Abstract
Age-related hearing loss (ARHL) or presbyacusis is a progressive loss of hearing sensitivity that is predominately associated with sensory or transduction neuro-cell degeneration in the peripheral and central auditory systems. Increased production of reactive oxygen species (ROS) and inflammatory response were frequently found in aging cochleae. In addition, inflammasomes are likely responsible for the accumulation of ROS in immune cells, although whether they are in fact involved in the development of ARHL is unknown. In this study, Q-PCR, WB and ELASA demonstrated significantly increased levels of activated Caspase-1, interleukin-1β and interleukin-18 and even NLRP3 in the inner ears of aging mice compared to younger one. In addition, NLRP3, as a sensor protein of ROS, may contribute to inflammasome assembly and subsequent inflammation in the cochleae. In conclusion, inflammation triggered by the activation of inflammasomes in the cochleae of aging mice appears to be playing an important role in the pathological process of ARHL and may be a potential cause of presbyacusis.
Keywords: ARHL, NLRP3, inflammasome
Introduction
Age-related hearing loss (ARHL, also called presbyacusis), one of the most common illnesses affecting people over 65 years of age, is a progressive loss of hearing sensitivity that is predominately associated with sensory cell degeneration in the inner ear [1].
As a condition characterized by a decline in auditory function, ARHL usually develops as a result of interaction of multiple factors, most notably cochlear aging, environment, genetic predisposition, and health comorbidities. The primary pathology of ARHL includes hair cells loss, loss of spiral ganglion neurons, stria vascularis atrophy, and changes in central auditory pathways. Oxidative stress has been implicated as having a major role in the pathophysiology of ARHL. Excessive accumulation of reactive oxygen species (ROS) is toxic to cells and can cause apoptosis of cells in the inner ears [2-5]. Moreover, ROS accumulation can interact with other pathological activities, such as inflammatory response [6,7], to potentiate age-related sensory cell degeneration [8,9].
More recent research has found that ROS accumulation involves the inflammasomes, a multiprotein oligomer component of the innate immune system [10,11] that consists mainly of caspase 1, PYCARD, and pattern recognition receptors (PRRs) [12]. A number of inflammasome-forming PRRs have been identified, including the Nod-like receptor (NLR) family pyrin domain containing 1 (NLRP1), NLRP3, NLRP6, NLRP7, NLRP12, CARD domain containing 4 (NLRC4), AIM 2 (absent in melanoma 2), IFI16, and RIG-I [13]. Of all types of PRRs, NLRP3, a sensor protein of ROS, can recruit Asc via PYD-PYD domain interaction and allow Asc to bind with caspase-1 [14]. Caspase 1 is then activated in inflammasome assembly, which promotes the maturation of downstream inflammatory cytokines interleukin-1 beta (IL-1β) and IL-18. Both are some of the earliest and most important alarms of the inflammation response [15].
The implication of inflammasomes in the pathophysiology of degenerative processes in other aging-related diseases has been investigated [16]. However, there is little evidence concerning the role of inflammasomes in the development of ARHL.
We studied the activation of inflammasomes in cochleae of aging mice during the pathological process of ARHL and confirmed NLRP3 as an important contributor to inflammasomes as-sembly and subsequent inflammation in cochleae. The data also establish that inflammation in the inner ear triggered by the activation of inflammasomes is a potential cause of ARHL.
Materials and methods
Animal model
We bred mice at one year of age and monitored their hearing through auditory brainstem responses (ABRs) at different time points until most of them acquired phenotypes of ARHL. All experimental animals were looked after in a clear and silent animal room to avoid noise and other stimuli that may otherwise induce hearing loss. Each of these deaf mice underwent strict eardrum diagnosis to determine tympanitis-related hearing loss. Animal experimentation was performed in accordance with protocols approved by the Ethics Committee of the Experimental Animal Center at Xuzhou Medical University.
ABRs
Recording electrodes were inserted into the top of skulls of experimental animals. Reference electrodes were placed on earlobes on the recording side, while ground electrodes were connected to earlobes on the opposite side. Impedance between electrodes was kept less than 3 kU. Click stimulation started from 100 dB SPL and was gradually reduced to 20 dB SPL by 10 dB each time. Filter setting was 100e3000 Hz. ABR thresholds indicated the stimulus intensity associated with disappearance of wave I.
