Abstract
Metformin is an AMP-activated protein kinase activator that is widely prescribed for treating type 2 diabetes. Recently, metformin was reported to slow down the development and alleviate the severity of diabetic retinopathy (DR). However, the underlying mechanisms remain unclear. Here, we used an alloxan-induced diabetes mouse model to study the effects of metformin on the development of DR as well as the mechanisms. We found that DR was induced in alloxan-treated mice 10 weeks after alloxan treatment, and treatment of metformin did not prevent the occurrence of alloxan-induced diabetes. However, metformin significantly alleviated the severity of DR, seemingly through attenuating the retina neovascularization. Moreover, the total vascular endothelial cell growth factor A (VEGF-A) mRNA in mouse eyes was not altered by metformin, but the protein levels was decreased. Further analysis showed that metformin may inhibit the VEGF-A protein translation through inducing a VEGF-A-targeting microRNA, microRNA-497a-5p, resulting in reduced retina neovascularization. Thus, our study suggests a previously unappreciated role of metformin in the prevention of development of DR.
Keywords: Diabetic retinopathy (DR), vascular endothelial cell growth factor A (VEGF-A), miR-497a-5p, retina neovascularization
Introduction
Diabetes is one of the most popular chronic diseases worldwide, and the most diabetes cases are type 2 diabetes, resulting from insulin resistance and beta cell dysfunction [1]. Metformin is a FDA-approved first-line medicine for treating overweight or obese type 2 diabetes patients who were at high risk of developing cardiovascular complications [2,3]. Metformin reduces plasminogen activator inhibitor 1 to improve fibrinolytic activity. Moreover, metformin inhibits inflammation-mediated angiogenesis in a murine model and in clinic, seemingly through the effects of plasminogen activator inhibitor 1 on the activation of thrombospondin 1 [2]. However, there have been conflicting reports on the effects of metformin on vascularization, which seemingly results from the difference in time course at analysis and experimental model [4]. Nevertheless, the exact molecular mechanisms underlying the effects of metformin on vascularization remain ill-defined.
Diabetic retinopathy (DR) is one of the most important complications of diabetes [5-8]. DR affects retina blood vessels, and appears to be the most common cause of vision loss among people with diabetes and the leading cause of vision impairment and blindness among working-age adults [9]. Very recently, metformin has been shown to alleviate DR when combined with a traditional Chinese medicine [10]. However, the effects of metformin alone on DR are unknown and the mechanisms have not been elucidated.
Vascular endothelial growth factor A (VEGF-A) is the major regulator of vascularization, and the binding of VEGF-A to VEGF Receptor 2 (VEGFR2) mediates most of the biological effects of VEGF-A, including blood vessel growth and branching, endothelial cell survival and vessel permeability [11-18]. VEGF-A plays a critical role in the development of DR, while its regulation is not completely defined. Recently, it was shown that VEGF-A could be regulated by microRNAs at the protein translation level, which opened a new window for studying its biological effects [19].
Here, we used an alloxan-induced diabetes mouse model to study the effects of metformin on the development of DR as well as the underlying mechanisms.
Materials and methods
Mouse manipulation
All mouse experiments were approved by the Animal Research and Care Committee at the First Hospital of Jinzhou Medical University. Male C57BL/6 mice were purchased from the Jackson Lab (Bar Harbor, Maine, USA), and were used in the experiments at 10 weeks of age. Fasting blood glucose monitoring were performed at 9am in the morning. The beta cell toxin alloxan (Sigma-Aldrich, St Louis, MO, USA) was injected from the tail vein at 70 mg/kg body weight, which allowed development of sustained high blood glucose and a certain period of survival of the animals. Two week after alloxan treatment, mice in the metformin group were administered an intraperitoneal injection of metformin (120 mg/kg body weight) every day for 10 weeks. Mice in the saline-treated group mice (CTL) were administered an intraperitoneal injection of an equivalent volume of normal saline at same frequency. The mice were analyzed at 8 or 10 weeks after metformin (or control), or 10 or 12 weeks after alloxan. Fasting blood glucose monitoring, intraperitoneal glucose tolerance test (IPGTT) and serum insulin measurement were performed as described before [20].
