Abstract
Signal transducer and activator of transcription-3 (STAT3) is closely associated with tumorigenesis and is activated in tumor cells of a variety of cancers. The raised expression of cyclin D1 (CCND1) by activated STAT3 has been verified in some cancers. However, the relationship between STAT3 and CCND1 has yet to be studied in gastric cancer (GC) cells. In the present study, we found that STAT3 was constitutively activated in several GC cells together with overexpressed CCND1. In addition, IL-6 treatment enhanced the expression level of p-STAT3 and CCND1, accelerating the cell cycle progress and transferring from G1 to S phase. The increased proliferation, migration and invasion were also demonstrated by the treatment of IL-6 in HGC-27 and BGC-823 cells. While AG490 treatment, a Janus Kinase (JAK) inhibitor, showed the opposite effect. Therefore, our research demonstrated the positive correlation between p-STAT3 and CCND1 in GC cells. The constitutive activation of STAT3 and CCND1 overexpression accounted for the proliferation, migration and invasion in GC cells.
Keywords: STAT3, CCND1, gastric cancer, proliferation, migration, invasion
Introduction
Gastric carcinoma is one of the most common cancers which occurs worldwide with a relatively high mortality [1,2]. In addition, the severity of GC is hard to determine because of its metastasis and the lack of early diagnostic techniques. There are many factors leading to the migration and invasion of gastric cancer cells, including STAT3 and interleukin-6 (IL-6).
STAT3 belongs to a family of transcription factors which play an important role in cytokine signaling [3], including epidermal growth factor (EGF) and IL-6 [4]. In a typical STAT3 signal pathway, STAT3 is activated by Janus kinase (JAK) in response to the stimulation of growth factor or cytokine and phosphorylated in the site of Y705, a specific tyrosine residue in the C-terminal domain of STAT3 [5-7]. Subsequently, the phosphorylated STAT3 dimerizes through the mutual interaction of SH2 domain [8]. STAT3 dimers transfer their location from the cytoplasm to the nucleus and bind to specific DNA response elements, thereby, regulating the expression of target genes [5-7].
A number of endogenous protein regulators have been reported to inactivate the STAT signaling, such as the suppressors of cytokine signaling (SOCS) family [9]. Therefore, in normal conditions, the activation of STAT3 is transient and strictly controlled [10]. However, many potential upstream factors can contribute to the activation of STAT3 [9] and the IL-6/JAK/STAT3 pathway is closely related [11]. It is widely reported that STAT3 is consistently activated in a variety of cancers such as multiple myeloma, lung cancer, as well as several kinds of gastric cancer cells [12,13]. In addition, the effects of STAT3 in the initiation, development and progression of cancers have been verified, including proliferation, anti-apoptosis, invasion and angiogenesis [12].
The activated STAT3 complex promotes the transcription of many STAT3 target genes [14], and the increased expression of CCND1 associated with consistently activated STAT3 has been reported in head and neck cancer and breast cancer [15,16]. CCND1 is of vital importance in the cell cycle which interacted with the cyclin-dependent kinases 4/6 (CDK4/6) to form a complex and mediates the progress of progressing through the G1 phase [17]. Abnormal expression of CCND1, which accelerates cell cycle progression, is closely associated with a variety of tumors and may lead to tumorigenesis [18].
In this study, we investigate the expression of STAT3 and CCND1 in gastric cancer cells and normal gastric epithelial cells, and then explore the effect of JAK/STAT3 signaling pathway on the expression of CCND1 and proliferation, apoptosis, invasion of gastric cancer cells.
Materials and methods
Cell culture and treatment
Human GC cell lines HGC27, BGC823, MGC803, SGC7901 and one normal gastric epithelial cell GES-1 were obtained from the Chinese Academy of Sciences (Shanghai, China). RPMI1640 (HyClone) with 10% fetal bovine serum (FBS, GIBCO), 2 mM L-Glutamine, 100 U/ml, and 100 µg/ml streptomycin was used for the cell culture at 37°C in a humidified atmosphere containing 5% CO2. The medium was changed every 2 days. Cells were treated with IL-6 (100 ng/ml, sigma) or AG490 (50 µmol/l, sigma), and then harvested or detected at indicated time.
