Abstract
Apoptosis is a process of programmed cell death that occurs in multicellular organisms. The mitochondrial pathway plays a paramount role in apoptosis. In this study, the expression levels of key factors in the mitochondrial pathway and the cell proliferation factor (PCNA) were measured to evaluate the level of apoptosis and proliferation in keloid scars, physiological scars and normal skin tissue. Thirty samples were taken from 30 patients: 10 keloid patients, 10 physiological scar patients and 10 patients without obvious scarring. All 30 patients were selected randomly from the Department of Plastic Surgery at Peking Union Medical College Hospital from June 2016 to December 2016. Hematoxylin and eosin staining and Masson staining were used to observe the differences in histology and fiber tissue content. Mitochondrial pathway factors (caspase-3, caspase-8, caspase-9, Bcl-2, Bax, cytochrome-c) and PCNA expression levels were detected by immunohistochemistry and were analyzed as the percentage of positively stained cells in the epidermis and dermis. Relative protein expression levels were measured by western blotting. Compared with physiological scars and normal skin tissue, keloid tissue had an increase in fiber number and decrease in cell content. In our immunohistochemical and western blot analyses, all tissue types showed similar expression levels of the mitochondrial pathway factors. However, the percentage of PCNA-positive cells and the relative protein expression level of PCNA were significantly higher in keloid tissue. Keloid has a similar apoptosis level as physiological scars and normal skin but has a higher expression of PCNA, indicating that keloid scars have high levels of proliferation and normal apoptosis.
Keywords: Keloid, physiological scar, skin, apoptosis, proliferation
Introduction
Scars are areas of fibrous tissue that replace normal skin after an injury, and are a natural part of the healing process. A keloid is a pathological scar, considered to be a result of abnormal wound healing, that presents with excessive scar tissue beyond the area of the original skin injury, and occurs in predisposed individuals [1]. Keloid scars are benign and not contagious, but are often accompanied by severe itchiness, pain, functional limitations and disfigurement [2], which may seriously affect a patient’s quality of life.
Apoptosis is a process of programmed cell death [3]. Specific proteins, signaling pathways and enzymes are involved in cell apoptosis, and the mitochondrial pathway also plays an important role. Caspase-8 receives the cell apoptosis signal from FasL/Fas (CD95 L/CD95) and assembles Bax on the mitochondrial membrane, leading to the release of cytochrome-c (cyt-c) from the mitochondria into the cytoplasm, which activates caspase-9 and caspase-3 to manage the apoptosis process [4,5].
Keloids, physiological scars and normal skin are different types of skin tissue of varying morphological and histological appearance. In 1996, Appleton [6] first reported the degree of apoptosis and proliferation in keloid tissue. Since then, most studies have focused on apoptosis in keloid fibroblasts in vitro. However, no study has concentrated on the mitochondrial apoptosis pathway in keloid tissue, or compared keloids with physiological scars and normal skin tissue to elucidate their differences. In this study, we focused on the expression of key factors in the mitochondrial apoptosis pathway (caspase-3, caspase-8, caspase-9, Bcl-2, Bax and Cyt-c) and of proliferating cell nuclear antigen (PCNA), to assess the level of apoptosis and proliferation among keloids, physiological scars and normal skin tissues.
Materials and methods
Patients, grouping and sample management
The study protocol was reviewed and approved by the Bioethical Committee of Peking Union Medical College Hospital. All patients provided informed consent. Thirty patients were randomly selected from the Department of Plastic Surgery at Peking Union Medical College Hospital (from June 2016 to December 2016): 10 patients with keloids (5 female and 5 male), 10 patients with physiological scars (5 female and 5 male) and 10 patients without any obvious scars (5 female and 5 male). The average ages ranged from 19 to 54 years old (average age ± standard deviation [SD], K: 35.60 ± 9.02 years; C: 36.30 ± 10.83 years; S: 35.60 ± 9.94 years). There were no significant differences in age, gender and scar site between groups (P>0.05). The keloids and physiological scars were caused by trauma, diagnosed by at least three experienced plastic surgeons and confirmed through pathological examination. No patients had any known systemic diseases, were on drug treatment or were receiving other treatment that might affect the study results.
Specimens (Figure 1) were taken from the central part of the keloid, physiological scar or normal skin tissue. Excess fat tissue and hair were removed, and the samples were washed in phosphate-buffered saline (PBS) three times to clean off any blood. Samples were divided into two parts for further analysis. For hematoxylin and eosin (H&E) staining, Masson staining and immunohistochemical analysis, one part was placed in 10% formalin solution for 48 hours before being embedded in paraffin. The other part was stored in 2 ml tubes frozen in liquid nitrogen, for protein extraction and western blot assessments.
Figure 1.