Mouse epithelial cell line culture and transfection
A mouse epithelial cell line MS1 was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). MS1 cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA), and incubated in a humidified chamber with 5% CO2 at 37°C. MiR-497a-5p-expressing and antisense (as) plasmids were prepared with general method. The MS1 cells were also transfected with a null plasmid as a control (null). Transfection was performed with Lipofectamine 2000 reagent (Invitrogen), according to the instructions of the manufacturer.
Luciferase-reporter activity assay
Luciferase-reporters were successfully constructed using molecular cloning technology. Target sequence for VEGF-A miRNA 3’-UTR clone was purchased from Creative Biogene (Shirley, NY, USA). MiR-497a-5p-modified MS1 cells were seeded in 24-well plates for 24 hours, after which they were transfected with 1 μg of Luciferase-reporter plasmids per well. Luciferase activities were measured using the dual-luciferase reporter gene assay kit (Promega, Beijing, China), according to the manufacturer’s instructions.
Fluorescein angiography and analysis of retina vessel density
At the time of sacrifice, mice were anesthetized by 2% isoflurane inhalation, and fluorescein angiography images were obtained after intraperitoneal injection of 0.15 ml of 2% fluorescein sodium (Alcon Laboratories, Inc., Fort Worth, TX, USA). Mouse eyeballs were harvested and immersed in 4% paraformaldehyde at room temperature for 2 hours. Afterwards, sclera, uveal tissue, cornea, and lens were removed from the retina. The isolated retina was washed twice with phosphate buffered saline for 5 minutes. Retinal vessels were stained by storing the retina in tomato lectin (Molecular Probes, Carlsbad, CA, USA) at 4°C for 2 days. Stained retinal tissues were flat-mounted on glass slides with coverslips.
Retinopathy grading
Avascular areas were evaluated using Image J (NIH, Bethesda, MA, USA) by two experienced investigators blinded to group-identifying information. Retinopathy grade was assessed by two experienced investigators using the scoring system proposed described before [21]. This scoring system incorporates assessments of blood vessel growth, blood vessel tufts, extra-retinal neovascularization, central vasoconstriction, retinal hemorrhage, and degree of vessel tortuosity.
Enzyme-linked immunosorbent assay (ELISA) for VEGF-A
Whole eyes were homogenized in PBS and sonicated. After centrifugation, supernatants containing most soluble proteins were collected. VEGF-A levels in the supernatant were determined using an ELISA kit (RayBiotech, Inc., Norcross GA, USA). Absorbance values were obtained at 450-570 nm using an Emax spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
RNA isolation and RT-qPCR
Total RNA was extracted from mouse eyes with Trizol (Invitrogen, Carlsbad, California, USA). RNA was quantified with Nanodrop1000 (Thermo Scientific, Rockford, IL, USA) according to the manufacturer’s instructions, followed by cDNA synthesis (Qiagen, Valencia, CA, USA). Quantitative PCR primers were shown in the figure. RT-qPCR Reactions were performed in triplicates with QuantiTect SYBR Green PCR Kit (Qiagen). Specificity of the amplified products was determined by melting peak analysis. Quantification was performed with the 2-ΔΔCt method. Values of genes were normalized against tubulin, which proved to be stable across the samples.
Immunohistochemistry
All samples are fixed in 4% paraformaldehyde at room temperature for 2 hours, then cryo-protected in 30% sucrose overnight before further approaches. DAB staining was performed with a DAB chromogen system (Dako, Carpinteria, CA, USA). Primary antibodies for immunostaining are: guinea pig polyclonal anti-insulin (Dako) and rat polyclonal anti-CD31 (Becton-Dickinson Biosciences, San Jose, CA, USA). No antigen retrieval is necessary for these antigens. Secondary antibodies for indirect fluorescent staining are Cy2-conjugated antibodies generated from donkey (Jackson ImmunoResearch Labs, West Grove, PA, USA).
