Abstract
Sphingosine kinase 1 (SPHK1) has been found to be upregulated in many different types of human malignancy and plays a crucial role in cancer development and progression. However, the potential of SPHK1 to act as a predictive and prognostic biomarker in breast cancer remains to be clarified. In the present study, SPHK1 expression was evaluated in breast cancer cell lines and 224 breast cancer tissue samples using immunohistochemical staining. Compared to the normal mammary epithelial cell line MCF-10A, SPHK1 mRNA and protein expression levels increased in the breast cancer cell lines SK-BR-3, MDA-MB-231, MDA-MB-436, and MCF-7. Immunohistochemical staining revealed SPHK1 expression to be significantly increased in breast cancer tissue compared to normal breast tissue, with 85 (37.9%) of the 224 invasive ductal carcinomas (IDC) exhibiting high SPHK1 expression. High SPHK1 expression in IDC showed a significant association with higher histological grade, distant metastasis, and triple negativity, and was shown to be an independent predictor for distant metastasis development. In addition, patients with high SPHK1 expression had significantly lower progression-free survival and overall survival rates compared to those with low SPHK1 expression. Our data suggest that SPHK1 is involved in the development and progression of breast cancer and can serve as a potential predictive biomarker of distant metastasis and patient outcome.
Keywords: Breast, invasive ductal carcinoma, sphingosine kinase 1, immunohistochemistry, metastasis, survival
Introduction
Breast cancer is one of the most commonly diagnosed cancers among females, accounting for approximately 30% of all new cancer cases [1]. In the Republic of Korea, breast cancer is the second most common cancer in females [2]. The majority of breast cancer patient deaths occur after the cancer metastasizes to distant organs and becomes a systemic disease. The metastatic potential of breast cancer cells is determined by complex and specific genetic alterations leading to a gain and/or loss of function, which enable cancer cell survival, proliferation, migration out of the primary site, and invasion of the host’s blood or lymphatic vasculature. In turn, this provides cancer cells access and a conduit to metastasize [3]. Although many researchers have suggested potential biomarkers to predict the development of breast cancer metastasis, no reliable biomarker has been identified to date. There is therefore an urgent need to establish novel predictive indicators to identify patients at higher risk of developing metastasis. This would also help enable oncologists with tailoring personalized therapeutic strategies for patients in order to improve their survival [4].
An emerging area of investigation within lipid research has been the role of sphingolipid metabolites in cancer etiology and pathogenesis [5]. A number of studies have established the roles of various enzymes involved in sphingolipid metabolism, of sphingolipid-binding proteins, and of transmembrane transporters in human cancers [6]. Among these proteins, members of the sphingosine kinase (SPHK) family have received the most attention as key enzymes in cancer pathophysiology because their catalytic activity lies at the critical intersection of sphingolipid metabolism regulation [5]. Two functional SPHK isoenzymes, SPHK1 and SPHK2, have been identified in humans [7]. Sphingosine-1-phosphate, a sphingolipid metabolite, plays a crucial role in various aspects of cellular survival, cellular growth, cellular proliferation, and apoptosis [8]. SPHK1 phosphorylates sphingosine to form sphingosine-1-phosphate, so as to prevent apoptosis and stimulate cellular proliferation and angiogenesis [5,9].
SPHK1 expression is upregulated in the cancers of many human organs, including the ovaries [10], uterine cervix [5], colon [11,12], stomach [9], lung [13], head and neck [8], and brain [14,15]. Previous studies have shown that SPHK1 is an oncogenic enzyme that is activated in close association with antiapoptotic activity, proliferation, survival, and transformation of tumor cells [11,14,16-18]. Multiple lines of evidence indicate that SPHK1 is involved in cancer development, progression, and metastasis, as well as neovascularization in the tumor microenvironment [19]. However, investigations of SPHK1 expression in breast cancer are very limited. In this study, we examined SPHK1 mRNA and protein expression levels in breast cancer cell lines. The expression pattern of SPHK1 in breast cancer tissue samples was further analyzed using a tissue microarray technique and immunohistochemical staining. In addition, we investigated the association between SPHK1 expression and the clinicopathological characteristics and outcomes of breast cancer patients. Our observations suggest SPHK1 is involved in breast cancer development and progression, and is a potential prognostic biomarker for patients with breast cancer.
Materials and methods
Cell lines
The normal human mammary epithelial cell line MCF-10A and the human breast cancer cell lines SK-BR-3, MDA-MB-231, MDA-MB-436, and MCF-7 were purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle medium (DMEM) or Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL; Gibco, Life Technologies, Grand Island, NY, USA). All cell lines were cultured at 37°C in a humidified atmosphere of 5% carbon dioxide.
cDNA synthesis
RNase-free DNase I treatment was carried out to remove contaminating genomic DNA from purified total RNA. Isolated total RNA was diluted to 1 mg/mL with sterile diethyl pyrocarbonate-treated water and 2.5 mL was added to reactions containing 1× DNase I buffer and 1 U DNase I (final volume, 10 mL). After incubation at 37°C for 30 min, the reactions were stopped by incubating at 70°C for 10 min. DNase I-treated RNA was reverse-transcribed into first-strand cDNA using random primers. DNase I-treated RNA (1 μg) and random primers (250 ng) were mixed in a 0.5 mL polymerase chain reaction (PCR) tube, brought to 11 mL with sterile diethyl pyrocarbonate-treated water, heated at 65°C for 5 min, and chilled quickly on ice. Other reagents were added to the 20 mL total reaction volume at the following final concentrations: 1× First-Strand Buffer, 10 mM dithiothreitol, 0.5 mM each dNTP, and 200 U Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Reactions were incubated at 42°C for 1 h, heated to 70°C for 10 min, and the products subsequently stored at -20°C.
