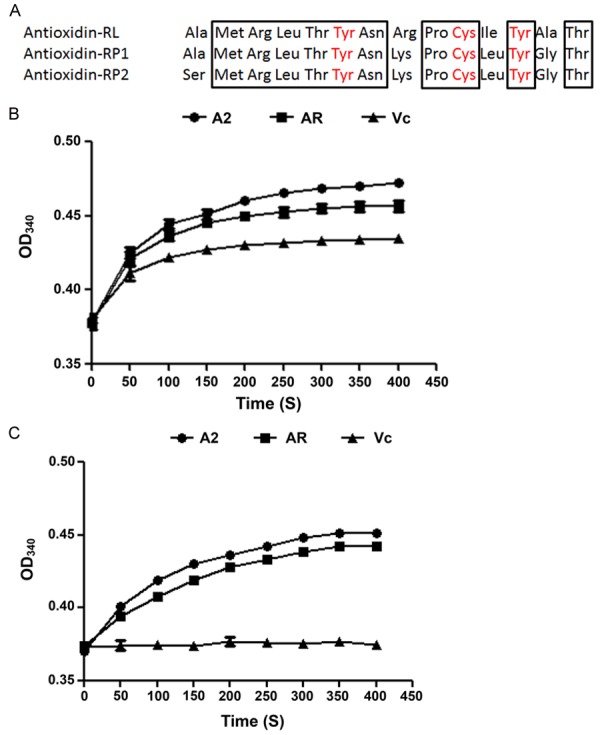
Amino acid sequence alignment among antioxidin peptides and ABTS+ free radical scavenging assay. (A) Amino acid sequence alignment between AR and its homologs antioxidin-RP1 and antioxidin-RP2. The consensus was highlighted in frame. (B) ABTS+ free radical scavenging was tested at 340 nm. The in vitro reaction was started by addition of AR, Vc or the mutants to ABTS solution. Final concentrations of antioxidants and ABTS+ free radical in the reaction mixture were 3 and 40 μM respectively. (C) The same as (B), except that samples was kept at room temperature and exposed to light and air for 1 week before the testing.
We postulated that adding the binding residue tyrosine might promote the reductive potency of the peptides on free radicals. Therefore, tyrosine was used to replace some non-conserved residues or inserted directly into the sequences at various locations. The reason why no more reductive residue cysteine was added to the sequence was to avoid the generation of intramolecular disulfide bond which would block the generation of cysteine anion and in turn hamper free radical reduction. Based on this rationale, five AR-derivatives were contrived (Table 1). However, 3 peptides A3, A4 and A5 had low solubility, and were excluded from the further studies.
Table 1.
Amino acid sequences and solubility of Antioxidin-RL and the mutants

|
In the reaction of the peptides with ABTS+ free radical, ABTS (maximal optical absorption at 340 nm) is formed as the reductive product from the free radical, accompanied by the intermediate product rendering maximal optical absorption at 550 nm, which is the complex of ABTS+ free radical and the peptide [11]. Considering the persistence of the free radical in the intermediate product, OD340 alone was examined during the assay to keep track of the reduction of free radicals. ABTS+ free radical assay showed that AR and A2 cleared ABTS+ free radicals faster than Vc, with A2 more effective than AR (Figure 1B). To compare the stability of A2, AR and Vc, the antioxidant in solution were kept at room temperature and exposed to light and air for 1 week before being used for the free radical assays. As shown in Figure 1C, the radical clearing efficiency dropped slightly for A2 and AR while Vc lost all activity (Figure 1C), suggesting that A2 and AR had excellent stability. As the only promising mutant among the cognates pool, A2 was selected to be further examined together with AR and Vc (as control).
AR and A2 attenuated PC12 cell injury induced by H2O2
CCK-8 kit was used to evaluate cell metabolic activity. First, we established the dosage range of AR, A2 and Vc in which the antioxidants did not harm cells. Vc at 100 μM significantly decreased cell metabolic activity, yet the antioxidant peptides AR and A2 showed no obvious toxicity to PC12 cells at 100 μM (data not shown). Therefore, all the subsequent experiments were performed by using concentrations 10 and 50 μM. As shown in Figure 2A, AR as well as A2 at 10 and 50 μM had no significant effect on cell metabolic activity, while Vc at 10 and 50 μM caused a modest decrease in cell metabolic activity.