RNA extraction and quantitative reverse transcription PCR (qRT-PCR)
Primers used for qRT-PCR are shown in Table 1. Cells were cultured, handled as indicated, and harvested at the indicated time. Total RNA was extracted from cell precipitation using TaKaRa MiniBEST Universal RNA Extraction Kit according to the manufacturer’s instructions. DNA was removed with gDNA Eraser. Well prepared RNA was reverse transcribed with a PrimeScriptTM RT reagent kit (Perfect Real Time, TaKaRa) according to the protocol. Quantitative PCR was performed on a CFX Connect system (BioRad) using FastStart Essential DNA Green Master (Roche). Each 20 µl PCR mixture included 1 µl cDNA, 10 µl 2×qPCR mix, and 250 nM forward and reverse primer. Reaction mixtures were denatured at 95°C for 5 min, followed by 40 three-step cycles of 95°C for 10 s, 60°C for 15 s and 72°C for 20 s. Melt curve analysis was carried out after the amplification. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard for target gene expression levels.
Table 1.
Primers used for qRT-PCR
| Gene | Oligonucleotide sequence | |
|---|---|---|
|
| ||
| Forward (5’-3’) | Reverse (5’-3’) | |
| CCND1 | GCGGAGGAGAACAAACAGAT | GAGGGCGGATTGGAAATGA |
| GAPDH | GGTGTGAACCATGAGAAGTATGA | GAGTCCTTCCACGATACCAAAG |
Western blot
Cells were treated with IL-6 (100 ng/ml) or AG490 (50 µmol/l) and then detected at indicated time. Cells used for Western Blot (WB) were lysed in cell lysis buffer (P0013, Beyotime, Shanghai, China) and total protein concentrations were determined using the BCA assay kit (Beyotime, Shanghai, China). The same amount of total cell protein was used to run SDS-PAGE and transferred to NC membrane (Millipore). The membranes were then blocked with 5% non-fat milk in TBST for 1 h at room temperature. Each membrane was then incubated with various primary antibodies overnight at 4°C, including anti-pSTAT3 (1:1000, CST, 8204, rabbit), anti-STAT3 (1:1000, AtaGenix, ata17461, rabbit), anti-CCND1 (1:1000, AtaGenix, ata11263, rabbit), anti-GAPDH (1:5000, AtaGenix, ata17456, rabbit) at 4°C overnight. After washing for five times with TBST, the membrane was incubated with the corresponding secondary antibody conjugated with HRP (1:5000, AtaGenix, ata17817) for 1 h at room temperature. GAPDH was used for the internal control.
MTT assay
The effects of IL-6 and AG490 on gastric cell HGC27 and BGC823 proliferation were assessed by MTT. Cells were seeded in a 96-well plate (5×103 cells/well, five wells per group) and allowed to attach overnight. Cells were treated with IL-6 (100 ng/ml) or AG490 (50 µmol/l) for 24-72 h. Then, the proliferative activity was determined by adding 20 µl MTT solution [3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolum bromide, 5 mg/ml, Sigma, St. Louis, MO, USA] to each well. After incubation at 37°C for 4 h, the supernatant was discarded and 150 µl DMSO (Dimethyl sulfoxide, Sigma) was added to each well. The plate was then gently shaken for 10 min. The optical density (OD) value was obtained at a wavelength of 490 nm.
