Abstract
Cancer-derived mesenchymal stem cells (MSCs) seem to play an important role in mediating tumor angiogenesis. Recently, curcumin has been shown to display multiple therapeutic properties, including anticancer activity. In the present study, we have tried to explore the role of curcumin in regulating gastric cancer cells-derived mesenchymal stem cells (GC-MSCs) mediated angiogenesis. Our results showed that curcumin attenuated the high expression levels of fibroblast proteins (α-SMA & Vimentin) in GC-MSCs. Its treatment also inhibited GC-MSCs induced human umbilical vein endothelial cells (HUVEC) tube formation, migration and colony formation. Furthermore, it was noticed that curcumin abrogated NF-κB signaling activity and VEGF production in GC-MSCs. Next, to establish the link between regulation of NF-κB/VEGF signaling by curcumin, and its influence on GC-MSC-derived angiogenesis, we pretreated GC-MSCs with either NF-κB inhibitor PDTC or a neutralizing antibody against VEGF (NA-VEGF), and then collected conditioned media (CM). The HUVEC cells were then cultured in this conditioned media to test their ability to form tubes, migrate and form colonies. Our results demonstrated that NF-κB/VEGF signaling is important for GC-MSCs induced tube formation, migration and colony formation in HUVEC cells. Moreover, we also observed that NF-κB/VEGF signaling regulated VEGF expression of gastric cancer cells both in vitro and in vivo. Overall, our study indicated that curcumin may serve as a novel therapeutic target for GC-MSCs derived angiogenesis, by inhibiting NF-κB/VEGF signaling.
Keywords: Curcumin, MSC, NF-κB, VEGF, tumor angiogenesis
Introduction
Tumor angiogenesis, characterized by formation of new capillary blood vessels in the tumor microenvironment, play an important role in tumor growth and metastasis. Multiple reports have shown a correlation between tumor angiogenesis and poor clinical prognosis [1,2]. Among the many pro-angiogenic signaling molecules, vascular endothelial growth factor (VEGF) has been well studied and been shown to play a central role in angiogenesis pathway. It is highly expressed in many human cancers. Consistent with this, initial clinical efforts of developing anti-angiogenic treatments, have largely focused on inhibition of VEGF signaling [3-5]. In addition, recently the role of NF-κB signaling has also been highlighted in tumor angiogenesis. Infact, both NF-κB & VEGF signaling pathways seems to be closely related to angiogenesis and inflammatory response in the tumor environment [6,7].
Bone marrow derived stem cells (BM-MSC) have exhibited strong tropism for tumor sites and subsequently transform into cancer associated stroma cells [8]. In this context, BM-MSCs-derived exosomes carrying Platelet-derived growth factors D (PDGFD) have been observed to promote lung cancer progression [9]. Also, the mesenchymal stem cells have been shown to differentially regulate the invasion of distinct glioblastoma cell lines [10]. Overall, accumulating evidences indicates that tumor associated stromal cells, including fibroblasts and mesenchymal stem cells (MSCs) play an important role in cancer progression [11,12]. In our earlier study, we have isolated mesenchymal stem cell-like cells from human gastric cancer tissues (GC-MSC), and demonstrated their involvement both during in vitro and in vivo tumor growth [13,14]. However, the effect of these GC-MSCs on tumor angiogenesis was not clear.
Curcumin, a bioactive compound found in the famous Indian spice turmeric and obtained from a plant Curcuma longa, has been identified as a potent anti-inflammatory substance with antimicrobial activities. It plays an important role in several inflammatory diseases [15,16], along with depicting anti-cancer properties. Various studies have shown that curcumin treatment leads to growth inhibition of androgen-independent prostate cancer cells, and effectively restrain stemness property of liver cancer cells, through modulating of NF-κB signaling pathway [17,18]. More recently, curcumin has been demonstrated to inhibit invasion of cancer-associated fibroblast-driven prostate cancer cells by regulating MAOA/mTOR/HIF-1α signaling. Thus, curcumin appears to regulate tumor progression by multiple biological pathways [19].
Here in our current study, we have tried to investigate the involvement of NF-κB/VEGF signaling in GC-MSC derived angiogenesis, and if curcumin has any inhibitory effect on the GC-MSC-driven angiogenesis.