Quantitative real-time reverse-transcriptase PCR
Total RNA was isolated using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA) and used for cDNA synthesis with a ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan). The resulting amount of cDNA was determined spectrophotometrically was and thereafter used for quantitative real-time reverse-transcriptase PCR (qRT-PCR) using the Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) with a C1000 Thermal Cycler (Bio-Rad Laboratories). PCR was carried out in a 20 μL reaction containing 0.5 μM of each primer, 10 μL of 2× SsoAdvanced SYBR Green Supermix (Bio-Rad Laboratories), and 2 μL of template cDNA. PCR for SPHK1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were initiated with a denaturing step at 95°C for 3 min, followed by 40 cycles at 95°C for 30 s and 60°C for 30 s. Amplification patterns were analyzed and threshold cycle numbers (Ct) determined for each sample using CFX Manager Software (Bio-Rad Laboratories). The ΔΔCt method was used to calculate relative target gene expression after normalization to β-actin [20]. Amplification of the target gene was confirmed by melting curve analysis and target amplicon size was confirmed by agarose gel electrophoresis. Each sample was assayed in triplicate.
Western blot analysis
Cells (5×104 cells/well) in 6-well plates were incubated at 37°C in 5% CO2 in DMEM or RPMI containing 10% heat-inactivated FBS. Whole-cell lysates were prepared in radioimmunoprecipitation assay buffer [50 mM Tris-hydrogen chloride, pH 8, 150 mM sodium chloride, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulphate] containing protease inhibitors (cOmplete Protease Inhibitor Cocktail Tablet; Roche Applied Science, Basel, Switzerland), and cleared by microcentrifugation (10,000×g for 20 min at 4°C). The resulting lysate was assessed for protein concentration, and 20-30 μg of each protein sample was resolved using 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (Bio-Rad Laboratories) and electroblotted onto nitrocellulose membranes (GE Healthcare, Little Chalfont, UK). After 1 h incubation in blocking solution (5% non-fat dry milk in Tris-buffered saline with Tween; Pierce, Rockford, IL, USA), the membranes were exposed to the following primary antibodies overnight at 4°C: anti-SPHK1 antibody (1:200, polyclonal; Abcam, Cambridge, MA, USA) and anti-GAPDH (1:1,000; Abcam). The blots were washed three times in Tris-buffered saline with Tween and incubated with the horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, Beverly, MA, USA) for 1 h at room temperature. Protein bands were visualized using enhanced chemiluminescence reagents (iNtRON Biotechnology, Seongnam, Republic of Korea).
Tissue specimens
This study (2017-08-017) was reviewed and approved by the Institutional Review Board of Kangbuk Samsung Hospital (Seoul, Republic of Korea). We selected 224 cases of invasive ductal carcinoma (IDC) and 35 cases of ductal carcinoma in situ (DCIS) from archival cases in Kangbuk Samsung Hospital (Seoul, Republic of Korea). Twenty normal breast tissue samples were used as controls. Tissues resected by surgeons were initially examined by pathologists before fixation in 10% neutral-buffered formalin. After fixation for 12-24 h, the tissues were thoroughly examined macroscopically and sectioned. After processing with an automatic tissue processor, the sections were embedded in paraffin blocks and 4 μm thick slices were cut from each formalin-fixed, paraffin-embedded tissue block using a rotary microtome. Tissue slices were subsequently stained with hematoxylin and eosin using an automatic staining instrument. After staining, the slides were covered with a glass coverslip and sent to two board-certified pathologists who examined the slides by light microscopy and made definitive pathological diagnoses. Clinical and pathological information was obtained from electrical medical information systems and pathology reports. The clinicopathological characteristics reviewed included age of the patients, histological grade, tumor size, pathological T stage (pT), pathological N stage (pN), distant metastasis, stage group, lymphovascular invasion, extensive intraductal component, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, triple negativity, tumor recurrence, follow-up period, and death from IDC. Histological grades were assigned based on the modified Bloom-Richardson grading system [21].
Tissue microarray construction
Tissue microarray blocks were constructed as previously described [4]. Briefly, all hematoxylin and eosin-stained slides were reviewed thoroughly and the two most representative tumor areas were marked on the corresponding formalin-fixed, paraffin-embedded tissue blocks. Two 2 mm diameter tissue cores were obtained from each block and manually arrayed into recipient tissue microarray blocks. The assembly was held in an X-Y position guide, with a 1 mm increment between the individual cores, and the instrument was used to create holes in a recipient block with defined array cores. The appropriate needle was used to transfer the cores into the recipient block. The percentage of tumor volume in each core was greater than 70%. A pair of tissue microarray blocks was made for each case.
Immunohistochemical staining
The 4 μm thick, formalin-fixed, paraffin-embedded slices were deparaffinized, dehydrated with xylene, and then rehydrated in a graded series of alcohol solutions. Immunohistochemical staining was performed using an automatic immunostainer, with a compact polymer method (Bond Intense Detection Kit, Leica Biosystems, Newcastle upon Tyne, UK), according to the manufacturer’s recommendations [4,5,22-26]. The primary antibodies used were specific for ER (1:200, clone SP1, Lab Vision Corporation, Fremont, CA, USA), PR (1:200, clone PgR 636, Dako, Glostrup, Denmark), HER2 (1:200, clone SP3, Lab Vision Corporation), and SPHK1 (1:100, polyclonal, Abgent, Inc., San Diego, CA, USA). After chromogenic visualization using peroxidase/DAB (EnVision+ Detection Systems, Dako), slices were counterstained with hematoxylin and coverslipped. The expression status of ER and PR was assessed using the Allred scoring method [27]. HER2 expression status was evaluated using American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations [28].