Inner ear staining
Mice were exsanguinated by transcardial perfusion with phosphate-buffered saline (PBS) and then fixed with 4% paraformaldehyde in phosphate buffer (4% PFA-PB). Cochlea was dissected away from the skull and immersed in 4% PFA-PB overnight and decalcified with 100 mM EDTA in PBS for 2 days at 25°C. The cochlea was then embedded in paraffin. Cochlear sections were cut and mounted on poly-l-lysin-coated glass slides and hematoxylin and eosin (HE) staining was performed as described previously [17].
Quantitative real-time PCR
Total RNA was extracted from mouse cochleae using Trizol (TIANGEN, China) reagent according to the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized from 1 mg of total RNA using the ImProm-II TM Reverse Transcription System (Promega, USA). To assess possible gene amplification, quantitative RT-PCR was performed with the use of SYBR green chemistry. β-Actin as an internal standard was arbitrarily assigned a value of 1.0. The sequence-specific primers in quantitative RT-PCR were: IL-6: F: CTGCAAGAGACTT-CCATCCAG, R: AGTGGTATAGACAGGTCTGTTGG; TNF-α: F: CTTCTCATTCCTGCTTGTGG, R: CACTTGGTGGTTTGCTACG; IL1β: F: GAAATGCCACCTTTTGACAGTG, R: TGGATGCTCTCATCAGGACAG; NLRP3: F: ATTACCCGCCCGAGAAAGG, R: CATGAGTGTGGCTAGATCCAAG; Actin: F: CTGAGAGGGAAATCGTGCGT, R: ACCGCTCGTTGCCAATAGT.
Western blotting
Tissues from mouse cochleae were lysed with RIPA. Western blotting was performed in a manner similar to previous studies adopting the same procedure [18]. In brief, proteins resolved in the SDS-PAGE gel were transferred onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA). Blots were immunoreacted with anti-Caspase 1 (ab1872, Abcam, USA), anti-IL-18 (ab71495, Abcam) or anti-NLRP3 (ab91413, Abcam) antibody. Each antibody preparation was diluted in 5% skim milk, and protein bands were visualized using the chemiluminescence detection system ECL plus (GE Healthcare, USA). Signals in the immunoblots were analyzed based on their exposure on pictures.
Immunofluorescence
Mice were exsanguinated by transcardial perfusion with phosphate-buffered saline (PBS) and then fixed with 4% paraformaldehyde in phosphate buffer (4% PFA-PB). The Cochlea was dissected away from the skull and immersed in 4% PFA-PB overnight, and decalcified with 100 mM EDTA in PBS for 2 days at 25°C. Decalcified cochleae were embedded in low melting point paraplast and sliced into 6-um sections, followed by overnight incubation at 4°C with primary antibody to Caspase-1 (ab1872, Abcam) or NLRP3 (ab91413, Abcam). Negative controls were prepared in parallel under identical conditions but incubated without primary antibodies. Sections were washed three times in PBS and incubated with a corresponding secondary antibody (Alexa Fluor 488 or 594, IgG, ThermoFisher, USA) diluted in PBS for 1 h. Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI; Invitrogen, USA). Images of immunolabeled specimens were obtained by confocal fluorescence microscopy.
Detection of intraellular ROS
Generation of intracellular ROS was measured by monitoring the increasing fluorescence of 2070-dichlorofluorescein (DCF). 2070-Dichlorodihydorofluorescein diacetate (DCFH-DA; Sigma-Aldrich, USA) can enter the cell. Subsequently, intracellular esterases cleave off the diacetated group. The resulting DCFH is retained in the cytoplasm and oxidized to DCF by ROS. SGN cells were dissected from the inner ears of young and aging mice inner ear were seeded into each well of a 48-well plate. After 48 h, the cells were then washed once with phenol red-free medium and incubated in 200 µL working solution of DCFH-DA (20 mM) at 37°C for 30 min. The cells were observed under by fluorescence microscopy (Olympus, Japan). The fluorescence of DCF was monitored at excitation and emission wavelengths of 485 nm and 530 nm, respectively.
Enzyme-linked immunosorbent assay (ELISA)
IL-1β and IL-18 in inner ear lymph were measured using commercial ELISA kits (Uscn Life Science, China).
