Abstract
The Cryptococcus neoformans/Cryptococcus gattii species complex is a group of fungal pathogens with different phenotypic and genotypic diversity that cause disease in immunocompromised patients as well as in healthy individuals. The immune response resulting from the interaction between Cryptococcus and the host immune system is a key determinant of the disease outcome. The species C. neoformans causes the majority of human infections, and therefore almost all immunological studies focused on C. neoformans infections. Thus, this review presents current understanding on the role of adaptive immunity during C. neoformans infections both in humans and in animal models of disease.
Keywords: Cryptococcus, adaptive immunity, dendritic cells, CD4, helper T cell type 1 (Th1), helper T cell type 2 (Th2), helper T cell type 17 (Th17), cytokines, immune reconstitution inflammatory syndrome (IRIS)
1. Introduction
The Cryptococcus species complex is a group of related fungal pathogens that cause disease in both healthy and immunocompromised patients. A recent study proposed to separate the Cryptococcus species complex into seven species; C. neoformans, C. deneoformans, C. gattii, C. bacillisporus, C. deutorogattii, C. tetragattii, and C. decagattii [1]. The first two species (C. neoformans and C. deneoformans) were previously known as C. neoformans var grubii and C. neoformans var neoformans respectively, while the other five species (C. gattii, C. bacillisporus, C. deutorogattii, C. tetragattii and C. decagattii) represent what was previously known as C. gattii VGI to VGIV [1]. C. neoformans and C. deneoformans mainly cause infections in profoundly immunosuppressed patients such as individuals with advanced HIV-AIDS, various T cell defects, patients with chronic lung, renal and hepatic diseases, and patients receiving immunosuppressive therapy before organ transplantation [2,3,4]. However, the five C. gattii species can cause disease both in immunocompetent and immunocompromised individuals [5,6,7,8]. In C. gattii infections, approximately 60% of affected populations have underlying diseases including respiratory diseases, diabetes or hematological malignancy [9]. These differences in patient populations suggest that the Cryptococcus species may exhibit subtle differences in their interaction with the host. However, the vast majority of studies exploring immune responses in both humans and animal models have utilized C. neoformans infections. Thus, this review will primarily focus on C. neoformans.
1.1. Dendritic Cells and Macrophages Connect the Innate and Adaptive Immune Systems during C. Neoformans Infection
C. neoformans is found in the environment throughout the world, and has been extracted from soil, bird droppings and decaying wood [10,11]. C. neoformans infection starts following inhalation of fungal spores. Upon entering the lungs, Cryptococcus cells are recognized by host innate immune cells such as dendritic cells (DCs), epithelial cells, endothelial cells, and alveolar macrophages. These DCs and macrophages ingest and destroy invading Cryptococcus, present Cryptococcus antigens to T cells, and produce mediators (cytokines and chemokines) that initiate and direct the adaptive immune response [12,13,14,15]. Depletion of resident pulmonary DCs and alveolar macrophages results in rapid deterioration and death of mice infected with C. neoformans [16]. Thus, alveolar macrophages and DCs play an important role in the initiation of anti-cryptococcal immune responses, and link the innate and adaptive immune system during C. neoformans infection. In addition to their role as a physical barrier, epithelial and endothelial cells also act as effector cells during C. neoformans infection. Epithelial cells produce cytokines in response to C. neoformans [17], while endothelial cells augment the anti-cryptococcal activity of polymorphonuclear leukocytes [18].
1.2. Dendritic Cells Are the Primary Antigen Presenting Cells during Cryptococcal Infection
The respiratory tract contains a dense network of DCs with antigen uptake and presentation as their primary function [19,20]. Mature DCs migrate to T-cell-rich areas of secondary lymphoid organs, and present antigens to naïve T cells [20,21]. In addition to presenting antigens to naïve T cells, DCs also produce cytokines that regulate the adaptive immune response [21]. The expression of major histocompatibility complex class II molecules on DCs is sufficient to stimulate naïve T cells [20], and mice lacking DCs fail to generate cytotoxic T lymphocyte responses to intracellular pathogens [22]. Depending on the type of co-stimulatory molecules expressed on DCs, they can induce differential helper T cell responses. Helper T lymphocytes (Th cells) that respond to fungal pathogens can be divided into three main groups; (a) helper T cell type 1 (Th1) that produce pro-inflammatory responses to kill intracellular pathogens, (b) helper T cell type 2 (Th2) associated with the promotion of antibody, eosinophilic, and anti-inflammatory immune responses, and (c) helper T cell type 17 (Th17) cells associated with mucosal immunity and autoimmune diseases [23,24,25]. Dendritic cells matured in the presence of IFNγ induce the formation of IL-12 producing Th1 cells, and IL-12 secreted by DCs promotes the formation of IFNγ-producing cells [26,27]. On the other hand, DCs expressing costimulatory molecules CD86 and OX40L induce the development of Th2 cells that produce IL-4, IL-5 and IL-13 cytokines [26,28,29]. This Th2 immune response leads to eosinophilic airway inflammation [30]. Confirming these observations, blocking CD86 decreased Th2 immune responses, demonstrated by low IL-4 and IL-5 cytokines as well as low airway eosinophilia [31]. The formation of IL-17-producing Th17 cells requires the costimulatory molecules CD28 and ICOS [32]. In the absence of IL-4 and IFNγ cytokines, IL-23 induce naïve cells to differentiate into Th17 cells, and the presence of IL-4 and IFNγ blocks this differentiation [32,33]. Th17 immunity has been associated with both protective and non-protective roles during fungal infections [34,35,36,37,38,39]. During C. neoformans infection, Th1 immune responses are beneficial and support pathogen clearance, whereas Th2 immunity enhances disease, and Th17 cells have been associated with both protection and increased disease, depending upon the model used [39,40,41,42,43,44,45,46,47,48].
In a mouse model of C. neoformans infection, lung DCs internalize cryptococcal cells within 2 h post-infection, and following this internalization, lung DCs express the maturation markers CD80, CD86 and major histocompatibility class II [14]. The stimulated DCs induce production of high levels of IL-2 cytokine in vitro when co-incubated with C. neoformans antigen-specific T cells compared to naïve T cells, demonstrating C. neoformans antigen presentation and T cell activation [14]. Myeloid DCs and Langerhans cells, but not lymphoid DCs, are the antigen presenting cells needed to induce a protective immune response during C. neoformans infection [49]. A subset of lung resident DCs, CD11+ conventional DCs, mediates the accumulation of pathological Th2 cells following pulmonary C. neoformans infection, and lymphoid priming is not required for pulmonary Th2 cell accumulation [47]. These data demonstrate that the type of antigen-presenting DCs is important in the polarization of Th-mediated adaptive immune responses.
