Abstract
Although glomerular and vascular damage have been considered the main characteristics of diabetic kidney disease (DKD), accumulating data now indicate that tubular atrophy also plays a major role. Cathepsin D (CatD) is the major aspartate protease within lysosomes. The current study demonstrated that CatD expression was altered in the renal tubular epithelium in patients with diabetes mellitus (DM). In contrast to its low and uniform distribution in the tubular epithelium in normal kidney tissues, CatD demonstrated flecked and increased expression in tubules with relatively integral structures, and disappeared in disordered tubules in DM kidney tissues. In vitro studies demonstrated that CatD protected HK2 cells from the damage induced by high glucose and advanced glycation end-products (AGEs), independent of its enzymatic activity. In addition, the current study demonstrated that AGEs induced lysosome membrane permeabilization (LMP) and loss of mitochondrial membrane potential (MMP). Overexpression of CatD prevented LMP and maintained the MMP in HK2 cells exposed to AGEs. In addition, the catalytic activity of CatD was not required for its role in LMP prevention and MMP maintenance. These results indicate, for the first time that CatD may improve the viability of renal tubular cells in the presence of diabetic mediators independent of its enzymatic activity by preventing LMP and stabilizing the MMP.
Keywords: Cathepsin D, diabetic mellitus, tubular epithelial cell, lysosome membrane permeabilization, mitochondrial membrane potential
Introduction
Diabetic kidney disease (DKD) is one of the leading causes of end-stage renal disease (ESRD), the prevalence of which has gradually increased in recent years [1]. Pathological changes in DKD include glomerular hyperfiltration, abnormal permeability of the glomerular sieve to albumin, and cellular and extracellular changes in the glomerulus and tubulointerstitium, causing renal dysfunction and ultimately developing into ESRD [2]. As in most chronic renal diseases, even if the primary damage is restricted to the glomerulus, injury to the renal tubule and interstitium becomes progressively more involved with disease progression. It is now believed that the stage and prognosis of chronic renal diseases correlate better with the severity of tubulointerstitial damage than with glomerular sclerosis [3,4].
In DKD, tubular injury is due to several factors, particularly high glucose levels and the presence of advanced glycation end-products (AGEs). Apoptosis, a form of programmed cell death, contributes to renal tubular injury mediated by AGEs and accelerates the progression of DKD via various mechanisms [5]. Both mitochondria and lysosomes play crucial roles in the regulation of the apoptotic process. During the early phase of apoptosis, these two organelles exhibit an increase in membrane permeability, resulting in the release of some contents [6-9]. Cathepsin D (CatD), a 52-kDa protein and the major aspartate protease within lysosomes, is one of the hydrolytic enzymes released, and has been implicated in the regulation of apoptotic processes [9,10]. During the process of apoptosis, mature CatD released from the lysosome into the cytosol may in turn lead to the mitochondrial release of cytochrome C into the cytosol and subsequently promote apoptosis [11]. On the contrary, there is increasing evidence that CatD can prevent apoptosis. It has been reported CatD upregulation protects against doxorubin-induced apoptosis in neuroblastoma [12], and that CatD deficiency results in extensive apoptotic neuronal death [13,14]. CatD has been reported to be implicated in some renal diseases such as Goodpasture disease, poststreptococcal GN, and passive Heymann nephritis [15,16]. In addition, CatD has been reported to affect the renin-angiotensin system by functioning as a renin-like enzyme to catalyze the breakdown of angiotensinogen to angiotensin I [17,18]. Nevertheless, the role of CatD in DKD has not been studied. The current study first examined the expression of CatD in DKD, and demonstrated that the expression and distribution of CatD was altered in the tubular epithelium in DKD. Using lentiviral vectors containing the CatD construct, the current study demonstrated that CatD protected tubular epithelial HK2 cells from the damage induced by high-glucose conditions. The current study also confirmed that the protective role of CatD was not dependent on its enzymatic activity. Furthermore, we showed that CatD protected HK2 cells from lysosome membrane permeability (LMP) and loss of mitochondrial membrane potential (MMP) induced by high glucose in a catalytic activity-independent manner. Therefore, our data establish CatD as a potential target for the prevention of tubular damage in DKD.