Materials and methods
Isolation and culturing of GC-MSCs, gastric cancer cell lines and HUVEC cells
GC-MSCs were isolated from gastric cancer tissues after informed consent, in accordance with institutional guidelines with approved protocol, as described previously [13]. HUVECs and HGC-27 cells were purchased from China Academia Sinica Cell Repository (Shanghai, China), and were cultured in RPMI 1640 medium (Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA).
Western blot analysis
Cell lysates were prepared in RIPA buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.1% SDS, 1 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 1 mg/ml aprotinin and 1 g/ml leupeptin) and were centrifuged at 10,000 rpm for 20 min (Kubota6930, Tokyo, Japan). Later protein samples were separated using 12% SDS-PAGE and then transferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). Next, the membrane was blocked with 5% fat-free milk in TBST buffer (20 mmol/L Tris-HCl, 137 mmol/L NaCl and 0.1% Tween 20) for 2 hr at room temperature (RT), followed by incubation with primary and secondary antibodies (Santa Cruz Biotechnology, Inc., USA). Finally, the signal was visualized using HRP substrate (Millipore), and analyzed using MD ImageQuant Software. GAPDH (Kangcheng, China) expression was used as loading control. The following primary antibodies were used: α-SMA, vimentin, (Abcam, USA); IκBα, t-NF-κB p65, p-NF-κB p65 (Cell Signaling Technology, Beverly, MA, USA); VEGF (Santa Cruz Biotechnology, Inc., USA); cyclinD, cyclinE, proliferating cell nuclear antigen (PCNA), BCL-2, and BCL-XL (Bioworld Technology, Louis Park, MN, USA).
Collection of conditioned media
Curcumin (Sigma, USA) was dissolved in olive oil, while NF-κB inhibitor Pyrrolidine dithiocarbamic acid (PDTC) (Sigma, USA) was dissolved in sterilized PBS, and then were aliquoted to store at -20°C for future experiments. GC-MSCs were pretreated with Curcumin (30 μM) or NF-κB inhibitor PDTC (20 μM) for 2 hr in the control culture medium. The conditioned media (CM) (including GC-MSC-CM, cur-GC-MSC-CM, GC-MSC+PDTC-CM) were collected, and centrifuged at 1,000 g for 5 min. Later the CM was filtered using 0.45-µm membrane to subsequently culture cells.
Enzyme-linked immunosorbent assay
The VEGF levels in the conditioned media from GC-MSC and cur-GC-MSC groups were measured using ELISA kit (R&D Systems, USA), according to the manufacturer’s instructions. All assays were performed in duplicate, and VEGF levels were assessed by comparing the values with standard curve, obtained using standard proteins provided in the kit.
Tube formation assay
The tube formation assay was conducted using HUVEC cells. These cells were first cultured in serum-free RPMI 1640 media for 24 hr. Next, matrigel (50 μl) was added to each well of a 96-well plate and allowed to polymerize. Thereafter, HUVEC cells (2×104 cells/well) were seeded onto a 96-well plate with matrigel, and conditioned medium derived from different GC-MSC groups was added for 12 hr for stimulation. The HUVEC cell tube formation was quantitatively measured using Image J software.
Migration assay
The HUVEC cells treated with following conditioned media GC-MSC-CM, cur-GC-MSC-CM, GC-MSC+PDTC-CM, GC-MSC+NA-VEGF, and GC-MSC+Ctrol-VEGF for 72 hrs, were seeded (2×105/well) into the upper chamber (8 mm) (Corning, NY, USA) of Transwell plate in serum-free medium. In the lower chamber, complete medium was placed as chemoattractant. Following incubation at 37°C in 5% CO2 for 10 hrs, the cells on the top of the polycarbonate membrane were wiped off using cotton swabs, while cells migrated to the lower surface of the membrane were fixed with methanol for 30 min. These migrated cells were later stained with crystal violet and counted in six random fields under the microscope (Olympus, Japan) during each assay.
Colony formation assay
After exposure of HUVEC cells with conditioned medium derived from different treatment groups of GC-MSCs for 72 hrs, they were trypsinized and suspended at a concentration of 2,000 cells/3 ml in high Glucose DMEM (HG-DMEM) media containing 10% FBS, and were incubated for two weeks. Later the cells were fixed with methanol for 30 min and stained with crystal violet dye for 15 min. The cell colonies were photographed and their numbers were counted.