Interpretation of immunohistochemical staining
The degree of SPHK1 expression, as determined by immunohistochemical staining, was evaluated by combining scores for the proportion of positively stained cancer cells and staining intensity, as previously described [5,15,29]. Briefly, the area of stained cancer cells was scored as follows: 0, no staining; 1, 1-9%; 2, 10-49%; and 3, ≥ 50% of all cancer cells. Staining intensity was determined as follows: 0, absent; 1, weak; 2, moderate; and 3, strong. The final score was calculated as the product of the proportion score and intensity score, resulting in scores of 0, 1, 2, 3, 4, 6, and 9. The optimal cutoff value for high and low SPHK1 expression levels were chosen on the basis of distribution of the staining results and a measure of heterogeneity with the log-rank test with respect to overall survival (OS). A final score of 4 or more was used to define tumors with high SPHK1 expression, and a final score less than 4 indicated low SPHK1 expression. All slides were examined and scored by two board-certified pathologists, who were blinded to the clinicopathological data and patient identity. Disagreements between the two pathologists were resolved by consensus.
Statistical analysis
We used the unpaired student’s t-test to compare the expression levels of SPHK1 between normal mammary epithelial and breast cancer cell lines. The chi-square test, Fisher’s exact test, or linear-by-linear association test were performed to determine the association between SPHK1 expression status and clinicopathological characteristics. Multivariate logistic regression analysis with a backward stepwise elimination method was used to identify the independent predictors of distant metastasis. Univariate and multivariate survival analyses were used to examine the prognostic significance of SPHK1 expression. Curves for OS and recurrence-free survival (RFS) were drawn according to the Kaplan-Meier method and differences were analyzed by applying the log-rank test for univariate survival analysis. Multivariate survival analysis was performed for parameters that achieved statistical significance in univariate survival analysis, using the Cox proportional hazards model (95% confidence interval) with a backward stepwise elimination method. Statistical analyses were performed using PASW Statistics for Windows (version 18.0; IBM SPSS Inc., Chicago, IL, USA). Statistical significance was defined as a P-value less than 0.05.
Results
Patient demographics
The patients’ age ranged from 28-92 years (median, 51 years). Among 224 patients, 57 (25.4%), 93 (41.5%), and 74 (33.0%) had an IDC according to the modified Bloom-Richardson grade of 3, 2, and 1, respectively; 112 (50.0%), 101 (45.1%), and 11 (4.9%) had pT1, pT2, and pT3 disease, respectively; 104 (46.4%) patients had lymph node metastasis; 65 (62.5%), 21 (20.2%), and 18 (17.3%) had pN1, pN2, and pN3 disease, respectively; and 76 (33.9%), 106 (47.3%), and 42 (18.8%) had stage I, II, and III disease, respectively. Twenty (8.9%) patients developed distant metastasis in the central nervous system, lung, liver, pancreas, adrenal gland, peritoneum, or bone. Lymphovascular invasion was detected in 89 (39.7%) patients. Forty (17.9%) patients exhibited an extensive intraductal component and 30 (13.4%) patients had triple negative tumors. The median follow-up of survivors was 63 months. Nine (4.0%) patients died by the time of the final follow-up, with a median time of 48 months from surgery to death. One hundred and sixteen (51.8%) patients survived more than 5 years. Tumor recurrence during the follow-up period occurred in 30 (13.4%) patients.
Sphingosine kinase 1 expression in breast cancer cells
SPHK1 was expressed at varying levels in different breast cancer cell lines. The breast cancer cell lines SK-BR-3, MDA-MB-231, MDA-MB-436, and MCF-7 exhibited increased SPHK1 expression compared to the normal mammary epithelial cell line MCF-10A (Figure 1A). Consistent with these findings, SK-BR-3 (normalized expression ratio, 1.7), MDA-MB-231 (normalized expression ratio, 1.5), MDA-MB-436 (normalized expression ratio, 3.4), and MCF-7 (normalized expression ratio, 2.7) showed higher SPHK1 mRNA expression levels than MCF-10A (Figure 1B).
Figure 1.
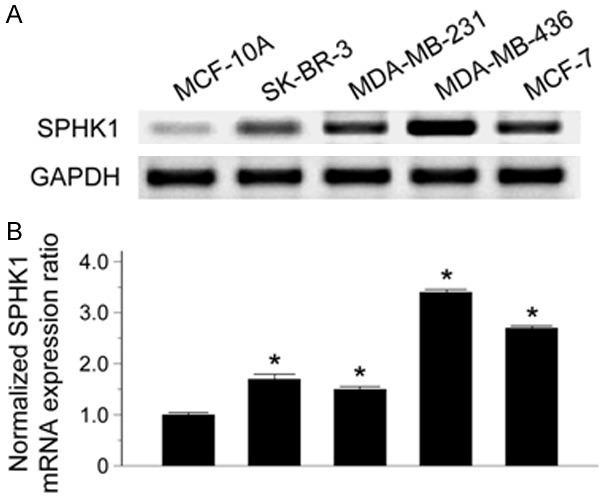
SPHK1 expression in breast cancer cell lines. A. Western blot for SPHK1. B. Normalized SPHK1 mRNA expression ratio analyzed by qRT-PCR. *P < 0.05 versus MCF-10A.