Materials and methods
Subjects
105 patients who underwent percutaneous renal biopsy between June 2008 and June 2013 and diagnosed as pre-existing T2DM were enrolled in this study. The subjects included 73 males and 32 females with ages ranging from 23 to 76 years and a mean age of 51 years. The duration of diabetes mellitus (DM) ranged from 0.5 to 25 years, with the average duration of DM being 7.76 years. Patients with current illness, including infectious disease or tumor, were excluded from the study. As controls, five individuals, including three males and two females, were chosen. Five patients were diagnosed as having renal trauma or benign renal tumor. Tissues were obtained from the farthest section from the region of the lesion. Based on serological examination and past history, the diagnosis of DM was excluded for these five patients. All the patients were from Shengjing Hospital of China Medical University. This study was conducted following approval from the Ethics Committee of Shengjing Hospital of China Medical University. Written informed consent was obtained from all participants.
Immunohistochemistry
Immunoperoxidase staining was performed on 10% formalin-fixed paraffin sections (2 μm in thickness). Deparaffinized tissue sections were subjected to antigen retrieval by microwave oven heating in 0.1 M sodium citrate (pH 6.0) for 10 min and then incubated with 3% hydrogen peroxide to block endogenous peroxidase. Tissue sections were then incubated with 10% normal goat serum for 40 min, followed by an overnight incubation at 4°C with anti-cathepsin D antibody (1:100) (Abcam). After washing, the sections were incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit polyclonal antibody for 20 min at 37°C and then developed with DAB (Sigma) to facilitate the color reaction. The sections were then counterstained with hematoxylin, dehydrated, and mounted. After that, the immunostained sections were observed under a light microscope.
Cell culture and reagents
An immortalized proximal tubule epithelial cell line, HK2 (human kidney 2), was grown in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin, and maintained at 37°C in a 5% CO2 water-saturated atmosphere. Bovine serum albumin (BSA) and advanced glycation end-product-BSA (AGEs) were purchased from Sigma and Calbiochem, respectively.
Transduction of CatD into HK2 cells using lentiviral vectors
Lentiviral plasmids containing CatD cDNA and a GFP expression cassette were produced by GeneChem Corporation (Shanghai, China). HK2 cells were incubated with vector supernatants for 8 h and transduction efficiency was determined by the observation of GFP+ cells by fluorescence microscopy 2 days later.
Cell viability analysis
Cell viability was determined by measuring the metabolism of MTT according to the manufacturer’s instructions. The absorbance was measured with a microplate reader at 550 nm.
RTCA proliferation assays
Cells were seeded in RTCA E-plates (ACEA Bioscience, San Diego, CA) and changes in electrical impedance were measured in a label-free real-time setting using xCELLigence RTCA DP (ACEA Bioscience, San Diego, CA), in which the increase in electrical impedance on the plate reflects the proliferative rate of cells [19,20].
5-Ethynyl-2-deoxyuridine (EdU) incorporation analysis
The rate of DNA synthesis in HK2 cells was determined by EdU incorporation analysis using the Click-iTTM EdU Alexa Fluor 555 Imaging Kit (InvitrogenTM, OR, USA) according to the manufacturer’s protocols. Briefly, BSA or AGE-treated HK2 cells were incubated with EdU at a final concentration of 10 μM for 4 h, and at least 500 cells were counted in each experiment. EdU-positive cells were expressed as a percentage of the total cells. Each experiment was performed in triplicate.
Viable cell count
Cells were stained with trypan blue, and the numbers of viable (negative staining) and dead (positive staining) cells were counted.
Analysis of LMP
LMP was analyzed using acridine orange (AO) staining. AO concentrated within lysosomes emits a granular red fluorescence, whereas AO concentrated in the cytosol emits a diffuse green fluorescence. A reduction in granular red fluorescence combined with an increased diffuse cytosolic green fluorescence indicates induction of LMP. Briefly, HK2 cells were treated with BSA or AGEs, and incubated with AO at final concentration of 5 μg/ml; images were subsequently acquired with a fluorescence microscope (Olympus).
Analysis of MMP
MMP was measured based on the uptake of tetramethylrhodamine ethyl ester (TMRE). Briefly, HK2 cells were treated with BSA or AGEs, then incubated with 5 nM TMRE for 15 min, and images were acquired using a fluorescence microscope (Olympus).
Statistical analysis
All data were obtained from at least three individual experiments. Values are expressed as the mean ± SD. Statistical analysis between groups was performed using one-way ANOVA. The statistical significance was defined at P<0.05.
Results
Expression of CatD is altered in renal tissues from patients with diabetes mellitus
CatD expression was investigated by immunohistochemical staining of renal tissues from 105 diabetes mellitus (DM) patients and 5 non-DM patients, and the representative results are shown in Figure 1. CatD is distributed uniformly with relatively low expression levels in the renal tubules of non-DM, while flecked distribution of CatD was observed in the renal tubules of DM (Figure 1). CatD expression was significantly enhanced in renal tubules that exhibited a relatively integral structure, but was reduced in disordered tubules (Figure 1). In addition, CatD expression was mainly restricted to the tubulointerstitium, with a minority expression in the glomeruli (Figure 1).