2. Cell-Mediated Immunity: T Cells
2.1. Importance of T Cells during Cryptococcal Infections
C. neoformans cause infections in immunocompromised patients, mainly individuals with HIV-AIDS. In non-HIV individuals, most patients who present with cryptococcosis have other underlying diseases or immunosuppression. These include patients receiving immunosuppressive medication before organ transplantation, patients on glucocorticosteroid therapy, patients with chronic hepatic, renal and lung diseases, and patients with idiopathic CD4 lymphocytopenia [3,4,50,51,52]. C. neoformans also infects patients suffering from X-linked hyper IgM syndrome with defects in their circulating T cells [53,54,55]. These observations clearly demonstrate that CD4 T cell-mediated immunity plays a critical role in controlling human cryptococcal infections.
In vitro and animal studies complement observations from human infections, and allow further identification of the role of T cells in immunity against C. neoformans. Different T cell subsets are involved in the immune response to C. neoformans infection. Both CD4 and CD8 T cells can inhibit the growth of C. neoformans cells by either direct killing [56,57], or production of pro-inflammatory cytokines that recruit and activate other phagocytes to kill C. neoformans cells [58,59,60,61,62]. Regulatory T cells promote protection against Cryptococcus infection by suppressing detrimental Th2 immune responses [63,64,65]. Other T cell subsets such as Natural Killer (NK), Natural Killer T (NKT) and gamma delta T (γδ T) cells are also involved in the development of a protective immune response against Cryptococcus infection [66,67,68,69,70,71]. However, γδ T cells can also downregulate the protective Th1 immune response [72].
2.2. Cryptococcal Antigens Activate T Cell Maturation and Proliferation
Whole C. neoformans cells or cell extracts (membrane, cell walls and proteins) induce the proliferation of human naïve T cells [73,74]. Phagocytosis and protein processing by accessory cells are necessary for the presentation of C. neoformans antigen to T lymphocytes [75]. Previous studies demonstrated that both CD4 and CD8 T cells proliferate in response to various C. neoformans antigens [59,60], and that immunity to C. neoformans infection requires both CD4 and CD8 T cells [76]. In addition, both CD4 and CD8 T cells directly inhibit the growth of cryptococcal cells in vitro [77,78], and both types of cells can use granulysin to kill C. neoformans [56,57]. A number of studies found divergent observations in addressing whether CD8 T cell responses depend upon CD4 T cells. Studies in mice found that neither CD8 T cell expansion and recruitment, nor their ability to produce IFNγ cytokine required CD4 T cells [58,61]. However, the loss of CD4 T cells resulted in a hyperexpansion of CD8 T cells [59]. On the other hand, studies using human cells showed that (a) proliferation of CD8 T cells requires CD4 T cells [60], and (b) the ability of CD8 T cells to kill C. neoformans through granulysin requires CD4 T cells, accessory cells and IL-15 [56]. These conflicting observations suggest that either the activity of CD4 and CD8 T cells in response to C. neoformans is different between humans and mice, or that the recruitment/expansion and function of CD8 T cells are regulated differently with CD4 T cells regulating the ability of CD8 T cells to kill C. neoformans through granulysin, but having no effect on how CD8 T cells develop and are recruited.
During cryptococcal infection, stimulated lung-infiltrating T lymphocytes secrete both Th1 (IFNγ, IL-2) and Th2 (IL-4, IL-5, IL-10) cytokines [61]. The absence of CD4 T cells is accompanied by the loss of T cells secreting IL-4, IL-5 and IL-10, but residual CD8 still produces IFNγ and IL-2 cytokines [61]. On the other hand, T cells from CD8 deficient mice produced similar IL-4, IL-5 and IL-10 levels as the control mice, but secreted lower levels of IFNγ [61]. Both CD4 and CD8 T cells produce IFNγ and TNFα cytokines in the lungs of infected mice [59]. These data suggest that both CD4 and CD8 T cells produce Th1 cytokines; however, CD4 T cells are the main or sole source of Th2 cytokines during C. neoformans infection.
2.3. Regulatory T Cells (Tregs)
Tregs are a subset of CD4 T cells that suppress immune responses [79,80]. Tregs have been found to play both beneficial and harmful roles during most common fungal infections. In Histoplasma and Candida albicans infections, reduced Tregs were associated with increased pro-inflammatory cytokines that promoted fungal clearance [81,82]. However, another study found that Tregs enhance Th17 responses and clearance of C. albicans cells [83]. In addition, Tregs were found to suppress Th2 immune responses in the lungs of mice infected with Pneumocystis [84].
Similar to their role during Pneumocystis infection, recent studies showed that Treg cells suppress harmful Th2 immune responses during murine C. neoformans infection [47,63,64,65]. Treg-depleted mice showed increased mucus production, eosinophilia, IgE production, Th2 cytokines (IL-4, IL-5, IL-13), as well as increased fungal burden [63]. Confirming these observations, increasing Tregs during pulmonary cryptococcal infection resulted in reduced IgE production, decreased mucus production and Th2 cytokines [64]. The C-C chemokine receptor type 5 (CCR5) and IFN regulatory factor 4 (IRF4) are required for the localization of Tregs to infected lungs and subsequent suppression of Th2 effector cells [65].
2.4. Natural Killer T(NKT) Cells
NKT cells play an important role in inducing protective Th1 immune responses during C. neoformans infection. C. neoformans infection is followed by an accumulation of NKT cells in the lungs and MCP-1 chemokine contributes to this NKT cell induction, especially Vα14+ NKT cells [66]. The activation of NKT cells is thought to be through the presentation of cryptococcal lipid antigens by DCs [68]. NKT cells induce delayed type hypersensitivity after immunization with cryptococcal culture filtrate antigen [69], and play an important role in the development of protective Th1 immune responses following C. neoformans infection [66,67,68]. In addition, activation of Vα14+ NKT cells with α-galactosylceramide resulted in Th1 immune responses, shown by increased IFNγ production, and enhanced local resistance to C. neoformans infection [67].
2.5. Gamma Delta (γδ) T Cells
During pulmonary C. neoformans infection, γδ T cells accumulate in the lungs, a process that does not involve MCP-1 chemokine [72]. Deficiency in γδ T cells was followed by an increase in IFNγ production, suggesting that they downregulate protective Th1 immune responses [72]. In addition, the absence of both Th cells and CD8 T cells leads to γδ T cell overproduction associated with neutrophilia and enhanced disease [39]. However, in the absence of neutrophils, γδ T cells produce IL-17A cytokine associated with protective immune responses in mice immunized with an IFNγ-producing C. neoformans strain [71]. The discrepancies in the roles of γδ T cells during C. neoformans infection observed in the above studies [39,71,72] might be due to the use of different C. neoformans and mouse strains.
2.6. Memory T Cells
Defects in CARD9 are accompanied by impaired accumulation of Natural Killer (NK) and memory T cells in the lungs and increased susceptibility to C. neoformans infection, suggesting that both NK and memory T cells contribute to the protective immune response against C. neoformans [70].