Sphingosine kinase-1 expression in normal breast tissue, ductal carcinoma in situ, and invasive ductal carcinoma tissues
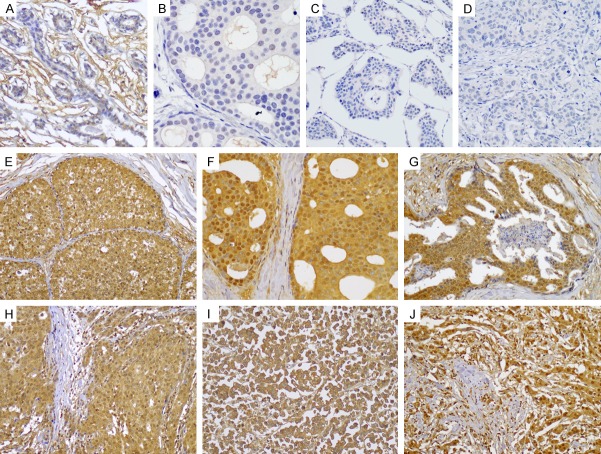
Representative photomicrographs of SPHK1 immunostaining in normal breast tissue, DCIS, and IDC are shown in Figure 2. No staining was observed in all 20 normal breast tissue samples. By contrast, both DCIS and IDC tissues exhibited SPHK1 expression of varying staining intensity and proportion. Seven of the 35 DCIS tissue samples (20%) exhibited high SPHK1 expression, while the remaining 28 samples (80.0%) exhibited low SPHK1 expression. Among the 224 IDC tissues, 85 (37.9%) showed high SPHK1 expression, whereas the remaining 137 (61.2%) had low SPHK1 expression. In DCIS and IDC tissues, SPHK1 expression was primarily observed in the cytoplasm of tumorous cells, although tumor cells with strong cytoplasmic SPHK1 expression displayed weak-to-moderate nuclear SPHK1 immunoreactivity. The frequency of high SPHK1 expression in IDC was significantly higher than that in DCIS (P = 0.039) or normal breast tissue (P < 0.001). Similarly, the frequency of high SPHK1 expression in DCIS was significantly higher than that in normal breast tissue (P = 0.040).
Figure 2.
Immunohistochemical expression of SPHK1 in normal and cancerous breast tissue. Negative SPHK1 expression in (A) the ductal epithelial cells of normal breast, (B) ductal carcinoma in situ, and (C and D) invasive ductal carcinoma. High SPHK1 expression in (E-G) ductal carcinoma in situ and (H-J) invasive ductal carcinoma (compact polymer method).
Association between sphingosine kinase 1 expression and clinicopathological characteristics of invasive ductal carcinoma of the breast
Relationships between SPHK1 expression status and clinicopathological characteristics of IDC are summarized in Table 1. Significant correlations were observed between high SPHK1 expression and higher histological grade (P < 0.001), development of distant metastasis (P < 0.001), ER negativity (P = 0.013), PR negativity (P = 0.018), HER2 negativity (P = 0.046), and triple negativity (P = 0.002). There were marginally significant associations between high SPHK1 expression and advanced stage (P = 0.085). Associations between SPHK1 expression and patients’ age, tumor size, pT, pN, stage group, lymphovascular invasion, and extensive intraductal component were not statistically significant.
Table 1.
Association between sphingosine kinase 1 (SPHK1) expression and clinicopathological characteristics of invasive ductal carcinoma of the breast
| Characteristic | Total | SPHK1 expression | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| High (%) | Low (%) | ||||
| Age (years) | >50 | 111 | 39 (35.1) | 72 (64.9) | 0.390 |
| ≤50 | 113 | 46 (40.7) | 67 (59.3) | ||
| Histological grade | 1 | 57 | 14 (24.6) | 43 (75.4) | < 0.001* |
| 2 | 93 | 31 (33.3) | 62 (66.7) | ||
| 3 | 74 | 40 (54.1) | 34 (45.9) | ||
| Tumor size (cm) | >2.0 | 112 | 43 (38.4) | 69 (61.6) | 0.890 |
| ≤2.0 | 112 | 42 (37.5) | 70 (62.5) | ||
| Pathological T stage | pT1 | 112 | 42 (37.5) | 70 (62.5) | 0.213 |
| pT2 | 101 | 34 (33.7) | 67 (66.3) | ||
| pT3 | 11 | 9 (81.8) | 2 (18.2) | ||
| Pathological N stage | pN0 | 120 | 45 (37.5) | 75 (62.5) | 0.385 |
| pN1 | 65 | 21 (32.3) | 44 (67.7) | ||
| pN2 | 21 | 11 (52.4) | 10 (47.6) | ||
| pN3 | 18 | 8 (44.4) | 10 (55.6) | ||
| Distant metastasis | Present | 20 | 15 (75.0) | 5 (25.0) | < 0.001* |
| Absent | 204 | 70 (34.3) | 134 (65.7) | ||
| Stage group | I | 76 | 26 (34.2) | 50 (65.8) | 0.085 |
| II | 106 | 37 (34.9) | 69 (65.1) | ||
| III | 42 | 22 (52.4) | 20 (47.6) | ||
| Lymphovascular invasion | Present | 89 | 39 (43.8) | 50 (56.2) | 0.141 |
| Absent | 135 | 46 (34.1) | 89 (65.9) | ||
| Extensive intraductal component | Present | 40 | 12 (30.0) | 28 (70.0) | 0.253 |
| Absent | 184 | 73 (39.7) | 111 (60.3) | ||
| Estrogen receptor status | Negative | 63 | 32 (50.8) | 31 (49.2) | 0.013* |
| Positive | 161 | 53 (32.9) | 108 (67.1) | ||
| Progesterone receptor status | Negative | 76 | 37 (48.7) | 39 (51.3) | 0.018* |
| Positive | 148 | 48 (32.4) | 100 (67.6) | ||
| Human epidermal growth factor receptor 2 status | Negative | 165 | 69 (41.8) | 96 (58.2) | 0.046* |
| Positive | 59 | 16 (27.1) | 43 (72.9) | ||
| Triple negativity | Yes | 30 | 19 (63.3) | 11 (36.7) | 0.002* |
| No | 194 | 66 (34.0) | 128 (66.0) | ||
Statistically significant.
