Abstract
Background
The antifibrinolytic agent tranexamic acid (TXA) is widely used for the prevention and treatment of hyperfibrinolytic states, such as in severe polytrauma. It can also be used for the systemic prevention of hemorrhage in elective orthopedic procedures. In this review, we assess the efficacy and risks of the prophylactic administration of tranexamic acid before major endoprosthetic surgery of the hip and knee.
Methods
This review is based on pertinent articles retrieved by a selective literature search in the PubMed and Cochrane Library databases.
Results
Endoprosthetic surgery of the hip and knee is often associated with perioperative blood losses exceeding 500 mL. The prophylactic administration of tranexamic acid immediately before such procedures has been shown in randomized, controlled trials to lessen the quantity of intra- and postoperative bleeding and to reduce the likelihood of blood transfusion (number needed to treat [NNT] 3.7–5.7 for knee replacement and 4.1–8.2 for hip replacement). The rate of thromboembolic events did not differ significantly from the rate in the placebo groups. No reliable data are available on the frequency of epileptic seizures as a complication of TXA use in knee and hip endoprosthetic surgery. On the basis of data from other types of surgery, one may reasonably conclude that the doses of TXA used for knee and hip endoprosthetic procedures are unlikely to cause this problem.
Conclusion
The prophylactic intravenous administration of tranexamic acid lessens the amount of bleeding in endoprosthetic knee and hip procedures and reduces the likelihood of blood transfusion. According to the current state of the evidence, complications are rare. Nonetheless, consideration of the risks and benefits implies that tranexamic acid should not be given for this purpose to patients who have recently had urogenital bleeding, pulmonary embolism, or a myocardial infarction, who have recently undergone percutaneous transluminal coronary angioplasty or stenting, or who are known to have epilepsy.
Hip or knee replacement surgery are among the most common procedures in orthopedic and trauma surgery. As the surgical sites are well vascularized, the procedure can lead to substantial blood loss and a high incidence of blood transfusion. Indeed, 42% of patients undergoing total hip replacement, and 34% of patients undergoing total knee replacement, received at least one perioperative blood transfusion (1, 2).
Blood transfusions carry a variety of risks, including transfusion reactions and the transmission of infectious diseases, such as human immunodeficiency virus (HIV), hepatitis B/C, Zika, and potentially as-yet-unknown pathogens. Immune modulation and suppression effects have also been described (3– 5).
In addition, retrospective studies have shown that blood transfusions result in longer hospital stay and higher mortality and morbidity (6). Allogeneic blood transfusions are considered to be an independent risk factor for a prosthesis infection (7, 8).
Various strategies are therefore used during clinical routine for patient blood management (9, 10) to reduce intraoperative and postoperative blood loss: improved techniques for surgical hemostasis, minimally invasive surgical procedures, autotransfusion, and heat preservation to optimize coagulation function. The long-used pharmacological therapies for treating apparent coagulation disorders, such as desmopressin for an unwanted drug-induced inhibition of platelet aggregation, or tranexamic acid (TXA) for an identified systemic hyperfibrinolysis, are increasingly being complemented with pharmacological prophylactic strategies.
The purpose of this review is to demonstrate the efficacy of TXA as a prophylactic for hip and knee replacement surgery, to describe the possible risks and complications of this pharmacological strategy, and finally, to highlight some economic aspects. We would like to point out that prophylactic administration of TXA prior to surgical intervention is only a partial aspect of the patient blood management in the perioperative period (9, 10).