Figure 1.
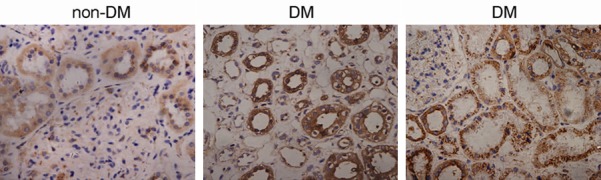
Expression of CatD in renal tissues. Renal tissue sections from 105 diabetes mellitus (DM) patients and five non-DM patients were immunohistochemically stained with anti-cathepsin D antibody. Representative images for DM and non-DM patients are provided.
CatD overexpression has no obvious influence on HK2 cell proliferation under normal glucose condition
To explore the influence of CatD on the growth and proliferation of tubular epithelial cells, the CatD gene was transduced into HK2 cells via lentiviral vectors. Observation of GFP-positive cells by fluorescence microscopy indicated that transduction efficiency for both the empty vector and the CatD construct in HK2 cells was approximately 90% (Figure 2A). Western blot confirmed that the lentiviral vector containing the CatD construct, but not the empty lentiviral vector, significantly increased CatD expression in HK2 cells (Figure 2B). Cell counts demonstrated that CatD exhibited no obvious influence on the proliferation of HK2 cells cultured with media containing normal levels of glucose (Figure 2C). Cell growth was also measured using a real-time analyzer (RTCA) system, and the results indicated that CatD did not affect the cell index of HK2 cells under normal culture conditions (Figure 2D). EdU incorporation experiments demonstrated that CatD had no obvious effect on DNA synthesis in HK2 cells (Figure 2E and 2F). In addition, cell cycle analysis showed that overexpression of CatD had no influence on the percentage of cells in the G1, S, and G2/M phases (Figure 2G).
Figure 2.
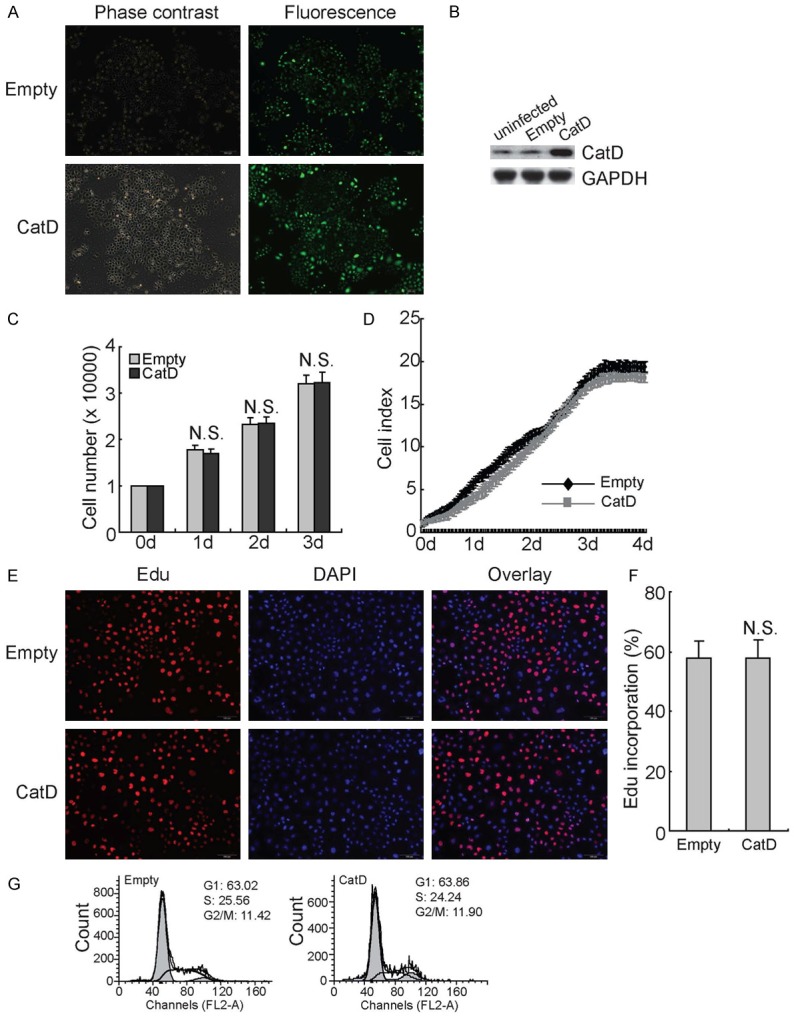
No effects of CatD upregulation on HK2 proliferation under culture with normal glucose. (A) HK2 cells were infected with lentiviral vectors containing empty or CatD construct for 12 h, then cultured for additional 48 h. Transfection efficiency was analyzed by measurement of GFP+ positive cells under a fluorescence microscopy, and representative images are provided. (B) HK2 cells were infected with lentiviral vectors containing empty or CatD construct for 12 h and cultured for additional 48 h, western blot was performed using the indicated antibodies. (C) HK2 cells were infected with lentiviral vectors containing empty or CatD construct for 12 h and cultured for additional 48 h. Viable cells were counted using trypan blue exclusion experiment daily. (D) HK2 cells were infected with lentiviral vectors containing empty or CatD construct for 12 h and cultured for additional 48 h. Cell proliferation was analyzed using RTCA in a real-time pattern. (E) HK2 cells were infected with lentiviral vectors containing empty or CatD construct for 12 h and cultured for additional 48 h. DNA synthesis was analyzed using the EdU incorporation assay, and representative images are provided. (F) Total and EdU-positive cells in (E) were counted, and the rate of EdU incorporation was calculated. (G) HK2 cells were infected with lentiviral vectors containing empty or CatD construct, and cell cycle progression was analyzed using flowcytometry. N.S., not significant.