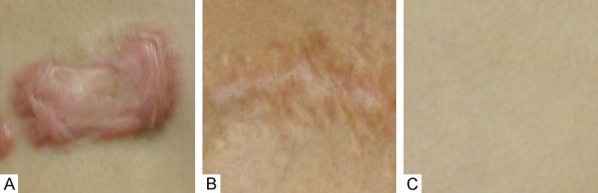
Sampling conditions of the 3 different groups of patients: A: Keloid tissue from keloid patients; B: Physiological scar tissue from scar patients; C: Normal skin tissue from patients without obvious scarring.
H&E and Masson staining
After paraffin embedding, specimens were sectioned and mounted on slides. For H&E and Masson staining, three slides from each sample were used to evaluate the morphological and histological appearance of the three groups (90 slides were stained with either H&E or Masson for analysis).
For H&E staining, all 90 slides were dewaxed in a slide drier (70°C for 30 min) and then washed twice in xylene for 30 min (15 min each). After being rehydrated in an ethanol gradient (100%, 95%, 85%, 75% and deionized water, 3 min each), the slides were stained with hematoxylin for 5 min and differentiated by 1% hydrochloric acid for 10 s. Slides were then washed in running water for 15 min to show the blue stain and counterstained with eosin for 1 min. Next, the slides were dehydrated with an alcohol gradient (backward concentration) and xylene and mounted. A Masson staining kit (Heart Biological Technology, Xian, China) was used to identify cell and fiber content. The procedures were conducted according to the kit instructions. In the H&E-stained sections, the cytoplasm and other components were red, and the nuclei were stained blue. In the Masson staining, cells were stained in red, and fibrils were stained blue.
Immunohistochemical analysis
Three slides of each factor for each sample were used in the immunohistochemical analysis (90 slides in total for each single factor). Slides were routinely dewaxed and rehydrated according to the procedures above and incubated in the dark for 10 min in 3% H2O2 to block endogenous catalase. For antigen retrieval, unstained slides were heated in citrate buffer to 95°C for 15 min. After cooling naturally, slides were incubated with normal goat serum at 37°C for 30 min to block non-specific staining. Then, slides were incubated with anti-caspase-3 (1:200, Abcam, Cambridge, United Kingdom), anti-caspase-8 (1:100, Abcam), anti-caspase-9 (1:100, Abcam), anti-Bcl-2 (1:100, Abcam), anti-Bax (1:100, Abcam), anti-cyt-c (1:100, Abcam) or anti-PCNA (1:100, Abcam) antibodies in a humidified chamber at 4°C overnight (12-16 hours). After washing with PBS three times (3 min each), the slides were incubated with horseradish peroxidase-conjugated secondary antibody (ZSGB-BIO, Beijing, China) at 37°C to mark the primary antibody (incubation time was based on secondary antibody instructions). Then, slides were rinsed with PBS (three times, 3 min each), stained with 3,3’-diaminobenzidine (DAB, 1×), counterstained with hematoxylin (5 min), and differentiated in 1% hydrochloric acid for 10 s. Slides were observed by microscopy with a computer-controlled digital camera and imaging software. Positive cells were measured by estimating the color: brown staining implied a positive expression area and the shade of the color represented the expression level of the target factor. The procedure was modified according to Zhang’s study [7].
Western blot assessment
Either the Tissue or Cell Total Protein Extraction Kit (Bio-Rad Laboratories, Hercules, CA, USA) was used to extract protein from 50-mg samples according to the manufacturer’s instructions. Samples were incubated on ice for 10 min in cell lysis buffer (246 μl of lysis buffer, 1.25 μl of phosphatase inhibitor, 0.25 μl of protease inhibitor, 2.5 μl of PMSF) and then centrifuged (4°C, 14000 rpm, 15 min). Equal amounts of supernatant protein (60 μg) were separated on a 10% SDS-PAGE gel and transferred to nitrocellulose membranes for immunoblotting (transfer time was based on the molecular weight of the protein of interest and ranged from 40 to 150 mins). Then, the membranes were blocked with 5% blocking buffer (Li-cor, Lincoln, NE, USA) for 2 hours at room temperature on a shaker and incubated with anti-caspase-3 (1:500, Abcam), anti-caspase-8 (1:500, Abcam), anti-caspase-9 (1:500, Abcam), anti-Bcl-2 (1:500, Abcam), anti-Bax (1:500, Abcam), anti-cyt-c (1:500, Abcam) or anti-PCNA (1:500, Abcam), antibodies at 4°C overnight (12-16 hours) on a shaker. The membranes were washed with TBST (TBS buffer + Tween 20, three times, 5 min each), incubated with secondary antibodies (Li-cor) at a 1:10000 dilution in the dark for 1 hour at room temperature on a shaker and then flushed with TBST (three times, 5 min each) and TBS (once, 5 min). The membranes were imaged with a double-color infrared laser imaging system (Odyssey, Li-cor). The procedure was modified according to Zhang’s study [7].