Statistical analysis
Statistical analysis was carried out using SPSS 16.0 software (SPSS, USA). All data are expressed as mean ± standard error (SEM) and analyzed by one-way ANOVA followed by Student’s t test. Morris water maze data were analyzed by two-way ANOVA. Linear regression analysis was used to evaluate relations between variables. Statistical significance level was defined at P<0.05.
Results
Degradation of peripheral hearing systems in aging mouse cochlear and loss of hearing
To establish the model of ARHL, mice were bred for 12 months (Figure 1A) until their hearing deteriorated significantly compared to 1-month-old (younger) mice (Figure 1C). The average ABR levels of each group are shown in Figure 1B. Hair cells and SGN cells in the cochleae of one-year-old (aging) mice deteriorated markedly (Figure 1D). Moreover, excessive ROS has been detected in SGN cells from aging mice cochleae by DCF fluorescence staining.
Figure 1.
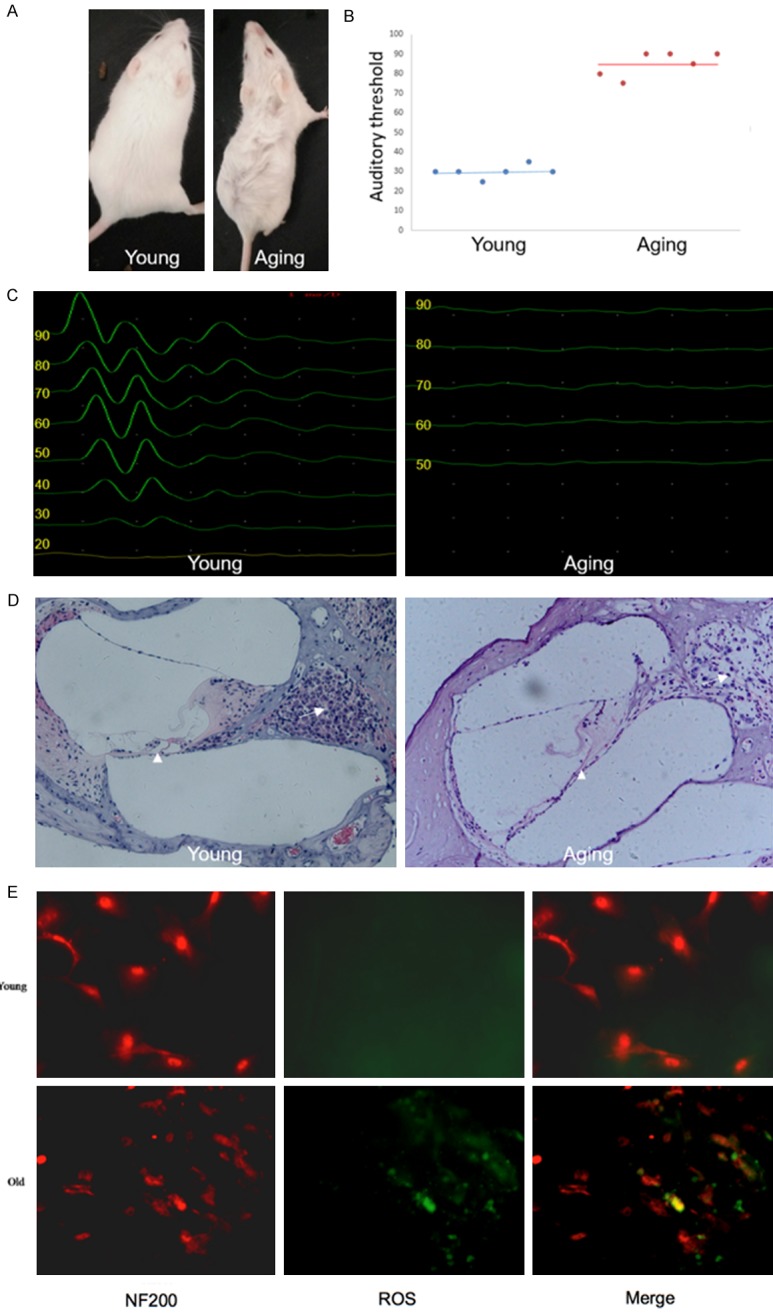
Degradation of phenotypes and function in aging mouse cochleae. A. Representative photograph of a young and aging mouse. B. Statistical analysis of ABR thresholds in young and aging mice (n = 3; ***P<0.001 vs. young mice). C. Representative images of ABR thresholds in young and aging mice. D. Degradation of hair cells and SGN cells in aging mice cochleae detected through HE staining. The organ of Corti and SGN cells are indicated by the arrow head and arrow, respectively. E. DCF fluorescence staining showing the increased ROS in SGN cells derived from cochleae of aging mice compare to young mice.