CatD overexpression protects HK2 cells from cytotoxicity induced by high glucose
To further investigate the potential effect of CatD, cells were cultured in a high-glucose environment. The results from the RTCA proliferative assay demonstrated that the cell index of HK2 cells infected with empty lentiviral vector began to decrease rapidly after two days in high-glucose culture. In contrast, reduction of cell index was significantly suppressed in HK2 cells transduced with the CatD construct (Figure 3A). High glucose significantly decreased the rate of EdU incorporation in HK2 cells transduced with empty lentiviral vector, whereas no significant difference was detected in CatD-transduced HK2 cells cultured under normal and high-glucose conditions (Figure 3B). The MTT assay confirmed that AGEs suppressed the viability of HK2 cells in a dose-dependent manner (Figure 3C). Importantly, overexpression of CatD significantly protected HK2 cells from growth inhibition mediated by AGEs (Figure 3C). Flow cytometry demonstrated that AGEs increased the apoptosis of HK2 cells in a dose-dependent manner, which was significantly prohibited by overexpression of CatD (Figure 3D).
Figure 3.
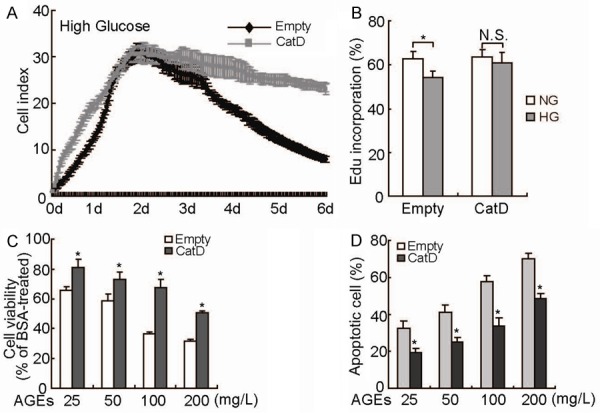
Suppression of high-glucose-mediated damage by CatD upregulation in HK2 cells. A: HK2 cells transduced with empty or CatD-containing lentivirus were cultured with 25 mM glucose, and cell proliferation was analyzed using RTCA in a real-time pattern. B: HK2 cells transduced with empty or CatD-containing lentivirus were cultured with 5 mM (NG) or 25 mM (HG) glucose, and DNA synthesis was analyzed using EdU incorporation experiments. C: HK2 cells transduced with empty or CatD-containing lentivirus were treated with various concentrations of AGEs or a comparable amount of BSA for 24 h, and cell viability was measured using MTT assay. D: HK2 cells transduced with Empty or CatD-containing lentivirus were treated with various concentrations of AGEs or a comparable amount of BSA for 24 h, and apoptotic cells were measured using flow cytometry. *, P<0.05.
Catalytic activity of CatD is not necessary for its protective effect in HK2 cells under high-glucose conditions
To assess whether the catalytic activity of CatD is necessary for its protective effect in HK2 cells cultured with high glucose, CatD-transduced HK2 cells were treated with pepstatin A, a specific CatD inhibitor. RTCA analysis demonstrated that pepstatin A had no obvious influence on the proliferation of CatD-transduced HK2 cells cultured in both normal glucose (Figure 4A) and high glucose (Figure 4B) conditions. EdU incorporation experiments demonstrated that DNA synthesis in CatD-transduced HK2 cells was not affected by pepstatin A under both normal and high-glucose conditions (Figure 4C). The MTT assay found that CatD overexpression increased the cell viability of HK2 cells exposed to 100 μg/ml of AGEs even in the presence of pepstatin A (Figure 4D).