Cell-cycle assays
Cells of HGC27 and BGC823 were seeded in a 24-well plate (1×105 cells/well, three wells per group) and allowed to attach overnight. Cells were treated with IL-6 (100 ng/ml) or AG490 (50 μmol/l) for 48 h. The cells were then harvested and washed with PBS. This was followed by fixation with 70% ethanol overnight at 4°C. After washing with PBS, the cells were re-suspended in PBS containing 20 ug/ml propidium iodide (Biolegend), 0.1% Triton X-100 (Sigma) and 200 ug/ml RNase A (Sangon Biotech) for 30 min at room temperature in the dark. Samples were analyzed for DNA content by flow cytometry. The cell-cycle phases were analyzed using CytExpert software. The experiments were performed at least three times and representative results were shown.
Cell migration and invasion assays
24-well plates with 8-μm polycarbonate membranes transwells (Costar, 3422) were used in the assay to test migration and invasion. The transwells were coated with or without MatrigelTM (20 μg/well, BD Biosciences, 356234) for cell migration or invasion. To test the ability of cell invasion induced by IL-6 and AG490, the treated or untreated HGC27 and BGC823 in RPMI-1640 with 1% FBS were seeded in the upper chamber (1×105/well). The lower chamber was filled with 600 μl RPMI-1640 with 20% FBS. After 48 h incubation at 37°C, cells in the upper chambers were erased by cotton swab. Then 4% PFA was used to fix the transwell chambers for 30 min and the cells were stained with crystal violet (Beyotime, Shanghai, China). Cells on the bottom chamber were photographed and the numbers of cells were counted in 5 random visual fields.
Results
STAT3 is constitutively phosphorylated and CCND1 is overexpressed in GC cells
To investigate STAT3 activation in GC cells, cell lysates from GC cell lines HGC27, BGC823, MGC803, SGC7901 and one normal gastric epithelial cell GES-1 were analyzed by WB. The expression of p-STAT3 was dramatically higher in GC cells than GES-1 while the amount of STAT3 was almost the same (Figure 1A). In addition, the protein of CCND1 was overexpressed in GC cells (Figure 1A). The qRT-PCR assay also indicated the RNA level of CCND1 was increased when compared with GES-1 (Figure 1B). Therefore, our research suggested the constitutive activation of STAT3 and the positive association between p-STAT3 and CCND1 in several GC cells.
Figure 1.
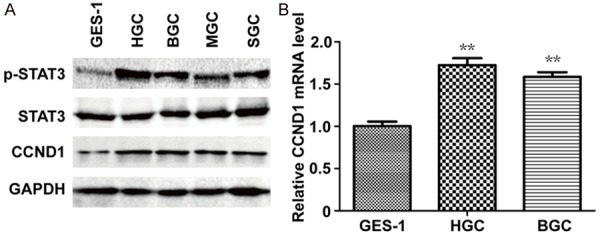
STAT3 is constitutively phosphorylated and CCND1 is overexpressed in GC cells. A: The expression of p-STAT3 (Tyr705), STAT3 and CCND1 protein was analyzed by Western blot. B: The expression of CCND1 mRNA in GES-1, HGC and BGC. 2-ΔΔCT were shown. *P < 0.05, **P < 0.01; Student’s t test, compared to GES-1.
STAT3 signaling mediates the expression of CCND1 and cell proliferation in HGC27 and BGC823 cells
Subsequently, we examined the effect of STAT3 signaling pathway on the expression of CCND1 and cell proliferation. IL-6, an activator of the STAT3 signaling pathway and AG490, a Janus Kinase (JAK) inhibitor, were used in HGC27 and BGC823 cells. In accordance with expectations, the result of WB showed the level of phosphorylated STAT3 was upregulated or downregulated respectively in a time dependent manner by the treatment of IL-6 or AG490 (Figure 2A). Interestingly, the expression of CCND1 shared the same changing trend with pSTAT3 (Figure 2A). Furthermore, a MTT assay was conducted to observe the effects of IL-6 and AG490 on the proliferation of HGC27 and BGC823 cells. The promoted proliferation was found in IL-6 treatment but the inhibited proliferation was shown in AG490 treatment and there was a significant difference at both 48 h and 72 h after treatment (Figure 2B). Thus, the STAT3 signaling positively mediates the expression of CCND1 and cell proliferation in HGC27 and BGC823 cells.
