Abstract
Background: To further explore the role of PPARγ in QL treatment, ISO-induced mice model and following methods were established. Methods: Cardiac remodeling on mice model was induced by isoproterenol (ISO) infusion or saline infusion as control for two weeks then divided into 4 groups, after that divided in 5 different treatment methods to investigate the role of PPARγ in QL therapy. Echocardiography and Masson’s trichrome staining were respectively used to determine cardiac function and fibrosis. Immunoblotting was applied to evaluate the expression levels of proliferator-activated receptor-γ (PPARγ), Bax, Bcl, phospho-Akt (Ser473), Akt, phospho-P38 and P38, phosphor-ERK and ERK. Results: QL treatment improved left ventricular function, decreased apoptosis, and prevented myocardial fibrosis at the same time. Meanwhile, the PPARγ level was elevated with QL treatment in ISO-injected mice hearts. Inhibition of PPARγ activity blocked the protective effects of QL, while the activator of PPARγ did not provide additional benefit. Specifically, the results indicated a decline in PPARγ in ISO-infused mice and QL decreased the toxicity of ISO by improving the level of PPARγ. Conclusions: Our study demonstrated that QL treatment provided cardioprotection against ISO-induced cardiac remodeling by improving PPARγ level, which could be as the potential therapeutic target in reversing cardiac remodeling and heart failure.
Keywords: Qiliqiangxin, heart failure, PPARγ, cardiac dysfunction, isoproterenol, cardiac fibrosis
Introduction
Cardiac remodeling is defined as changes in size, shape and function of the heart resulting from cardiac load or injury, which is regarded as a determinant of the clinical course of heart failure (HF) [1]. Whereas patients with major remodeling undergo progressive worsening of cardiac function, slowing or preventing cardiac remodeling has become a new goal of heart failure therapy [2,3]. One of the major components of cardiac remodeling is due to the neurohormonal over-activation. The therapeutic antagonism of neurohormonal systems, as we all know, β-adrenergic blocking agents, angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and mineralocorticoid-receptor antagonists (MRAs), has become the cornerstone of contemporary pharmacotherapy for heart failure [1,4,5]. Isoproterenol-induced cardiac injury includes activation of inflammation, necrosis of myocardium, and disarrangement of energy reserves in cardiomyocytes, interstitial fibrosis and cardiac remodeling occur, and eventually caused cardiac dysfunction [6,7]. Qiliqiangxin (QL), a traditional Chinese medicine (TCM), is an extract of 11 herbs. Our previous study showed reduing of plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) level after treatment with QL in 512 patients with chronic heart failure, among those 20% of the patients have hypertension [8]. Our group previously reported that QL protected against cardiac remodeling after experimental acute myocardial infarction (AMI) in animal models [9], and enhanced metabolism in cardiomyocytes by increasing mitochondrial content and biogenesis [10]. Although the evidence of beneficial effects with QL on chronic heart failure is established, the underlying mechanism remains unclear.
Materials and methods
Animals
C57/BL6 male mice, 8-10 weeks old, weighing 22-28 g, were obtained from Nanjing University. Mice maintained in specific pathogen-free (SPF) conditions under a 12 h light/dark cycle, and fed ad libitum on a standard rodent diet. The study was approved by the ethical committees of the Nanjing Medical University and all animal experiments were conducted under the guidelines on humane use and care of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996). ISO was dissolved in sterile saline and was intraperitoneally injected (60 mg/kg/day) once daily for 14 consecutive days. The day after final injection, cardiac function were measured by echocardiography.
Echocardiography
The systolic heart function of the mice was measured by echocardiography. Mice were anesthetized with 2% isoflurane, maintained in the decubitus position and were allowed to breathe spontaneously during the procedure. Echocardiography was performed with a 35-MHz phased-array ultrasound system Vevo 2100 (Visual Sonics Inc, Toronto, Ontario, Canada). The following parameters were measured from M-mode images taken from the parasternal short-axis view at papillary muscle level: interventricular septum (IVS), left ventricular internal dimension (LVID), Left ventricular volume (LV vol), left ventricle mass (LV mass), left ventricular fractional shortening (FS) and left ventricular ejection fraction (EF).