Statistical analysis
Study data are presented as means ± SD. SPSS 24.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Statistical significance was set at P<0.05. Analysis was by one-way analysis of variance (ANOVA) followed by least significant difference t-test.
Results
Morphological and histological observations
Morphological and histological differences were observed by H&E and Masson staining. Compared with the other groups, the epidermis was relatively thicker, the dermis had an increased number of bulky collagen fibrils that were arranged intricately and closely, and there was less cellular content in the K group. However, the dermis of group C contained slender collagen fibrils that were distributed more loosely. Collagen fibrils constituted the majority of physiological scars. In contrast, the normal skin dermis showed more cellular content and less fibrils, and were arranged relatively loosely compared with the K and C groups. In addition, inflammatory cell infiltration could barely be observed in the C and S groups, but more inflammatory cells were found in the keloid dermis (Figure 2).
Figure 2.
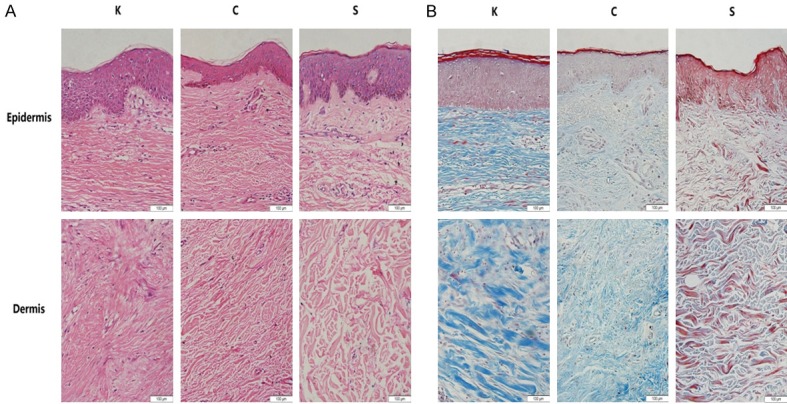
The results of epidermal and dermal H&E staining (A) and of Masson staining (B) in all groups (200×). In the K group, the epidermis was thicker, there were an increased number of bulky collagen fibrils intricately arranged in the dermis, and there was a reduction in cellular content. The C group had slender collagen fibrils which composed the majority of the dermis. In contrast, normal skin dermis showed increased cellular content and less fibrils, which were relatively loosely arranged compared with that of the K and C group.
Immunohistochemical studies
Immunohistochemical staining qualitatively reflects the expression of key factors in the mitochondrial apoptosis pathway, such as caspase-3, caspase-8, caspase-9, Bcl-2, Bax and cyt-c, and the expression of the proliferation factor PCNA. In the keloid epidermal layer, caspase-3, caspase-9 and Bax showed higher expression levels; however, caspase-8, Bcl-2 and cyt-c expression levels were similar in the C and S groups. PCNA expression was much higher in the keloid epidermal layer. In the keloid dermal layer, Bcl-2 and Bax showed higher expression levels; however, caspase-3, caspase-8, caspase-9 and cyt-c expression levels were similar in the C and S groups. Dermal PCNA expression was substantially higher in the K group (Figure 3).
Figure 3.
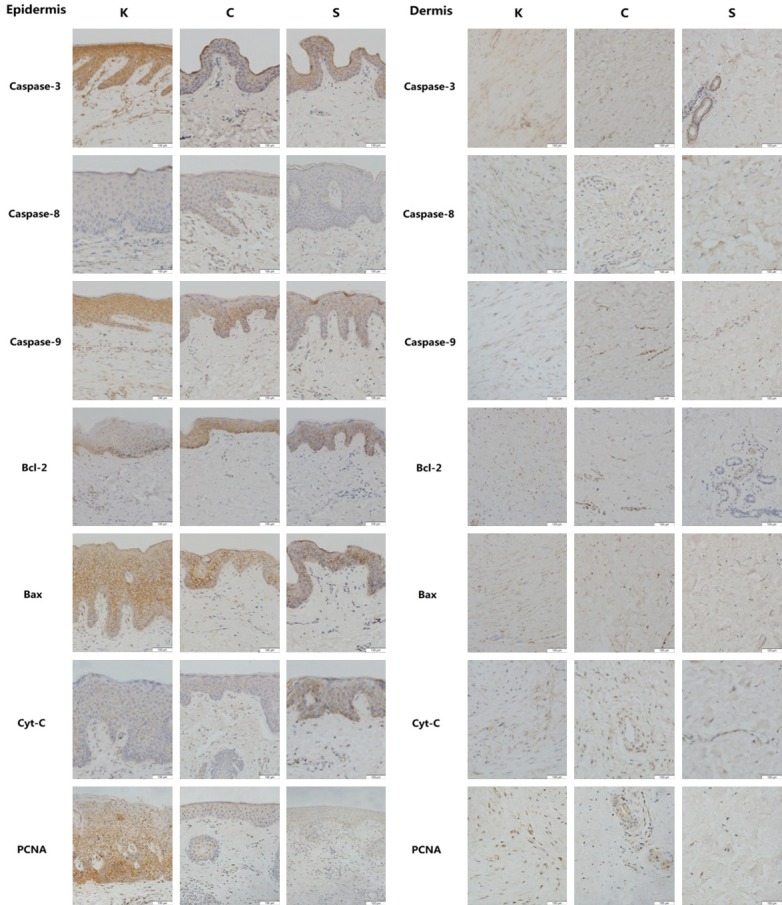
Representative immunohistochemistry micrographs (200×) of epidermal and dermal skin tissue for caspase-3, caspase-8, caspase-9, Bcl-2, Bax, cyt-c and PNCA in all groups. Brown staining indicates the positive expression areas, and the shade of the color represents the expression level of the target protein. In the epidermal layer of the K group, caspase-3, caspase-9 and Bax showed higher expression levels; however, caspase-8, Bcl-2 and cyt-c expression levels were similar to those of the C and S group. PCNA expression was much higher in the keloid epidermal layer than in the non-keloid tissues. In the K dermal layer, Bcl-2 and Bax showed higher expression levels than those in the non-keloid tissues; however, caspase-3, caspase-8, caspase-9 and cyt-c expression levels were similar to those of the C and S group. Dermal PCNA expression was remarkably higher in the K group than in the non-keloid tissues.