Increased inflammatory factors in aging mouse inner ears
The role of inflammation in the development of age-induced hearing loss has been addressed [18]. Presently, activated inflammatory factors including IL-6, tumor necrosis factor-alpha (TNF-α) and most notably IL-1β in the inner ears of aging mice were all detected by quantitative real-time PCR (Figure 2A), Western blotting (Figure 2B) and ELISA (Figure 2C).
Figure 2.
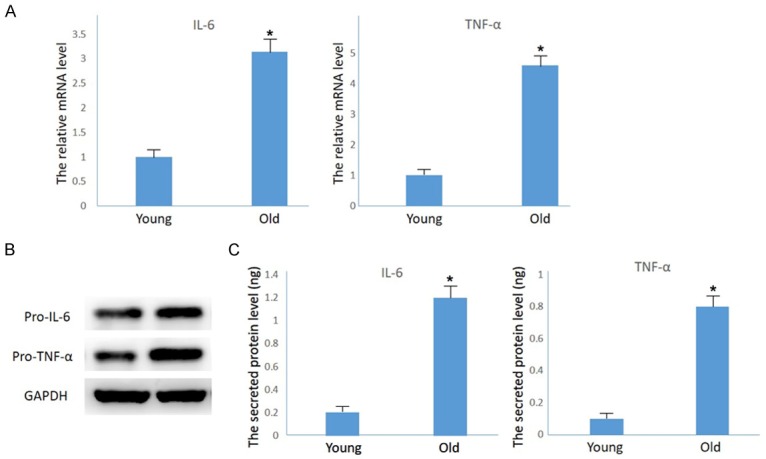
Up-regulation of inflammatory factors in the inner ears of aging mice. A. Quantitative real-time PCR results showing increased levels of IL-6 and TNF-α mRNA in aging mouse cochleae compared to the one-month-old mouse cohort (n = 3; *P<0.01 vs. young mice). B. Western blotting results showing increased accumulation the precursor protein of IL-6 and TNF-α in aging mouse cochleae. C. ELASA results showing increased accumulation of secreted IL-6 and TNF-α in aging mouse cochleae (n = 3; ***P<0.001 vs. young mice).
Assembling inflammasomes in mice cochleae in the development of ARHL
Having detected increased activity of inflammatory factors in the cochleae of aging mice, we next studied whether inflammasomes-related factors are activated in the development of ARHL. Markedly increased expression of mRNA (Figure 3A) and cleaved proteins (Figure 3B) of Caspase-1 were evident. Most proteins were revealed in SGN cells using immunofluorescence (Figure 3C). Cleaved IL-1β and IL-18, which are two substrates of Caspase-1, were also up-regulated and activated in aging cochleae (Figure 3A-C), indicating that inflammasomes were activated and played an important role in triggering inflammation during the ARHL process.
Figure 3.
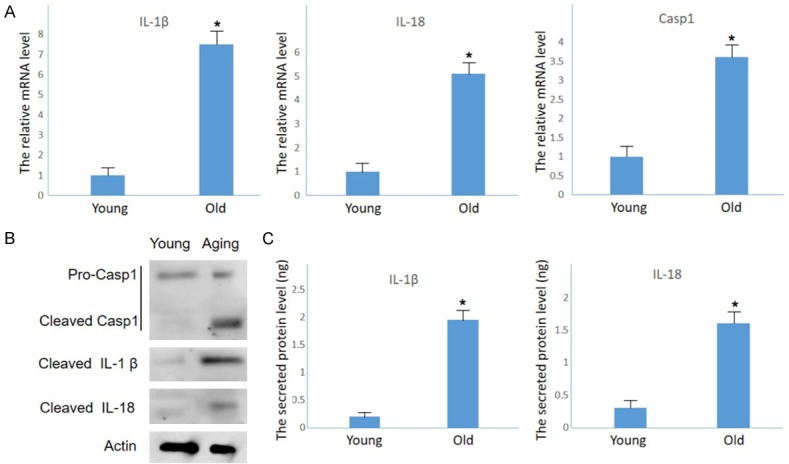
Inflammasome activation in the inner ear of aging mice. A. Increased mRNA levels of Caspase-1, IL-1β and IL-18 detected by Q-PCR (n = 3; #P<0.05 vs. the young group). B. Cleaved Caspase-1 and downstream factors IL-1β and IL-18 detected by Western blot. C. ELISA showing increased accumulation of secreted IL-1β and IL-18 in aging mouse cochleae (n = 3. ***P<0.001 vs. young mice).