Pharmacology
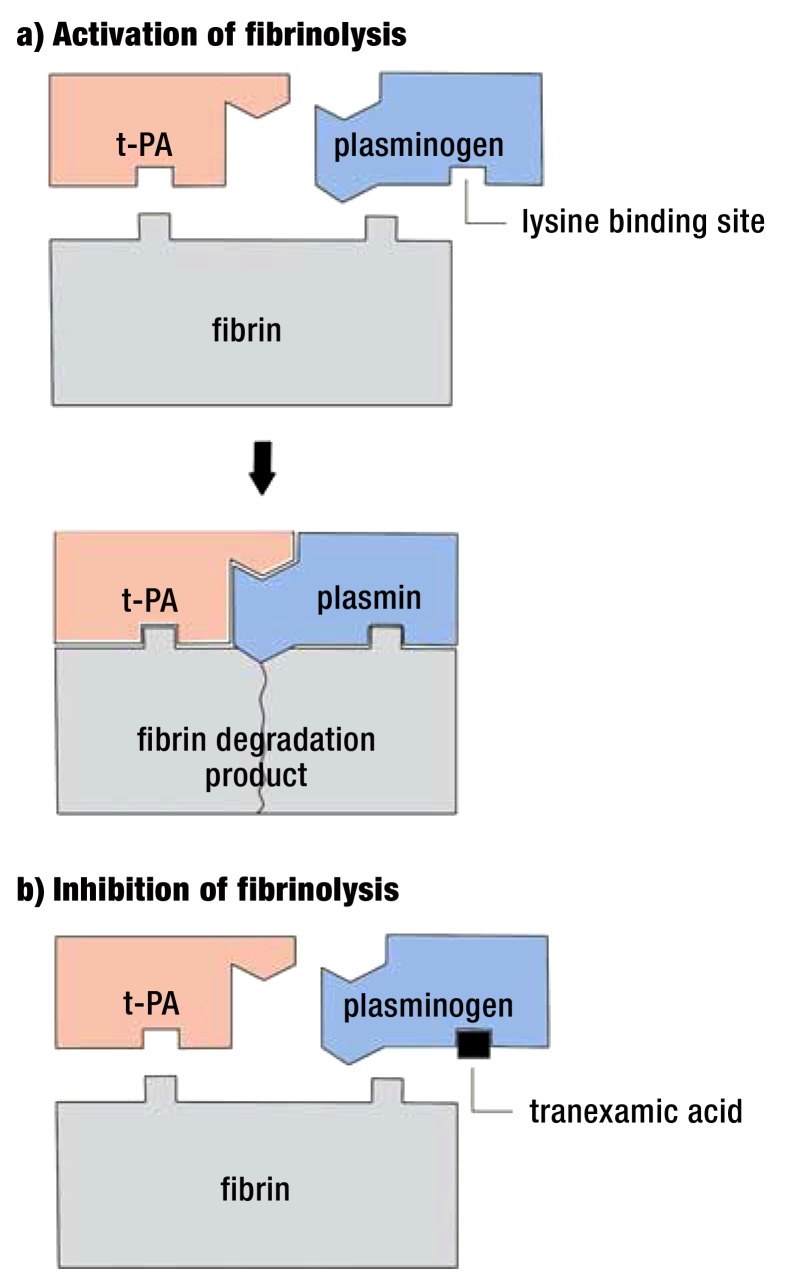
TXA belongs to the group of antifibrinolytics and has been the only substance of this group available in Germany since the suspension of aprotinin (decision of November 2007; Federal Institute for Drugs and Medical Devices [Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM]) (11). TXA and the chemically related substance e-aminocaproic acid (not available in Germany) belong to the group of e-carboxylic acids. Both substances inhibit the binding of plasminogen to fibrin, thereby also inhibiting formation of an active serine protease plasmin and subsequent fibrinolysis (12). TXA is about a 10-fold stronger inhibitor of fibrinolysis than the related e-aminocaproic acid. The conversion of inactive plasminogen into the fibrinolytically active plasmin by tissue-type plasminogen activator (t-PA) is catalyzed by the binding of t-PA–plasminogen to the lysine residues of fibrin (figure). As a result, plasmin is formed locally within the blood clot. TXA binds reversibly to the lysine binding sites of plasminogen and thus blocks its conversion to plasmin; as a result, fibrin is not cleaved in the clot (13).
FIGURE.
Effects of tranexamic acid, modified according to Mannucci (37)
a) Activation of fibrinolysis: tissue-type plasminogen activator (t-PA) and plasminogen bind to fibrin. This catalyzes the conversion of plasminogen into the fibrinolytic plasmin by a factor of 1000.
b) Inhibition of fibrinolysis: TXA inhibits fibrinolysis by blocking the binding site of plasminogen and fibrin.
In vivo and in vitro experiments have shown that after intravenous bolus administration of TXA (30 mg/kg body weight [BW]), the maximum inhibition of plasmin in blood plasma is reached after about 30 minutes (14).
The substance can be administered enterally (with an oral bioavailability of approximately 33%), parenterally, or topically (14, 15).
Due to its molecular properties, TXA can cross the blood-brain barrier and promote cerebral seizures. It is believed that TXA causes hyperexcitability through an antagonistic action on ?-aminobutyric acid type A (GABAA) receptors in the brain—the usual level of TXA in the cerebrospinal fluid is approximately one-tenth of its serum concentration (14, 16, 17). Due to the pleiotropic effects of plasmin and its pharmacological inhibition by TXA, further potential indications as well as possible complications could be discovered in the future. For example, TXA can have an anti-inflammatory effect in cardiac surgery (18) but can also worsen the healing of chronic wounds with long-term use (12, 19). TXA is almost not at all metabolized in the body but rather is completely excreted via the kidney. After bolus administration of 10 mg/kg BW, more than 90% of the substance quantity is excreted in the urine within 24 hours. As the elimination half-life of TXA is about 3 hours, accumulation effects are expected only in cases of renal insufficiency but not in those of hepatic insufficiency (13). The dose and dose interval should therefore be adjusted according to the manufacturer’s instructions in the case of renal insufficiency (creatinine >1.35 mg / dL) (20) (table 1).
Table 1. Manufacturer´s recommended dosage (20).
| Serum creatinine | Tranexamic acid | ||
| µmol/L | mg/dL | dose i.v. | administration |
| <119 | to 1.35 | 15 mg/kg BW | every 8 hours |
| <249 | to 2.82 | 10 mg/kg BW | every 12 hours |
| <499 | to 5.65 | 10 mg/kg BW | every 24 hours |
| ≥500 | >5,65 | 5 mg/kg BW | every 24 hours |
i.v., intravenous; BW, body weight
The systemic antifibrinolytic effect of TXA can be assayed in the laboratory using reduction of D-dimers (fibrin cleavage products) or rotational elastometry (reduction of the clot lysis index) (21, 22).