3. Antibody-Mediated Immunity against Cryptococcal Infections
The fact that both humans and rodents produce antibody responses reactive with cryptococcal proteins [85] suggests that antibody-mediated immunity might play an important role against C. neoformans infection. Naïve laboratory mice and rats have no serum antibodies reactive with cryptococcal proteins, but produce antibody responses after C. neoformans infection [85]. Interestingly, adult human sera contain antibodies to cryptococcal proteins and GXM regardless of the person’s HIV status, previous history of C. neoformans infection, or whether the sera came from individuals with or without potential exposure to C. neoformans [85,86]. In addition, by the age of 5, sera from immunocompetent children also contain antibodies reactive with various cryptococcal proteins [87], suggesting that exposure to C. neoformans and subsequent development of antibody responses occurs early in life.
3.1. B Cells and Antibody-Mediated Immune Responses in Human Cryptococcal Infections
Previous studies reported conflicting observations about the role of antibody-mediated immune responses in controlling cryptococcal infections. Deficiency in B cells and antibody immune responses have been associated with a greater risk for developing cryptococcal infections both in HIV and non-HIV patients [5,88,89]. In HIV-patients, decrease in B cells expressing IgM is associated with cryptococcal infections [88]. In addition, lower IgG counts are associated with cryptococcal meningitis in non-HIV patients with normal T cell counts and ratios [5,89]. Cryptococcal meningitis also occurs in patients with X-linked hyper IgM syndrome, characterized by lower IgG, IgA and IgE [53,54,90,91,92]. X-linked hyper IgM syndrome is a genetic defect in the gene encoding CD40 ligands on activated CD4 T cells, and is required for normal B cells activation [90,91,93]. C. neoformans infections are also linked to a total absence of B cells, such as in Burton’s agammaglobulinaemia in non-HIV patients [94,95]. These examples show that B cells and antibodies play an important role in controlling cryptococcal infection.
Yet, antibody responses can also enhance cryptococcal disease in humans. Autoantibodies, such as anti-IFNγ and anti-GM-CSF, are associated with infections in non-HIV patients with normal CD4 counts [96]. Specifically, anti-GM-CSF autoantibodies were associated with cryptococcal meningitis in one patient [96].
3.2. B Cells and Antibody-Mediated Immune Responses in Non-Human Cryptococcal Infections
Early mouse studies concluded that antibodies were not involved in protection against C. neoformans because B cell deficient mice had no differences in mortality or organ CFUs when compared to wild-type mice [97]. However, subsequent studies demonstrated that B cells and antibodies can play a role in either resistance or susceptibility to cryptococcal infection. Deficiency in B cells and IgM antibodies has been associated with increased lung fungal burden and enhanced susceptibility to C. neoformans infection [98,99,100]. In addition, IgG antibodies enhance the ability of murine splenic NK cells to kill C. neoformans [101]. Another study showed that although B cells are dispensable for the development of acquired resistance to C. neoformans, they play an important role in protection against systemic infection when T cell immunity is impaired [102]. Divergence in the observed role of B cells and antibodies during C. neoformans infection might be due to the use of different C. neoformans and mouse strains. For example, studies showing that B cells and antibodies have a protective role against C. neoformans used C57BL/6J, BALB/c and CBA/J mouse strains [98,99,100], while the study that showed that antibody responses are dispensable during C. neoformans infection uses Swiss albino mice [97], and the C. neoformans strains used in these studies were also different [97,98,99,100].
Antibodies can also act as opsonins to enhance phagocytosis of Cryptococcus cells [103,104,105,106,107,108,109,110]. Anti-β-glucan monoclonal antibody with the ability to bind C. neoformans cell wall inhibits cryptococcal growth and increases in vitro killing by human and murine peritoneal macrophages [111]. In addition, administration of this anti-β-glucan antibody to infected mice reduces fungal burden in the brain and liver [111], showing that passive administration of anti-Cryptococcus antibodies can protect against C. neoformans infection. The efficacy of passive administration of anti-Cryptococcus antibodies depends on the antibody dose and mouse strain. In BALB/c mice, the efficacy of passive antibody decreases with higher doses, while in CBA/J mice protection against C. neoformans infection is only observed at high antibody doses [100]. In addition to antibody dose and host genetics, the type of antibody also affects the ability to confer protection against C. neoformans infection. Antibodies such as IgG, IgM and IgA have been associated with protection against C. neoformans infection [100,101,103,108,109,110], while increased IgE is associated with a Th2 immune response that exacerbates disease [100,112,113,114]. The above observations show that antibody-mediated immunity against Cryptococcus infection is a complex process where protective or non-protective roles of antibodies against C. neoformans depend on the type of C. neoformans strain, host genetics, and immunoglobulin class.
4. Cytokine Responses during C. neoformans Infections
Cytokines are small secreted proteins that mediate the interaction and communication between different immune cell types. Different cell types can secrete the same cytokines and similar functions can be induced by different types of cytokines. In addition, a single cytokine can act on many cell types, thus deciphering the impact of a specific cytokine can be challenging. A wide range of cytokines and chemokines (cytokines with chemotactic activities) play a crucial role in protecting mice against cryptococcal infection. A common feature of these protective cytokines and chemokines is that they either induce the Th1 immune response, enhance the activity of other Th1-inducing cytokines, and/or suppressing Th2 immune responses (Table 1). Th2 cytokines mainly induce non-protective immune responses that enhance cryptococcal disease, whereas Th17 cells are associated with both protection and disease enhancement during C. neoformans infection (Table 1).
Table 1.
Schematic representation of cytokine function based on tissue analyzed.
| Human | Mice | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Classification | Cytokines/Chemokines | Blood/Plasma/Serum | CSF | Lungs | Spleen | Brain | |||||
| Protective cytokines | IFNγ | [40,118,121,122,127,128,130,136] | |||||||||
| IL-12 | [40,100,116,124] | ||||||||||
| IL-2 | NA | NA | [119,120,123] | ||||||||
| Protection support cytokines | IL-6 | NA | NA | [100,117,128,129,130] | |||||||
| IL-18 | NA | NA | NA | [134,135] | |||||||
| IL-23 | NA | NA | NA | [133] | |||||||
| IP10 | NA | [127,130,136,137] | |||||||||
| G-CSF | NA | NA | [71,128,130,138,139] | ||||||||
| GM-CSF | NA | NA | NA | [128,131,138] | |||||||
| TNFα | NA | [41,121,128,130,136,138] | |||||||||
| Non-protective cytokines | IL-5 | NA | NA | [47,112,136,140] | |||||||
| IL-13 | NA | NA | [42,47,112,115,141] | ||||||||
| Cytokines/ chemokines with varying roles |
IL-1β | NA | NA | [136,142,143] | |||||||
| IL-4 | [42,112,115,117,124,131,136,138,144,145] | ||||||||||
| IL-8 | NA | NA | NA | [124,128,129,130,146] | |||||||
| IL-10 | NA | [47,100,117,124,127,129,131,142,146] | |||||||||
| IL-17 | NA | [39,45,112,124,131,138] | |||||||||
| MCP-1 | NA | NA | [131,136,147,148] | ||||||||
| MIP-1α | NA | NA | NA | [40,131,136,148] | |||||||
| RANTES | NA | NA | NA | [47,136] | |||||||
Blue (protective), yellow (neutral), red (non-protective), NA: not available.