Figure 2.
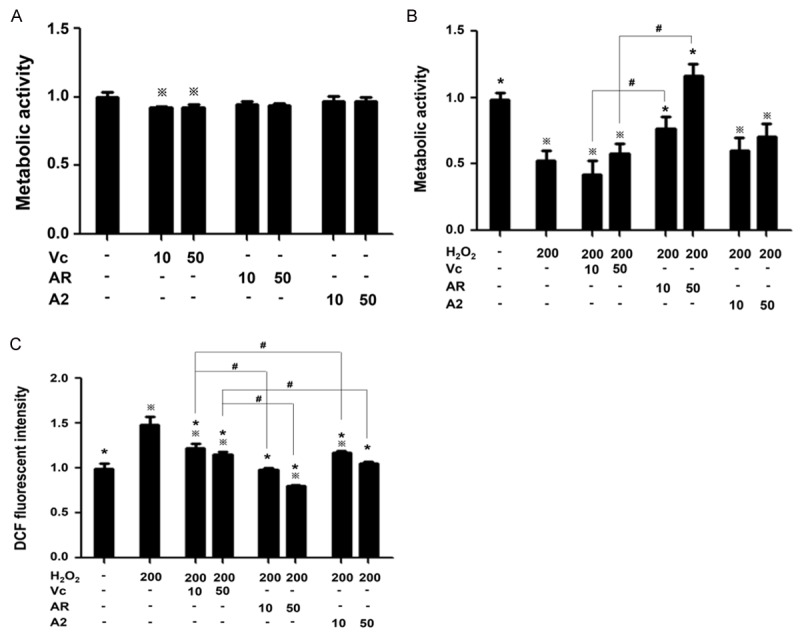
PC12 Cell metabolic activity examined with CCK-8 kit and intracellular ROS level examined with DCFH-DA probe. A. Metabolic activity of cells treated with antioxidants in the absence of H2O2. B. Metabolic activity of H2O2-insulted cells with antioxidants. C. intracellular ROS accumulation of H2O2-insulted cells with antioxidants. All experiments were carried out in triplicates. Data were shown as the percentage of the values corresponding to the uninjured cells and represented as mean ± SD. *P<0.05 compared to H2O2-treated cells. ※P<0.05 compared to untreated cells. #P<0.05 compared to cells treated with H2O2 and Vc at respective concentration.
H2O2 treatment decreased cell metabolic activity by 50% (Figure 2B). Vc failed to rescue the effect of H2O2. AR was the most effective, showing a dose-dependency in rescuing cells, and AR at 50 μM completely abolished the adverse effect of H2O2. A2 also displayed the protective effect, however, weaker than its native counterpart AR, which posed a contrast with its advantage over AR in removing ABTS+ free radical.
AR and A2 suppress intracellular ROS content boosted by H2O2
DCFH-DA was used to evaluate intracellular ROS level. Consistent with the results of the cell metabolic activity assay, Vc only slightly decreased ROS level, while both AR and A2 decreased intracellular ROS in a dose-dependent manner, with AR most effective (Figure 2C). These results suggest that the protective effect of the antioxidant peptides could be attributed to the suppression of intracellular ROS induced by oxidative stress. However, other mechanisms by which the peptides work to protect oxidatively stressed cells remains to be investigated.