Immunofluorescence staining
The GC-MSCs or cur-GC-MSC, along with HGC-27 cells treated with conditioned medium derived from each treatment group, were washed 3 times with cold PBS. Next, they were fixed with 4% paraformaldehyde for 20 min, and permeabilized with 0.1% Triton X-100 for 5 min. Later the antigen blocking was performed using 5% BSA (Boster Bioengineering, China), and later cells were incubated with anti-vimentin, anti-FAP, anti-α-SMA primary antibodies (Abcam, USA) at 4°C overnight. This was followed by incubation of these cells with Cy3-conjugated anti-rabbit secondary antibody (Sigma, USA). Finally, the cells were stained with DAPI for nuclear staining, and images were acquired using Nikon Eclipse Ti-S microscope.
Immunohistochemistry
Formalin-fixed paraffin-embedded mouse tumor tissue sections were first deparaffinized in xylene, and then rehydrated through graded ethanol. The antigen retrieval of the tissue sections was performed by boiling in citrate buffer (pH 6.0, 10 mM) for 10 min. The endogenous peroxidase activity was inhibited by exposing the tissue section to 3% hydrogen peroxide for 10 min. This was followed by blocking of the tissue sections with 5% BSA and then subsequent incubation with diluted VEGF primary antibody (1:200) at 37°C for 1 hr. After PBS washing, the sections were incubated with diluted secondary antibody for 20 min. Finally, the tissue sections were treated with 3,3’-diaminobenzidine (DAB) reagent and counterstained with hematoxylin for developing the staining signal, which was later examined under a light microscope (Olympus, Japan).
Animal study
Four to five-week-old BALB/c nude mice were purchased from Slac Laboratory Animal Center (Shanghai, China). All the animals were maintained according to the institutional policy, and study was performed with approval from the University Committee on Use and Care of Animals, Jiangsu University. Animals were randomly divided into 4 groups, and were injected subcutaneously with HGC-27 cell cultured with conditioned medium derived from variably treated GC-MSCs groups. Tumors were then surgically removed after 40 days of injection, photographed, and then sliced into tumor tissue sections for further immunohistochemistry analysis.
Statistical analyses
All experiments were conducted at least in triplicate, and data have been expressed as means ± SD. The data analysis has been performed using SPSS 11.0 software. The means from different treatment groups were compared by two-way ANOVA or Student’s t-test. P-value of < 0.05 represented statistically significant difference.
Results
Curcumin attenuated the expression of fibroblast proteins in GC-MSCs
The isolated GC-MSCs appeared as homogeneous population of fibroblast-like cells, and displayed spindle shape and plastic adherent characteristics (Figure 1A). In addition, high expression levels of fibroblast proteins, like α-SMA, vimentin (Figure 1A, 1B) were observed in these cells. Inter-estingly, curcumin (30 μmol/L) treatment of GC-MSCs, attenuated high expression of α-SMA protein. The immunofluorescence and western blot analysis revealed that the expression of α-SMA and vimentin proteins were decreased in curcumin treated GC-MSCs (Figure 1A, 1B).
Figure 1.
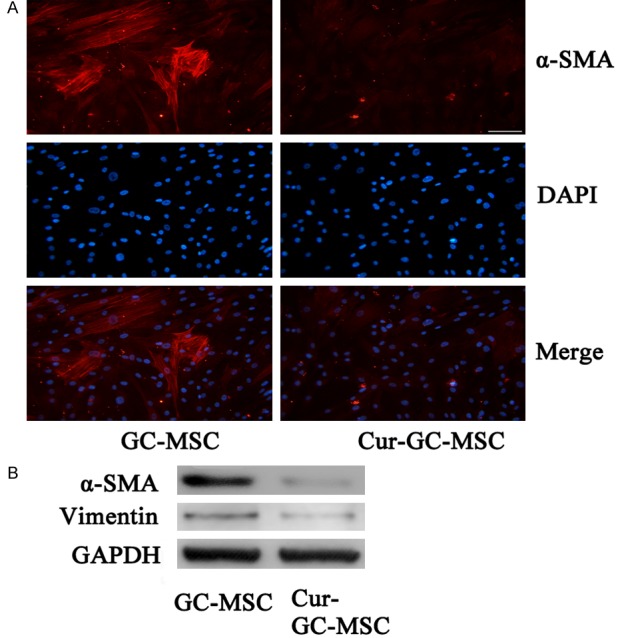
Curcumin attenuated the expression of fibroblast proteins in GC-MSCs. A. Immunofluorescence analyses of α-SMA protein in GC-MSCs ± curcumin treatment. Magnification, ×200; scale bar = 50 μm. B. Western blot analyses of α-SMA and Vimentin protein in GC-MSCs ± curcumin treatment (30 μmol/L).