NLRP3 is responsible for inflammasome activation during ARHL
For many aging-related diseases, ROS-induced overexpression of NLRP3 is responsible for regulating caspase-1-dependent maturation of IL-1β and IL-18 [19]. Presently, the expression of NLRP3 was markedly increased in aging mouse cochleae at both the mRNA and protein levels (Figure 4A and 4B). The proteins were primarily located in SGN cells (Figure 4C), similar fashion to that of Caspase-1 proteins. Therefore, NLRP3 is likely involved in inflammasomes activation during ARHL.
Figure 4.
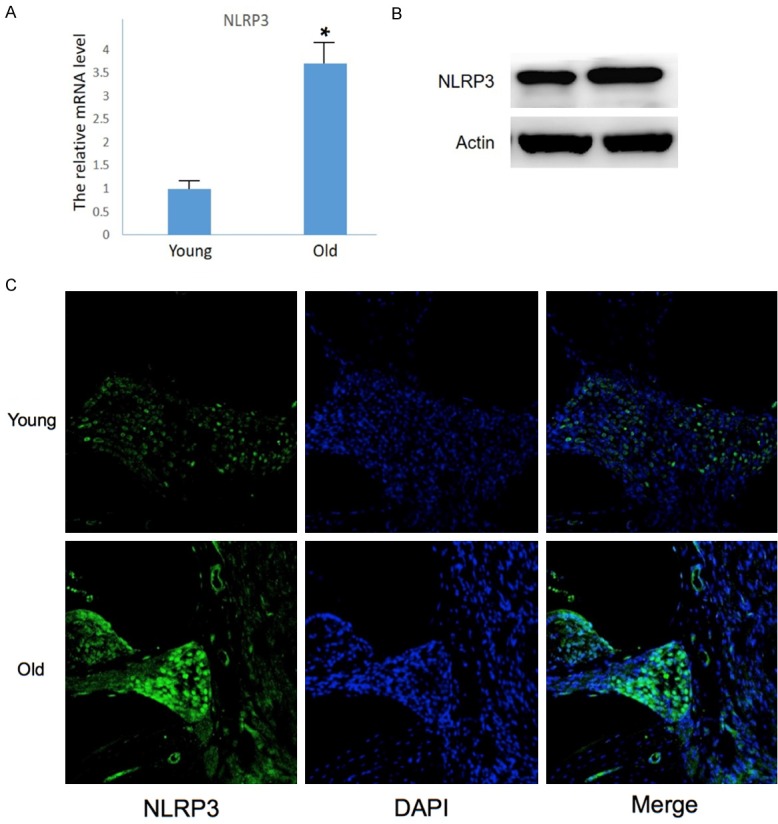
NLRP3 responsible for inflammasomes activation in the inner ears of aging mice. A. Up-regulated mRNA detected by Q-PCR (n = 3, #P<0.05 vs. young mice). B. Upregulated NLRP3 proteins detected by Western blot. C. Immunofluorescence showing increased NLRP3 in SGN cells.