AR and A2 maintained mitochondria membrane potential (MMP) and mitochondrial morphology in H2O2-treated PC12 cells
The energy producing metabolism of mitochondria depends critically on the rigorous permeability of mitochondrial membrane system that holds up MMP formed by the proton gradient between the intermembrane space and mitochondrial matrix. MMP is the energy source for ATP synthase localized on mitochondrial inner membrane to convert ADP and phosphate into ATP. Oxidative damage often results in lossing permeability of the membrane system, which causes dissipation or even loss of MMP, not only impeding or terminating ATP generation but mediating the generation of large amount of ROS as the byproduct of the inefficient phosphorylation and meanwhile the signal cascade to apoptosis will be activated [12,13]. Therefore, MMP was used to evaluate mitochondria function and test the protective effect of antioxidants peptides on cells suffering from oxidative stress. As shown in Figure 3A, H2O2 treatment decreased MMP by 50%. Vc at both concentrations had modest effect, restoring MMP by 10%. 50 μM AR and A2 recovered MMP by 30%, while 10 μM of AR as well as A2 rescued MMP by 20-25%.
Figure 3.
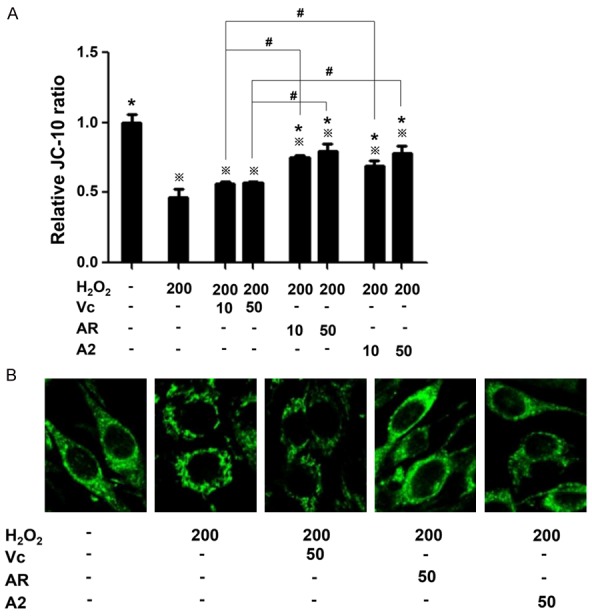
MMP evaluation with JC-10 probe and mitochondrial morphology with MitoTracker Green. A. Relative JC-10 ratio representing MMP. JC-10 ratio equals the intensity at 590 nm divided by that at 525 nm, and relative JC-10 ratio is represented as the percentage of values corresponding to the uninjured cells. All experiments were carried out in triplicates and the data were shown as mean ± SD. ※P<0.05, *P<0.05 and #P<0.05 as indicated in Figure 2. B. Confocal microscopy image of mitochondrial morphology.
Since MMP reflects the functional state of mitochondria, it was expected that mitochondrial morphology might also show difference under oxidative stress. The correlation of MMP to mitochondrial morphology was validated by the fluorescent imaging with laser confocal microscope at 40 x objective. As shown in Figure 3B, the uninjured cells adopted a polar shape with mitochondria distributing homogeneously and successively while the H2O2 injured cells appeared much rounder with mitochondria agglomerating to tiny particles and distributing discretely. The mitochondria morphology of the impaired cells treated with AR was the closest to the untreated cells, and A2 treatment had more mitochondria recovery than Vc. Altogether, MMP measurement and mitochondrial imaging demonstrated that the antioxidant peptides were able to rescue mitochondrial function and morphology in H2O2 injured cells, which also accounted for their maintenance of cell metabolic activity under oxidative stress.
AR and A2 protect mitochondria by downregulating Drp1 expression
Drp1, the member of the large GTPase of the dynamin superfamily, serves to mediate mitochondrial fission and also stimulate oligomerization of proapoptotic Bcl-2 family members such as Bax on mitochondrial outer membrane to enhance the membrane permeability and thus enable the release of proteins including cytochrome c from the intermembrane space, which subsequently triggers apoptosis [14-16]. Therefore, the overexpression of Drp1 promotes apoptosis and decreases cell metabolic activity. Western blotting analysis indicated H2O2 treatment significantly boosted Drp1 expression and that AR, A2 and Vc all damped the expression, with AR most effective (Figure 4). The apparent difference in Drp1 expression between cells with and without AR or A2 suggested the antioxidant peptides might inhibit oxidative stress induced apoptosis by downregulating Drp1 expression. Together with the foregoing MMP evaluation and mitochondrial morphology imaging, Drp1 expression once again highlighted mitochondria as a crucial target for the antioxidant peptides to protect cells from oxidative stress.