Curcumin inhibited GC-MSC induced tube formation, migration and colony formation in HUVEC cells
We further tested the effect of curcumin on GC-MSCs mediated angiogenesis by analyzing HUVEC cells tube formation, migration and colony formation. As seen in Figure 2A, the HUVEC cells formed tubes after they were exposed to conditioned media from GC-MSCs (GC-MSC-CM). However, in parallel, when these HUVEC cells were treated with conditioned media from curcumin treated GC-MSCs (Cur-GC-MSC-CM), we did not observe the tube formation. Similarly, HUVEC cells cultured in GC-MSC-CM showed enhanced migration, whereas Cur-GC-MSC-CM attenuated their migration (Figure 2B). In addition, conditioned media from curcumin treated GC-MSCs also reduced HUVEC cell colony formation, in comparison to control treatment (Figure 2C).
Figure 2.
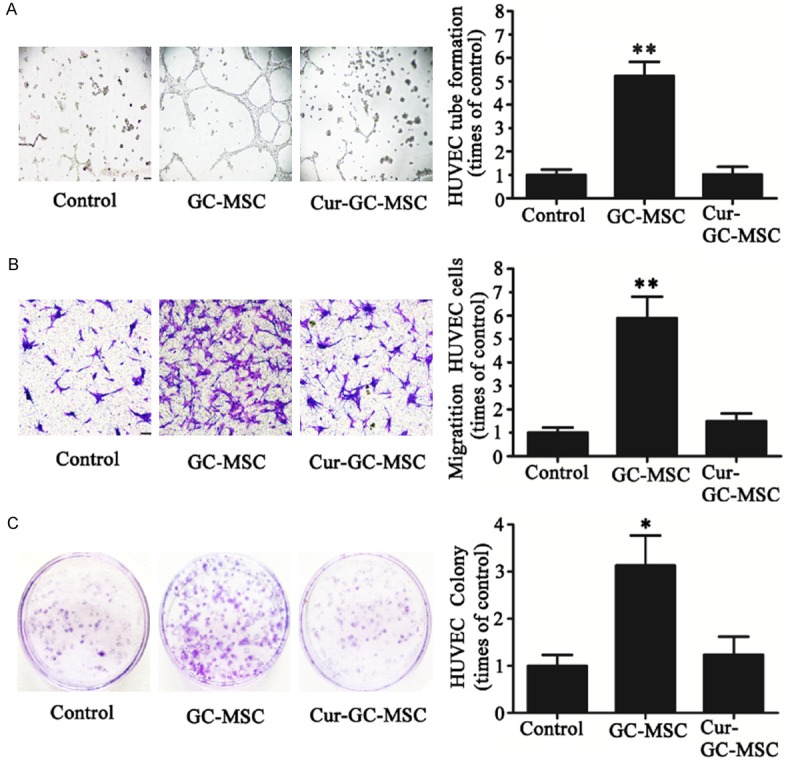
Curcumin inhibited GC-MSCs induced HUVECs tube formation, migration and colony formation. A. Human umbilical vein endothelial cells (HUVECs) were seeded on growth factor-reduced matrigel and stimulated for 12 hrs with either control culture medium, or conditioned media (CM) from GC-MSCs and cur-GC-MSCs. Representative images demonstrating the in vitro HUVECs tube formation. Bar graphs showing the quantifications of the tube formation assay (**P < 0.01). B. Representative images showing the migration of HUVEC cells cultured in conditioned media derived from GC-MSCs treated with or without curcumin. Magnification, ×100; scale bar = 50 μm (**P < 0.01). C. Colony formation assay showing the effect of curcumin on proliferation ability of GC-MSC-induced HUVEC cells. (**P < 0.01).