The percentage of positive cells shown by the immunohistochemical staining was calculated and statistically evaluated (Figure 4; Table 1). Caspase-3 showed a higher expression level in the keloid epidermal layer. The percentage of positive caspase-3 cells was not significantly different among the three groups and neither was the percentage of Bax-positive cells in the epidermal and dermal layers. However, the percentage of epidermal caspase-9-positive and dermal Bcl-2-positive cells was significantly different between the keloid and non-keloid groups, which was consistent with the immunohistochemical staining results. Other key factors in the mitochondrial apoptosis pathway showed differences among the groups, but none of them were significant. The percentage of PCNA-positive cells in both the epidermal and dermal layers showed substantial differences between the keloid group and non-keloid groups.
Figure 4.

Results of the percentage of positive cells. In both the epidermis and the dermis, the expression levels of most key factors of the mitochondrial pathway showed no significant differences between the K group and those of the other groups, except for caspase-9 in the epidermis and Bcl-2 in the dermis. The percentage of PCNA-positive cells in the keloid tissue was significantly higher than in the non-keloid tissues. Values are shown as the means ± SD; (n=10 in each group; *P<0.05, **P<0.01, ***P<0.001 vs. the K group).
Table 1.
Percentage of immunohistochemical staining positive cells in each group
| Factor | Percentage of positive cells | P value | |||
|---|---|---|---|---|---|
|
| |||||
| K | C | S | |||
| Caspase-3 | Epidermis | 80.9443 ± 11.7670 | 67.3050 ± 17.2652 | 76.3617 ± 10.9651 | P>0.05 |
| Dermis | 55.3417 ± 13.5683 | 55.7233 ± 18.5395 | 49.4783 ± 10.7742 | P>0.05 | |
| Caspase-8 | Epidermis | 53.0233 ± 20.4949 | 52.2617 ± 10.5576 | 51.6267 ± 7.9021 | P>0.05 |
| Dermis | 46.2633 ± 6.9158 | 46.2300 ± 13.5720 | 40.9833 ± 8.8916 | P>0.05 | |
| Caspase-9 | Epidermis | 81.7783 ± 11.8048 | 69.1783 ± 8.2896 | 61.8250 ± 4.9463 | K vs. C, P<0.05 |
| K vs. S, P<0.01 | |||||
| Dermis | 36.4517 ± 11.0440 | 35.4333 ± 7.9662 | 32.4850 ± 6.7351 | P>0.05 | |
| Bcl-2 | Epidermis | 38.0183 ± 9.2773 | 47.1867 ± 15.4039 | 44.9933 ± 13.4954 | P>0.05 |
| Dermis | 58.3417 ± 11.5927 | 44.2400 ± 6.9644 | 42.1333 ± 5.4587 | K vs. C, P<0.05 | |
| K vs. S, P<0.01 | |||||
| Bax | Epidermis | 76.1283 ± 17.2829 | 73.8200 ± 16.8697 | 70.0883 ± 21.1146 | P>0.05 |
| Dermis | 50.9450 ± 11.9813 | 45.0900 ± 13.1078 | 40.9417 ± 7.1781 | P>0.05 | |
| Cyt-C | Epidermis | 59.4183 ± 8.4284 | 58.5417 ± 12.8049 | 60.7667 ± 5.9783 | P>0.05 |
| Dermis | 63.1500 ± 7.5588 | 69.7633 ± 11.2946 | 65.1450 ± 7.1470 | P>0.05 | |
| PCNA | Epidermis | 90.3867 ± 4.6494 | 44.8450 ± 10.0372 | 33.5750 ± 17.2094 | K vs. C & S, P<0.001 |
| Dermis | 79.0317 ± 11.6562 | 43.9517 ± 15.2434 | 37.3883 ± 12.4482 | K vs. C & S, P<0.001 | |
Values are means ± SD.