Western blot
Protein was extracted using RIPA buffer (Sigma-Aldrich) for Western Blot. The supernatants were collected after centrifugation at 12000×g at 4°C for 20 min. Protein concentration was determined using BCA protein assay, and whole lysates were mixed with 4×SDS loading buffer at a ratio of 1:3. Samples were heated at 100°C for 5 min and were separated on SDS-polyacrylamide gels. The separated proteins were then transferred to a PVDF membrane. The membrane blots were first probed with a primary antibody. After incubation with horseradish peroxidase-conjugated second antibody, autoradiograms were prepared using the enhanced chemiluminescent system to visualize the protein antigen. Primary antibodies for Western Blot are anti-VEGF-A and anti-α-tubulin (all purchased from Cell Signaling, San Jose, CA, USA). Secondary antibody is HRP-conjugated anti-rabbit (Jackson ImmunoResearch Labs). Images shown in the figure were representative from 5 repeats. Densitometry of Western blots was quantified with ImageJ software (NIH).
Statistical analysis
All statistical analyses were carried out using GraphPad prism 6.0 (GraphPad Software Inc. La Jolla, CA, USA). All values are depicted as mean ± SD and are considered significant if P<0.05. All data were statistically analyzed using one-way ANOVA with a Bonferroni correction, followed by Fisher’s Exact Test for comparison of two groups.
Results
Experimental model
In order to study the effects of metformin on DR, we used alloxan to induce diabetes in mice. The beta cell toxin alloxan was injected to develop sustained high blood glucose. Two week after alloxan treatment, mice in the metformin group (alloxan + Met) were administered an intraperitoneal injection of metformin (120 mg/kg body weight) every day for 10 weeks. Mice in the saline-treated group mice (alloxan) were administered an intraperitoneal injection of an equivalent volume of normal saline at same frequency. The mice were analyzed at 8 weeks after metformin treatment for Retinopathy score, and at 10 weeks after metformin treatment for retina vessel density and mechanisms (Figure 1A).
Figure 1.
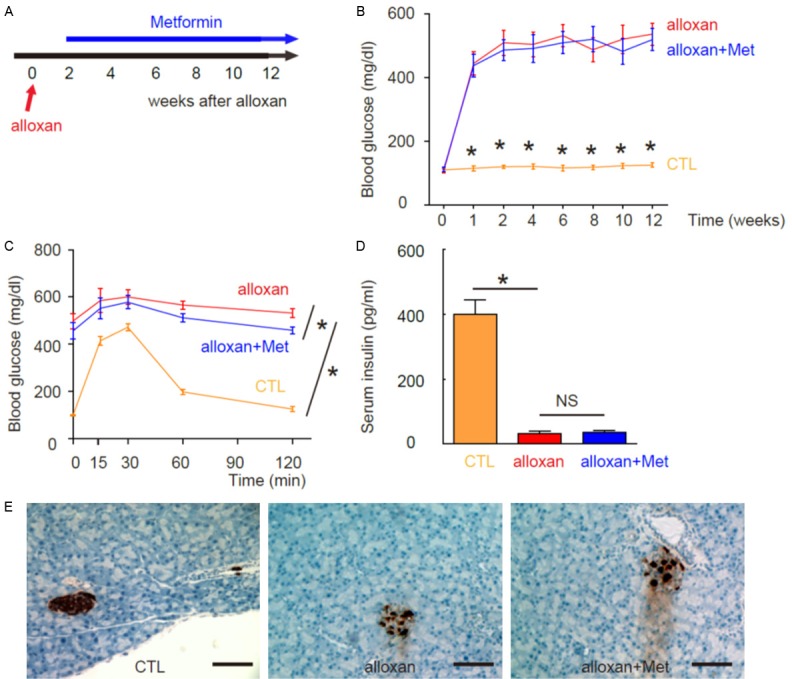
Experimental model and Induction of diabetes in mice by alloxan administration. A: Experimental model. Two week after beta-cell toxin alloxan injection, mice in the metformin group (alloxan + Met) were administered an intraperitoneal injection of metformin every day for 10 weeks. Mice in the saline-treated group mice (alloxan) were administered an intraperitoneal injection of an equivalent volume of normal saline at same frequency. The mice that did not received alloxan and metformin were used as negative controls (CTL). The mice were analyzed at 8 weeks after metformin treatment for Retinopathy score, and at 10 weeks after metformin treatment for retina vessel density and mechanisms. B: Fasting blood glucose. C: IPGTT. D: Serum insulin. E: The analysis of pancreas sections at 12 weeks after alloxan showed significant loss of islet beta cells in both groups, based on insulin immunostaining. *P<0.05. N=10. Scale bars are 50 µm.