Figure 4.
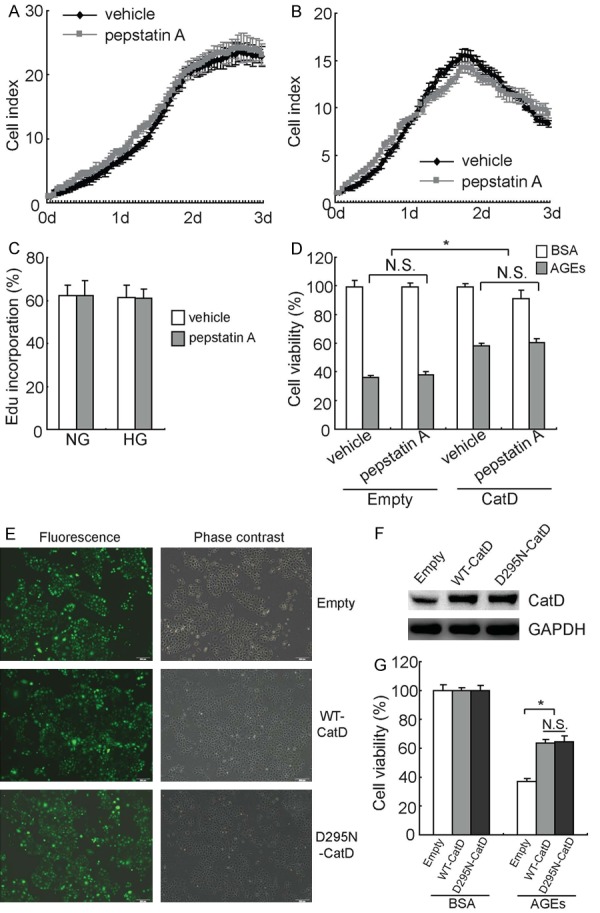
Protection of HK2 cells by CatD, independent of its catalytic activity. (A, B) CatD-transduced HK2 cells were cultured with 5 mM (A), or 25 mM (B) glucose in the absence or presence of pepstatin A, and cell proliferation was analyzed using RTCA in a real-time manner. (C) CatD-transduced HK2 cells were cultured with 5 mM (NG), or 25 mM (HG) glucose in the absence or presence of pepstatin A, and DNA synthesis was measured using EdU incorporation experiments. (D) HK2 cells were treated with AGEs in the absence or presence of pepstatin A, and cell viability was measured using the MTT assay. (E) HK2 cells were infected with empty lentivirus vector, lentivirus containing wild-type CatD (WT-CatD), or lentivirus containing an inactive mutant of CatD (D295N-CatD), and transduction efficiency was observed using fluorescence microscopy. (F) HK2 cells were infected with empty lentivirus vector, lentivirus containing wild-type CatD (WT-CatD), or lentivirus containing an inactive mutant of CatD (D295N-CatD), and CatD expression was analyzed using western blot analysis. (G) HK2 cells were infected with empty lentivirus vector, lentivirus containing wild-type CatD (WT-CatD), or lentivirus containing an inactive mutant of CatD (D295N-CatD) for 72 h, and treated with AGEs for another 24 h, following which cell viability was measured using the MTT assay. *, P<0.05; N.S., not significant.
To further confirm the involvement of catalytic activity of CatD, a lentiviral vector containing cDNA that encodes an inactive mutant of CatD, in which Asp295 is substituted by Asn (D295N), was generated. Measurement of GFP+ cells demonstrated that the transduction efficiency was about 90% for empty lentiviral vector, lentiviral vector containing wild-type CatD (WT-CatD), and lentiviral vector containing the D295N mutant CatD (D295N-CatD) (Figure 4E). Western blot confirmed that both WT-CatD and D295N-CatD significantly increased CatD expression in HK2 cells (Figure 4F). MTT assay demonstrated that both WT-CatD and D295N-CatD significantly increased the viability of HK2 cells exposed to AGEs (Figure 4G). Importantly, no obvious difference was observed between cells transfected with WT-CatD and D295N-CatD (Figure 4G).