Factors independently predicting distant metastasis in patients with invasive ductal carcinoma of the breast
Relationships between distant metastasis, clinicopathological characteristics, and SPHK1 expression are summarized in Table 2. Higher pT (P = 0.017), higher pN (P = 0.016), PR negativity (P = 0.037), and high SPHK1 expression were found to closely associate with distant metastasis in patients with IDC. To identify the factors that independently predict the development of distant metastasis, these four covariates were entered into a multivariate logistic regression analysis. High SPHK1 expression was the only independent predictive factor for distant metastasis (P = 0.001, relative risk = 5.841; 95% confidence interval = 2.024-16.858); pT, pN, and PR status did not independently predict the development of distant metastasis.
Table 2.
Factors predicting distant metastasis in patients with invasive ductal carcinoma of the breast
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Distant metastasis | P-value | P-value | Relative risk (95% confidence interval) | |||
|
| ||||||
| Present (%) | Absent (%) | |||||
| Age (years) | >50 | 6 (5.4) | 105 (94.6) | 0.067 | Not applicable | |
| ≤50 | 14 (12.4) | 99 (87.6) | ||||
| Histological grade | 1 | 2 (3.5) | 55 (96.5) | 0.092 | Not applicable | |
| 2 | 9 (9.7) | 84 (90.3) | ||||
| 3 | 9 (12.2) | 65 (87.8) | ||||
| Tumor size (cm) | >2.0 | 12 (10.7) | 100 (89.3) | 0.349 | Not applicable | |
| ≤2.0 | 8 (7.1) | 104 (92.9) | ||||
| Pathological T stage | pT1 | 8 (7.1) | 104 (92.9) | 0.017* | 0.699 | 1.218 (0.447-3.321) |
| pT2 | 7 (6.9) | 94 (93.1) | ||||
| pT3 | 5 (45.5) | 6 (54.5) | ||||
| Pathological N stage | pN0 | 7 (5.8) | 113 (94.2) | 0.016* | 0.085 | 2.383 (0.888-6.396) |
| pN1 | 5 (7.7) | 60 (92.3) | ||||
| pN2 | 5 (23.8) | 16 (76.2) | ||||
| pN3 | 3 (16.7) | 15 (83.3) | ||||
| Lymphovascular invasion | Present | 12 (13.5) | 77 (86.5) | 0.052 | Not applicable | |
| Absent | 8 (5.9) | 127 (94.1) | ||||
| Extensive Intraductal component | Present | 1 (2.5) | 39 (97.5) | 0.138 | Not applicable | |
| Absent | 19 (10.3) | 165 (89.7) | ||||
| Estrogen receptor status | Negative | 13 (8.1) | 148 (91.9) | 0.474 | Not applicable | |
| Positive | 7 (11.1) | 56 (88.9) | ||||
| Progesterone receptor status | Negative | 9 (6.1) | 139 (93.9) | 0.037* | 0.135 | 2.103 (0.794-5.573) |
| Positive | 11 (14.5) | 65 (85.5) | ||||
| Human epidermal growth factor receptor 2 status | Negative | 4 (6.8) | 55 (93.2) | 0.603 | Not applicable | |
| Positive | 16 (9.7) | 149 (90.3) | ||||
| Triple negativity | Yes | 5 (16.7) | 25 (83.3) | 0.110 | Not applicable | |
| No | 15 (7.7) | 179 (92.3) | ||||
| Sphingosine kinase 1 expression | High | 15 (17.6) | 70 (83.3) | < 0.001* | 0.001* | 5.841 (2.024-16.858) |
| Low | 5 (3.6) | 134 (96.4) | ||||
Statistically significant.
Prognostic significance of sphingosine kinase 1 expression in patients with invasive ductal carcinoma of the breast
Univariate analysis for OS revealed that higher pT (P = 0.026), lymph node metastasis (P = 0.012), distant metastasis (P < 0.001), advanced stage (P < 0.001), lymphovascular invasion (P = 0.024), PR negativity (P = 0.029), triple negativity (P = 0.029), and high SPHK1 expression (P = 0.048) significantly predicted poor OS (Table 3). The 5-year OS rates were 97.7% for patients with SPHK1-high IDC and 89.4% for patients with SPHK1-low IDC (Figure 3). Multivariate analysis of OS was performed using pT, lymph node metastasis, distant metastasis, stage group, lymphovascular invasion, PR status, triple negativity, and SPHK1 expression status as covariates. Only distant metastasis independently predicted OS (P < 0.001; Table 3). SPHK1 expression by itself did not predict OS (P = 0.671).
Table 3.
Factors predicting shorter overall survival of patients with invasive ductal carcinoma of the breast
| Characteristic | Overall survival | ||
|---|---|---|---|
|
| |||
| Univariate | Multivariate | ||
|
| |||
| P-value | P-value | Hazard ratio (95% confidence interval) | |
| Age (years): >50 vs. ≤50 | 0.747 | Not applicable | |
| Histological grade: 3-2 vs. 1 | 0.069 | Not applicable | |
| Pathological T stage: pT2-3 vs. pT1 | 0.026* | 0.169 | 4.329 (0.536-34.999) |
| Pathological N stage: pN1-3 vs. pN0 | 0.012* | 0.090 | 6.157 (0.752-50.411) |
| Distant metastasis: Present vs. absent | < 0.001* | < 0.001* | 11.909 (3.145-45.101) |
| Stage group: III vs. I-II | < 0.001* | 0.765 | 1.341 (0.196-9.187) |
| Lymphovascular invasion: Present vs. absent | 0.024* | 0.598 | 1.582 (0.287-8.712) |
| Estrogen receptor status: Negative vs. positive | 0.213 | Not applicable | |
| Progesterone receptor status: Negative vs. positive | 0.029* | 0.605 | 1.564 (0.286-8.543) |
| Human epidermal growth factor receptor 2 status: Negative vs. positive | 0.248 | Not applicable | |
| Triple negativity: Yes vs. no | 0.029* | 0.096 | 3.409 (0.804-14.455) |
| Sphingosine kinase 1 expression: High vs. low | 0.048* | 0.671 | 1.411 (0.287-6.937) |
Statistically significant.