TXA is approved in Germany for intravenous administration “for the prevention and treatment of bleeding due to local or systemic hyperfibrinolysis in adults and children over the age of 1 year” (20).
Methods
This work is based on a selective literature search from the PubMed database and the Cochrane Library. The keywords “tranexamic acid”, “antifibrinolytic” linked to “endoprothetic surgery”, “knee arthroplasty”, “hip surgery”, and “hip arthroplasty” were used. From the resulting search hits, works were selected in which the systemic prophylactic use of TXA in endoprosthetics of the knee and the hip was examined in systematic reviews or meta-analyses. Additionally, the keywords “complications“, “thromboembolism”, and “seizure” associated with surgery were searched for, to assess the frequency of typical side effects of TXA.
State of evidence for knee replacements
Knee replacement surgery is currently one of the most commonly performed orthopedic procedures. In 2011, 168 486 knee endoprostheses were implanted in Germany (23).
The average total blood loss (intraoperative and postoperative) in the absence of TXA administration is between 762 and 1789 mL for primary total knee endoprosthesis (TKE) implantation (15). Current data show that transfusion of red blood cell concentrates is necessary for about 25% of patients (with a median of 2.2 units) (24).
Since then, numerous studies have been published in which TXA was administered (mainly as an intravenous bolus shortly before surgery). A meta-analysis by Alshryda et al. analyzed 18 randomized controlled trials (15). They found that overall blood loss was reduced by 591 mL when TXA was administered (95% confidence interval [536; 647]). Further, the rate of intra- and postoperative blood transfusions in the included studies (14 studies, with 824 patients) demonstrated that the relative risk (2.56 [2.1, 3.1]) increased significantly when no TXA was administered. The transfusion rate in the control group was 47.1% (190/403) and in the prophylactic TXA study group, 23.5% (99/421). The absolute risk reduction for a necessary transfusion therapy was thus 23.6%, which corresponds to a number needed to treat (NNT) of 4.2 [3.3; 5.8]. It should be noted, however, that the studies used widely differing transfusion triggers (e.g., hemoglobin levels of 7–10 g/dL). Nevertheless, a reduced likelihood of transfusion was consistently detectable in the included studies.
The articles included in this meta-analysis examined the use of TXA in very different clinical procedures. Some of the studies used thigh tourniquet, while others did not. Likewise, the TXA doses (bolus dose, bolus dose plus continuous dose, multiple bolus dose) were not easily comparable, so that the effect of the drug has still been insufficiently assessed. Studies with and without chemical thrombosis prophylaxis were included. Table 2 shows an overview of selected meta-analyses.
Table 2. Tranexamic acid in knee arthroplasty—selected meta-analyses.
| Study |
Reduction of total bleeding volume (mL) [95% CI] |
Reduction of intraoperative bleeding volume (mL) |
Reduction of postoperative bleeding volume mL) [95% CI] |
NNT [95% CI] |
Transfusion rate TXA group vs. control group (number of transfusions / total number of patients) | Number of studies for transfusions / patient number | Risk of bias* |
| Alshryda et al. 2011 (15) |
−591 [−536; –647] (I2 = 89%) |
n/a | −245 [−213; –278] (I2 = 78%) |
4.2 [3.3; 5.8] (I2 = 75%) |
23.5% (99/421) vs. 47.1% (190/403) |
14/824 | moderate |
| Tan et al. 2013 (38) |
−570 [−663; –478) (I2 = 77%) |
n/a | −290 [−385; –196] (I2 = 93%) |
3.9 [3.2; 5.0] (I2 = 92%) |
29.2% (149/511) vs. 55.0% (295/536) |
17/1047 | moderate to high |
| Wei & Liu 2015 (28) |
−322 [−413; −230] (I2 = 95%) |
n/a | n/a | 5.7 [4.6; 7.5] (I2 = 69%) |
14.1% (104/736) vs. 31.7% (212/668) |
18/1404 | moderate |
| Wu et al. 2015 (39) |
n/a | n/a | n/a | 3.7 [3.1; 4.5] (I2 = 42.3%) |
26.5% (201/758) vs. 53.3% (396/743) |
23/1501 | moderate |
All studies included in the meta-analyses are randomized controlled trials. Bleeding volumes are given (in mL) for comparison between TXA and controls (mean difference, 95% confidence interval).
CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; I 2 values, given as an indicator of heterogeneity; n/a, data missing or not calculable; NNT, number needed to treat; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; TXA, tranexamic acid
*The authors of this article first determined the bias risk for the individual studies in the meta-analyses using the GRADE system and the PRISMA statements (or the Cochrane risk of bias tool) and then averaged a mean value for every meta-analysis.
State of evidence for hip replacement
Hip replacement is likewise one of the most common procedures in orthopedic surgery. In 2011, 232 320 hip endoprostheses were implanted in Germany (23).