Figure 4.
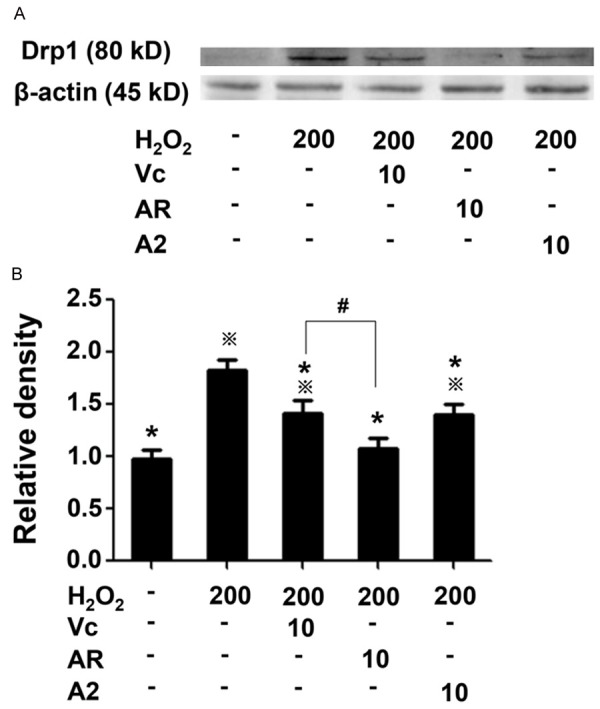
Drp1 expression by Western blotting analysis. (A) Images of the Western blot. β-actin was used as the internal reference. (B) Bar graph showing quantified densitometry from (A). Relative density was represented as the percentage of values corresponding to the uninjured cells. All experiments were carried out in triplicates. Data were shown as mean ± SD. ※P<0.05, *P<0.05 and #P<0.05 as indicated in Figure 2.
AR and Vc inhibited the change in SOD1 and GPx1 expression caused by H2O2 stimulation
To our knowledge, cells may exploit the inherent defensive mechanism to resist oxidative stress by regulating oxidative stress-responsive genes, SOD1 and GPx1. SOD1 reduces superoxide to H2O2 that is then converted by GPx1 to H2O and O2, with the formation of disulfide-containing oxidized glutathione from reduced glutathione [17]. Obviously, the sequential transformation by the two enzymes relieved cells from oxidative stress from superoxide and H2O2. Some antioxidants like liraglutide, piper sarmentosum and oleuropein, have been reported to relieve cells from oxidative stress by regulating the expression of GPx1 and SOD1 [18-20]. To test whether AR and A2 could also function in the same way, qPCR was done to test the expression of SOD1 and GPx1 in H2O2-treated PC12 cells. H2O2 treatment led to >2.5 folds upregulation of SOD1, which might have likely conduced to the further accumulation of H2O2 in cells. AR and Vc inhibited H2O2-induced SOD1 upregulation, whereas A2 at this concentration only partially inhibited SOD1 upregulation (Figure 5A). Therefore, AR, Vc and A2 could inhibit the possible H2O2 accumulation in the injured cells by limiting SOD1 upregulation caused by extracellular H2O2 stimulation.
Figure 5.