Figure 2.
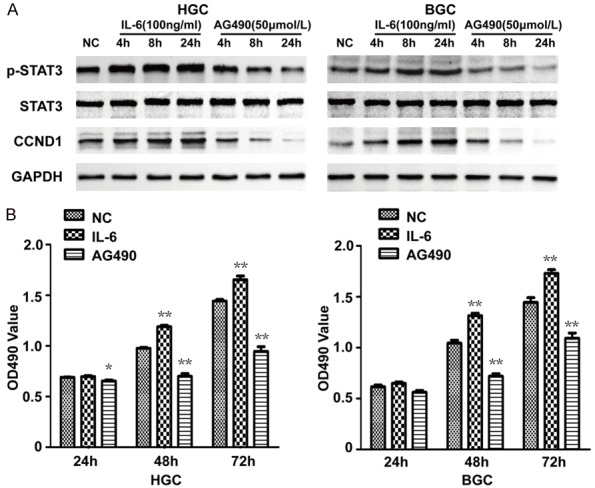
Effects of IL-6 and AG490 on the expression of CCND1 and cell proliferation of HGC27 and BGC823. A: Western blot analysis suggests IL-6 promotes the expression of p-STAT3 and CCND1, and AG490 inhibits the expression of p-STAT3 and CCND1. B: MTT result suggests IL-6 promotes cell proliferation and AG490 inhibits cell proliferation in HGC27 and BGC823. *P < 0.05, **P < 0.01.
IL-6 promotes cell cycle progress of HGC27 and BGC823 while AG490 inhibits
To determine the relationship between STAT3 signaling pathway and cell cycle progress, HGC27 and BGC823 cells were treated with IL-6 or AG490 for 48 h and a cell cycle assay was performed using a flow cytometer. The ratio of G0/G1 phase was deceased while the S phase was increased by IL-6 treatment (Figure 3), indicating the positive role of IL-6 in the cell cycle transfer from G1 to S phase. But the result of AG490 was opposite (Figure 3). These results suggest the positive role of STAT3 signaling pathway in the cell cycle progress, leading to the cell cycle transition from G1 to S phase.
Figure 3.
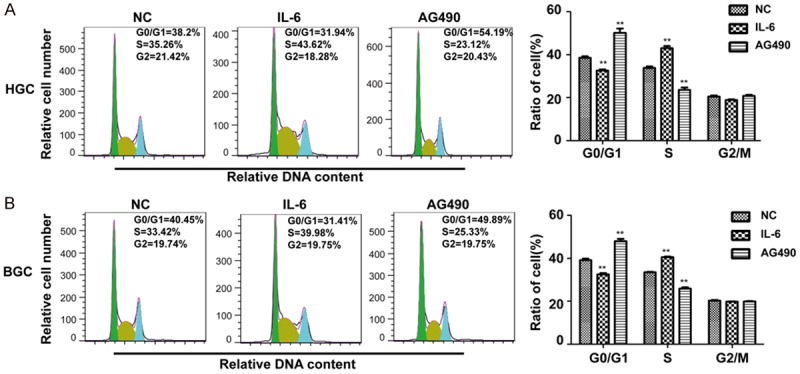
Effects of IL-6 and AG490 on cell cycle of HGC27 and BGC823. A: Cell-cycle analysis of IL-6 or AG490 treated HGC after 48 h and the percentages of cell-cycle phases are shown. B: Cell-cycle analysis of IL-6 or AG490 treated BGC after 48 h and the percentages of cell-cycle phases are shown. *P < 0.05, **P < 0.01, compared to NC.