The total blood loss in hip replacement surgery varies between 1200 and 2100 mL and is approximately 1600 mL on average (depending on the surgical technique) (10, 25). Approximately 30% of patients receive at least one blood unit (with a median of 2.2 units) (24).
In a meta-analysis of randomized controlled trials, Sukeik et al. investigated 11 studies with primary total hip replacement surgery (26). The total blood loss in the TXA group was reduced by 289 mL [138; 440 ml]. The same effect was observed in the meta-analysis of Farrow and colleagues (27). The rate of intra- and postoperative blood transfusions was significantly lower in the included randomized controlled trials (of seven studies with 750 patients). The relative risk of blood transfusion was 1.9 [1.2; 2.9] for the control group as compared to the TXA group. TXA administration resulted in an absolute risk reduction of the transfusion rate of 12.2% (85/321 in the TXA group compared to 166/429 in the control group). This corresponds to a NNT of 8.2 [5.3; 18,4] (27). It should be noted that Sukeik et al. found a linear relationship between the TXA dose and the postoperative blood loss, but not between the dose and the intraoperative blood loss or the rate of transfusion (26).
As for knee replacement surgery, the state of evidence for the intravenous application of TXA is very heterogeneous. Very different dosages were used in the published work; for instance, the randomized controlled trial included in the meta-analysis by Farrow et al. investigated doses of 1–4 g/24 hours (27). Studies were included with and without chemical thrombosis prophylaxis. Table 3 shows an overview of selected meta-analyses.
Table 3. Tranexamic acid in hip arthroplasty—selected meta-analyses.
| Study |
Reduction of total bleeding volume (mL) [95% CI] |
Reduction of intraoperative bleeding volume (mL) [95% CI] |
Reduction of postoperative bleeding volume (mL) [95% CI] |
NNT [95% CI] |
Transfusion rate TXA group vs. control group (number of transfusions/ total patient number) | Number of studies for transfusions / patient number | Risk of bias* |
| Sukeik et al. 2011 (26) |
−289 [−440; –138] (I2 = 54%) |
−104 [−44; –164] (I2 = 0%) |
−172 [−263; –81] (I2 = 63%) |
5 [3.4; 9.1] (I2 = 15%) |
n/a | 7/346 | low |
| Farrow et al. 2016 (27) |
n/a | −190 [−495; –115] (I2 = 91%) |
−341 [−672; −9.87] (I2 = 100%) |
8.2 [5.3; 18.4] (I2 = 78%) |
26.5% (85/321) vs. 38.7% (166/429) |
7/750 | moderate to high |
| Zhou et al. 2013 (40) |
−305 [−398; –213] (I2 = 32%) |
−86 [−152; –20] (I2 = 67%) |
−177 [−237; –116] (I2 = 60%) |
7.8 [5.8; 12.2] (I2 = 11%) |
10.9% (55/507) vs. 23.6% (128/543) |
21/1050 | moderate |
| Huang et al. 2015 (e1) |
−369 [−482; –259] (I2 = 73%) |
n/a | n/a | 4,1 [3,3; 5,4] (I2 = 18%) |
16% (65/405) vs. 40,6% (167/411) |
14/816 | moderate |
| Moskal et al. 2016 (e2) |
total bleeding volume: 1089 (± 1 251) vs. 1 410 (± 1 111) (P <0.001 |
total bleeding volume: 668 (± 1 169) vs. 793 (± 1 805) (P = 0.002) |
total bleeding volume: 449 (± 620) vs. 597 (± 1012) (P = 0.003) |
4.5 [3.5; 6.4] | 16.6% (55/332) vs. 38.6% (131/339) |
10/671 | moderate |
All studies included in the meta-analyses are randomized controlled trials. Bleeding volumes are given (in mL) for comparison between TXA and controls (mean difference, 95% confidence interval). CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; I2 values, given as an indicator of heterogeneity; n/a, data missing or not calculable; NNT, number needed to treat; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SD, standard deviation; TXA, tranexamic acid
* The authors of this article first determined the bias risk for the individual studies in the meta-analyses using the GRADE system and the PRISMA statements (or the Cochrane risk of bias tool) and then averaged a mean value for every meta-analysis.
Complications
Based on its inhibition of fibrinolysis, using TXA is theoretically assumed to active coagulation with an increased tendency for thrombosis.
In the meta-analysis of Alshryda et al. for TXA use in knee arthroplasty, a total of five pulmonary embolisms occurred in 971 patients, with one in the TXA group and four in the control group; this difference was not significant. In the same meta-analysis, no difference was found for the outcome of leg vein thrombosis (15). A recent meta-analysis included 39 randomized controlled trials of TXA in hip and knee replacement procedures (28). In this study, thromboembolic events in the population with perioperative TXA administration also did not increase significantly. The CRASH-2 trial examined TXA administration (with an initial 1 g TXA followed by a continuous dose of 1 g/8 hour) that had been randomized and blinded for over 20 000 patients in severe trauma with hemorrhage in 40 countries, as compared to a placebo group that received no TXA. In addition to the primary endpoints of clinical effectiveness (such as mortality and transfusion rates), the complications were also recorded in detail in this study. The relative risk (RR) values were given as follows:
Deep vein thrombosis: 0.98 [0.63; 1.51] (41/10 067 for control group, compared to 40/10 060 for TXA group)
Pulmonary embolism: 1.01 [0.73; 1.41] (71/10 067 for control group, compared to 72/10 060 for TXA group)
Stroke: 0.86 [0.61; 1.23] (66/10 067 for control group, compared to 57/10 060 for TXA group).