Animal groups
QL was provided by Shijiazhuang Yiling Pharmaceutical Co., Ltd. (Shijiazhuang, Hebei, China). QL were given intragastrically after ISO-infusion to the mice with the optimum dose (0.5 g/kg/d) found in our previous study [9]. Mice were randomly divided into four groups: (1) saline; (2) Saline+QL; (3) ISO; (4) ISO+QL.
To prove whether PPARγ provide beneficial effects on ISO-induced heart failure, mice were randomly divided into five groups and were treated as follows: (1) saline; (2) ISO; (3) ISO+QL; (4) ISO+QL+PPARγ activator (Rosiglitazone, 1 mg/kg/d); (5) ISO+QL+PPARγ inhibitor (T0070907, 1 mg/kg/d), PPARγ activator and inhibitor were intraperitoneally injected just after ISO injection.
Immunochemistry
To assess the degree of fibrosis, sections were stained with Masson-Trichrome and scanned with computer-assisted video densitometry; the fibrotic fraction was obtained by calculating the ratio of blue (fibrotic) to total myocardial area using Image J (NIH).
Immunoblotting
Heart tissues were lysed in RIPA buffer (P0013C, Beyondtime) supplemented with 1 mM PMSF (ST505, Beyondtime). 30 μg of total protein was subjected to electrophorese on 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes which incubated with 5% milk and with primary antibody at 4°C overnight, and then washed three times with TBST buffer before adding secondary antibody. Specific protein bands were visualized by using ECL (Invitrogen) reagents.
Primary antibodies as follows: transforming growth factor beta (TGF-β, 1:1000 dilution, Cell Signaling Technology), metalloproteinase-2 (MMP-2, 1:1000 dilution, Abcam), matrix metallopeptidase 9 (MMP-9, 1:1000 dilution, Abcam), B-cell lymphoma 2 (Bcl-2, 1:1000 dilution, Cell Signaling Technology), Bcl-2-associated X protein (Bax, 1:1000 dilution, Cell Signaling Technology), proliferator-activated receptor-γ (PPARγ, 1:500 dilution, Abcam), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α, 1:1000 dilution, NOVUS), protein kinase B (Akt, 1:1000 dilution, Cell Signaling Technology), phospho-Akt (Ser473, 1:1000 dilution, Cell Signaling Technology), extracellular signal regulated kinases (ERK, 1:1000 dilution, Cell Signaling Technology) and phospho-ERK (Thr202/Tyr204, 1:1000 dilution, Cell Signaling Technology), phospho-P38 MAPK (p-P38, 1:1000 dilution, Cell Signaling Technology), protein 38 mitogen activated protein kinases (P38, 1:1000 dilution, Cell Signaling Technology), Glyceraldehyde 3-phosphate dehydrogenase antibody (GAPDH, 1:1000 dilution, Kangchen, Shanghai, China).
Quantitative real time polymerase chain reaction (qRT-PCR)
Total RNAs were isolated from heart tissues using TRIZOL RNA extraction kit (Invitrogen, USA) and reverse transcribed to cDNAs using iScript™ cDNA Synthesis Kit (Bio-Rad, USA) according to the manufacturer’s instructions. Real-Time PCRs were used to determine gene expression levels by using an ABI 7900HT fast Real-Time PCR System (Applied Biosystems) with SYBR-Green supermix Kit (Bio-Rad).
The primer sequences (forward and reverse) used in this study are listed as follows: atrial natriuretic peptide (ANP): CTC CCA GGC CAT ATT GGAG, TCC AGG TGG TCT AGC AGGTT; brain natriuretic peptide (BNP): TGG GAA GTC CTA GCC AGT CTC, TCT GAG CCA TTT CCT CTGAC Relative mRNA expression was presented using the 2-ΔΔct method.