IL-6 promotes migration and invasion of HGC27 and BGC823 while AG490 inhibits
The effects of IL-6 and AG490 on migration and invasion ability of HGC27 and BGC823 cells were analyzed using a transwell assay. The numbers of migrated and invaded cells were counted in five random fields after being treated with or without IL-6 or AG490 for 48 h. The total number of cells of which migrated or invaded in the IL-6 group was significantly higher than the number of migrating or invading cells in the control group (Figure 4). However, the total number cells of migrating or invading in the AG490 group was significantly lower than the number of migrating or invading cells in the control group (Figure 4). This shows that the presence of STAT3 in the signaling pathway promotes migration and invasion in HGC27 and BGC823 cells.
Figure 4.
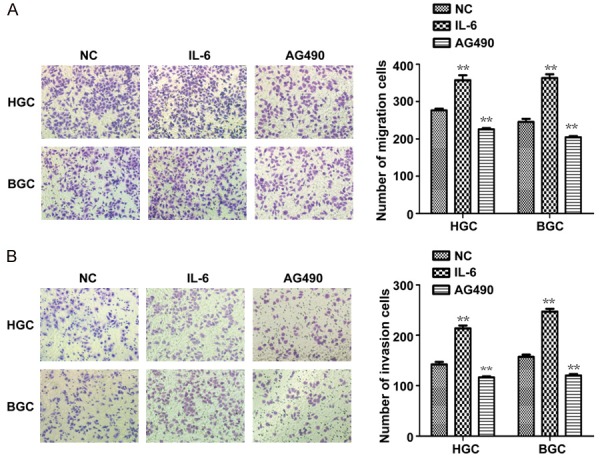
Effects of IL-6 and AG490 on GC cell migration and invasion. A, B: Representative migration or invasion images of HGC-27 and BGC-823 treated with IL-6 or AG490. Cell numbers were counted in five random fields. *P < 0.05, **P < 0.01, compared to NC.
Discussion
The abnormal activation of STAT3 has attracted a lot of attention in cancer research. Phosphorylation of STAT3 is required for its activation and results in dimerization, nuclear translocation, DNA binding, and transcriptional activation of target genes. The p-STAT3 may not only promote proliferation, anti-apoptosis, invasion and angiogenesis but can also produce resistance to conventional therapies [12,19]. Therefore, it is important to conduct further study on the STAT3 signaling pathway.
In this study, we initially investigated the expression of p-STAT3 in several GC cell lines as well as one normal gastric epithelial cell line, GES-1. Results showed the p-STAT3 level was obviously higher in GC cells than GES-1. But the STAT3 was also slightly phosphorylated in GES-1. Activation of STAT3 signaling regulates the expression of numerous genes [20,21]. Consistently activated STAT3 and its correlation with upregulation of CCND1 has been reported before [15,16], and Leslie et al. [16] verified that activated STAT3 associates with the cyclin D1 promoter and regulates its expression. Our research has also confirmed the positive relationship between activated STAT3 and overexpressed CCND1 in RNA and protein level in GC cells.
Next, we investigated whether activating or inhibiting the STAT signaling pathway affected the expression of CCND1, cell proliferation and the cell cycle. IL-6 and AG490, two different factors playing opposite roles in STAT signaling pathway, were used. Our results demonstrated that raising the expression of CCND1 correlated with accelerated cell proliferation and cell cycle in the IL-6 group compared with the NC group. However, the treatment of AG490 showed the opposite effect.
The JAK/STAT pathway plays an important role in GC and the disordered activation of STAT may be due to multiple reasons. For example, To et al. reported downregulation of SOCS-1 by hypermethylation activates the JAK/STAT pathway in GC [22]. Ouyang et al found that over-expression of GKN2 can increase apoptosis, reduce proliferation and invasion ability of gastric cancer cells as a result of down-regulated JAK2/STAT3 signaling pathway [23]. Furthermore, the role of IL-6 in mediating the pathway of JAK-STAT3 has also been verified [24]. The increased cell proliferation by p-STAT3 has been widely reported [25-27]. In this study, we found a positive association between activated STAT3 and the expression of CCND1, which may be responsible for the promoting effect of proliferation and cell cycle progress by the STAT3 signaling. Our results also showed the promoted migration and invasion in HGC27 and BGC823 cells. However, further studies are required to clarify the cause of increased migration and invasion by activated STAT3.