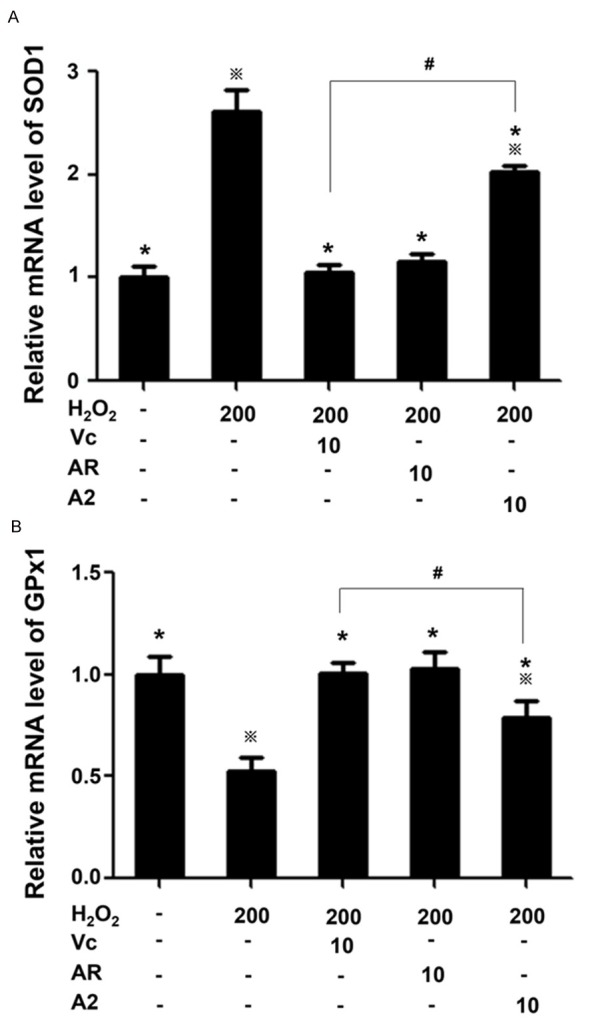
Oxidative stress responsive gene expression detected by qPCR. A. SOD1 mRNA expression level. B. GPx1 mRNA expression level. ※P<0.05, *P<0.05 and #P<0.05 as indicated in Figure 2.
In contrast, H2O2 treatment downregulated GPx1 level by half compared with that in untreated cells (Figure 5B), which might compromise the removal of H2O2 catalyzed by GPx1. AR and Vc raised GPx1 expression from that of H2O2 insulted cells to the level in untreated cells. Although A2 also raised GPx1 level in the injured cells, but the extent of the increase was much smaller than that caused by AR and Vc. Consequently, through hampering SOD1 upregulation and GPx1 downregulation induced by H2O2 treatment, AR, A2 and Vc were able to inhibit intracellular H2O2 accumulation, but A2 was not as effective as AR and Vc for this mechanism.
Conclusion
The antioxidant peptide AR with the binding residue tyrosine and the catalyzing residue cysteine scavenges ROS in vitro at a high efficiency. We introduced additional tyrosine residue(s) into AR, but several mutants had poor solubility. Although the soluble mutant A2 cleared ABTS+ free radical more effectively than AR, AR exhibited its advantage over A2 and Vc in the tests with PC12 cells. The protective effect of AR and A2 was more than clearing intracellular ROS and inhibiting H2O2-stimulated expression change of oxidative stress responsive genes. AR and A2 targeted mitochondria to exert its antioxidant potency, considering its maintenance of MMP and mitochondrial morphology as well as downregulation of Drp1, which may keep cells from suffering apoptosis caused by mitochondrial dysfunction. Collectively, AR and its derivatives hold great promise for the treatment of ROS-related neuronal degeneration and many other diseases.
Acknowledgements
This work was supported by Foundation for State Key Program of Research and Development (No. 2016YFC1100202), Ministry of Science and Technology of the People’s Republic of China, and the support from the University of California, Los Angeles.
Disclosure of conflict of interest
None.