Figure 3.
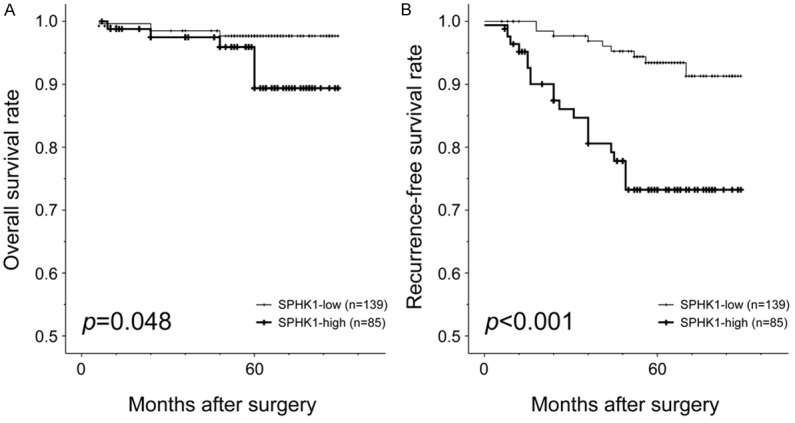
Kaplan-Meier curves illustrating (A) overall survival and (B) recurrence-free survival among patients with invasive ductal carcinoma with respect to SPHK1 expression status. Patients whose tumors showed high SPHK1 expression had shorter overall survival times (P = 0.048) and recurrence-free survival (P < 0.001) compared to those showing low SPHK1 expression (log-rank test).
Univariate analysis of RFS revealed that higher histological grade (P = 0.038), higher pT (P = 0.017), distant metastasis (P < 0.001), advanced stage (P = 0.044), PR negativity (P = 0.019), triple negativity (P = 0.003), and high SPHK1 expression (P < 0.001) were significant predictors of poor RFS (Table 4). The respective 3- and 5-year RFS rates were 96.9% and 93.4% for patients with SPHK1-high IDC, and 80.6% and 73.2% for patients with SPHK1-low IDC (Figure 3). Multivariate analysis of RFS revealed that higher pT (P = 0.047), distant metastasis (P < 0.001), triple negativity (P = 0.043), and high SPHK1 expression (P = 0.038) were independent predictors of shorter RFS (Table 4). The hazard ratio of high SPHK1 expression for RFS (2.426, 95% confidence interval = 1.052-5.595) was higher than that of high pT (2.300, 95% confidence interval = 1.012-5.228) and triple negativity (2.357, 95% confidence interval = 1.027-5.408).
Table 4.
Factors predicting shorter recurrence-free survival of patients with invasive ductal carcinoma of the breast
| Characteristic | Recurrence-free survival | ||
|---|---|---|---|
|
| |||
| Univariate | Multivariate | ||
|
| |||
| P-value | P-value | Hazard ratio (95% confidence interval) | |
| Age (years): >50 vs. ≤50 | 0.338 | Not applicable | |
| Histological grade: 3-2 vs. 1 | 0.038* | 0.529 | 1.482 (0.435-5.054) |
| Pathological T stage: pT2-3 vs. pT1 | 0.017* | 0.047* | 2.300 (1.012-5.228) |
| Pathological N stage: pN1-3 vs. pN0 | 0.173 | Not applicable | |
| Distant metastasis: Present vs. absent | < 0.001* | < 0.001* | 10.189 (4.623-22.453) |
| Stage group: III vs. I-II | 0.044* | 0.615 | 0.762 (0.264-2.198) |
| Lymphovascular invasion: Present vs. absent | 0.174 | Not applicable | |
| Estrogen receptor status: Negative vs. positive | 0.132 | Not applicable | |
| Progesterone receptor status: Negative vs. positive | 0.019* | 0.913 | 0.948 (0.369-2.482) |
| Human epidermal growth factor receptor 2 status: Negative vs. positive | 0.353 | Not applicable | |
| Triple negativity: Yes vs. no | 0.003* | 0.043* | 2.357 (1.027-5.408) |
| Sphingosine kinase 1 expression: High vs. low | < 0.001* | 0.038* | 2.426 (1.052-5.595) |
Statistically significant.
Discussion
In the present study, we analyzed the immunohistochemical expression of SPHK1 in human breast cancer cells and tissues. Consistent with previous reports [5,9,15,30-32], SPHK1 expression was significantly increased in breast cancer cells and tissues compared with normal mammary epithelial cells and breast tissues. In our study, 37.9% (85/224) of IDC tissue samples showed high SPHK1 expression, with significant differences in the frequency of high SPHK1 expression between DCIS and normal breast tissue (P = 0.040), and between IDC and DCIS (P = 0.039). These findings indicate that SPHK1 expression is upregulated in breast cancer and implicate SPHK1 as a potential diagnostic biomarker for breast cancer.