CatD attenuates AGE-induced LMP and loss of MMP independent of its enzymatic activity
We next investigated whether the lysosomal pathway was implicated in AGE-induced apoptosis in HK2 cells. The lysosomotropic agent AO accumulates in acidic compartments such as lysosomes, and emits bright red fluorescence. Upon LMP, lysosomes become more alkaline, resulting in a decrease in this red fluorescence. Fluorescence microscopy analysis of HK2 cells stained with AO revealed high levels of red fluorescence in HK2 cells transduced with empty, WT-CatD, or D295N-CatD vector constructs, indicating the accumulation of AO in acidic lysosomes under normal glucose conditions (Figure 5A). Exposure to AGEs led to a dissipation of red fluorescence, associated with an increase in green fluorescence, indicative of LMP, in empty vector-transduced HK2 cells (Figure 5A). By contrast, both WT-CatD and D295N-CatD significantly blocked the loss of red fluorescence induced by exposure to AGEs (Figure 5A).
Figure 5.
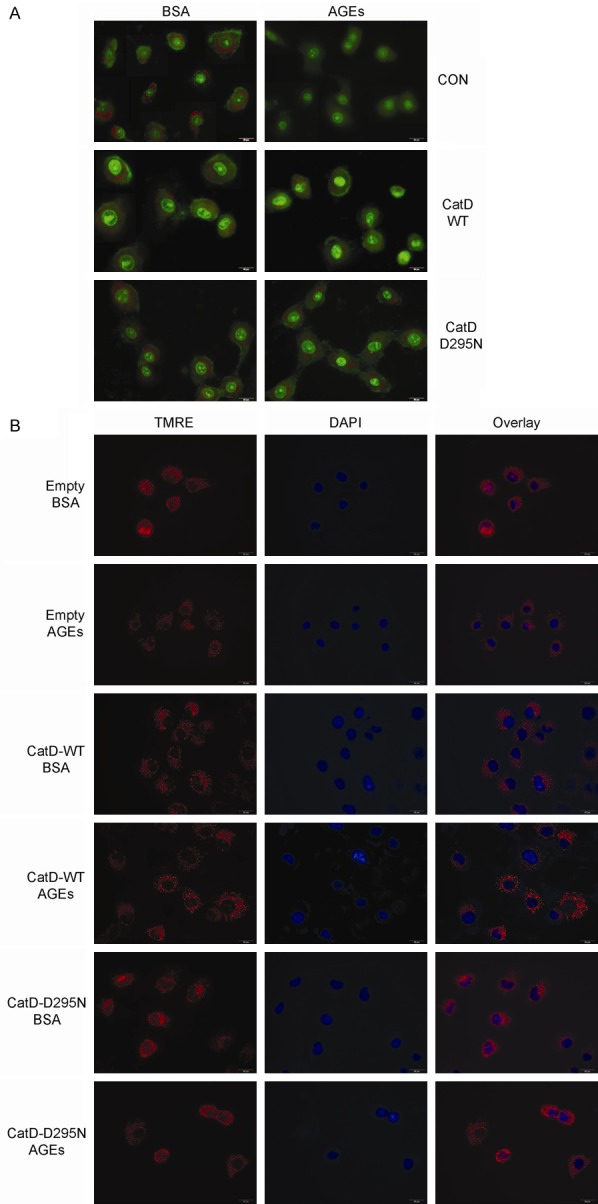
Prevention of AGE-mediated LMP and MMP loss by CatD independent of its catalytic activity. A: Empty, WT-CatD, or D295N-CatD vector-transduced HK2 cells were treated with BSA or AGEs for 24 h, LMP was measured by AO staining, and representative images are presented. B: Empty, WT-CatD, or D295N-CatD vector-transduced HK2 cells were treated with BSA or AGEs for 24 h, MMP was measured by TMRE staining, and representative images are presented.
To further investigate the function of CatD in regulating the MMP, the mitochondria in HK2 cells were marked by the addition of tetramethylrhodamine ethyl ester (TMRE), which emits red fluorescence. AGEs induced a decline in this red fluorescence in HK2 cells, and this decline was significantly reversed by both WT-CatD and D295N-CatD (Figure 5B).