Induction of diabetes in mice by alloxan administration
We found that ever since 1 week after alloxan injection, the mice developed sustained hyperglycemia, in both alloxan and alloxan + Met groups. There are no difference in the levels of fasting blood glucose (Figure 1B), slightly differences in glucose tolerance (Figure 1C) and no difference in serum insulin (Figure 1D) between two groups. The analysis of pancreas sections at 12 weeks after alloxan showed significant loss of islet beta cells in both groups, based on insulin immunostaining (Figure 1E). Thus, alloxan induces diabetes in mice and metformin does not revert hyperglycemia.
Induction of DR in mice by alloxan administration
Next, we examined whether alloxan may induce DR in these diabetic mice. We found that 10 weeks after alloxan treatment, DR was induced in alloxan-treated mice, regardless treatment of metformin, based on retinopathy score. However, metformin seemed to alleviate the severity of DR (Figure 2A). Interestingly, metformin treatment did not alter the avascular area in eyes at this point, shown by quantification (Figure 2B), and by representative images (Figure 2C). These data suggest that metformin may not affect retinopathy at the initial ischemic period. Thus, we hypothesize that metformin may affect the neovascularization in the development of DR.
Figure 2.
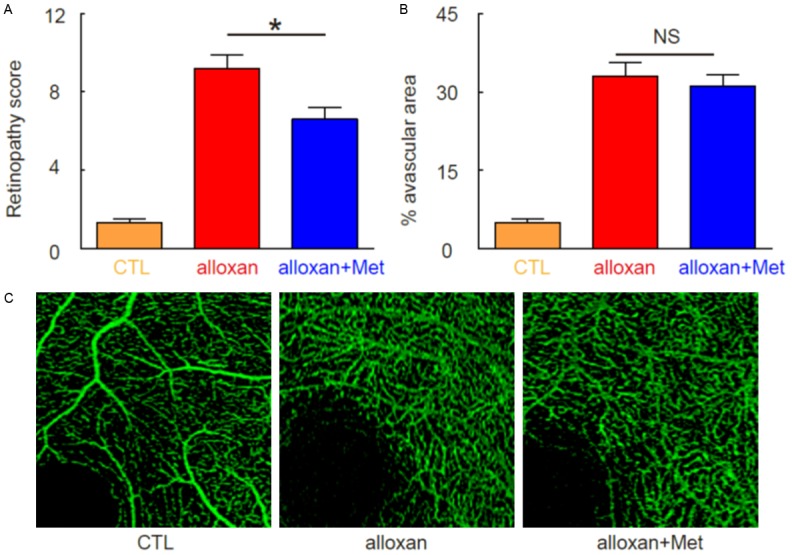
Induction of DR in mice by alloxan administration. (A) Retinopathy scoring at 10 weeks after alloxan. (B, C) Avascular area in eyes at 10 weeks after alloxan, by quantification (B), and by representative images (C). *P<0.05. NS: non-significant. N=10.
Metformin attenuates neovascularization in the development of DR through suppressing VEGF-A
Next, we examined the mice at 12 weeks after alloxan, when the neovascularization occurs. We found that metformin significantly attenuated vessel density at this time point, shown by representative images (Figure 3A), and by quantification (Figure 3B). Since VEGF-A is the most potent neovascularization initiator, we analyzed VEGF-A protein levels by ELISA and mRNA levels by RT-qPCR in the mouse eyes. Surprisingly, although we found that metformin reduced the VEGF-A protein in eyes by alloxan (Figure 3C), we did not detect such an effect of metformin on VEGF-A mRNA (Figure 3D). Hence, Metformin attenuates neovascularization in the development of DR through suppressing VEGF-A protein, but not mRNA.
Figure 3.