Expression of mitochondrial pathway factors and PCNA protein
Western blotting was used for quantitatively analyzing the protein expression levels of mitochondrial pathway factors and PCNA. In these results, there was a significant difference in caspase-3 protein expression between the K and S groups and in caspase-8 expression between the keloid and non-keloid groups. PCNA expression was substantially and significantly different between the keloid and non-keloid groups. However, no significant differences were found in the protein expression levels of other factors of the mitochondrial apoptosis pathway among the three groups (Figure 5; Table 2).
Figure 5.
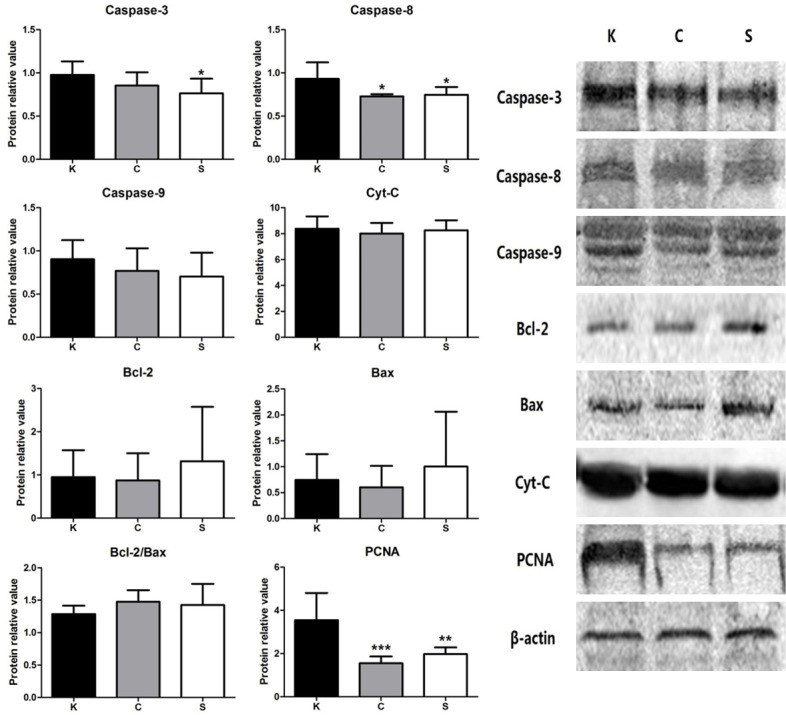
Relative protein amounts for all target proteins. The expression levels of most key factors in the mitochondrial apoptosis pathway were not significantly different between the K group and the other groups, except caspase-3 and caspase-8. The highest expression level of PCNA was in the K group. Representative images of the western blots for caspase-3, caspase-8, caspase-9, Bcl-2, Bax, cyt-c and PCNA are shown on the right. Values are shown as the means ± SD; (n=10 in each group; *P<0.05, ***P<0.001 vs. the K group).
Table 2.
Protein relative value in all groups
| Factor | Protein relative value | P value | ||
|---|---|---|---|---|
|
| ||||
| K | C | S | ||
| Caspase-3 | 0.9771 ± 0.1564 | 0.8531 ± 0.1538 | 0.7634 ± 0.1702 | K vs. S, P<0.05 |
| Caspase-8 | 0.9306 ± 0.1917 | 0.7298 ± 0.0245 | 0.7471 ± 0.0894 | K vs. C and S, P<0.05 |
| Caspase-9 | 0.9030 ± 0.2216 | 0.7693 ± 0.2611 | 0.7035 ± 0.2763 | P>0.05 |
| Bcl-2 | 0.9522 ± 0.6222 | 0.8742 ± 0.6263 | 1.3152 ± 1.2608 | P>0.05 |
| Bax | 0.7454 ± 0.4978 | 0.6035 ± 0.4125 | 1.0041 ± 1.0581 | P>0.05 |
| Bcl-2/Bax ratio | 1.2877 ± 0.1286 | 1.4752 ± 0.1803 | 1.4264 ± 0.3266 | P>0.05 |
| Cyt-C | 8.3758 ± 0.9523 | 8.0049 ± 0.8225 | 8.2587 ± 0.7709 | P>0.05 |
| PCNA | 3.5482 ± 1.2597 | 1.5505 ± 0.3113 | 1.9752 ± 0.3105 | K vs. C, P<0.001 |
| K vs. S, P<0.01 | ||||
Values are means ± SD.