4.1. Protective Cytokines
The main protective cytokines produced during cryptococcal infections include IFNγ, IL-12 and IL-2. These cytokines have been associated with protection both in humans and mouse models of C. neoformans infection. In mice, the absence or reduction of IFNγ, IL-12 and IL-2 correlated with increased lung and brain fungal burden, increased lung eosinophilia, reduced numbers of macrophages expressing inducible nitric oxide synthase, increased fungal dissemination to the brain and overall increased susceptibility to infection [40,42,44,115,116,117,118]. Treating mice with anti-CD40 and IL-2 increases protection from disseminated C. neoformans infection through IFNγ activation of microglial cells [119,120], and IL-2 activated T and NK cells directly inhibit the growth of C. neoformans in vitro [77]. In addition, the absence of IL-12 induces a switch from protective Th1 to non-protective Th2 cytokines [40,44]. Human studies validate the role of IFNγ, IL-12 and IL-2 cytokines in protecting the host against Cryptococcus infection. Recombinant IFNγ and IL-2 were used as adjunct therapy to successfully treat cryptococcal meningitis (CM) patients [43,121,122,123]. Similar to observations in mice, adjunct IFNγ therapy was associated with an improved rate of fungal clearance in the CSF of HIV-CM patients with a trend towards improved mycological and clinical outcome [43,122], and restoration of immunological parameters and a sustained clinical recovery [121]. The importance of IL-12 in human CM was shown by (a) a correlation between higher CSF levels of IL-12 cytokine and increased survival in AIDS patients with CM, and (b) an increased ability to produce IFNγ when human PBMCs were treated with IL-12 [124]. All three cytokines are known to play a major role in the induction of Th1 immune responses. IFNγ and IL-12 cytokines from innate immune cells stimulate the differentiation of helper T cells into Th1 cells, while IL-2 induces proliferation of T cells [125,126].
In addition to these three major protective cytokines, there is another group of cytokines that we classified as supportive cytokines because they induce or promote the three major protective cytokines (IFNγ, IL-12 and IL-2) or their protective role has been shown in either human or animal models of cryptococcosis, but not in both. These supportive cytokines include TNFα, IL-6, IL-8, IL-18, IL-23 and IP10. The ability to produce TNFα, IL-8, IL-6 and IP10 cytokines was associated with improved outcome in AIDS patients with CM [124,127,128,129,130,131]. In addition, IL-6 and IL-1β are the main cytokines involved in anti-cryptococcal resistance in the brain of infected mice [132]. IL-23 and IL-18 cytokines were shown to play a protective role in mice against cryptococcal infection [133,134,135]; however, their role in human infection has yet to be determined. In mice, the absence of IL-23 was followed by impaired recruitment of inflammatory cells and cytokine responses [133], while defects in IL-18 correlated with increased lung fungal burden and reduced IFNγ and IL-12 cytokines [134,135].
4.2. Non-Protective Cytokines
In general, Th2 cytokines such as IL-5 and IL-13 promote cryptococcal disease. In mice, both IL-5 and IL-13 are associated with increased lung fungal burden, pulmonary eosinophilia, and overall increased sensitivity to C. neoformans infection [42,47,112,115,136,140,149]. In addition, the absence of IL-13 correlated with an increase in production of IFNγ and IL-17, cytokines known to be protective against C. neoformans infection [112]. Confirming observations in mice, high IL-13 levels in the CSF are associated with increased mortality in HIV-infected patients with CM [141]. IL-5 has not yet been associated with human C. neoformans infection.
Cytokines Associated with IRIS
Immune reconstitution inflammatory syndrome (IRIS) is characterized by pathological excessive inflammatory responses that result from a rapid recovery of immune responses in HIV-CM patients after antiretroviral therapy (ART) initiation [150]. Two different types of cryptococcal IRIS have been recognized; “paradoxical” and “unmasking” IRIS. Paradoxical IRIS presents as a worsening of disease or recurrent disease despite microbiological evidence of effective antifungal treatment (negative cultures) [151,152,153]. Unmasking IRIS is characterized by the development of Cryptococcus disease after ART initiation emerging from previous asymptomatic sub-clinical infection during immune reconstitution [150,154,155]. Various cytokines have been associated with cryptococcal IRIS. The absence/reduction of serum pro-inflammatory cytokines such as TNFα, G-CSF, GM-CSF and VEGF (vascular-endothelial growth factor), and increase in serum IL-17 and IL-4, predispose AIDS patient with CM to developing subsequent CM-IRIS [138]. Increased risk of CM-IRIS is also associated with low CSF inflammation at the time of diagnosis [130]. However, at the time of CM-IRIS, there are significant increases in CSF levels of IFNγ, TNFα, G-CSF, VEGF and eotaxin compared to baseline levels within AIDS patients [130]. These observations demonstrate that the types of sample (serum vs. CSF) and the time of analysis (diagnosis, during or after treatment) play an important role in relating cytokine responses to either a protective or non-protective role during Cryptococcus infection.
4.3. Cytokines/Chemokines with Varying/Conflicting Roles
Cytokines/chemokines, such as IL-4, IL-8, IL-10, IL-1β, MCP-1 (monocyte chemoattract protein 1), MIP-1α (macrophage inflammatory protein form 1 alpha) and RANTES (CCL5) have been associated with both protection and disease exacerbation during Cryptococcus infection.
4.3.1. Cytokines/Chemokines with Beneficial Role in Mice, but Detrimental in Humans
Decreased levels of MCP-1 in the lungs of infected mice correlated with impaired macrophages and CD4 T cell recruitment, reduced TNFα and IL-6 production, and inhibition of fungal clearance [147], and early expression of MCP-1 is associated with protection against C. neoformans infection [136,148]. Similarly, mice defective in MIP-1α production showed eosinophilic pneumonia, high levels of Th2 cytokines (IL-4, IL-13), and a reduction in protective cytokines IFNγ and IL-12 [40,136,148]. In contrast, human studies show that high MIP-1α and MCP-1 are associated with less peripheral CD4, lower CSF lymphocytes number, high risk for developing IRIS and increased mortality within AIDS patients with CM [131,141]. Observations from these studies suggest that MCP-1 and MIP-1α play antagonistic roles in inducing a protective immune response against C. neoformans infection in humans and mice. An alternative explanation could be that these chemokines play different roles at different sites of infection. This alternative explanation is supported by the fact that cytokines and chemokines were measured in CSF and/or serum in humans [131,141], but in lung and brain tissues in infected mice [40,136,147,148].