We examined the association between SPHK1 expression and the clinicopathological characteristics of patients with IDC and found a significant relationship between high SPHK1 expression and aggressive oncogenic behaviors, including higher histological grade, distant metastasis, ER negativity, PR negativity, HER2 negativity, and triple negativity. These findings implicate SPHK1 as a potentially important contributing factor in breast cancer progression and are consistent with recent data showing that increased SPHK1 expression is associated with lymph node metastasis and distant metastasis of breast cancer [33]. Our results are also in agreement with those of previous studies demonstrating an association between increased SPHK1 expression and aggressive oncogenic behaviors, such as larger tumor size, deeper invasion depth, advanced stage, worse histological differentiation, higher invasive capacity, and/or chemotherapeutic resistance in cervical cancer [5], head and neck cancer [34], thyroid cancer [29], salivary duct cancer [30], esophageal cancer [35], colorectal cancer [36], and bladder cancer [37]. Furthermore, we observed that high SPHK1 expression was the only independent factor for predicting the development of distant metastasis.
The extent of primary tumor and lymph node metastasis is the most important prognostic factor in breast cancer and one of the main determinants for predicting tumor progression and aggressive oncogenic behavior. Surprisingly, in this study, the relative risk of distant metastasis associated with high SPHK1 expression (5.841, 95% confidence interval = 2.024-16.858) was higher than the risk associated with pT (1.218, 95% confidence interval = 0.447-3.321) and pN (2.383, 95% confidence interval = 0.888-6.396). To the best of our knowledge, the use of SPHK1 expression to predict distant metastasis has not been previously investigated. Our data suggest that SPHK1 immunostaining provides clinically useful information for patients with IDC of the breast and that SPHK1 expression is a strong and novel predictive biomarker for the identification of patients at high risk of developing distant metastasis.
We further observed that IDC patients whose tumors showed high SPHK1 expression had shorter OS and RFS than those with SPHK1-low tumors. Moreover, high SPHK1 expression was an independent factor for predicting shorter recurrence-free survival of patients with IDC. These results are in line with those of a recent study demonstrating that increased SPHK1 expression is associated with both decreased overall and disease-specific survival of patients with IDC, and independently predicts worse OS [33]. The prognostic significance of SPHK1 has been documented in several types of human malignancy, including glioblastoma [14], head and neck cancer [8], salivary duct cancer [30], esophageal cancer [35], gastric cancer [9], and colorectal cancer [36]; with all these studies suggesting that patients with SPHK1-high tumors had shorter survival times whereas those with SPHK1-low tumors survived longer. Although the traditional staging system successfully grades patients with respect to their prognosis according to clinicopathological characteristics, it does not provide critical information that may influence treatment strategy. Many biomarkers have been investigated to overcome the limitations of the traditional system and have shown potential predictive significance. However, reliable biomarkers that can stratify patients with IDC are substantially limited. In our analysis, high SPHK1 expression had a clear prognostic value for RFS in patients with IDC. SPHK1 expression in IDC can be used as a novel prognostic marker for poor outcomes in breast cancer patients.
In conclusion, we demonstrated that, compared with normal breast tissue, SPHK1 expression was significantly upregulated in breast cancer tissues, and upregulated SPHK1 expression showed significant association with aggressive oncogenic behavior of breast cancer, including higher histological grade, distant metastasis, and hormone receptor negativity. High SPHK1 expression was also found to be an independent factor predicting the development of distant metastasis and shorter recurrence-free survival. Thus, alterations in SPHK1 expression potentially promote tumor development, progression, and metastasis of breast cancer, and SPHK1 expression status may serve as a predictive biomarker for distant metastasis and patient outcomes.
Acknowledgements
This research was supported by the Medical Research Funds from Kangbuk Samsung Hospital, a faculty research grant of Yonsei University College of Medicine (6-2017-0036), and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03935584).
Disclosure of conflict of interest
None.
References
- 1.Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S, Takabe K. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Min SY, Kim Z, Hur MH, Yoon CS, Park EH, Jung KW. The basic facts of korean breast cancer in 2013: results of a nationwide survey and breast cancer registry database. J Breast Cancer. 2016;19:1–7. doi: 10.4048/jbc.2016.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoyagi T, Nagahashi M, Yamada A, Takabe K. The role of sphingosine-1-phosphate in breast cancer tumor-induced lymphangiogenesis. Lymphat Res Biol. 2012;10:97–106. doi: 10.1089/lrb.2012.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do SI, Yoon G, Kim HS, Kim K, Lee H, Do IG, Kim DH, Chae SW, Sohn JH. Increased brahma-related gene 1 expression predicts distant metastasis and shorter survival in patients with invasive ductal carcinoma of the breast. Anticancer Res. 2016;36:4873–4882. doi: 10.21873/anticanres.11051. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Yoon G, Ryu JY, Cho YJ, Choi JJ, Lee YY, Kim TJ, Choi CH, Song SY, Kim BG, Bae DS, Lee JW. Sphingosine kinase 1 is a reliable prognostic factor and a novel therapeutic target for uterine cervical cancer. Oncotarget. 2015;6:26746–26756. doi: 10.18632/oncotarget.