Discussion
While the relationships between presbyacusis in mice with ROS accumulation [19] and inflammatory cell infiltration in inner ears [20] have been studied, the exact molecular mechanism behind ROS-induced inflammatory response in aging cochleae is a considerably less researched subject. We explored the assumed involvement of inflammasomes activation in ARHL and found that the inflammasomes assembly process was dependent on NLRP3 as an inflammasomes-forming PRR. The NLRP3-related inflammasomes is an intracellular platform that helps activate pro-inflammatory cytokines IL-1β and IL-18 upon receiving host- or pathogen-derived “danger” signals, and mediates pyroptosis, a form of inflammatory cell death. Initially known for playing a role in rare autoinflammatory syndromes called cryopyrinopathies, this important component of innate immune systems soon came to be recognized as a mediator of injurious inflammation in a broad array of diseases. While inflammasomes activation is a phenomenon most likely to occur in immune cells like macrophages, dendritic cells, as well as essential kidney cells like renal tubular epithelium, NLRP3-related inflammasomes also have proven implications in the pathogenesis of renal conditions, among them acute kidney injury, chronic kidney disease, diabetic nephropathy and crystal-related nephropathy [21]. Abundant evidence shows that similar pathological pathways might also have contributed to age-related cochleae diseases.
The hypothesis that inflammasomes play a pivotal role in hearing lesions as a result of excessive immune reaction is supported by research findings for cytomegalovirus infection-induced sensorineural hearing loss [18] and Mucklewell syndrome [22], another rare hearing disease triggered by a mutation in the NLRP3 gene that leads to excessive production of interleukin IL-1β. In both of these syndromes, IL-1β causes inflammatory manifestations accompanied by progressive sensorineural hearing loss [23]. Therefore, the attenuation of inflammasomes activation may provide a new strategy to contain hearing impairments in-duced by excessive immune responses.
In this study, we confirmed inflammasomes as a key initiator of inflammation in inner ears in the lead-up to ARHL. Possible signaling pathways are diagrammatically illustrated in Figure 5. In addition to ROS accumulation that may directly trigger hearing system lesions, the NLRP3 ROS sensor can engage with ASCs to form an inflammasomes and regulate caspase-1-dependent activation of IL-1β and IL-18, as well as downstream inflammation in aging mouse cochlea. These inflammations can potentially lead to tissue damage and ARHL.
Figure 5.
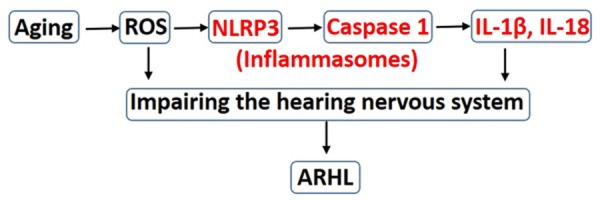
Diagram of possible pathways of inflammasomes involved in ARHL.
We proposed a novel strategy for ARHL treatment. Inhibiting inflammasomes activation may avoid organ and tissue damage as a result of a persistent inflammatory state. Supporting this view, drugs like anakinra are used to treat sensory deafness by inhibiting activity of cryopyrin inflammasomes for patients with Muckle-Wells syndrome [24]. The strategy is recommended for alleviating clinical and laboratory manifestations of ARHL.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (81470684, 21405130, 81670940), Postdoctoral Science Foundation of China (2015M571818). Clinical Special Fund of Jiangsu Province (b12014032), Six Major Categories Talent (2014-WSN-043, 2011-WS-074), Jiangsu Provincial University Fund (16621632), Innovation and Entrepreneurship Training Program for College Students in Jiangsu Province (KYLX14-1455, 201610313002Z), Colleges and universities Foundation in Jiangsu Province (16621632, 16KJB320016), the National Nature Science Foundation of Xuzhou (2017).
Disclosure of conflict of interest
None.