4.3.2. Cytokines with Contradictory Roles in Both Mouse and Human C. neoformans Infections
Three cytokines IL-4, IL-10 and IL-17 have been reported to be protective against C. neoformans infection, detrimental or not having any effect on the course of disease progression. Increased levels of IL-4 are associated with slower clearance of C. neoformans cells and increased death of infected mice [115,144], and IL-4 was absent in the brain of immune (protected) mice [136]. Similarly, high levels of serum IL-4 correlated with development of IRIS and subsequent death in AIDS-CM patients from Brazil and Uganda [124,138]. In contrast, high CSF IL-4 levels have been associated with a protective immune response in AIDS-CM patients from South Africa [131]. In addition to this, several other studies reported that IL-4 cytokine has no effect on C. neoformans disease whether in mice [42,117] or humans [145]. Similar observations have been made for IL-10. IL-10 has been associated with (a) enhanced disease in both mice [100,117,136] and humans [124,142], (b) protection against C. neoformans disease in humans [129,131], and (c) having no effect on C. neoformans disease in human patients [146]. Reduced IL-17 levels correlated with increased susceptibility in mice [112], and high IL-17 levels in the CSF of AIDS patients with CM correlated with better fungal clearance and improved clinical outcome [124,131]. In addition, IL-17 activates anticryptococcal macrophage functions [45]. In contrast, high serum IL-17 levels, in the absence of pro-inflammatory cytokines, predispose AIDS patients with CM to subsequent IRIS and death [138]. These observations suggest that for IL-4, IL-10 and IL-17, the type of host (human or mouse) and/or site of infection (lungs, blood, CSF) are important in determining whether these cytokines play a protective or non-protective role during Cryptococcus infection. In addition, the above human studies were done in different countries and continents, suggesting that different patient populations respond differently to C. neoformans infections.
4.3.3. Cytokines with Contradictory Roles in Only One System
IL-8 and RANTES have been associated with Cryptococcus disease only in human patients and infected mice respectively. High CSF IL-8 levels are associated with increased survival among AIDS patients with CM in several studies [124,128,129]; however, similar plasma IL-8 levels were observed between AIDS patients with CM and control individuals in another study [146]. Similarly, increased expression of RANTES in the brain is associated with protection against CM in mice [136], but high levels of RANTES in the lungs correlated with increased Th2 immune responses and enhanced disease in another study [47]. These observations again suggest a differential role of cytokines/chemokines at different sites during Cryptococcus infection.
5. Current Model of the Adaptive Immune Response to Cryptococcus Infection
A summary of our current understanding of the initiation, development, and function of adaptive immunity during Cryptococcus infection is presented in Figure 1. The model is based on information derived from C. neoformans experiments in mice (Figure 1A) as many aspects of the model are yet to be explored in humans (Figure 1B). Following infection, innate immune cells, mainly macrophages and DCs, recognize and phagocytose C. neoformans cells. Cryptococcal antigens are processed and presented to naïve T cells by antigen presenting cells (APCs). Naïve T cells will then differentiate into mature helper T cells. The type of cryptococcal antigens, co-stimulatory molecules on antigen presenting cells and presence of cytokines produced by innate cells determine whether naïve T cells differentiate into Th1, Th2 or Th17 cells. The presence of IFNγ and IL-12 induces the differentiation of naïve CD4 T cells into Th1 cells, while the presence of IL-4 and expression of costimulatory molecules CD86 and OX40L on APCs induce the formation of Th2 cells. In the absence of IFNγ and IL-4 cytokines, IL-23 induces the formation of Th17 cells, and these IL-17 producing T cells typically enhance protection in a healthy host. However, the Th17 cells exacerbate cryptococcal disease when both CD4 and CD8 T cells are lacking, such as in individuals with HIV/AIDS. IL-17 cytokine has also been associated with increased risk of developing CM-IRIS in HIV infected patients. Cytokines produced by Th1 and Th2 cells can in turn activate and enhance macrophage function. The presence of IFNγ induces the development of classically activated macrophages (M1), while IL-4 and IL-13 direct macrophage polarization into alternatively activated macrophages (M2) [112,114,137,156]. M1 macrophages are associated with protection against C. neoformans infection, whereas M2 macrophages enhance disease by increasing intracellular cryptococcal proliferation. In addition to Th cells, increased Tregs in the lungs of infected mice promote protection against C. neoformans infection by blocking detrimental Th2 immune responses [63,64,65].
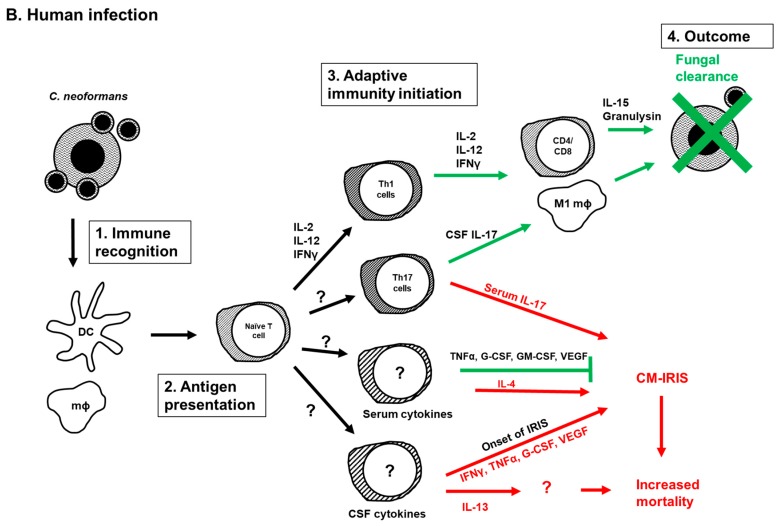
Figure 1.
Adaptive immunity during Cryptococcus infection. Current understanding of initiation, development and function of adaptive immunity during C. neoformans infection in the mouse model (A) or in humans (B). (1) C. neoformans pathogen-associated molecule patterns (PAMPS) are recognized by innate immune cells (macrophages and DCs). This recognition triggers phagocytosis, antigen processing and presentation of C. neoformans antigens to naïve T cells by antigen presenting cells. (2) Antigen presentation induces activation and differentiation of naïve T cells into Th1, Th2, Tregs and Th17 cells. (3) The presence of IFNγ and IL-12 promotes Th1 differentiation, while the presence of IL-4, as well as expression of costimulatory molecules CD86 and OX40L induces Th2 differentiation. IL-23 induces Th17 differentiation in the absence of IFNγ and IL-4. (4) Th1 cells promote cryptococcal killing either by direct contact, or by producing the Th1 cytokines IFNγ, IL-12 and IL-2 that stimulate phagocyte recruitment and polarization to classically activated macrophages that eliminate C. neoformans cells. In contrast, Th2 immune responses mediated by IL-4, IL-5 and IL-13 result in increased eosinophilia and polarization of alternatively activated macrophages, and ultimately lead to the dissemination of C. neoformans cells and disease exacerbation. These Th2 immune responses can be blocked by the action of Tregs. In the absence of Th cells, IL-17 production intensifies cryptococcal disease through neutrophilia. Green arrows denote beneficial immune responses, whereas red arrows denote detrimental immune responses. CM (Cryptococcus meningitis), DCs (dendritic cells), IFNγ (interferon gamma), IL- (interleukin, IL-12: interleukin-12), IRF4 (IFN regulatory factor 4), IRIS (immune reconstitution inflammatory syndrome), Th (helper T cell), Tregs (regulatory T cells), Ø (macrophage), M1Ø (classically activated macrophage), M2Ø (alternatively activated macrophage), CD4 (helper T cell), CD8 (cytotoxic T cell), CD86 (costimulatory molecule 86), OX40L (costimulatory molecule OX40L, ligand of OX40 receptor on T cells).