Statistical analysis
Results were presented as mean ± SEM. Independent-samples t-test was used for comparisons between two groups. One-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test were used to compare among three (or more) groups. A value of p<0.05 was considered statistically significant. The data were analyzed by GraphPad Prism 7 software.
Results
QL attenuates cardiac remodeling in ISO-induced cardiac dysfunction mice
Cardiac dysfunction was induced by ISO (60 mg/Kg/d) intraperitoneal injection, and then QL or saline administered intragastrically for 2 weeks, following echocardiography was performed for evaluating cardiac function. The echocardiography revealed significant impairment in LV function after ISO infusion, including EF and FS (Figure 1A).
Figure 1.
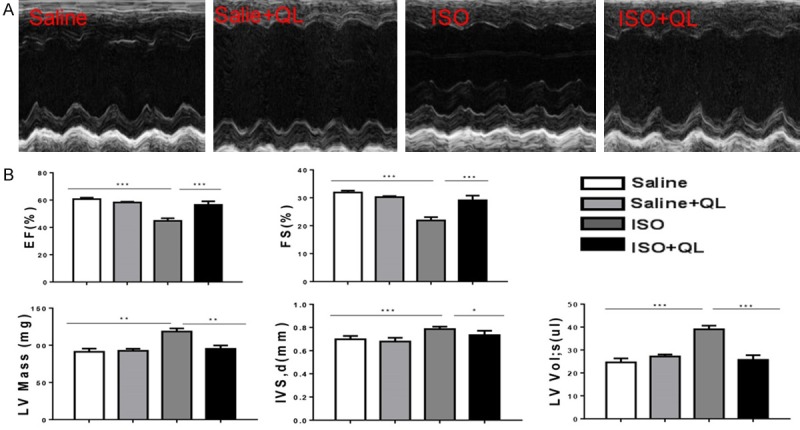
QL attenuates cardiac remodeling after ISO infusion. A. Left ventricular fractional shortening (%) (EF) and ejection fraction (%) (FS) as measured by echocardiography. B. Echocardiography revealed significant impairment in LV function after ISO infusion, including EF and FS, QL treatment improved cardiac function in EF and FS. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and expressed as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. n=6 per group.
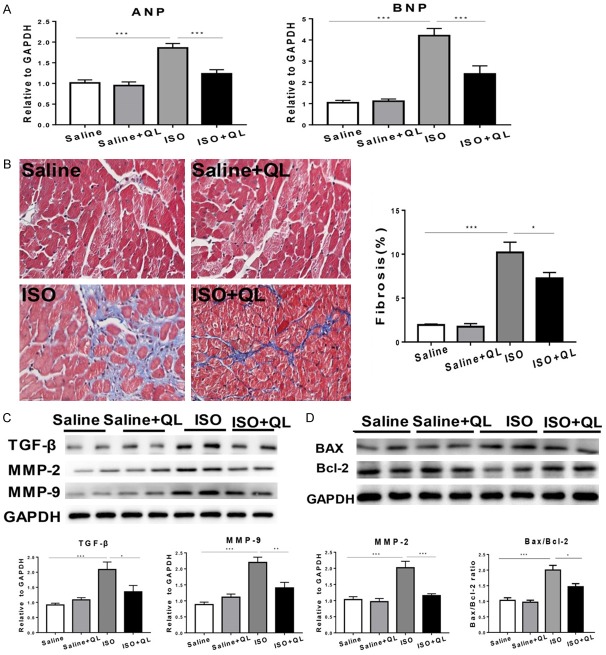
On the other side, two consecutive weeks apply of ISO in mice displayed a marked increase in heart size, such as interventricular septum (IVS), left ventricular volume (LV vol), left ventricle mass (LV mass) (Figure 1B), and also increased expressions of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) showed in real-time qPCR (Figure 2A), which these physiological changes were prevented by QL treatment.