Curcumin abrogated NF-κB signaling and VEGF secretion/levels in GC-MSCs
To investigate the effect of curcumin on NF-κB signaling activity in GC-MSCs, we treated them with curcumin (30 μmol/L) for 2 hrs. Western blot analysis indicated significant increase in IκBα levels after curcumin treatment, while the phosphorylation of NF-κB (p-NF-κB) remarkably decreased, in comparison to control treatment (Figure 3A). In addition, we also assessed the effect of curcumin on VEGF secretion in GC-MSCs. ELISA analyses revealed higher levels of VEGF in GC-MSCs. However, curcumin treatment reduced VEGF levels (Figure 3B). Similarly, immunohistochemical analysis also confirmed that VEGF protein levels were remarkably inhibited by curcumin treatment in GC-MSCs (Figure 3C).
Figure 3.

Curcumin abrogated NF-κB signaling activity and VEGF production in GC-MSCs. A. Western blot analysis showing the expression pattern of IκBα and NF-κB phosphorylation (p-NF-κB) in GC-MSCs treated with or without curcumin. B. ELISA based assessment of VEGF levels in conditioned media from GC-MSCs treated with or without curcumin (**P < 0.01). C. Immunohistochemical analysis of VEGF protein expression in GC-MSC treated with or without curcumin.
NF-κB/VEGF signaling positively contributed into GC-MSCs mediated angiogenesis
In order to decipher if NF-κB/VEGF signaling was important in GC-MSCs mediated angiogenesis, we specifically pretreated GC-MSCs with NF-κB inhibitor PDTC (20 μM) for 2 hrs and collected the conditioned medium. In another set, we added either a neutralizing antibody against VEGF (NA-VEGF) or an isotype-matched normal antibody (CtrolA-VEGF) in the conditioned media from GC-MSCs. Subsequent assay using HUVEC cells, showed notable inhibition in their tube formation ability, when exposed to conditioned media from GC-MSCs treated with either PDTC or NA-VEGF, in comparison to CtrolA-VEGF (Figure 4A). Similar trends were also observed in Transwell migration and cell colony formation assays involving HUVEC cells (Figure 4B, 4C). Furthermore, western blot analysis showed that HUVEC cells cultured in conditioned media from GC-MSCs treated with PDTC and NA-VEGF, had reduced cyclinD and cyclinE expression. Also, the expression of other antiapoptotic proteins, like BCL-XL & BCL-2, along with cell proliferation protein PCNA was decreased in these HUVEC cells, in comparison to HUVECs cultured with conditioned media from GC-MSCs with no treatment. The expression of all these proteins hardly changed in HUVEC cell cultured with conditioned media from GC-MSCs treated with control antibody (Figure 4D). Overall, these results indicated that NF-κB/VEGF signaling is critical for GC-MSC derived angiogenesis.
Figure 4.

NF-κB/VEGF signaling regulated GC-MSC-induced tube formation, migration and colony formation in HUVEC cells. A. Bar graphs showing quantifications of tube formation in HUVEC cells cultured in conditioned media from GC-MSCs treated with either NF-κB inhibitor PDTC (20 μM) or VEGF-specific blocking antibody or no treatment or treated with control antibody (**P < 0.01). B. Transwell Migration Assay showing the migration ability of HUVECs cultured in conditioned media from GC-MSCs treated either with NF-κB inhibitor, PDTC or VEGF-specific blocking antibody (**P < 0.01). C. Cell colony formation assay showing the effect of NF-κB inhibitor, PDTC and VEGF-specific blocking antibody on HUVEC proliferation GC-MSC-induced (*P < 0.05). D. Western blot analysis of the effects of NF-κB inhibitor PDTC and VEGF-specific blocking antibody on PCNA, cyclinD/cyclinE and antiapoptotic proteins BCL-XL, BCL-2 in HUVECs.
NF-κB/VEGF signaling in GC-MSCs induced both in vitro and in vivo VEGF expression in gastric cancer cells
Finally, we tested the contribution of NF-κB/VEGF signaling in GC-MSCs, to regulate VEGF expression in gastric cells. The VEGF expression, as assessed by immunofluorescent staining in HGC-27 gastric cancer cells, was reduced when these cells were cultured in conditioned media from GC-MSCs treated with either PDTC or NA-VEGF, in contrast to GC-MSC alone. However, the VEGF expression was not changed in HGC-27 cells exposed to conditioned media from GC-MSCs treated with control antibody (Figure 5A). Further to evaluate the effect of NF-κB/VEGF signaling on VEGF expression of gastric cancer cells in vivo, we performed an animal experiment. HGC-27 cells treated with conditioned media from GC-MSCs, GC-MSCs+PDTC, GC-MSCs+NA-VEGF, and GC-MSCs+Ctrol-antibody groups, were injected into mice to form xenograft tumors. Later the VEGF expression was analyzed by immunohistochemical staining in tumor tissue sections from each group. Representative immunohistochemical images showed markedly less number of VEGF-positive cells with reduced intensity in tumor sections from CM/GC-MSC+PDTC, CM/NA-VEGF groups, as compared to CM/GC-MSC and CM/Ctrol-VEGF groups (Figure 5B). The overall percentages of VEGF-positive cells from different groups were consistent with observed immunohistochemistry pattern (Figure 5C).