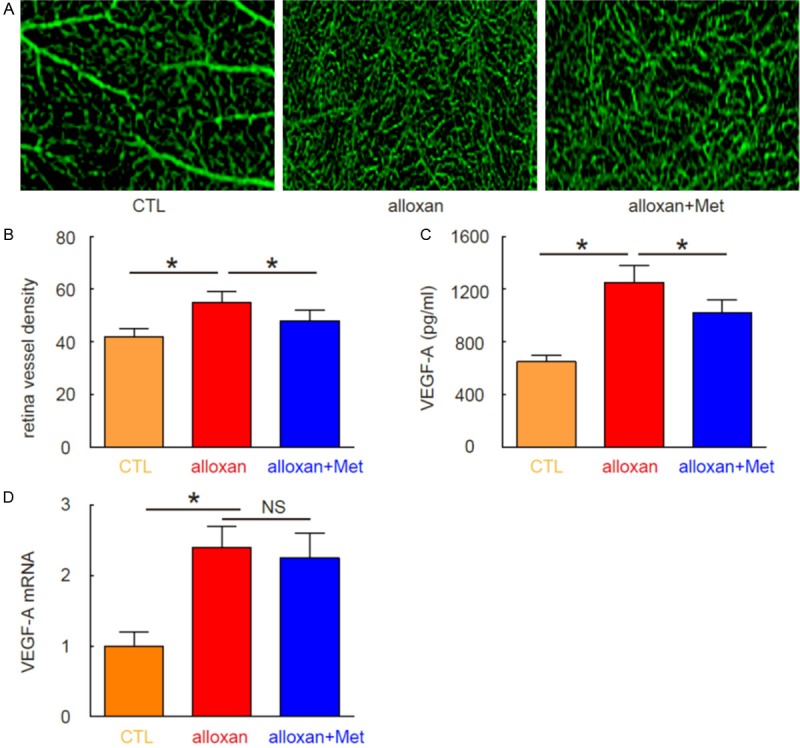
Metformin attenuates neovascularization in the development of DR through suppressing VEGF-A. (A, B) Vessel density was analyzed at 12 weeks after alloxan, shown by representative images (A), and by quantification (B). (C) ELISA for VEGF-A protein in mouse eyes. (D) RT-qPCR for VEGF-A RNA in mouse eyes. *P<0.05. NS: non-significant. N=10.
MiR-497a-5p targets 3’-UTR of VEGF-A mRNA to inhibit its expression
Since our data suggest presence of a post-transcriptional regulation of VEGF-A induced by metformin, we hypothesized that metformin may alter expression of a VEGF-A-targeting miRNA. We then screened all VEGF-A-targeting miRNAs and specifically found that miR-497a-5p targeted VEGF-A mRNA by bioinformatics analyses (Figure 4A), and metformin significantly increased the levels of miR-497a-5p in mouse eyes (Figure 4B). In order to examine whether miR-497a-5p may regulate VEGF-A in eye epithelial cells, we either overexpressed miR-497a-5p, or inhibited miR-497a-5p in MS1 cells by transfection of the cells with a miR-497a-5p-expressing plasmid, or with a plasmid carrying miR-497a-5p antisense (as-miR-497a-5p). The MS1 cells were also transfected with a null plasmid as a control (null). The overexpression or inhibition of miR-497a-5p in NPC cells was confirmed by RT-qPCR (Figure 4C). MiR-497a-5p-modified MS1 cells were then transfected with 1 μg of VEGF-A-3’-UTR luciferase-reporter plasmid. The luciferase activities were quantified in these cells, suggesting that miR-497a-5p targets 3’-UTR of VEGF-A mRNA to inhibit its translation (Figure 4D).
Figure 4.