Figure 2.
QL treatment diminishes isoproterenol (ISO)-induced cardiac fibrosis and cell death. A. Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were increased in the ISO-induced mice which were reversed by QL treatment. B. Effects of QL on interstitial fibrosis assessed by masson trichrome staining, 400× magnifications. C. Western blotting analysis showed protein level of TGF-β, MMP-2 and MMP-9 was decreased by QL treatment. D. Western blotting analysis showed the Bcl-2/Bax ratio was decreased by QL in the ISO-induced mice. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and expressed as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. n=6 per group.
QL reduces ISO-induced cardiac apoptosis and fibrosis
Apoptosis and fibrosis are considered to be the main elements for cardiac remodeling [11,12]. The application of ISO increased the amount of apoptosis and fibrosis. Western blot indicated that the ratio of pro-apoptotic molecule Bax to anti-apoptotic molecule Bcl-2 was decreased with QL treatment (Figure 2D and Supplementary Figure 1A). Masson trichrome staining showed collagen deposition in left ventricle section was decreased with QL treatment compared to ISO infusion (Figure 2B). The developmental markers of cardiac remodeling, TGF-β, MMP-9 and MMP-2 levels were markedly improved by ISO-infusion; on the contrary, QL treatment significantly reduced ISO-induced improvements of TGF-β, MMP-9 and MMP-2 levels (Figure 2C and Supplementary Figure 1B).
Increased PPARγ and PGC1-α with QL treatment in ISO-induced cardiac dysfunction mice
The mechanism of the protective effect of QL treatment in ISO-induced cardiac dysfunction mice was further explored. According to our previous research, PPARγ plays an important role in QL treatment after AMI in mice. This study was conducted to explore whether PPARγ is involved in sustained ISO activation with QL treatment.
Based on the immunoblotting analysis, deceased level of PPARγ and PGC1-α were found in ISO-induced mice with vehicle-treated by QL treatment (Figure 3A and Supplementary Figure 1C). However, QL treatment did not alter the expression of AKT, p-ERK1/2/ERK1/2 and p-P38/P38 in ISO-induced cardiac dysfunction, suggesting these signaling pathways were not participated in QL-mediated improvement in cardiac remodeling in ISO-induced heart failure (Figure 3B and Supplementary Figure 1C).
Figure 3.
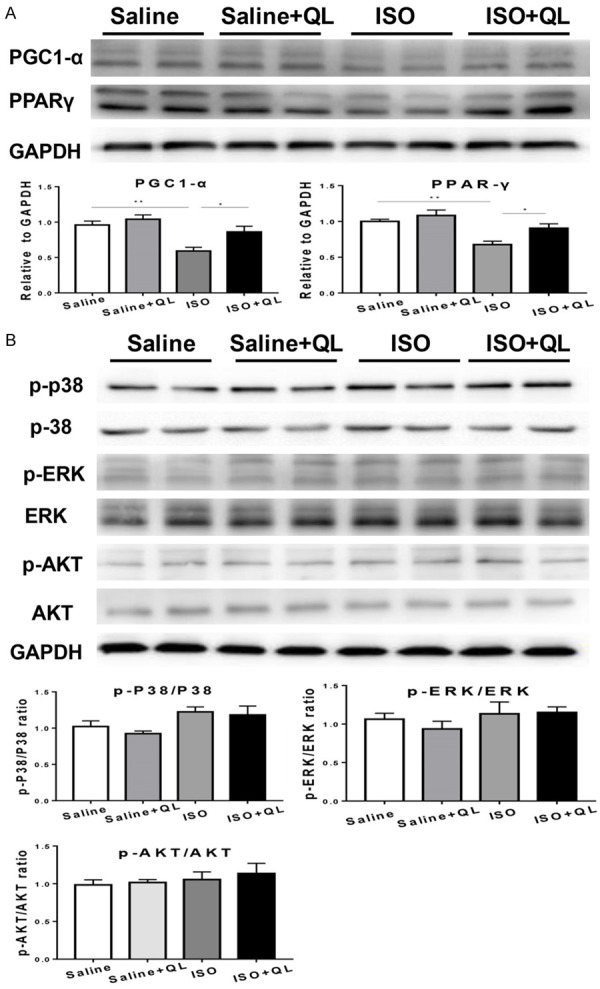
Expression of PPARγ, p-AKT, AKT, p-ERK, ERK, p-P38, P38 in western blotting. A. The protein level of PPARγ and PGC1-α was down-regulated in the ISO-induced mice, which was reversed by QLQX treatment. B. QL has no effect on AKT, P-38 and ERK pathways. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and expressed as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. n=6 per group.