Figure 5.

NF-κB/VEGF signaling regulated GC-MSCs induced VEGF expression in gastric cancer cells in vitro and in vivo. A. Immunofluorescence staining showing the expression level of VEGF in HGC-27 cells cultured in conditioned media from GC-MSCs, GC-MSCs+PDTC, GC-MSCs+NA-VEGF and GC-MSCs+Ctrol-VEGF. Magnification, ×200; scale bar, 50 μm. B. Immunohistochemical analysis of VEGF expression in xenograft tumor tissues derived from HGC-27 cells cultured before injection, in conditioned media from GC-MSCs, GC-MSCs+PDTC, GC-MSCs+NA-VEGF and GC-MSCs+Ctrol-VEGF. Magnification, ×100; scale bar, 50 μm. C. Analysis of the percentage of VEGF-positive cells from different groups (**P < 0.01).
Discussion
Curcumin has been shown to be a promising antitumor compound that inhibits tumor progression [20,21]. Also multiple studies have indicated about the positive association between mesenchymal stem cells and solid tumor progression [22,23]. Since, there are no studies linking curcumin to its regulation of cancer derived mesenchymal stem cells, we in our study have thus tried to understand this link. Our results indicated that GC-MSC (gastric cancer associated stroma cells) typically express high levels of fibroblast proteins, α-SMA and vimentin, and curcumin treatment attenuated this high expression level, thereby indicating altered cellular function of these cells. In general, MSCs have been shown to promote tumor growth by stimulating tumor cell proliferation, inducing epithelial-mesenchymal transition of tumor cells, supporting the increase of cancer stem cells, and promoting tumor angiogenesis and metastasis [24-26]. In addition, as tumor growth specifically require formation of new blood vessels for additional nutrition and oxygen, and tumor metastasis and invasion also seems to be dependent on angiogenesis [27,28], we further tried to elucidate the link between curcumin and regulation of GC-MSCs mediated angiogenesis. Here, we observed that GC-MSCs indeed enhanced HUVECs tube formation, migration and colony formation ability, which was consistent with supporting role of MSCs in angiogenesis. However, curcumin treatment of GC-MSCs inhibited their pro-angiogenesis role.
To further investigate the specific mechanism of curcumin mediated inhibition of GC-MSC-driven angiogenesis, we initially focused on NF-κB signaling pathway. This pathways has been shown to be regulated by curcumin [29]. As activation of NF-κB signaling has been frequently associated with tumor progression, specifically in inflammatory cancers [18], we thus tested if this pathway played any role. Moreover, from angiogenesis perspective, the role of vascular endothelial growth factors (VEGFs) could also not be overlooked, as it directly stimulated the formation of new blood vessels [30,31]. So finally we focused our attention on understanding the role and contribution of both NF-κB and VEGF pathways, in curcumin mediated inhibition of GC-MSCs driven angiogenesis. Our data did indicate that curcumin treatment abrogated NF-κB signaling activity and VEGF production in GC-MSC. Further to establish a link between these signaling pathways and angiogenesis regulation, we specifically inhibited these pathways in GC-MSCs, either by specific NF-κB inhibitor (PTDC) or VEGF neutralizing antibody. The conditioned media from GC-MSCs treated with these inhibitors was used to culture HUVEC cells, and then test their ability to form tubes and colonies. Notably, the tube formation ability, transwell migration, and cell colony formation capacity of these HUVEC cells was inhibited by PDTC and VEGF-specific blocking antibody groups. These observations led us to conclude that curcumin indeed abrogated the ability of cancer-derived mesenchymal stem cells to drive HUVEC tube formation, migration and colony formation, through inhibiting NF-κB/VEGF signaling.