References
- 1.Gates GA, Caspary DM, Clark W, Pillsbury HC 3rd, Brown SC, Dobie RA. Presbycusis. Otolaryngol Head Neck Surg. 1989;100:266–271. doi: 10.1177/019459988910000403. [DOI] [PubMed] [Google Scholar]
- 2.Esterberg R, Linbo T, Pickett SB, Wu P, Ou HC, Rubel EW, Raible DW. Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. J Clin Invest. 2016;126:3556–3566. doi: 10.1172/JCI84939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Chen Y, Qi J, Zhang Y, He Y, Ni W, Li W, Zhang S, Sun S, Taketo MM, Wang L, Chai R, Li H. Wnt activation protects against neomycininduced hair cell damage in the mouse cochlea. Cell Death Dis. 2016;7:e2136. doi: 10.1038/cddis.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YR, Kim MA, Cho HJ, Oh SK, Lee IK, Kim UK, Lee KY. Galangin prevents aminoglycosideinduced ototoxicity by decreasing mitochondrial production of reactive oxygen species in mouse cochlear cultures. Toxicol Lett. 2016;245:78–85. doi: 10.1016/j.toxlet.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Rousset F, Carnesecchi S, Senn P, Krause KH. NOX3-targeted therapies for inner ear pathologies. Curr Pharm Des. 2015;21:5977–5987. doi: 10.2174/1381612821666151029112421. [DOI] [PubMed] [Google Scholar]
- 6.Kaur T, Borse V, Sheth S, Sheehan K, Ghosh S, Tupal S, Jajoo S, Mukherjea D, Rybak LP, Ramkumar V. Adenosine A1 receptor protects against cisplatin ototoxicity by suppressing the NOX3/STAT1 inflammatory pathway in the cochlea. J Neurosci. 2016;36:3962–3977. doi: 10.1523/JNEUROSCI.3111-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fetoni AR, Eramo SL, Paciello F, Rolesi R, Samengo D, Paludetti G, Troiani D, Pani G. The redox protein p66 (shc) mediates cochlear vascular dysfunction and transient noise-induced hearing loss. Sci Rep. 2016;6:25450. doi: 10.1038/srep25450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto C, Yamasoba T. Oxidative stresses and mitochondrial dysfunction in age-related hearing loss. Oxid Med Cell Longev. 2014;2014:582849. doi: 10.1155/2014/582849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riva C, Donadieu E, Magnan J, Lavieille JP. Age-related hearing loss in CD/1 mice is associated to ROS formation and HIF target proteins up-regulation in the cochlea. Exp Gerontol. 2007;42:327–336. doi: 10.1016/j.exger.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 11.Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011;21:558–560. doi: 10.1038/cr.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolli D, Velayutham TS, Casola A. Host-viral interactions: role of pattern recognition receptors (PRRs) in human pneumovirus infections. Pathogens. 2013;2:232–263. doi: 10.3390/pathogens2020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder K, Muruve DA, Tschopp J. Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol. 2009;19:R262–R265. doi: 10.1016/j.cub.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 15.LaRock CN, Nizet V. Inflammasome/IL-1β responses to streptococcal pathogens. Front Immunol. 2015;6:518. doi: 10.3389/fimmu.2015.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Liu RT, Cao S, Cui JZ, Wang A, To E, Matsubara JA. NLRP3 inflammasome: activation and regulation in age-related macular degeneration. Mediators Inflamm. 2015;2015:690243. doi: 10.1155/2015/690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Hoshino T, Iwasaki S, Wu R. Photochemically induced focal cochlear lesions in the guinea pig: I. DAB staining and SEM study. Microsc Res Tech. 1998;41:323–333. doi: 10.1002/(SICI)1097-0029(19980515)41:4<323::AID-JEMT5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Shi X, Dong YF, Li Y, Zhao ZL, Li H, Qiu SW, Li YH, Guo WW, Qiao YH. Inflammasome activation in mouse inner ear in response to MCMV induced hearing loss. J Otology. 2015;10:143–149. doi: 10.1016/j.joto.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki M, Harris JP. Expression of intercellular adhesion molecule-1 during inner ear inflammation. Ann Otol Rhinol Laryngol. 1995;104:69–75. doi: 10.1177/000348949510400111. [DOI] [PubMed] [Google Scholar]
- 21.Hutton HL, Ooi JD, Holdsworth SR, Kitching AR. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology (Carlton) 2016;21:736–744. doi: 10.1111/nep.12785. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607–612. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- 23.Kuemmerle-Deschner JB, Haug I. Canakinumab in patients with cryopyrin-associated periodic syndrome: an update for clinicians. Ther Adv Musculoskelet Dis. 2013;5:315–329. doi: 10.1177/1759720X13502629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki T, Masumoto J, Agematsu K, Sawai N, Kobayashi S, Shigemura T, Yasui K, Koike K. Anakinra improves sensory deafness in a Japanese patient with Muckle-Wells syndrome, possibly by inhibiting the cryopyrin inflammasome. Arthritis Rheum. 2008;58:864–868. doi: 10.1002/art.23261. [DOI] [PubMed] [Google Scholar]