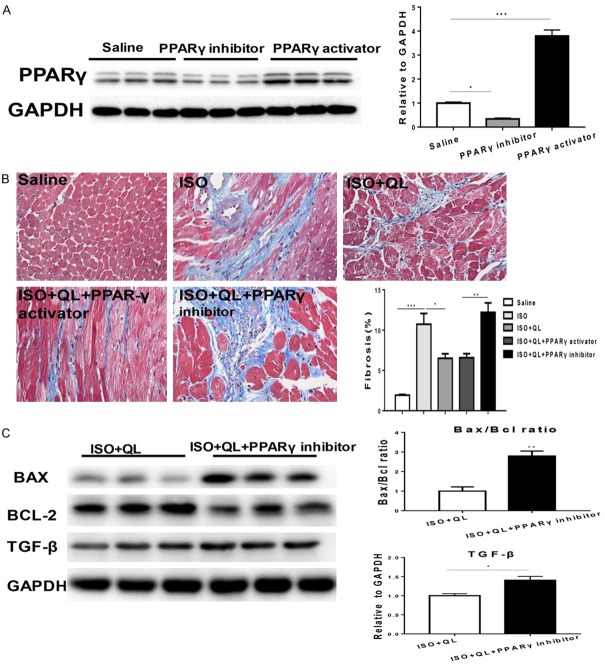
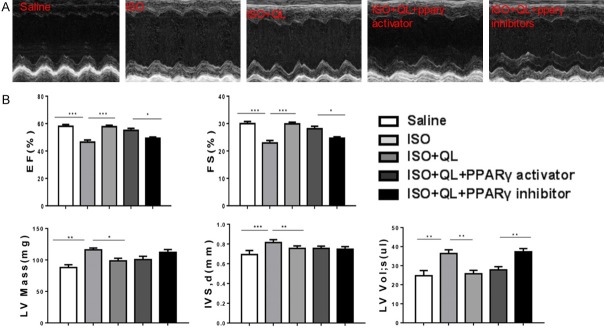
QL attenuates cardiac remodeling in sustained ISO infusion via PPARγ
To further explore the contribution of PPARγ to the beneficial effects of QL in ISO infusion, we treated mice with T0070907, an inhibitor of PPARγ (1 mg/kg/d), combinated with QL and ISO. The effects of PPARγ inhibitor or PPARγ activator were detected by the decreased or raised PPARγ expression levels (Figure 5A and Supplementary Figure 1D). Consequently, PPARγ inhibitor prevented the beneficial effects of QL on cardiac function (evaluated by EF and FS) after ISO infusion (Figure 4A, 4B). Moreover, PPARγ activator failed to provide extra profits in cardiac function in the presence of QL (Figure 4A, 4B), suggesting that PPARγ activation is necessary for its beneficial effects in attenuating cardiac remodeling after ISO infusion. Meanwhile, inhibition of PPARγ activity potently blocked the protective effects of QL against ISO-induced cardiac dysfunction, which revealed in cardiac fibrosis determined by Masson trichrome staining (Figure 5B). PPARγ inhibitor abolished the effects of QL in decreasing ISO-induced apoptosis which indicated by the ratio of Bax/Bcl-2 (Figure 5C and Supplementary Figure 1E). Consequently, these data suggested that the increased levels of PPARγ and PGC1-α could contribute to the beneficial effects of QL in attenuating cardiac remodeling after sustained ISO activation.