Interestingly, it has been observed that to facilitate angiogenesis, sometimes tumor cells directly release angiogenic factors like VEGFs to stimulate formation of new blood vessels [32]. VEGF signaling in tumor cells markedly affects tumor cell function and can be an essential component of VEGF-mediated angiogenesis and vascular permeability [33]. Our immunofluorescent analysis revealed that VEGF expression in HGC-27 gastric cancer cells was decreased after these cells were cultured in conditioned media from GC-MSCs treated with PDTC and NA-VEGF. However, VEGF expression in HGC-27 cells did not change significantly, when these cells were cultured in conditioned media from GC-MSCs treated with control antibody. These results were consistent with VEGF immunohistochemical staining pattern observed in tissue sections from different treatment groups. Thus, it illustrated that NF-κB/VEGF signaling also governed the GC-MSC-induced VEGF expression in gastric cancer cells both in vitro and in vivo.
In summary, we showed that curcumin inhibited not only the NF-κB/VEGF signaling in GC-MSCs, but also their ability to drive HUVECs tube formation, migration and colony formation. Furthermore, we also confirmed the direct involvement of NF-κB and VEGF signaling in GC-MSCs driven angiogenesis by using NF-κB inhibitor, PDTC and VEGF neutralizing antibody. This led us to conclude that curcumin could inhibit GC-MSCs driven angiogenesis by inhibiting NF-κB/VEGF signaling. Therefore, targeting of GC-MSCs driven angiogenesis by curcumin may represent a novel therapeutic strategy for gastric cancer progression.
Acknowledgements
This work was supported by Social Development Science and Technology Special Project to KunShan (Grant No. KS1645), and Natural Science Fund of Education Department of Anhui province (Grant No. KJ2017A226).
Disclosure of conflict of interest
None.
References
- 1.Wu S, Singh S, Varney ML, Kindle S, Singh RK. Modulation of CXCL-8 expression in human melanoma cells regulates tumor growth, angiogenesis, invasion, and metastasis. Cancer Med. 2012;3:306–317. doi: 10.1002/cam4.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Contois LW, Akalu A, Caron JM, Tweedie E, Cretu A, Henderson T, Liaw L, Friesel R, Vary C, Brooks PC. Inhibition of tumor-associated αvβ3 integrin regulates the angiogenic switch by enhancing expression of IGFBP-4 leading to reduced melanoma growth and angiogenesis in vivo. Angiogenesis. 2015;18:31–46. doi: 10.1007/s10456-014-9445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist. 2015;20:660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, Yang YH, Basile JR. The Semaphorin 4D-Plexin-B1-RhoA signaling axis recruits pericytes and regulates vascular permeability through endothelial production of PDGF-B and ANGPTL4. Angiogenesis. 2014;17:261–274. doi: 10.1007/s10456-013-9395-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang JR, Cho YJ, Lee Y, Park Y, Han HD, Ahn HJ, Lee JH, Lee JW. The C-terminus of IGFBP-5 suppresses tumor growth by inhibiting angiogenesis. Sci Rep. 2016;6:39334. doi: 10.1038/srep39334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang KS, Li J, Wang Z, Mi C, Ma J, Piao LX, Xu GH, Li X, Jin X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol Immunotoxicol. 2017;39:28–36. doi: 10.1080/08923973.2016.1267744. [DOI] [PubMed] [Google Scholar]
- 8.Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrowmesenchymal stem cells as key players. Biochim Biophys Acta. 2013;1836:321–335. doi: 10.1016/j.bbcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Huang F, Yao Y, Wu J, Yu L, Wu S, Pu X, Xu L, Wang M, Xia L. Effect of mesenchymal stem cell derived exosomes carrying PDGFD on lung cancer. Int J Clin Exp Pathol. 2017;10:224–232. [Google Scholar]