Discussion
Keloids are considered a result of pathological wound healing after injury and inflammation [8-10]. The main symptoms, such as itchiness (86% [11]), pain (46% [11]), functional limitations and significant psychological morbidities, seriously affect the quality of life of keloid patients [12,13]. Unlike hypertrophic or physiological scars, keloids tend to outgrow the original boundary of the wound. Furthermore, keloids do not regress, and have been classified as a type of benign dermal tumor [6]. So far, the major treatment approach is surgical excision with either a single adjunctive therapy, such as radiotherapy [14], steroid injection [15,16], comprehensive therapy [17], silicone gel sheeting [18] or 5-fluorouracil treatment [19], or combined adjunctive therapies [20-22], to decrease the recurrence rate.
Programmed cell death, known as apoptosis, is controlled by many signaling pathways, and the mitochondrion is a central location for its occurrence, indicating that activation of the mitochondrial pathway is a key procedure during the process. The mitochondrial pathway can be activated by inflammatory factors and stress conditions. Caspase-8 expression increases when FasL combines with Fas on the cell membrane and cuts the Bid protein, magnifying the apoptotic signal and transferring the signal to the mitochondria [23]. Then, the expression ratio of Bcl-2/Bax decreases, causing the release of cyt-c from the mitochondria to the cytoplasm. The cytoplasmic cyt-c-Apaf-1 complex and dATP/ATP unite to become the apoptosome, leading to the activation of caspase-9. Furthermore, caspase-3 is activated and causes apoptosis as a result of cell damage, such as chromosome condensation, nuclear DNA fragmentation and nuclear membrane rupture [24].
The balance between cell proliferation and cell death is central to the etiology of most pathologies [6]. Tissues usually maintain a stable condition when proliferation and apoptosis are balanced. PCNA is a homotrimer that achieves its activity by acting as a scaffold that encircles DNA and recruits proteins involved in DNA replication, DNA repair, chromatin remodeling and epigenetics [25]. PCNA expression is substantially upregulated in proliferating tissue, especially tumors [26-28], making it an effective indicator of the level of proliferation in the tissue.
In this study, three different kinds of skin tissue (keloid, physiological scars and normal skin) were investigated to assess the levels of apoptosis and proliferation. H&E and Masson staining were used to evaluate the morphological and histological differences among these tissues. In our results, the fiber content was reduced in normal skin compared to keloid tissue, but the cellular content was higher in the normal tissue compared with keloid scars. The excessive deposition of collagens and other extracellular matrix components was observed histologically in keloid tissue and an increased number of blood vessels and cells was also observed. However, collagen fibrils are arranged in bundles parallel to the epidermis. Irregular orientation of large collagenous fibrils was found in keloid tissue. This histological appearance is similar to that observed in Marneros’ report1. Physiological scars are a kind of mature scar formation. Sometimes, physiological scars atrophy with fewer cell components but more fiber than normal skin tissue, which presents an intermediate state between keloid and normal skin tissue.
The apoptosis process starts with the increased expression of caspase-8 after FasL combines with Fas. In this case, caspase-8 is a key initiation factor that is located upstream of the mitochondrial apoptosis pathway. In our study, caspase-8 showed higher expression in keloid tissue, demonstrating that the initiation link of apoptosis is stronger in keloid than other skin types. In our former study [7], we found that keloid tissue expressed more inflammatory factors (IL-6, IL-8, IL-18, TGF-β) and contained more inflammatory cells than physiological scars and normal skin, suggesting that inflammatory stimuli may be the main factors determining the level of apoptosis. In the mitochondrial apoptosis pathway, one pair of proteins is of paramount importance: the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax. These two proteins can combine to form a heterodimer, and the Bcl-2/Bax ratio determines the apoptotic fate of cells [29]. Our results showed that, although there were differences in the expression of Bcl-2 and Bax among the three skin types, most of the differences were insignificant. Cyt-c release is one of the necessary procedures in the mitochondrial apoptosis pathway. None of the immunohistochemical images, analyses of positive cell percentages or western blot results showed any significant differences in cyt-c expression. Caspase-9 and caspase-3 are downstream factors in the mitochondrial apoptosis pathway. Caspase-9 is another initiating factor that plays a role after cyt-c release, while caspase-3 is an effected factor. In our western blot results, there was a significant difference in caspase-3 expression between keloid tissue and normal skin, and the percentage of caspase-9-positive cells between keloid and non-keloid tissue was significantly different, indicating that the apoptosis effect is higher in keloid tissue. In all, most mitochondrial apoptosis pathway factors showed no significant differences in expression among these three skin types. In these results, the apoptosis level of keloid tissue is statistically similar to that of physiological scars and normal skin.