Figure 5.
QL attenuates cardiac fibrosis and apoptosis via PPARγ. (A) PPARγ inhibitor abolishes the effects of QL in decreasing cardiac fibrosis. (B) PPARγ inhibitor decreases the expression of PPARγ and PPARγ activator increases the expression of PPARγ. (B) Masson trichrome staining for cardiac fibrosis, 400× magnification. (C) PPARγ inhibitor abolishes the effects of QL in decreasing apoptosis as indicated by the ratio of Bax/Bcl-2. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test (A and B), independent-samples t-test was used for comparisons between two groups (C) and expressed as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. n=3 per group.
Figure 4.
QL improves cardiac function via increasing PPARγ in ISO-induced mice. A. Left ventricular fractional shortening (%) (EF) and ejection fraction (%) (FS) as measured by echocardiography. B. Echocardiography parameters show that PPARγ inhibitor abolish the beneficial effects of QL on improving cardiac function, while PPARγ activator failed to provide any further improvement in cardiac function in the presence of QL. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and expressed as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. n=6 per group.
Discussion
With use of QL in ISO-induced cardiac remodeling, this study found that QL-mediated cardiac protection in isoproterenol-induced cardiac remodeling by inducing PPARγ, decreasing apoptosis and reducing cardiac fibrosis are involved in the pharmacologic effects. The peroxisome proliferation-activated receptor γ (PPARγ) is from the nuclear receptor superfamily of ligand-inducible transcription factor family [13]. Study showed PPARγ activation can prevent cardiac hypertrophy, inhibit cardiac remodeling, and reduce infarct size in several animal models; the underlying protective effects of PPARγ activation have been attributed to reducing inflammation, oxidative stress and cell death [14-16]. However, the limitation of PPARγ as a therapeutic measure in cardiovascular disease attributes to the risk for fluid retention, weight gain, bone loss and congestive heart failure [17]. Therefore, some aspects of PPARγ function are poorly understood. Designing or identifying the selective PPARγ modulators with respect to therapeutic applications is highly desirable and is a major challenge to date [18].Only parts of the cardioprotective mechanism of QL on variety of pathological animal models have been demonstrated in the line of the previous studies [10,19-21]. As increased sympathetic nerve activity in the myocardium is a central feature of patients with heart failure [4,22].Therefore, ISO-infused mice were established to develop cardiac dysfunction. Following with echocardiographic parameters revealed significant impairment in cardiac function; immunohistochemical staining indicated that myocardial fibrosis was significantly improved in ISO group. However, QL-treated mice significantly improved cardiac function, decreased myocardial fibrosis and apoptosis. Based on the immunoblotting analysis, the PPARγ and PGC1-α levels were shown to be deceased in ISO-induced mice compared to vehicle-treated mice, and these decreases were prevented by QL treatment. In addition, inhibition of PPARγ activity potently prevented the protective effects of QL against ISO-induced cardiac dysfunction. Moreover, PPARγ activator was unable to provide additional profits in cardiac function with QL treatment, suggesting that PPARγ and QL function predominantly through the same pathway, which is fully activated with either treatment alone. Fluid retention may be the major contributor to the negative impact of PPARγ agonist thiazolidinediones in heart and the underlying mechanism has not been fully elucidated [23]. While some studies reported that some new PPARγ activators showed a promising therapeutic effect with lesser adverse effects such as fluid retention and heart failure [24,25]. Non-genomic and genomic mechanisms have been crucial in helping to analyze the relative contributions of PPARγ activation in the therapeutic effects of QL, as well as determining which cell types in myocardial tissue are involved in PPARγ transformation and would be expected to have a role in the cardioprotective effect of PPARγ on the heart [23]. Further studies are needed to clarify the role of PPARγ in heart and to understand the molecular mechanism of PPARγ-dependent and PPARγ-independent pathways [17]. It is also important to identify the exact active ingredients of QL responsible for the therapeutic effects of QL in the future [26].