- 10.Breznik B, Motaln H, Vittori M, Rotter A, Turnšek TL. Mesenchymal stem cells differentially affect the invasion of distinct glioblastoma cell lines. Oncotarget. 2017;8:25482–25499. doi: 10.18632/oncotarget.16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Huang F, Wang M, Yang T, Cai J, Zhang Q, Sun Z, Wu X, Zhang X, Zhu W, Qian H, Xu W. Gastric cancer-derived MSC-secreted PDGF-DD promotes gastric cancer progression. J Cancer Res Clin Oncol. 2014;140:1835–1848. doi: 10.1007/s00432-014-1723-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, Wang S, Wu X, Yang T, Huang F, Cai J, Zhu Q, Zhu W, Qian H, Xu W. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110:1199–1210. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park CH, Hahm ER, Park S, Kim HK, Yang CH. The inhibitory mechanism of curcumin and its derivative against beta-catenin/Tcf signaling. FEBS Lett. 2005;579:2965–2971. doi: 10.1016/j.febslet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Xiang ST, Zhang QH, Wu JJ, Tang Q, Zhou JF, Yang LJ, Chen ZQ, Hann SS. Combination of curcumin and bicalutamide enhanced the growth inhibition of androgen-independent prostate cancer cells through SAPK/JNK and MEK/ERK1/2-mediated targeting NF-κB/p65 and MUC1-C. J Exp Clin Cancer Res. 2015;34:46. doi: 10.1186/s13046-015-0168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D, Breuhahn K, Conner EA, Galle PR, Andersen JB, Factor VM, Thorgeirsson SS. Curcumin effectively inhibits oncogenic NF-kB signaling and restrains stemness features in liver cancer. J Hepatol. 2015;63:661–669. doi: 10.1016/j.jhep.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Y, Long Q, Zhang L, Shi Y, Liu X, Li X, Guan B, Tian Y, Wang X, Li L, He D. Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling. International Journal of Oncology. 2015;47:2064–2072. doi: 10.3892/ijo.2015.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taverna S, Giallombardo M, Pucci M, Flugy A, Manno M, Raccosta S, Rolfo C, De Leo G, Alessandro R. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget. 2015;6:21918–21933. doi: 10.18632/oncotarget.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thacker PC, Karunagaran D. Curcumin and emodin down-regulate TGF-β signaling pathway in human cervical cancer cells. PLoS One. 2015;10:e0120045. doi: 10.1371/journal.pone.0120045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liotta F, Querci V, Mannelli G, Santarlasci V, Maggi L, Capone M, Rossi MC, Mazzoni A, Cosmi L, Romagnani S, Maggi E, Gallo O, Annunziato F. Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br J Cancer. 2015;112:745–754. doi: 10.1038/bjc.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan XL, Jia YL, Chen L, Zeng Q, Zhou JN, Fu CJ, Chen HX, Yuan HF, Li ZW, Shi L, Xu YC, Wang JX, Zhang XM, He LJ, Zhai C, Yue W, Pei XT. Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: role of the S100A4-miR155-SOCS1-MMP9 axis. Hepatology. 2013;57:2274–2286. doi: 10.1002/hep.26257. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z, Wang S, Zhao RC. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J Hematol Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johann PD, Muller I. Multipotent mesenchymal stromal cells: possible culprits in solid tumors? Stem Cells Int. 2015;2015:914632. doi: 10.1155/2015/914632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabashima-Niibe A, Higuchi H, Takaishi H, Masugi Y, Matsuzaki Y, Mabuchi Y, Funakoshi S, Adachi M, Hamamoto Y, Kawachi S, Aiura K, Kitagawa Y, Sakamoto M, Hibi T. Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci. 2013;104:157–164. doi: 10.1111/cas.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu Z, Chen X, Guan S, Yan Y, Lin H, Hua ZC. Curcumin inhibits angiogenesis and improves defective hematopoiesis induced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling pathway. Oncotarget. 2015;6:19469–19482. doi: 10.18632/oncotarget.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35:75–91. doi: 10.1007/s10555-016-9618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS One. 2013;8:e57218. doi: 10.1371/journal.pone.0057218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, Eliceiri BP, Reisfeld RA. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8:1369–1375. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- 31.Yamagishi N, Teshima-Kondo S, Masuda K, Nishida K, Kuwano Y, Dang DT, Dang LH, Nikawa T, Rokutan K. Chronic inhibition of tumor cell-derived VEGF enhances the malignant phenotype of colorectal cancer cells. BMC Cancer. 2013;13:229. doi: 10.1186/1471-2407-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen L, You XL, Al Moustafa AE, Batist G, Hynes NE, Mader S, Meloche S, Alaoui-Jamali MA. Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis. Oncogene. 2000;19:3460–3469. doi: 10.1038/sj.onc.1203685. [DOI] [PubMed] [Google Scholar]
- 33.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]