In conclusion, STAT3 is constitutively activated in human GC cell lines HGC27, BGC823, MGC803 and SGC7901. Activation of the JAK/STAT3 pathway promotes the expression of CCND1 at RNA and protein level. The overexpression of CCND1 may be a reason for the accelerated proliferation and cell cycle progress. The JAK/STAT3 pathway also mediates the migration and invasion in HGC27 and BGC823 cell lines. The inhibition of STAT3 signaling may be a potential treatment on gastric cancer.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Levy DE, Inghirami G. STAT3: a multifaceted oncogene. Proc Natl Acad Sci U S A. 2006;103:10151–10152. doi: 10.1073/pnas.0604042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong Z, Wen Z, Darnell JE Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102:311–319. doi: 10.1002/jcb.21475. [DOI] [PubMed] [Google Scholar]
- 8.Darnell JE Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 9.Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromberg JF. Activation of STAT proteins and growth control. Bioessays. 2011;23:161–169. doi: 10.1002/1521-1878(200102)23:2<161::AID-BIES1023>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang SW, Sun YM. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (Review) Int J Oncol. 2014;44:1032–1040. doi: 10.3892/ijo.2014.2259. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Crowe PJ, Goldstein D, Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review) Int J Oncol. 2012;41:1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- 13.Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y, Sekikawa A, Kawada M, Suzuki K, Kayahara T, Fukui H, Sawada M, Chiba T. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921–4929. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- 14.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 15.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 16.Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, Sakamaki T, Pestell R, Bromberg J. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res. 2006;66:2544–2552. doi: 10.1158/0008-5472.CAN-05-2203. [DOI] [PubMed] [Google Scholar]
- 17.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Zhang Y, Zhao L, Liu S, Yu S, Ma Y, Sun G. MicroRNA-193b inhibits the proliferation, migration and invasion of gastric cancer cells via targeting cyclin D1. Acta Histochem. 2016;118:323–330. doi: 10.1016/j.acthis.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. Int J Oncol. 2007;30:437–442. [PubMed] [Google Scholar]
- 20.Burke WM, Jin X, Lin HJ, Huang M, Liu R, Reynolds RK, Lin J. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- 21.Real PJ, Sierra A, De Juan A, Segovia JC, Lopez-Vega JM, Fernandez-Luna JL. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene. 2002;21:7611–7618. doi: 10.1038/sj.onc.1206004. [DOI] [PubMed] [Google Scholar]
- 22.To KF, Chan MW, Leung WK, Ng EK, Yu J, Bai AH, Lo AW, Chu SH, Tong JH, Lo KW, Sung JJ, Chan FK. Constitutional activation of IL-6-mediated JAK/STAT pathway through hypermethylation of SOCS-1 in human gastric cancer cell line. Br J Cancer. 2004;91:1335–1341. doi: 10.1038/sj.bjc.6602133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouyang J, Pan X, Lin H, Hu Z, Xiao P, Hu H. GKN2 increases apoptosis, reduces the proliferation and invasion ability of gastric cancer cells through down-regulating the JAK/STAT signaling pathway. Am J Transl Res. 2017;9:803–811. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, Zhuang J, Ye J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. 2016;35:1787–1795. doi: 10.3892/or.2016.4544. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Shaw PE. Autocrine-mediated activation of STAT3 correlates with cell proliferation in breast carcinoma lines. J Biol Chem. 2002;277:17397–17405. doi: 10.1074/jbc.M109962200. [DOI] [PubMed] [Google Scholar]
- 26.Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K, Beug H, Ohlschläger P, Schütz A, Halbhuber KJ, Friedrich K. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding BB, Yu JJ, Yu RY, Mendez LM, Shaknovich R, Zhang Y, Cattoretti G, Ye BH. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–1523. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]