In conclusion, the present study showed that QL administration offers notable benefits on cardiac function and attenuates the progression of heart remodeling induced by ISO, meditating in activation of PPARγ and PGC-1α, which could provide favorable evidence of clinical therapeutic effects on reversing cardiac remodeling and heart failure
Acknowledgements
This work was supported by the grants the Natural Science Foundation of Jiangsu Province (BK2012648 to Hui Wang), “Six talent peaks” project in Jiangsu Province (2015-WSN-033 to Hui Wang), the National Natural Science Foundation of China (81370332 and 81170201 to XL Li), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD20102013 to XL Li), Twelve-Fifth National Key Technology R&D Program (2011BAL11B08 to XL Li). Dr XL Li is an Associate Fellow at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Piek A, de Boer RA, Sillje HH. The fibrosiscell death axis in heart failure. Heart Fail Rev. 2016;21:199–211. doi: 10.1007/s10741-016-9536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 3.Koitabashi N, Kass DA. Reverse remodeling in heart failure--mechanisms and therapeutic opportunities. Nat Rev Cardiol. 2011;9:147–157. doi: 10.1038/nrcardio.2011.172. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez A, Ravassa S, Beaumont J, Lopez B, Diez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol. 2011;58:1833–1843. doi: 10.1016/j.jacc.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 5.Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30–38. doi: 10.1038/nrcardio.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann JE, Heale J, Bieraugel M, Ramos M, Fisher RL, Vickers AE. Isoproterenol effects evaluated in heart slices of human and rat in comparison to rat heart in vivo. Toxicol Appl Pharmacol. 2014;274:302–312. doi: 10.1016/j.taap.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Zhang J, Huang J, Ma A, Yang J, Li W, Wu Z, Yao C, Zhang Y, Yao W, Zhang B, Gao R Efficacy and Safety of Qili Qiangxin Capsules for Chronic Heart Failure Study Group. A multicenter, randomized, double-blind, parallelgroup, placebo-controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J Am Coll Cardiol. 2013;62:1065–1072. doi: 10.1016/j.jacc.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Tao L, Shen S, Fu S, Fang H, Wang X, Das S, Sluijter JP, Rosenzweig A, Zhou Y, Kong X, Xiao J, Li X. Traditional Chinese Medication Qiliqiangxin attenuates cardiac remodeling after acute myocardial infarction in mice. Sci Rep. 2015;5:8374. doi: 10.1038/srep08374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Li S, Zhou Q, Sun Q, Shen S, Zhou Y, Bei Y, Li X. Qiliqiangxin attenuates phenylephrine-induced cardiac hypertrophy through downregulation of MiR-199a-5p. Cell Physiol Biochem. 2016;38:1743–1751. doi: 10.1159/000443113. [DOI] [PubMed] [Google Scholar]
- 11.Schirone L, Forte M, Palmerio S, Yee D, Nocella C, Angelini F, Pagano F, Schiavon S, Bordin A, Carrizzo A, Vecchione C, Valenti V, Chimenti I, Falco E, Sciarretta S, Frati G. A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxid Med Cell Longev. 2017;2017:3920195. doi: 10.1155/2017/3920195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 13.Kelly DP. PPARs of the heart: three is a crowd. Circ Res. 2003;92:482–484. doi: 10.1161/01.RES.0000064382.46274.95. [DOI] [PubMed] [Google Scholar]