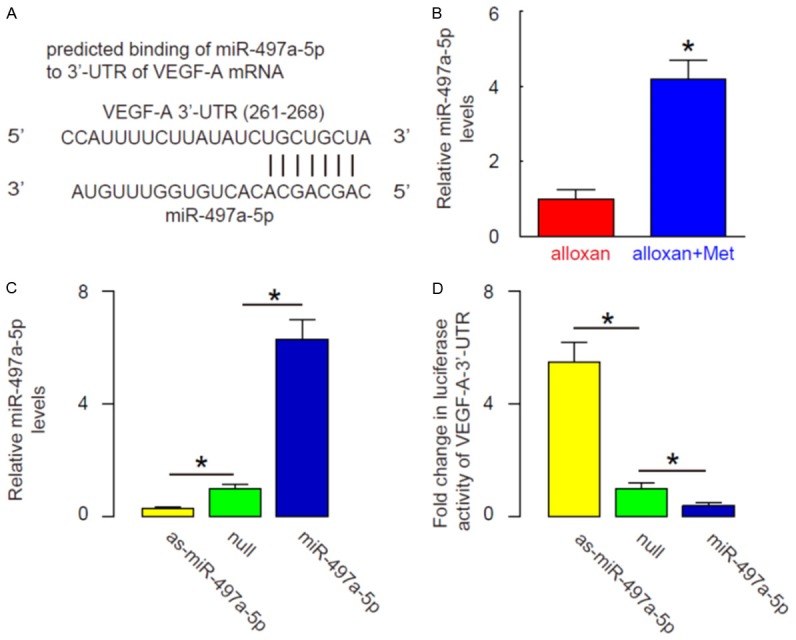
MiR-497a-5p targets 3’-UTR of VEGF-A mRNA to inhibit its expression. A: Bioinformatics analyses show that miR-497a-5p targets VEGF-A mRNA. B: Metformin significantly increased the levels of miR-497a-5p in mouse eyes, shown by RT-qPCR. C: We either overexpressed miR-497a-5p, or inhibited miR-497a-5p in MS1 cells by transfection of the cells with a miR-497a-5p-expressing plasmid, or with a plasmid carrying miR-497a-5p antisense (as-miR-497a-5p). The MS1 cells were also transfected with a null plasmid as a control (null). The overexpression or inhibition of miR-497a-5p in NPC cells was confirmed by RT-qPCR. D: MiR-497a-5p-modified MS1 cells were then transfected with 1 μg of VEGF-A-3’-UTR luciferase-reporter plasmid. The luciferase activities were quantified in these cells. *P<0.05. N=10.
MiR-497a-5p inhibits VEGF-A protein translation in MS1 cells
We found that alteration of miR-497a-5p in MS1 cells did not change mRNA levels of VEGF-A (Figure 5A). However, overexpression of miR-497a-5p significantly decreased VEGF-A protein levels, while inhibition of miR-497a-5p significantly increased VEGF-A protein levels in MS1 cells, by Western blot (Figure 5B). These data suggest that MiR-497a-5p inhibits VEGF-A protein translation, as a mechanism for the suppression of eye neovascularization by metformin.
Figure 5.
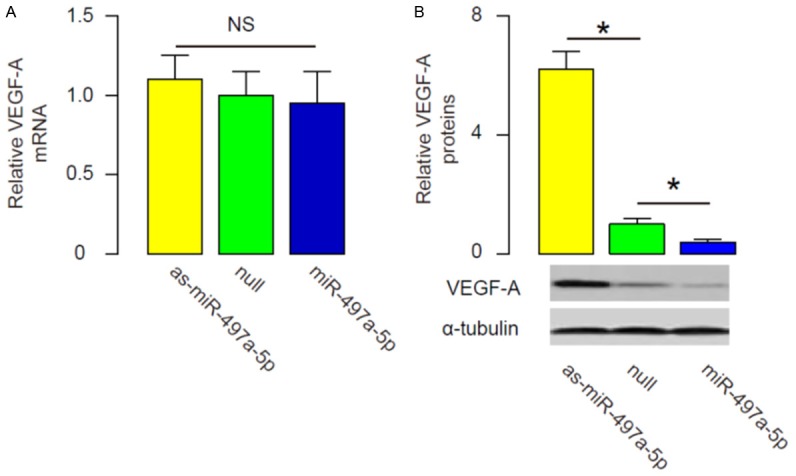
MiR-497a-5p inhibits VEGF-A protein translation in MS1 cells. (A, B) RT-qPCR (A) and Western blot (B) for VEGF-A in miR-497a-5p-modified MS1 cells. *P<0.05. NS: non-significant. N=10.
Discussion
In clinic, metformin improves blood glucose control of the patients through lowering fasting plasma insulin concentrations and enhancing insulin sensitivity, to increase glucose uptake by peripheral tissues and decreasing hepatic glucose output.