Myocardial infarction was rare in the TXA group, with a relative risk of 0.64 [0.42; 0.97] (55/10 067 for control group, compared to 35/10 060 for TXA group). However, only clear complications were recorded, so that minor thromboembolic events may not have been documented, and a so-called underreporting bias could be present (29).
In a retrospective study of patients with a history of venous thrombosis, Sabbag et al. investigated whether thrombosis occurred with TXA administration. They found no significant differences (RR for TXA: 1.24 [0.51; 3.00]; 2.3% [6/258], compared to RR for placebo: 1.9% [25/1337]) (30).
However, there is still a great need for research on the safe use of TXA in patients with a recent myocardial infarction, percutaneous transluminal coronary angioplasty (PTCA), or stent implantation. For these patients, TXA should be given perioperatively for endoprosthetic surgery only after individual risk assessment.
On the other hand, various studies on the use of TXA in coronary artery bypass surgery have shown that thromboembolic complications did not increase. Karski et al. showed no increased incidence of myocardial infarction (RR: 0.75 [0.13; 4.42]; 3/165 in control versus 2/147 in TXA group) (31).
Seizures after TXA administration are additional much-discussed complications. Good studies on seizures are missing for TXA in knee and hip arthroplasty. This important side effect of the drug has been observed especially in high dose applications, for example a bolus of 30 mg/kg BW plus a subsequent continuous dose over several days of 15 mg/hour per kg BW with severely impaired renal function. These high-dose applications are often chosen for procedures using the heart-lung bypass machine to inhibit fibrinolysis. Retrospective analyses from this particular group of patients show that the incidence of postoperative seizures is approximately 2.7% for moderate to high doses of TXA (32, 33). The incidence of seizures in the groups that did not receive antifibrinolytic agents was 0.5%. In a retrospective study published in 2017 by Counture et al., the incidence of seizures was 0.62% (46/7452) for patients who received 1 g TXA bolus intraoperatively and another 400 mg/hour TXA (34).
Nevertheless, it seems plausible that a one-time dose of TXA, as recommended by the manufacturer (table 1), is generally considered to be relatively safe with respect to this complication (14, 16, 32).
In patients with known epilepsy, however, intravenous administration of TXA should be considered with great caution in terms of benefits and risks.
Discussion
The meta-analyses of the use of TXA in total hip and knee replacement have a high degree of heterogeneity in some cases (I2 values) (Table 2 and Table 3). Further, a publication bias in the meta-analyses cannot be ruled out. The meta-analyses also summarized studies with different dosages and clinical procedures.
The patient blood management concept was inconsistent in the primary studies. Nevertheless, the blood-sparing effects of TXA are consistently observed in all primary studies.
The meta-analyses from Table 2 and Table 3 show similar results, partly due to the use of similar primary studies. eTable 1 and eTable 2 provide a detailed overview of the overlaps of the individual meta-analysis.
eTable 1. Overlap of primary studies in meta-analyses on the use of tranexamic acid in knee arthroplasty.
| Alshryda et al. 2011 (15) | Tan et al. 2013 (38) | Wei & Liu 2015 (28) | Wu et al. 2015 (39) |
| Primary studies that were used in 4 meta-analyses: | |||
| Good 2003 | Good 2003 | Good 2003 | Good 2003 |
| Ellis 2001 | Ellis 2001 | Ellis 2001 | Ellis 2001 |
| Camarasa 2006 | Camarasa 2006 | Camarasa 2006 | Camarasa 2006 |
| Alvarez 2008 | Alvarez 2008 | Alvarez 2008 | Alvarez 2008 |
| Tanaka 2001 | Tanaka 2001 | Tanaka 2001 | Tanaka 2001 |
| Kakar 2009 | Kakar 2009 | Kakar 2009 | Kakar 2009 |
| Orpen 2005 | Orpen 2005 | Orpen 2005 | Orpen 2005 |
| Veien 2002 | Veien 2002 | Veien 2002 | Veien 2002 |
| Zohar 2004 | Zohar 2004 | Zohar 2004 | Zohar 2004 |
| Primary studies that were used in 3 meta-analyses: | |||
| Benoni 1996 | Benoni 1996 | Benoni 1996 | |
| Jansen 1999 | Jansen 1999 | Jansen 1999 | |
| Hiippala 1995 | Hiippala 1995 | Hiippala 1995 | |
| Hiippala 1997 | Hiippala 1997 | Hiippala 1997 | |
| Ido 2000 | Ido 2000 | Ido 2000 | |
| Engel 2001 | Engel 2001 | Engel 2001 | |
| Zhang 2007 | Zhang 2007 | Zhang 2007 | |
| Molloy 2007 | Molloy 2007 | Molloy 2007 | |
| Cchareancholvanich 2011 | Cchareancholvanich 2011 | Cchareancholvanich 2011 | |
| Primary studies in which 2 meta-analyses were used: | |||
| Wong 2010 | Wong 2010 | ||
| Martin 2014 | Martin 2014 | ||
| Maniar 2012 | Maniar 2012 | ||
| Georgiadis 2013 | Georgiadis 2013 | ||
| McConnell 2012 | McConnell 2012 | ||
| Ishida 2011 | Ishida 2011 | ||
| Roy 2012 | Roy 2012 | ||
| Primary studies that were used in 1 meta-analyses: | |||
| Zohar 1999 | Lin 2011 | Oremus 2014 | Pachauri 2013 |
| Gautam 2013 | Aguilera 2013 | ||
| Raviraj 2012 | Seo 2013 | ||
| Yamasak 2005 | Lee 2013 | ||
| MacGillivray 2011 | Alshryda 2013 | ||
| Konig 2013 | Kim 2014 | ||
| Dahuja 2013 | Onodera 2012 | ||
| Alipour 2013 | Gautam 2011 | ||
| Wong 2010 | |||
| Abrishami 2010 | |||
eTable 2. Overlap of primary studies in meta-analyses on the use of tranexamic acid in hip arthroplasty.