6. Concluding Remarks
Current understanding of adaptive immunity against Cryptococcus mainly comes from studies that used mouse models of disease. The availability of a wide range of genetically defined (knockout and transgenic) mouse strains makes mice an invaluable tool to study different aspects of the interaction between the host immune system and the pathogen. However, observations in mice do not always translate to human infection. For example, Th2 immune responses correlate with increased C. neoformans disease in mice [47,112,136,140,157], but various studies in humans do not associate a Th2 immune response to enhanced disease [131,145,158]. One reason for this difference could be the different types of samples collected in humans (blood and CSF) and mice (predominantly lungs). In addition, we do not know the protective immune response in healthy humans because the only human studies to date focus on patients with C. neoformans infection.
Human studies focused on infection of the central nervous system because the majority of patients present to hospitals with CM. However, the recognition and immune responses to C. neoformans in human lungs is not known, although the lungs are the initial site of cryptococcal infection. Studies in mice show that change in C. neoformans morphologies such as titan cell formation in the lungs of infected mice affect C. neoformans virulence [159,160,161,162,163]. Specifically, increases in cell wall chitin content are associated with detrimental Th2 immune responses in the lungs that worsen cryptococcal disease [47]. It is not known whether changes in cell surface components affect human immune responses to Cryptococcus. Extensive work has been done in understanding the role and origin of various cytokines in anti-cryptococcal immunity in the mouse model of infection. However, the role and origin (immune cell subsets) of cytokines during human Cryptococcus infection are not well known. Although various cytokines have been associated with either improved patient survival or worsening of disease, their specific role in immunity against Cryptococcus is not well understood. It is also not known whether these cytokines are produced by innate or adaptive immune cells. Additionally, different subsets of immune cells play different roles during Cryptococcus infection in mice (Figure 1A). However, it is not known whether similar mechanisms occur in human patients. Further studies are needed to identify mechanisms underlying protective immune responses in humans and address unanswered questions.
It is important to note that the majority of immunological studies have focused on C. neoformans infections because they are the most prevalent. Yet recent studies suggest that C. gattii infections in both mouse models and humans do not behave the same as C. neoformans [143,164,165,166,167]. This is not surprising, based on the observation that C. gattii often causes disease in immunocompetent individuals. Thus, additional studies on immune responses during C. gattii infection are desperately needed to better understand the similarities and differences between the Cryptococcus species and how they cause diseases.
Acknowledgments
The writing of this review was supported by National Institute of Health grants AI080275 and AI122352 to Kirsten Nielsen.
Author Contributions
Liliane Mukaremera and Kirsten Nielsen wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hagen F., Khayhan K., Theelen B., Kolecka A., Polacheck I., Sionov E., Falk R., Parnmen S., Lumbsch H.T., Boekhout T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015;78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Park B.J., Wannemuehler K.A., Marston B.J., Govender N., Pappas P.G., Chiller T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 3.Pappas P.G., Perfect J.R., Cloud G.A., Larsen R.A., Pankey G.A., Lancaster D.J., Henderson H., Kauffman C.A., Haas D.W., Saccente M., et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin. Infect. Dis. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 4.Pyrgos V., Seitz A.E., Steiner C.A., Prevots D.R., Williamson P.R. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS ONE. 2013;8:e56269. doi: 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marr K.A., Datta K., Pirofski L.A., Barnes R. Cryptococcus gattii infection in healthy hosts: A sentinel for subclinical immunodeficiency? Clin. Infect. Dis. 2012;54:153–154. doi: 10.1093/cid/cir756. [DOI] [PubMed] [Google Scholar]
- 6.Galanis E., Hoang L., Kibsey P., Morshed M., Phillips P. Clinical presentation, diagnosis and management of Cryptococcus gattii cases: Lessons learned from British Columbia. Can. J. Infect. Dis. Med. Microbiol. 2009;20:23–28. doi: 10.1155/2009/719659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoang L.M., Maguire J.A., Doyle P., Fyfe M., Roscoe D.L. Cryptococcus neoformans infections at Vancouver Hospital and Health Sciences Centre (1997–2002): Epidemiology, microbiology and histopathology. J. Med. Microbiol. 2004;53:935–940. doi: 10.1099/jmm.0.05427-0. [DOI] [PubMed] [Google Scholar]
- 8.Kidd S.E., Hagen F., Tscharke R.L., Huynh M., Bartlett K.H., Fyfe M., MacDougall L., Boekhout T., Kwon-Chung K.J., Meyer W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc. Natl. Acad. Sci. USA. 2004;101:17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips P., Galanis E., MacDougall L., Chong M.Y., Balshaw R., Cook V.J., Bowie W., Steiner T., Hoang L., Morshed M., et al. Longitudinal clinical findings and outcome among patients with Cryptococcus gattii infection in British Columbia. Clin. Infect. Dis. 2015;60:1368–1376. doi: 10.1093/cid/civ041. [DOI] [PubMed] [Google Scholar]
- 10.Bennett J.E., Kwon-Chung K.J., Howard D.H. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am. J. Epidemiol. 1977;105:582–586. doi: 10.1093/oxfordjournals.aje.a112423. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa M.M., Lazera M.S., Barbosa G.G., Trilles L., Balassiano B.R., Macedo R.C., Bezerra C.C., Perez M.A., Cardarelli P., Wanke B. Serotyping of 467 Cryptococcus neoformans isolates from clinical and environmental sources in Brazil: Analysis of host and regional patterns. J. Clin. Microbiol. 2003;41:73–77. doi: 10.1128/JCM.41.1.73-77.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luster A.D. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 2002;14:129–135. doi: 10.1016/S0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 13.Svanborg C., Godaly G., Hedlund M. Cytokine responses during mucosal infections: Role in disease pathogenesis and host defence. Curr. Opin. Microbiol. 1999;2:99–105. doi: 10.1016/S1369-5274(99)80017-4. [DOI] [PubMed] [Google Scholar]