The degree of proliferation was evaluated by the expression of PCNA in three skin types. In immunohistochemical images, the expression of PCNA appeared as a darker brown color, and there were more positive areas in the K group. Keloid tissue also presented with the highest percentage of PCNA-positive cells, in both the epidermal and the dermal layers. According to the western blot analysis, the high PCNA expression in the K group was significantly different than that in the non-keloid groups. Based on the PCNA results, keloid tissue has a substantially higher proliferation level than either physiological scars or normal skin tissue.
As mentioned above, inflammation may play a part in promoting keloid apoptosis; if this is true, then why is the difference in apoptosis level between keloid and non-keloid tissue not more obvious? The balance between cell proliferation and apoptosis is crucial for tissue development and homeostasis. In Melo’s study [30] on PCNA and apoptosis during post-spawning ovarian remodeling in the teleost Oreochromis niloticus, PCNA mainly labeled the nuclei of oocytes and follicular cells in a high proportion of follicles, but a low occurrence of apoptosis was detected. Melo concluded that PCNA and apoptosis work cooperatively to ensure the success of follicle development and the maintenance of tissue homeostasis. Yu also reported that PCNA-positive cells were significantly increased and apoptotic cells were significantly decreased in the frontal cortical neurons of rats after stress [31]. Er’s study also mentioned that a group with low PCNA expression showed a higher number of apoptotic cells [32]. According to these findings, we speculate that the high expression level of PCNA in keloid tissue may negatively affect the apoptosis process.
Recent studies of keloid apoptosis have generally concentrated on fibroblasts derived from keloid tissue. Few studies have focused on the level of apoptosis, especially of the mitochondrial pathway, in keloid tissue. Our study investigated the expression levels of key factors in the mitochondrial pathway and of the classical proliferation factor in keloid tissue and compared this scar tissue with non-keloid tissue types. We found that the apoptosis level in keloid and non-keloid skin tissue is statistically similar but that PCNA expression is significantly higher in keloid scars than in the non-keloid tissues. The phenomenon of high PCNA expression and normal apoptosis level can be explained by the continuous growth and non-regression characteristics of keloid tissue. The opposite relationship between PCNA and apoptosis indicates that PCNA may become a target for keloid treatment. This study was mainly observational; the negative relationship between high PCNA expression and keloid apoptosis should be defined in further experiments.
Disclosure of conflict of interest
None.
References
- 1.Marneros AG, Krieg T. Keloids-clinical diagnosis, pathogenesis, and treatment options. J Dtsch Dermatol Ges. 2004;2:905–913. doi: 10.1046/j.1439-0353.2004.04077.x. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557–568. doi: 10.1097/PRS.0b013e3181c82dd5. [DOI] [PubMed] [Google Scholar]
- 3.Green DR. Means to an end: apoptosis and other cell death mechanisms. Cold Spring Harbor Laboratory Press; 2011. [Google Scholar]
- 4.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21:871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Chalah A, Khosravi-Far R. The mitochondrial death pathway. Adv Exp Med Biol. 2008;615:25–45. doi: 10.1007/978-1-4020-6554-5_3. [DOI] [PubMed] [Google Scholar]
- 6.Appleton I, Brown NJ, Willoughby DA. Apoptosis, necrosis and proliferation: possible implications in the etiology of keloids. Am J Pathol. 1996;149:1441–1447. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M, Xu Y, Liu Y, Cheng Y, Zhao P, Liu H, Wang Y, Ma X. Chemokine-like factor 1 (CKLF-1) is overexpressed in keloid patients: a potential indicating factor for keloid-predisposed individuals. Medicine. 2016;95:e3082–e3089. doi: 10.1097/MD.0000000000003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollack SV, Goslen JB. The surgical treatment of keloids. J Dermatol Surg Oncol. 1982;8:1045–1049. doi: 10.1111/j.1524-4725.1982.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 9.Kelly AP. Keloids. Dermatol Clin. 1988;6:407–412. [PubMed] [Google Scholar]
- 10.Peacock EE Jr, Madden JW, Trier WC. Biologic basis for the treatment of keloids and hypertrophic scars. South Med J. 1970;63:755–760. doi: 10.1097/00007611-197007000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Lee SS, Yosipovitch G, Chan YH. Pruritus, pain and small nerve fiber function in keloids: a controlled study. J Am Acad Dermatol. 2004;51:1002–1006. doi: 10.1016/j.jaad.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 12.Datubo-Brown DD. Keloids: a review of the literature. Br J Plast Surg. 1990;43:70–77. doi: 10.1016/0007-1226(90)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Lahiri A, Tsiliboti D, Gaze NR. Experience with difficult keloids. Br J Plast Surg. 2001;54:633–635. doi: 10.1054/bjps.2001.3665. [DOI] [PubMed] [Google Scholar]