| Sukeik et al. 2011 (26) | Farrow et al. 2016 (27) | Zhou et al. 2013 (40) | Huang et al. 2015 (e1) | Moskal et al. 2016 (e2) |
| Primary studies that were used in 4 meta-analyses: | ||||
| Johansson 2005 | Johansson 2005 | Johansson 2005 | Johansson 2005 | |
| Benoni 2001 | Benoni 2001 | Benoni 2001 | Benoni 2001 | |
| Ekbäck 2000 | Ekbäck 2000 | Ekbäck 2000 | Ekbäck 2000 | |
| Garneti 2004 | Garneti 2004 | Garneti 2004 | Garneti 2004 | |
| Niskanen 2005 | Niskanen 2005 | Niskanen 2005 | Niskanen 2005 | |
| Claeys 2007 | Claeys 2007 | Claeys 2007 | Claeys 2007 | |
| Benoni 2000 | Benoni 2000 | Benoni 2000 | Benoni 2000 | |
| Primary studies that were used in 3 meta-analyses: | ||||
| Yamasaki 2004 | Yamasaki 2004 | Yamasaki 2004 | ||
| Husted 2003 | Husted 2003 | Husted 2003 | ||
| Lemay 2004 | Lemay 2004 | Lemay 2004 | ||
| Kazemi 2010 | Kazemi 2010 | Kazemi 2010 | ||
| Primary studies that were used in 2 meta-analyses: | ||||
| Rajesparan 2009 | Rajesparan 2009 | |||
| Yamasaki 2004 | Yamasaki 2004 | |||
| Malhotra 2011 | Malhotra 2011 | |||
| Ido 2000 | Ido 2000 | |||
| McConnell 2011 | McConnell 2011 | |||
| Primary studies that were used in 1 meta-analysis: | ||||
| Lee 2015 | Singh 2010 | Yue 2014 | Hsu 2015 | |
| Sadeghi 2007 | Jamie 2011 | Oremus 2014 | Wei 2014 | |
| Zuffery 2010 | Norio 2012 | Imai 2012 | ||
| Emara 2014 | Clave 2012 | |||
| Mohib 2015 | Fu 2012 | |||
| Vijay 2013 | ||||
| Tengberg 2016 | ||||
Using TXA as prophylaxis for bleeding in large joint arthroplasty (hip, knee) seems to be effective. Preoperative TXA administration reduces intra- and postoperative bleeding as well as the amount of transfusions necessary. The current state of knowledge suggests that the thromboembolic risk does not increase with low-dose, short-term administration for these indications within normal clinical use of postoperative prophylaxis for drug thrombosis. The risk of seizures does not seem to increase significantly, although there is still insufficient data for this.
Topical application of TXA results in lower plasma levels and therefore may have a reduced risk of complications, according to theoretical considerations.
Alshryda et al. recently published a meta-analysis on topical application of TXA in total hip and knee replacement (35). They observed a marked reduction in both blood loss during knee replacement surgery and the need for transfusions (RR: 4.5 [3.0; 6.7]) as compared to placebo. Overall, only a few studies involving the systemic administration of TXA as a further comparison group could be evaluated, which have revealed no relevant differences in the transfusion rate between the two forms of application.
Economic aspects of the prophylactic administration of TXA
Blood is a scarce, valuable resource in medicine. In Germany, about 4 million units of blood per year are transfused. In addition to the risks already described, transfusion of red blood cells is also a relevant cost factor. The direct production costs of packed red blood cells are approximately 140 to 230 Euro per concentrate (10, 36). Comparing this with the cost of two applications of 1 g TXA (5 euros for 500 mg—about 20 euros for 2 g) for a 70 kg patient with normal kidney function, and using a NNT of 3.7–8.2, to avoid a necessary transfusion reveals a possible savings potential. The above-mentioned complications and risks of blood transfusions, and especially that of postoperative prosthetic joint infection, can lead to significantly prolonged hospital stays and considerable costs (6– 8).