- 14.Wozniak K.L., Vyas J.M., Levitz S.M. In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infect. Immun. 2006;74:3817–3824. doi: 10.1128/IAI.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vecchiarelli A., Dottorini M., Pietrella D., Monari C., Retini C., Todisco T., Bistoni F. Role of human alveolar macrophages as antigen-presenting cells in Cryptococcus neoformans infection. Am. J. Respir. Cell Mol. Biol. 1994;11:130–137. doi: 10.1165/ajrcmb.11.2.8049074. [DOI] [PubMed] [Google Scholar]
- 16.Osterholzer J.J., Milam J.E., Chen G.H., Toews G.B., Huffnagle G.B., Olszewski M.A. Role of dendritic cells and alveolar macrophages in regulating early host defense against pulmonary infection with Cryptococcus Neoformans. Infect. Immun. 2009;77:3749–3758. doi: 10.1128/IAI.00454-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guillot L., Carroll S.F., Badawy M., Qureshi S.T. Cryptococcus neoformans induces IL-8 secretion and CXCL1 expression by human bronchial epithelial cells. Respir. Res. 2008;9:9. doi: 10.1186/1465-9921-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roseff S.A., Levitz S.M. Effect of endothelial cells on phagocyte-mediated anticryptococcal activity. Infect. Immun. 1993;61:3818–3824. doi: 10.1128/iai.61.9.3818-3824.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schon-Hegrad M.A., Oliver J., McMenamin P.G., Holt P.G. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J. Exp. Med. 1991;173:1345–1356. doi: 10.1084/jem.173.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trombetta E.S., Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 21.Upham J.W. The role of dendritic cells in immune regulation and allergic airway inflammation. Respirology. 2003;8:140–148. doi: 10.1046/j.1440-1843.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- 22.Jung S., Unutmaz D., Wong P., Sano G., de los Santos K., Sparwasser T., Wu S., Vuthoori S., Ko K., Zavala F., et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/S1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger A. Th1 and Th2 responses: What are they? Br. Med. J. 2000;321:424. doi: 10.1136/bmj.321.7258.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guglani L., Khader S.A. Th17 cytokines in mucosal immunity and inflammation. Curr. Opin. HIV AIDS. 2010;5:120–127. doi: 10.1097/COH.0b013e328335c2f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Becker G., Moulin V., Tielemans F., de Mattia F., Urbain J., Leo O., Moser M. Regulation of T helper cell differentiation in vivo by soluble and membrane proteins provided by antigen-presenting cells. Eur. J. Immunol. 1998;28:3161–3171. doi: 10.1002/(SICI)1521-4141(199810)28:10<3161::AID-IMMU3161>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Vieira P.L., de Jong E.C., Wierenga E.A., Kapsenberg M.L., Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 28.Ranger A.M., Das M.P., Kuchroo V.K., Glimcher L.H. B7-2 (CD86) is essential for the development of IL-4-producing T cells. Int. Immunol. 1996;8:1549–1560. doi: 10.1093/intimm/8.10.1549. [DOI] [PubMed] [Google Scholar]
- 29.Ohshima Y., Yang L.P., Uchiyama T., Tanaka Y., Baum P., Sergerie M., Hermann P., Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- 30.Lambrecht B.N., de Veerman M., Coyle A.J., Gutierrez-Ramos J.-C., Thielemans K., Pauwels R.A. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Investig. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haczku A., Takeda K., Redai I., Hamelmann E., Cieslewicz G., Joetham A., Loader J., Lee J.J., Irvin C., Gelfand E.W. Anti-CD86 (B7.2) treatment abolishes allergic airway hyperresponsiveness in mice. Am. J. Respir. Crit. Care Med. 1999;159:1638–1643. doi: 10.1164/ajrccm.159.5.9711040. [DOI] [PubMed] [Google Scholar]
- 32.Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 34.Zelante T., de Luca A., D’Angelo C., Moretti S., Romani L. IL-17/Th17 in anti-fungal immunity: What’s new? Eur. J. Immunol. 2009;39:645–648. doi: 10.1002/eji.200839102. [DOI] [PubMed] [Google Scholar]
- 35.Huang W., Na L., Fidel P.L., Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 36.Van de Veerdonk F.L., Kullberg B.J., Verschueren I.C., Hendriks T., van der Meer J.W., Joosten L.A., Netea M.G. Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock. 2010;34:407–411. doi: 10.1097/SHK.0b013e3181d67041. [DOI] [PubMed] [Google Scholar]
- 37.Hernández-Santos N., Gaffen S.L. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11:425–435. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delsing C.E., Becker K.L., Simon A., Kullberg B.J., Bleeker-Rovers C.P., van de Veerdonk F.L., Netea M.G. Th17 cytokine deficiency in patients with Aspergillus skull base osteomyelitis. BMC Infect. Dis. 2015;15:140. doi: 10.1186/s12879-015-0891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiesner D.L., Smith K.D., Kashem S.W., Bohjanen P.R., Nielsen K. Different Lymphocyte Populations Direct Dichotomous Eosinophil or Neutrophil Responses to Pulmonary Cryptococcus Infection. J. Immunol. 2017;198:1627–1637. doi: 10.4049/jimmunol.1600821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoag K.A., Lipscomb M.F., Izzo A.A., Street N.E. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am. J. Respir. Cell Mol. Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 41.Herring A.C., Lee J., McDonald R.A., Toews G.B., Huffnagle G.B. Induction of interleukin-12 and γ interferon requires tumor necrosis factor α for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect. Immun. 2002;70:2959–2964. doi: 10.1128/IAI.70.6.2959-2964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoag K.A., Street N.E., Huffnagle G.B., Lipscomb M.F. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 1995;13:487–495. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 43.Jarvis J.N., Meintjes G., Rebe K., Williams G.N., Bicanic T., Williams A., Schutz C., Bekker L.G., Wood R., Harrison T.S. Adjunctive interferon-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis: A randomized controlled trial. Aids. 2012;26:1105–1113. doi: 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decken K., Kohler G., Palmer-Lehmann K., Wunderlin A., Mattner F., Magram J., Gately M.K., Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voelz K., Lammas D.A., May R.C. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect. Immun. 2009;77:3450–3457. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y., Wang F., Tompkins K.C., McNamara A., Jain A.V., Moore B.B., Toews G.B., Huffnagle G.B., Olszewski M.A. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am. J. Pathol. 2009;175:2489–2500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiesner D.L., Specht C.A., Lee C.K., Smith K.D., Mukaremera L., Lee S.T., Lee C.G., Elias J.A., Nielsen J.N., Boulware D.R., et al. Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection. PLoS Pathog. 2015;11:e1004701. doi: 10.1371/journal.ppat.1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murdock B.J., Huffnagle G.B., Olszewski M.A., Osterholzer J.J. Interleukin-17A Enhances Host Defense against Cryptococcal Lung Infection through Effects Mediated by Leukocyte Recruitment, Activation, and γ Interferon Production. Infect. Immun. 2014;82:937–948. doi: 10.1128/IAI.01477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauman S.K., Nichols K.L., Murphy J.W. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J. Immunol. 2000;165:158–167. doi: 10.4049/jimmunol.165.1.158. [DOI] [PubMed] [Google Scholar]