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gault CR, Obeid LM. Still benched on its way to the bedside: sphingosine kinase 1 as an emerging target in cancer chemotherapy. Crit Rev Biochem Mol Biol. 2011;46:342–351. doi: 10.3109/10409238.2011.597737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 8.Facchinetti MM, Gandini NA, Fermento ME, Sterin-Speziale NB, Ji Y, Patel V, Gutkind JS, Rivadulla MG, Curino AC. The expression of sphingosine kinase-1 in head and neck carcinoma. Cells Tissues Organs. 2010;192:314–324. doi: 10.1159/000318173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Yu CP, Xia JT, Zhang L, Weng GX, Zheng HQ, Kong QL, Hu LJ, Zeng MS, Zeng YX, Li M, Li J, Song LB. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- 10.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 11.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, Ai Y, Ristimaki AP, Fyrst H, Sano H, Rosenberg D, Saba JD, Proia RL, Hla T. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, Hannun YA, Obeid LM. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- 14.Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Guan HY, Gong LY, Song LB, Zhang N, Wu J, Yuan J, Zheng YJ, Huang ZS, Li M. Clinical significance of sphingosine kinase-1 expression in human astrocytomas progression and overall patient survival. Clin Cancer Res. 2008;14:6996–7003. doi: 10.1158/1078-0432.CCR-08-0754. [DOI] [PubMed] [Google Scholar]
- 16.Meng XD, Zhou ZS, Qiu JH, Shen WH, Wu Q, Xiao J. Increased SPHK1 expression is associated with poor prognosis in bladder cancer. Tumour Biol. 2013;35:2075–2080. doi: 10.1007/s13277-013-1275-0. [DOI] [PubMed] [Google Scholar]
- 17.Le Scolan E, Pchejetski D, Banno Y, Denis N, Mayeux P, Vainchenker W, Levade T, Moreau-Gachelin F. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 2005;106:1808–1816. doi: 10.1182/blood-2004-12-4832. [DOI] [PubMed] [Google Scholar]
- 18.Akao Y, Banno Y, Nakagawa Y, Hasegawa N, Kim TJ, Murate T, Igarashi Y, Nozawa Y. High expression of sphingosine kinase 1 and S1P receptors in chemotherapy-resistant prostate cancer PC3 cells and their camptothecin-induced up-regulation. Biochem Biophys Res Commun. 2006;342:1284–1290. doi: 10.1016/j.bbrc.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 19.Pyne NJ, Tonelli F, Lim KG, Long JS, Edwards J, Pyne S. Sphingosine 1-phosphate signalling in cancer. Biochem Soc Trans. 2012;40:94–100. doi: 10.1042/BST20110602. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. Lyon, France: IARC; 2012. [Google Scholar]
- 22.Kim EK, Yoon G, Kim HS. Chemotherapyinduced endometrial pathology: mimicry of malignancy and viral endometritis. Am J Transl Res. 2016;8:2459–2467. [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon N, Kim JY, Kim HS. Clinical outcomes of advanced-stage glassy cell carcinoma of the uterine cervix: a need for reappraisal. Oncotarget. 2016;7:78448–78454. doi: 10.18632/oncotarget.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon G, Koh CW, Yoon N, Kim JY, Kim HS. Stromal p16 expression is significantly increased in endometrial carcinoma. Oncotarget. 2017;8:4826–4836. doi: 10.18632/oncotarget.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon N, Yoon G, Park CK, Kim HS. Stromal p16 expression is significantly increased in malignant ovarian neoplasms. Oncotarget. 2016;7:64665–64673. doi: 10.18632/oncotarget.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Na K, Sung JY, Kim HS. Stromal p16 overexpression in adult granulosa cell tumors of the ovary. Anticancer Res. 2017;37:2437–2444. doi: 10.21873/anticanres.11583. [DOI] [PubMed] [Google Scholar]
- 27.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 28.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan H, Liu L, Cai J, Liu J, Ye C, Li M, Li Y. Sphingosine kinase 1 is overexpressed and promotes proliferation in human thyroid cancer. Mol Endocrinol. 2011;25:1858–1866. doi: 10.1210/me.2011-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G, Zheng H, Zhang Z, Wu Z, Xiong H, Li J, Song L. Overexpression of sphingosine kinase 1 is associated with salivary gland carcinoma progression and might be a novel predictive marker for adjuvant therapy. BMC Cancer. 2010;10:495. doi: 10.1186/1471-2407-10-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malavaud B, Pchejetski D, Mazerolles C, de Paiva GR, Calvet C, Doumerc N, Pitson S, Rischmann P, Cuvillier O. Sphingosine kinase-1 activity and expression in human prostate cancer resection specimens. Eur J Cancer. 2010;46:3417–3424. doi: 10.1016/j.ejca.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 32.Bayerl MG, Bruggeman RD, Conroy EJ, Hengst JA, King TS, Jimenez M, Claxton DF, Yun JK. Sphingosine kinase 1 protein and mRNA are overexpressed in non-Hodgkin lymphomas and are attractive targets for novel pharmacological interventions. Leuk Lymphoma. 2008;49:948–954. doi: 10.1080/10428190801911654. [DOI] [PubMed] [Google Scholar]
- 33.Zhu YJ, You H, Tan JX, Li F, Qiu Z, Li HZ, Huang HY, Zheng K, Ren GS. Overexpression of sphingosine kinase 1 is predictive of poor prognosis in human breast cancer. Oncol Lett. 2017;14:63–72. doi: 10.3892/ol.2017.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamashiro PM, Furuya H, Shimizu Y, Iino K, Kawamori T. The impact of sphingosine kinase-1 in head and neck cancer. Biomolecules. 2013;3:481–513. doi: 10.3390/biom3030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan J, Tao YF, Zhou Z, Cao BR, Wu SY, Zhang YL, Hu SY, Zhao WL, Wang J, Lou GL, Li Z, Feng X, Ni J. An novel role of sphingosine kinase-1 (SPHK1) in the invasion and metastasis of esophageal carcinoma. J Transl Med. 2011;9:157. doi: 10.1186/1479-5876-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan SS, Khin LW, Wong L, Yan B, Ong CW, Datta A, Salto-Tellez M, Lam Y, Yap CT. Sphingosine kinase 1 promotes malignant progression in colon cancer and independently predicts survival of patients with colon cancer by competing risk approach in South asian population. Clin Transl Gastroenterol. 2014;5:e51. doi: 10.1038/ctg.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng XD, Zhou ZS, Qiu JH, Shen WH, Wu Q, Xiao J. Increased SPHK1 expression is associated with poor prognosis in bladder cancer. Tumour Biol. 2014;35:2075–2080. doi: 10.1007/s13277-013-1275-0. [DOI] [PubMed] [Google Scholar]