Conclusion
From our point of view, the prophylactic use of systemic TXA for knee and hip arthroplasty can be used as an effective and relatively safe concept in practice. Contraindications and risks, embedded in a more extensive patient blood management concept, must be considered. This could result in significant reductions of intra- and postoperative blood loss as well as consecutive allogenic blood transfusions. However, whether a prophylactic strategy should be applied to all patients who undergo elective large joint arthroplasty, or whether stratified concepts should be favored in the future that consider underlying disease, baseline hemoglobin values, and local transfusion probabilities, needs to be clarified in further studies. Initial results from a retrospective study suggest that TXA administration may not be an absolute contraindication in patients with leg vein thrombosis. None-the-less, the prophylactic use of TXA for patients with knee and hip arthroplasty and urogenital bleeding, pulmonary embolism, recent heart attack, PTCA, or stent implantation, as well as known epilepsy, should be avoided according to the risk-benefit assessment.
Key Messages.
Endoprosthetic surgery of the knee and hip cause relevant bleeding and often require transfusions, which can lead to further complications (including transmission of infections, increased prosthetic infections, and higher mortality and morbidity).
The prophylactic administration of tranexamic acid is a useful part of the blood-saving perioperative patient blood management concept in the field of elective hip and knee arthroplasty.
Whether it is safe to use tranexamic acid after myocardial infarction, percutaneous transluminal coronary angioplasty, or stent implantation still needs considerable research. In these cases, therefore, the risks and benefits must be weighed individually.
The rate of thromboembolic complications is not higher as compared to control groups in randomized controlled trials.
Acknowledgments
Translated from the original German by Veronica A. Raker, PhD.
Footnotes
Conflict of interest statement
PD Dr. Wiesmann has received consultant honoraria from B. Braun Melsungen (Germany).
Prof. Wulf has received consultant honoraria from B. Braun and Sintetica, speaking honoraria from MSD, Grünenthal, and Edwards, und study support (third-party funds) von Teleflex.
The remaining authors declare that no conflict of interest exists.
References
- 1.Mohib Y, Rashid RH, Ali M, Zubairi AJ, Umer M. Does tranexamic acid reduce blood transfusion following surgery for inter-trochanteric fracture? A randomized control trial. J Pak Med Assoc. 2015;65:17–20. [PubMed] [Google Scholar]
- 2.Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96:576–582. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 3.Triulzi DJ, Vanek K, Ryan DH, et al. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32:517–524. doi: 10.1046/j.1537-2995.1992.32692367194.x. [DOI] [PubMed] [Google Scholar]
- 4.Lannan KL, Sahler J, Spinelli SL, et al. Transfusion immunomodulation: the case for leukoreduced and (perhaps) washed transfusions. Blood Cells Mol Dis. 2013;50:61–68. doi: 10.1016/j.bcmd.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101:299–308. doi: 10.1016/S0002-9343(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 6.Elsamadicy AA, Adogwa O, Vuong VD, et al. Association of intraoperative blood transfusions on postoperative complications, 30-day readmission rates, and 1-year patient-reported outcomes. Spine. 2017;42:610–615. doi: 10.1097/BRS.0000000000001803. [DOI] [PubMed] [Google Scholar]
- 7.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Innerhofer P, Klingler A, Klimmer C, Fries D, Nussbaumer W. Risk for postoperative infection after transfusion of white blood cell-filtered allogeneic or autologous blood components in orthopedic patients undergoing primary arthroplasty. Transfusion. 2005;45:103–110. doi: 10.1111/j.1537-2995.2005.04149.x. [DOI] [PubMed] [Google Scholar]
- 9.Meybohm P, Fischer D, Schnitzbauer A, et al. Patient blood management. Chirurg. 2015;87:40–46. doi: 10.1007/s00104-015-3011-3. [DOI] [PubMed] [Google Scholar]
- 10.Shander A, van Aken H, Colomina MJ, et al. Patient blood management in Europe. Br J Anaesth. 2012;109:55–68. doi: 10.1093/bja/aes139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bundesinstitut für Arzneimittel und Medizinprodukte. Trasylol® (Aprotinin): Umsetzung der Kommissionsentscheidung zum Ruhen der Zulassung. www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/DE/RI/2008/RI-aprotinin.html (last accessed on 22 June 2017) [Google Scholar]
- 12.Draxler DF, Medcalf RL. The fibrinolytic system—more than fibrinolysis? Transfus Med Rev. 2015;29:102–109. doi: 10.1016/j.tmrv.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Müller-Berghaus G, Pötzsch B. Heidelberg Springer-Verlag. Berlin: 2013. Hämostaseologie; 1 pp. [Google Scholar]
- 14.McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72:585–617. doi: 10.2165/11209070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93:1577–1585. doi: 10.1302/0301-620X.93B12.26989. [DOI] [PubMed] [Google Scholar]