- 14.Drosatos K, Khan RS, Trent CM, Jiang H, Son NH, Blaner WS, Homma S, Schulze PC, Goldberg IJ. Peroxisome proliferator-activated receptor-gamma activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ Heart Fail. 2013;6:550–562. doi: 10.1161/CIRCHEARTFAILURE.112.000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen T, Jin X, Crawford BH, Cheng H, Saafir TB, Wagner MB, Yuan Z, Ding G. Cardioprotection from oxidative stress in the newborn heart by activation of PPARgamma is mediated by catalase. Free Radic Biol Med. 2012;53:208–215. doi: 10.1016/j.freeradbiomed.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Liu HR, Tao L, Gao E, Qu Y, Lau WB, Lopez BL, Christopher TA, Koch W, Yue TL, Ma XL. Rosiglitazone inhibits hypercholesterolaemiainduced myeloperoxidase upregulation--a novel mechanism for the cardioprotective effects of PPAR agonists. Cardiovasc Res. 2009;81:344–352. doi: 10.1093/cvr/cvn308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra M, Miriyala S, Panchatcharam M. PPARgamma and its role in cardiovascular diseases. PPAR Res. 2017;2017:6404638. doi: 10.1155/2017/6404638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlaka E, Galatou E, Mellidis K, Ravingerova T, Lazou A. Role of pleiotropic properties of peroxisome proliferator-activated receptors in the heart: focus on the nonmetabolic effects in cardiac protection. Cardiovasc Ther. 2016;34:37–48. doi: 10.1111/1755-5922.12166. [DOI] [PubMed] [Google Scholar]
- 19.Zou Y, Lin L, Ye Y, Wei J, Zhou N, Liang Y, Gong H, Li L, Wu J, Li Y, Jia Z, Wu Y, Zhou J, Ge J. Qiliqiangxin inhibits the development of cardiac hypertrophy, remodeling, and dysfunction during 4 weeks of pressure overload in mice. J Cardiovasc Pharmacol. 2012;59:268–280. doi: 10.1097/FJC.0b013e31823f888f. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Fang H, Lin S, Shen S, Tao L, Xiao J, Li X. Qiliqiangxin protects against cardiac ischemia-reperfusion injury via activation of the mTOR pathway. Cell Physiol Biochem. 2015;37:454–464. doi: 10.1159/000430368. [DOI] [PubMed] [Google Scholar]
- 21.Lin S, Wu X, Tao L, Bei Y, Zhang H, Zhou Y, Shen S, Xiao J, Li X. The metabolic effects of traditional Chinese medication Qiliqiangxin on H9C2 cardiomyocytes. Cell Physiol Biochem. 2015;37:2246–2256. doi: 10.1159/000438580. [DOI] [PubMed] [Google Scholar]
- 22.Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J. 2015;36:1974–1982b. doi: 10.1093/eurheartj/ehv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmona MC, Louche K, Lefebvre B, Pilon A, Hennuyer N, Audinot-Bouchez V, Fievet C, Torpier G, Formstecher P, Renard P, Lefebvre P, Dacquet C, Staels B, Casteilla L, Pénicaud L Consortium of the French Ministry of Research and Technology. S 26948: a new specific peroxisome proliferator activated receptor gamma modulator with potent antidiabetes and antiatherogenic effects. Diabetes. 2007;56:2797–2808. doi: 10.2337/db06-1734. [DOI] [PubMed] [Google Scholar]
- 25.Dunn FL, Higgins LS, Fredrickson J, DePaoli AM. Selective modulation of PPARγ activity can lower plasma glucose without typical thiazolidinedione side-effects in patients with Type 2 diabetes. J Diabetes Complications. 2011;25:151–158. doi: 10.1016/j.jdiacomp.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Tang WH, Huang Y. Cardiotonic modulation in heart failure: insights from traditional Chinese medicine. J Am Coll Cardiol. 2013;62:1073–1074. doi: 10.1016/j.jacc.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.