Discussion
CatD is a 52-kDa lysosomal acid proteinase that has been shown to be involved in various biological activities. The level and activity of CatD varies among different tissues and organs. CatD synthesis is increased in multiple pathological conditions, including hypoxia, adidemia, and necrosis [21]. It has been reported that a small amount of CatD is expressed in renal tissues; however, the role of CatD in renal tissues is poorly understood [21,22]. Consistent with these previous reports [21,22], the current study demonstrated low-level expression and uniform distribution of CatD in the tubular epithelium in kidney tissues of non-diabetic patients. In kidney tissues of DM patients, CatD expression was increased in tubules that exhibited a relatively normal structure, whereas its expression disappeared in destroyed tubules. To investigate the potential involvement of CatD in tubular damage under DM conditions, we transferred the CatD gene into tubular epithelial HK2 cells via lentiviral vectors, and found that CatD upregulation prevented cytotoxicity induced by high glucose and AGEs in HK2 cells. CatD played a protective role even in the presence of its specific inhibitor pepstatin A. In addition, WT-CatD and D295N-CatD (a mutant construct without enzymatic activity) protected HK2 cells from high-glucose-mediated damage to similar extents. These data indicate that the catalytic activity of CatD might not be necessary for its protective role in tubular damage induced by high-glucose conditions.
It has been reported that under different apoptotic stimuli such as exposure to reactive oxygen species (ROS), TNF-α induces release of CatD from the lysosome to the cytosol, which subsequently triggers cytochrome C release from mitochondria and promotes cell apoptosis [10,11,23]. On the contrary, the anti-apoptotic effect of CatD has also been reported in neurons [13,14]. The current study reported that CatD upregulation improved cell viability and suppressed the apoptosis induced by high glucose and AGEs in HK2 cells. Together with the increased and decreased expression of CatD in tubules with relatively normal and destroyed structures, respectively, in DKD tissues, the current study indicates a potential role of CatD in the protection of tubular epithelium in DKD. This paradoxical effect of CatD on both pro- and anti-apoptotic functions may be ascribed to differences in cellular context.
The involvement of lysosomes and mitochondria in apoptotic cell death has attracted increased attention [24,25]. It has been suggested that LMP plays a key role in the release of CatD from the lysosome into the cytosol and subsequently triggering the mitochondrial apoptotic cascade [26]. In the present study, AGEs induced LMP and loss of MMP in HK2 cells, which was prevented to some degree by CatD upregulation, indicative of the potential involvement of lysosomes in tubular damage in DKD. The current study demonstrated that the enzymatic activity of CatD is not required for its protective effects on cell damage, LMP, and loss of MMP. Thus, the mechanisms by which CatD protects HK2 cells from apoptosis, LMP, and loss of MMP require further investigation.
In conclusion, we observed altered CatD expression in the tubulointerstitium of renal tissue in DKD patients. We found that overexpression of CatD improved cell viability in a high-glucose environment and ameliorated AGE-induced apoptosis in HK2 cells. In addition, CatD upregulation suppressed the LMP and the dissipation of MMP caused by AGEs. We also demonstrated that these effects of CatD are not dependent on its enzymatic activity. For the first time, the current study demonstrated that CatD might play a protective role in tubular damage induced by high glucose, thereby identifying CatD as a possible target for the treatment of DKD.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (81271292, 81301838 and 81470584) and Foundation of Liaoning Educational Committee (L2010581).
Disclosure of conflict of interest
None.
Abbreviations
- CatD
cathepsin D
- AGEs
advanced glycation end-products
- DKD
diabetic kidney disease
- ESRD
end-stage renal disease
- LMP
lysosome membrane permeabilization
- MMP
mitochondrial membrane potential
- DM
diabetic mellitus
References
- 1.White SL, Cass A, Atkins RC, Chadban SJ. Chronic kidney disease in the general population. Adv Chronic Kidney Dis. 2005;12:5–13. doi: 10.1053/j.ackd.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Vallon V, Komers R. Pathophysiology of the diabetic kidney. Compr Physiol. 2011;1:1175–1232. doi: 10.1002/cphy.c100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosius FC 3rd. New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord. 2008;9:245–254. doi: 10.1007/s11154-008-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC 3rd. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes. 2008;57:1439–1445. doi: 10.2337/db08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habib SL. Diabetes and renal tubular cell apoptosis. World J Diabetes. 2013;4:27–30. doi: 10.4239/wjd.v4.i2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2006;57:545–553. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 7.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bivik CA, Larsson PK, Kagedal KM, Rosdahl IK, Ollinger KM. UVA/B-induced apoptosis in human melanocytes involves translocation of cathepsins and Bcl-2 family members. J Invest Dermatol. 2006;126:1119–1127. doi: 10.1038/sj.jid.5700124. [DOI] [PubMed] [Google Scholar]