- 14.van de Kar AL, Kreulen M, van Zuijlen PP, Oldenburger F. The results of surgical excision and adjuvant irradiation for therapy-resistant keloids: a prospective clinical outcome study. Plast Reconstr Srug. 2007;119:2248–2254. doi: 10.1097/01.prs.0000260751.20217.28. [DOI] [PubMed] [Google Scholar]
- 15.Rosen DJ, Patel MK, Freeman K, Weiss PR. A primary protocol for the management of ear keloids: results of excision combined with intraoperative and postoperative steroid injections. Plast Reconstr Surg. 2007;120:1395–1400. doi: 10.1097/01.prs.0000279373.25099.2a. [DOI] [PubMed] [Google Scholar]
- 16.Donkor P. Head and neck keloid: treatment by core excision and delayed intralesional injection of steroid. J Oral Maxllofac Surg. 2007;65:1292–1296. doi: 10.1016/j.joms.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 17.Prasad KC, Karthik S, Prasad SC. A comprehensive study on lesions of the pinna. Am J Otolaryngol. 2005;26:1–6. doi: 10.1016/j.amjoto.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2013;12:CD003826. doi: 10.1002/14651858.CD003826.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uppal RS, Khan U, Kakar S, Talas G, Chapman P, McGrouther AD. The effects of a single dose of 5-fluorouracil on keloid scars: a clinical trial of timed wound irrigation after extralesional excision. Plast Reconstr Surg. 2001;108:1218–1224. doi: 10.1097/00006534-200110000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Chen XE, Liu J, Bin Jameel AA, Valeska M, Zhang JA, Xu Y, Liu XW, Zhou H, Luo D, Zhou BR. Combined effects of long-pulsed neodymium-yttrium-aluminum-garnet laser, diprospan and 5-fluorouracil in the treatment of keloid scars. Exp Ther Med. 2017;13:3607–3612. doi: 10.3892/etm.2017.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torii K, Maeshige N, Aoyama-Ishikawa M, Miyoshi M, Terashi H, Usami M. Combination therapy with butyrate and docosahexaenoic acid for keloid fibrogenesis: an in vitro study. An Bras Dermatol. 2017;92:184–190. doi: 10.1590/abd1806-4841.20176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XQ, Li ZN, Wang QM, Jin HY, Gao Z, Jin ZH. Lipid nano-bubble combined with ultrasound for anti-keloids therapy. J Liposome Res. 2016;23:1–9. doi: 10.1080/08982104.2016.1239633. [DOI] [PubMed] [Google Scholar]
- 23.Jiang XJ, Wang XD. Cytochrome C promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez J, Lazebnik Y. Caspase-9 and Apaf-1 form an active holoenzyme. Genes Dev. 1999;13:3179–3184. doi: 10.1101/gad.13.24.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Chen T, Huang H, Jiang Y, Yang L, Lin Z, He H, Liu T, Wu B, Chen J, Kamp DW, Liu G. miR-363-3p inhibits tumor growth by targeting PCNA in lung adenocarcinoma. Oncotarget. 2017;8:20133–20144. doi: 10.18632/oncotarget.15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu L, Li HL, Li WF, Chen JM, Yang JT, Gu JJ, Xin L. Clinical significance of expression of proliferation cell nuclear antigen and E-cadherin in gastric carcinoma. World J Gastroenterol. 2017;23:3721–3729. doi: 10.3748/wjg.v23.i20.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenner M, Demgensky A, Molls C, Hardt A, Luers JC, Grosheva M, Huebbers CU, Klussmann JP. Prognostic value of proliferating cell nuclear antigen in parotid gland cancer. Eur Arch Otorhinolaryngol. 2012;269:1225–1232. doi: 10.1007/s00405-011-1740-6. [DOI] [PubMed] [Google Scholar]
- 29.Ayatollahi SA, Ajami M, Reyhanfard H, Asadi Y, Nassiri-Kashani M, Firoozabadi MR, Davoodi SH, Habibi E, Pazoki-Toroudi H. Bcl-2 and bax expression in skin flaps treated with finasteride or azelaic acid. Iran J Pharm Res. 2012;11:1285–1290. [PMC free article] [PubMed] [Google Scholar]
- 30.Melo RM, Marins YS, Luz RK, Rizzo E, Bazzoli N. PCNA and apoptosis during postspawning ovarian remodeling in the teleost oreochromis niloticus. Tissue Cell. 2015;47:541–549. doi: 10.1016/j.tice.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Yu AY, Su QR, Wang L, Zhou J, Liu XH. Effects of citalopram on the expression of PCNA and C-fos and cell apoptosis in rat fronta cortical neurons after stress. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2014;30:439–442. [PubMed] [Google Scholar]
- 32.Er H, Acar N, Kipmen-Korgun D, Celik-Ozenci C, Ustunel I, Asar M, Korgun ET. Determination of PCNA, cyclin D3, p27, p57 and apoptosis rate in normal and dexamethasone-induced intrauterine growth restricted rat placentas. Acta Histochem. 2015;117:137–147. doi: 10.1016/j.acthis.2014.11.010. [DOI] [PubMed] [Google Scholar]