A recent study showed that a Chinese medicinal formula combined with metformin significantly reversed the prolongation of latency times of flash electroretinogram and oscillatory potentials in diabetic rats [10]. Moreover, this treatment showed a remarkable suppression of retinal neovascularization and amelioration of retinal internal limiting membrane morphology [10]. However, no mechanisms have been studies in this report.
VEGF-A is the most important regulator of neovascularization during development of DR. Thus, here we aimed to reproduce the anti-DR effects of metformin and further study the effects of metformin on VEGF-A and retinal vascularization. First, we used a mouse model of diabetes to study the effects of metformin on DR. We confirmed sustained diabetes and development of DR in this alloxan-induced diabetes and DR model. We indeed confirmed a modest protective effect of metformin against DR, seemingly at the second stage of DR when neovascularization occurs after ischemia period. These data are consistent with the previous report [19] and other literatures showing that metformin may activate protective mechanisms against angiogenesis during the progression of DR [22-24].
Next, we examined the VEGF-A levels after metformin treatment. Although VEGF-A mRNA levels were not affected by metformin, metformin indeed reduced the VEGF-A protein, suggesting that a post-transcriptional control of VEGF-A protein translation. Recently, it was shown that VEGF-A could be regulated by microRNAs at the protein translation level [19]. Gu et al. showed that compared to other cancers, non-small cell lung cancer had a significant higher ratio of VEGF-A protein vs mRNA, and significantly lower levels of miR-497. Moreover, bioinformatics analyses showed that miR-497 bound to 3’-UTR of VEGF-A mRNA in non-small cell lung cancer cells to inhibit its translation [19]. This study inspired us to examine the involvement of VEGF-A-targeting miRNAs in this model. Our results showed that metformin may inhibit the VEGF-A protein translation through inducing a VEGF-A-targeting miR-497a-5p, resulting in reduced retina neovascularization. Thus, our study suggests a previously unappreciated role of metformin in the prevention of development of DR.
The future studies may address patients’ specimens to figure out whether this mechanism is also functional in humans. To summarize, our findings are critical for generating novel medicine through miRNA-mediated modulation of VEGF-A for DR treatment.
Acknowledgements
This work is supported by Liaoning Province Doctor Startup Fund (grant NO.20170520129).
Disclosure of conflict of interest
None.
References
- 1.Gunasekaran U, Gannon M. Type 2 diabetes and the aging pancreatic beta cell. Aging (Albany NY) 2011;3:565–575. doi: 10.18632/aging.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alusik S, Paluch Z. Metformin: the past, presence, and future. Minerva Med. 2015;106:233–238. [PubMed] [Google Scholar]
- 3.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 4.Dallaglio K, Bruno A, Cantelmo AR, Esposito AI, Ruggiero L, Orecchioni S, Calleri A, Bertolini F, Pfeffer U, Noonan DM, Albini A. Paradoxic effects of metformin on endothelial cells and angiogenesis. Carcinogenesis. 2014;35:1055–1066. doi: 10.1093/carcin/bgu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Liu X, Guo H, Zhao Z, Li YS, Chen G. The significance of the increased expression of phosphorylated MeCP2 in the membranes from patients with proliferative diabetic retinopathy. Sci Rep. 2016;6:32850. doi: 10.1038/srep32850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Yan H. MicroRNA-126 contributes to Niaspan treatment induced vascular restoration after diabetic retinopathy. Sci Rep. 2016;6:26909. doi: 10.1038/srep26909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal R, Smart T, Nobre-Cardoso J, Richards C, Bhatnagar R, Tufail A, Shima D, Jones PH, Pavesio C. Assessment of red blood cell deformability in type 2 diabetes mellitus and diabetic retinopathy by dual optical tweezers stretching technique. Sci Rep. 2016;6:15873. doi: 10.1038/srep15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Li Y, Payne J, Srivastava S, Fan X, Fung J, Li X, Kern TS, Lin F. Presence of retinal pericyte-reactive autoantibodies in diabetic retinopathy patients. Sci Rep. 2016;6:20341. doi: 10.1038/srep20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veluthakal R, Kumar B, Mohammad G, Kowluru A, Kowluru RA. Tiam1-Rac1 Axis promotes activation of p38 MAP Kinase in the development of diabetic retinopathy: evidence for a requisite role for protein palmitoylation. Cell Physiol Biochem. 