Abbreviations
- ABTS
2,2’-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)
- AR
antioxidin-RL
- A2
antioxidin-2
- Vc
Vitamin C
- ROS
reactive oxygen species
- MMP
mitochondrial membrane potential
- Drp1
dynamin-related protein 1
References
- 1.Di Domenico F, Barone E, Perluigi M, Butterfield DA. Strategy to reduce free radical species in Alzheimer’s disease: an update of selected antioxidants. Expert Rev Neurother. 2015;15:19–40. doi: 10.1586/14737175.2015.955853. [DOI] [PubMed] [Google Scholar]
- 2.Sanders LH, Greenamyre JT. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic Biol Med. 2013;62:111–20. doi: 10.1016/j.freeradbiomed.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, Hong J, Yang H, Wu J, Ma D, Li D, Lin D, Lai R. Frog skins keep redox homeostasis by antioxidant peptides with rapid radical scavenging ability. Free Radic Biol Med. 2010;48:1173–81. doi: 10.1016/j.freeradbiomed.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Wang X, Liu X, Wu J, Liu C, Gong W, Zhao Z, Hong J, Lin D, Wang Y, Lai R. Antioxidant peptidomics reveals novel skin antioxidant system. Mol Cell Proteomics. 2009;8:571–83. doi: 10.1074/mcp.M800297-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X, Lai R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem Rev. 2015;115:1760–846. doi: 10.1021/cr4006704. [DOI] [PubMed] [Google Scholar]
- 7.Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Liu Y, Zhang T, Wu H, Lin M, Wang C, Zhan Y, Zhou Q, Qiao B, Sun X, Zhang Q, Guo X, Zhao G, Zhang W, Huang W. Synthetic Bax-Anti Bcl2 combination module actuated by super artificial hTERT promoter selectively inhibits malignant phenotypes of bladder cancer. J Exp Clin Cancer Res. 2016;35:3. doi: 10.1186/s13046-015-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aranda A, Sequedo L, Tolosa L, Quintas G, Burello E, Castell JV, Gombau L. Dichlorodihydro-fluorescein diacetate (DCFH-DA) assay: a quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol In Vitro. 2013;27:954–63. doi: 10.1016/j.tiv.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Y, Yu T, Wang W, Pan K, Shi D, Sun H. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochem Biophys Res Commun. 2014;448:15–21. doi: 10.1016/j.bbrc.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Akerstrom B, Maghzal GJ, Winterbourn CC, Kettle AJ. The lipocalin alpha1-microglobulin has radical scavenging activity. J Biol Chem. 2007;282:31493–503. doi: 10.1074/jbc.M702624200. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb E, Armour SM, Harris MH, Thompson CB. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–17. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- 13.Cosentino K, Garcia-Saez AJ. Mitochondrial alterations in apoptosis. Chem Phys Lipids. 2014;181:62–75. doi: 10.1016/j.chemphyslip.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:706–16. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Li L, Ling C, Li J, Pang JY, Lin YC, Liu J, Huang R, Wang GL, Pei Z, Zeng J. Marine compound Xyloketal B protects PC12 cells against OGD-induced cell damage. Brain Res. 2009;1302:240–7. doi: 10.1016/j.brainres.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Gillies LA, Kuwana T. Apoptosis regulation at the mitochondrial outer membrane. J Cell Biochem. 2014;115:632–40. doi: 10.1002/jcb.24709. [DOI] [PubMed] [Google Scholar]
- 17.Victor VM, Rocha M, Sola E, Banuls C, Garcia-Malpartida K, Hernandez-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15:2988–3002. doi: 10.2174/138161209789058093. [DOI] [PubMed] [Google Scholar]
- 18.Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, Kodama K, Sakamoto Y, Kotooka N, Hirase T, Node K. The glucagon-like peptide 1 analog liraglutide reduces TNF-alpha-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375–82. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Ugusman A, Zakaria Z, Hui CK, Nordin NA. Piper sarmentosum inhibits ICAM-1 and Nox4 gene expression in oxidative stress-induced human umbilical vein endothelial cells. BMC Complement Altern Med. 2011;11:31. doi: 10.1186/1472-6882-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi C, Chen X, Liu Z, Meng R, Zhao X, Liu Z, Guo N. Oleuropein protects L-02 cells against H2O2-induced oxidative stress by increasing SOD1, GPx1 and CAT expression. Biomed Pharmacother. 2017;85:740–748. doi: 10.1016/j.biopha.2016.11.092. [DOI] [PubMed] [Google Scholar]