- 16.Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: causes and treatment. Ann Neurol. 2016;79:18–26. doi: 10.1002/ana.24558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koscielny J, Jambor C. Perioperativer Einsatz von Antifibrinolytika. Vascular Care. 2008;15:32–51. [Google Scholar]
- 18.Later AFL, Sitniakowsky LS, van Hilten JA, et al. Antifibrinolytics attenuate inflammatory gene expression after cardiac surgery. J Thorac Cardiovasc Surg. 2013;145:1611–1616. doi: 10.1016/j.jtcvs.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Guo Y, Mikus P, et al. Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds. Blood. 2012;119:5879–5887. doi: 10.1182/blood-2012-01-407825. [DOI] [PubMed] [Google Scholar]
- 20.PFIZER PHARMA PFE GmbH. Fachinformation „Cyclocapron®“. Fa. Pfizer. www.pfizermed.de/fileadmin/produktdatenbank/pdf/008797_freigabe.pdf (last accessed on 21 February 2016) [Google Scholar]
- 21.Levrat A, Gros A, Rugeri L, et al. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth. 2008;100:792–797. doi: 10.1093/bja/aen083. [DOI] [PubMed] [Google Scholar]
- 22.Napolitano LM, Cohen MJ, Cotton BA, Schreiber MA, Moore EE. Tranexamic acid in trauma: how should we use it? Trauma Acute Care Surg. 2013;74:1575–1587. doi: 10.1097/TA.0b013e318292cc54. [DOI] [PubMed] [Google Scholar]
- 23.Wengler A, Nimptsch U, Mansky T. Hip and knee replacement in Germany and the USA—analysis of individual inpatient data from German and US hospitals for the years 2005 to 2011. Dtsch Arztebl Int. 2014;111:407–416. doi: 10.3238/arztebl.2014.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gombotz H, Rehak PH, Shander A, Hofmann A. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion. 2014;54:2646–2657. doi: 10.1111/trf.12687. [DOI] [PubMed] [Google Scholar]
- 25.Yamasaki S, Masuhara K, Fuji T. Tranexamic acid reduces postoperative blood loss in cementless total hip arthroplasty. J Bone Joint Surg Am. 2005;87:766–770. doi: 10.2106/JBJS.D.02046. [DOI] [PubMed] [Google Scholar]
- 26.Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93:39–46. doi: 10.1302/0301-620X.93B1.24984. [DOI] [PubMed] [Google Scholar]
- 27.Farrow LS, Smith TO, Ashcroft GP, Myint PK. A systematic review of tranexamic acid in hip fracture surgery. Br J Clin Pharmacol. 2016;82:1458–1470. doi: 10.1111/bcp.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25:151–162. doi: 10.1111/tme.12212. [DOI] [PubMed] [Google Scholar]
- 29.Shakur H, Roberts I, et al. CRASH-2 trial collaborators, Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 30.Sabbag OD, Abdel MP, Amundson AW, Larson DR, Pagnano MW. Tranexamic acid was safe in arthroplasty patients with a history of venous thromboembolism: a matched outcome study. J Arthroplasty. 2017;32:246–250. doi: 10.1016/j.arth.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Karski J, Djaiani G, Carroll J, et al. Tranexamic acid and early saphenous vein graft patency in conventional coronary artery bypass graft surgery: a prospective randomized controlled clinical trial. J Thorac Cardiovasc Surg. 2005;130:309–314. doi: 10.1016/j.jtcvs.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: A meta-analysis. Seizure. 2016;36:70–73. doi: 10.1016/j.seizure.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Keyl C, Uhl R, Beyersdorf F, et al. High-dose tranexamic acid is related to increased risk of generalized seizures after aortic valve replacement. Eur J Cardiothorac Surg. 2011;39:e114–e121. doi: 10.1016/j.ejcts.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 34.Counture P, Lebon JS, Laliberté É, et al. Low dose versus high dose tranexamic acid reduces the risk of non-ischemic seizures after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2017 doi: 10.1053/j.jvca.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B:1005–1015. doi: 10.1302/0301-620X.96B8.33745. [DOI] [PubMed] [Google Scholar]
- 36.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–765. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 37.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339:245–253. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 38.Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184:880–887. doi: 10.1016/j.jss.2013.03.099. [DOI] [PubMed] [Google Scholar]
- 39.Wu Q, Zhang H, Liu S, Meng T, Zhou X, Wang P. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol. 2015;25:525–541. doi: 10.1007/s00590-014-1568-z. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X, Tao L, Li J, Wu L. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133:1017–1027. doi: 10.1007/s00402-013-1761-2. [DOI] [PubMed] [Google Scholar]
- E1.Huang F, Wu Y, Yin Z, Ma G, Chang J. A systematic review and meta-analysis of the use of antifibrinolytic agants in total hip arthroplasty. Hip Int. 2015;25:502–509. doi: 10.5301/hipint.5000285. [DOI] [PubMed] [Google Scholar]
- E2.Moskal JT, Capps SG. Meta-analysis of intravenous tranexamic acid in primary total hip arthroplasty. Orthopedics. 2016;39:883–892. doi: 10.3928/01477447-20160526-02. [DOI] [PubMed] [Google Scholar]