2015;36:208–220. doi: 10.1159/000374065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WP, Wang YD, Ma Y, Zhang ZY, Hu LY, Lin JL, Lin BQ. Danhong Huayu Koufuye combined with metformin attenuated diabetic retinopathy in Zucker diabetic fatty rats. Int J Ophthalmol. 2015;8:1094–1100. doi: 10.3980/j.issn.2222-3959.2015.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao X, Prasadan K, Guo P, El-Gohary Y, Fischbach S, Wiersch J, Gaffar I, Shiota C, Gittes GK. Pancreatic duct cells as a source of VEGF in mice. Diabetologia. 2014;57:991–1000. doi: 10.1007/s00125-014-3179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao X, Guo P, Chen Z, El-Gohary Y, Wiersch J, Gaffar I, Prasadan K, Shiota C, Gittes GK. Hypoglycemia reduces vascular endothelial growth factor a production by pancreatic Beta cells as a regulator of Beta cell mass. J Biol Chem. 2013;288:8636–8646. doi: 10.1074/jbc.M112.422949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villalta SA, Lang J, Kubeck S, Kabre B, Szot GL, Calderon B, Wasserfall C, Atkinson MA, Brekken RA, Pullen N, Arch RH, Bluestone JA. Inhibition of VEGFR-2 reverses type 1 diabetes in NOD mice by abrogating insulitis and restoring islet function. Diabetes. 2013;62:2870–2878. doi: 10.2337/db12-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Gao Y, Yi X, Ding Y. Ginsenoside Rh2 suppresses neovascularization in xenograft psoriasis model. Cell Physiol Biochem. 2015;36:980–987. doi: 10.1159/000430272. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Sun K, Xia M, Li X, Lu Y. MMP13 regulates aggressiveness of pediatric multiple myeloma through VEGF-C. Cell Physiol Biochem. 2015;36:509–516. doi: 10.1159/000430116. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Wu X, Wang H, Huang C, Zhang L. Combined anti-PLGF and anti-endostatin treatments inhibit ocular hemangiomas. Cell Physiol Biochem. 2015;36:930–936. doi: 10.1159/000430267. [DOI] [PubMed] [Google Scholar]
- 17.Huo X, Li Y, Jiang Y, Sun X, Gu L, Guo W, Sun D. Inhibition of ocular neovascularization by co-inhibition of VEGF-A and PLGF. Cell Physiol Biochem. 2015;35:1787–1796. doi: 10.1159/000373990. [DOI] [PubMed] [Google Scholar]
- 18.Gao Q, Yao X, Zheng J. MiR-323 inhibits prostate cancer vascularization through adiponectin receptor. Cell Physiol Biochem. 2015;36:1491–1498. doi: 10.1159/000430313. [DOI] [PubMed] [Google Scholar]
- 19.Gu A, Lu J, Wang W, Shi C, Han B, Yao M. Role of miR-497 in VEGF-A-mediated cancer cell growth and invasion in non-small cell lung cancer. Int J Biochem Cell Biol. 2016;70:118–125. doi: 10.1016/j.biocel.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- 21.Higgins RD, Yu K, Sanders RJ, Nandgaonkar BN, Rotschild T, Rifkin DB. Diltiazem reduces retinal neovascularization in a mouse model of oxygen induced retinopathy. Curr Eye Res. 1999;18:20–27. doi: 10.1076/ceyr.18.1.20.5390. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Li G, Wang Y, Tang S, Sun X, Feng X, Li Y, Bao G, Li P, Mao X, Wang M, Liu P. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1alpha/VEGF secretion axis. Oncotarget. 2015;6:44579–44592. doi: 10.18632/oncotarget.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao S, Jiang J, Li P, Song H, Wang W, Li C, Kong D. Attenuating tumour angiogenesis: a preventive role of metformin against breast cancer. Biomed Res Int. 2015;2015:592523. doi: 10.1155/2015/592523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Pietro M, Parborell F, Irusta G, Pascuali N, Bas D, Bianchi MS, Tesone M, Abramovich D. Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model. Endocrinology. 2015;156:1453–1463. doi: 10.1210/en.2014-1765. [DOI] [PubMed] [Google Scholar]