- 9.Johansson AC, Steen H, Ollinger K, Roberg K. Cathepsin D mediates cytochrome c release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death Differ. 2003;10:1253–1259. doi: 10.1038/sj.cdd.4401290. [DOI] [PubMed] [Google Scholar]
- 10.Kagedal K, Johansson U, Ollinger K. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 2001;15:1592–1594. doi: 10.1096/fj.00-0708fje. [DOI] [PubMed] [Google Scholar]
- 11.Roberg K. Relocalization of cathepsin D and cytochrome c early in apoptosis revealed by immunoelectron microscopy. Lab Invest. 2001;81:149–158. doi: 10.1038/labinvest.3780222. [DOI] [PubMed] [Google Scholar]
- 12.Sagulenko V, Muth D, Sagulenko E, Paffhausen T, Schwab M, Westermann F. Cathepsin D protects human neuroblastoma cells from doxorubicin-induced cell death. Carcinogenesis. 2008;29:1869–1877. doi: 10.1093/carcin/bgn147. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi H, Zhang J, Koike M, Nishioku T, Okamoto Y, Kominami E, von Figura K, Peters C, Yamamoto K, Saftig P, Uchiyama Y. Involvement of nitric oxide released from microglia-macrophages in pathological changes of cathepsin D-deficient mice. J Neurosci. 2001;21:7526–7533. doi: 10.1523/JNEUROSCI.21-19-07526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shacka JJ, Klocke BJ, Young C, Shibata M, Olney JW, Uchiyama Y, Saftig P, Roth KA. Cathepsin D deficiency induces persistent neurodegeneration in the absence of Bax-dependent apoptosis. J Neurosci. 2007;27:2081–2090. doi: 10.1523/JNEUROSCI.5577-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karan A, Saatci U, Bakkaloglu A. The role of cathepsin D in pathogenesis of acute poststreptococcal glomerulonephritis. Acta Paediatr Scand. 1976;65:355–360. doi: 10.1111/j.1651-2227.1976.tb04897.x. [DOI] [PubMed] [Google Scholar]
- 16.Zou J, Hannier S, Cairns LS, Barker RN, Rees AJ, Turner AN, Phelps RG. Healthy individuals have Goodpasture autoantigen-reactive T cells. J Am Soc Nephrol. 2008;19:396–404. doi: 10.1681/ASN.2007050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graciano ML, Cavaglieri Rde C, Delle H, Dominguez WV, Casarini DE, Malheiros DM, Noronha IL. Intrarenal Renin-Angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol. 2004;15:1805–1815. doi: 10.1097/01.asn.0000131528.00773.a9. [DOI] [PubMed] [Google Scholar]
- 18.Peng H, Xing YF, Ye ZC, Li CM, Luo PL, Li M, Lou TQ. High glucose induces activation of the local reninangiotensin system in glomerular endothelial cells. Mol Med Rep. 2014;9:450–456. doi: 10.3892/mmr.2013.1855. [DOI] [PubMed] [Google Scholar]
- 19.Solly K, Wang X, Xu X, Strulovici B, Zheng W. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol. 2004;2:363–372. doi: 10.1089/adt.2004.2.363. [DOI] [PubMed] [Google Scholar]
- 20.Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol Biol. 2011;740:33–43. doi: 10.1007/978-1-61779-108-6_6. [DOI] [PubMed] [Google Scholar]
- 21.Minarowska A, Karwowska A, Gacko M. Quantitative determination and localization of cathepsin D and its inhibitors. Folia Histochem Cytobiol. 2009;47:153–177. doi: 10.2478/v10042-009-0073-4. [DOI] [PubMed] [Google Scholar]
- 22.Wu CC, Chen JS, Huang CF, Chen CC, Lu KC, Chu P, Sytwu HK, Lin YF. Approaching biomarkers of membranous nephropathy from a murine model to human disease. J Biomed Biotechnol. 2011;2011:581928. doi: 10.1155/2011/581928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith B, Randle D, Mezencev R, Thomas L, Hinton C, Odero-Marah V. Camalexin-induced apoptosis in prostate cancer cells involves alterations of expression and activity of lysosomal protease cathepsin D. Molecules. 2014;19:3988–4005. doi: 10.3390/molecules19043988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagedal K, Johansson AC, Johansson U, Heimlich G, Roberg K, Wang NS, Jurgensmeier JM, Ollinger K. Lysosomal membrane permeabilization during apoptosis--involvement of Bax? Int J Exp Pathol. 2005;86:309–321. doi: 10.1111/j.0959-9673.2005.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira C, Chaves S, Alves S, Salin B, Camougrand N, Manon S, Sousa MJ, Corte-Real M. Mitochondrial degradation in acetic acidinduced yeast apoptosis: the role of Pep4 and the ADP/ATP carrier. Mol Microbiol. 2010;76:1398–1410. doi: 10.1111/j.1365-2958.2010.07122.x. [DOI] [PubMed] [Google Scholar]
- 26.Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–2890. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]