- 50.Lee S.J., Choi H.K., Son J., Kim K.H., Lee S.H. Cryptococcal Meningitis in Patients with or without Human Immunodeficiency Virus: Experience in a Tertiary Hospital. Yonsei Med. J. 2011;52:482–487. doi: 10.3349/ymj.2011.52.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kofteridis D.P., Saridaki Z., Kazakou I., Lazaridou S., Alegakis D., Milaki G., Gikas A. Idiopathic CD4+ T lymphocytopenia disclosed by recurrent cryptococcal meningitis. First case report from Greece. Int. J. Infect. Dis. 2005;9:347–348. doi: 10.1016/j.ijid.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Sharma A., Lal V., Modi M., Khurana D., Bal S., Prabhakar S. Idiopathic CD4 lymphocytopenia presenting as refractory cryptococcal meningitis. Ann. Indian Acad. Neurol. 2010;13:136–138. doi: 10.4103/0972-2327.64646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jo E.K., Kim H.S., Lee M.Y., Iseki M., Lee J.H., Song C.H., Park J.K., Hwang T.J., Kook H. X-linked hyper-IgM syndrome associated with Cryptosporidium parvum and Cryptococcus neoformans infections: The first case with molecular diagnosis in Korea. J. Korean Med. Sci. 2002;17:116–120. doi: 10.3346/jkms.2002.17.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winkelstein J.A., Marino M.C., Ochs H., Fuleihan R., Scholl P.R., Geha R., Stiehm E.R., Conley M.E. The X-linked hyper-IgM syndrome: Clinical and immunologic features of 79 patients. Medicine. 2003;82:373–384. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 55.Jain A., Atkinson T.P., Lipsky P.E., Slater J.E., Nelson D.L., Strober W. Defects of T-cell effector function and post-thymic maturation in X-linked hyper-IgM syndrome. J. Clin. Investig. 1999;103:1151–1158. doi: 10.1172/JCI5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma L.L., Spurrell J.C., Wang J.F., Neely G.G., Epelman S., Krensky A.M., Mody C.H. CD8 T cell-mediated killing of Cryptococcus neoformans requires granulysin and is dependent on CD4 T cells and IL-15. J. Immunol. 2002;169:5787–5795. doi: 10.4049/jimmunol.169.10.5787. [DOI] [PubMed] [Google Scholar]
- 57.Zheng C.F., Ma L.L., Jones G.J., Gill M.J., Krensky A.M., Kubes P., Mody C.H. Cytotoxic CD4+ T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood. 2007;109:2049–2057. doi: 10.1182/blood-2006-03-009720. [DOI] [PubMed] [Google Scholar]
- 58.Lindell D.M., Moore T.A., McDonald R.A., Toews G.B., Huffnagle G.B. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. J. Immunol. 2005;174:7920–7928. doi: 10.4049/jimmunol.174.12.7920. [DOI] [PubMed] [Google Scholar]
- 59.Lindell D.M., Ballinger M.N., McDonald R.A., Toews G.B., Huffnagle G.B. Diversity of the T-Cell Response to Pulmonary Cryptococcus neoformans Infection. Infect. Immun. 2006;74:4538–4548. doi: 10.1128/IAI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syme R.M., Wood C.J., Wong H., Mody C.H. Both CD4+ and CD8+ human lymphocytes are activated and proliferate in response to Cryptococcus neoformans. Immunology. 1997;92:194–200. doi: 10.1046/j.1365-2567.1997.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huffnagle G.B., Lipscomb M.F., Lovchik J.A., Hoag K.A., Street N.E. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 62.Lindell D.M., Moore T.A., McDonald R.A., Toews G.B., Huffnagle G.B. Distinct Compartmentalization of CD4(+) T-Cell Effector Function Versus Proliferative Capacity during Pulmonary Cryptococcosis. Am. J. Pathol. 2006;168:847–855. doi: 10.2353/ajpath.2006.050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schulze B., Piehler D., Eschke M., von Buttlar H., Kohler G., Sparwasser T., Alber G. CD4(+) FoxP3(+) regulatory T cells suppress fatal T helper 2 cell immunity during pulmonary fungal infection. Eur. J. Immunol. 2014;44:3596–3604. doi: 10.1002/eji.201444963. [DOI] [PubMed] [Google Scholar]
- 64.Schulze B., Piehler D., Eschke M., Heyen L., Protschka M., Kohler G., Alber G. Therapeutic expansion of CD4+FoxP3+ regulatory T cells limits allergic airway inflammation during pulmonary fungal infection. Pathog. Dis. 2016;74:ftw020. doi: 10.1093/femspd/ftw020. [DOI] [PubMed] [Google Scholar]
- 65.Wiesner D.L., Smith K.D., Kotov D.I., Nielsen J.N., Bohjanen P.R., Nielsen K. Regulatory T Cell Induction and Retention in the Lungs Drives Suppression of Detrimental Type 2 Th Cells During Pulmonary Cryptococcal Infection. J. Immunol. 2016;196:365–374. doi: 10.4049/jimmunol.1501871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawakami K., Kinjo Y., Uezu K., Yara S., Miyagi K., Koguchi Y., Nakayama T., Taniguchi M., Saito A. Monocyte Chemoattractant Protein-1-Dependent Increase of Vα14 NKT Cells in Lungs and Their Roles in Th1 Response and Host Defense in Cryptococcal Infection. J. Immunol. 2001;167:6525–6532. doi: 10.4049/jimmunol.167.11.6525. [DOI] [PubMed] [Google Scholar]
- 67.Kawakami K., Kinjo Y., Yara S., Koguchi Y., Uezu K., Nakayama T., Taniguchi M., Saito A. Activation of Vα14(+) Natural Killer T Cells by α-Galactosylceramide Results in Development of Th1 Response and Local Host Resistance in Mice Infected with Cryptococcus neoformans. Infect. Immun. 2001;69:213–220. doi: 10.1128/IAI.69.1.213-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aoyagi T., Nakamura K., Miyazato A., Nakagawa K., Miyazawa T., Kinjyo Y., Kronenberg M., Kaku M., Kawakami K. CD1d-dependent activation of NKT cells by Cryptococcus neoformans-pulsed dendritic cells (B172) J. Immunol. 2007;178(Suppl. 1):LB36. [Google Scholar]
- 69.Blackstock R., Murphy J.W. Age-related resistance of C57BL/6 mice to Cryptococcus neoformans is dependent on maturation of NKT cells. Infect. Immun. 2004;72:5175–5180. doi: 10.1128/IAI.72.9.5175-5180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto H., Nakamura Y., Sato K., Takahashi Y., Nomura T., Miyasaka T., Ishii K., Hara H., Yamamoto N., Kanno E., et al. Defect of CARD9 leads to impaired accumulation of γ interferon-producing memory phenotype T cells in lungs and increased susceptibility to pulmonary infection with Cryptococcus neoformans. Infect. Immun. 2014;82:1606–1615. doi: 10.1128/IAI.01089-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wozniak K.L., Kolls J.K., Wormley F.L. Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by γ/δ T cells. BMC Immunol. 2012;13:65. doi: 10.1186/1471-2172-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uezu K., Kawakami K., Miyagi K., Kinjo Y., Kinjo T., Ishikawa H., Saito A. Accumulation of γδ T Cells in the Lungs and Their Regulatory Roles in Th1 Response and Host Defense against Pulmonary Infection with Cryptococcus neoformans. J. Immunol. 2004;172:7629–7634. doi: 10.4049/jimmunol.172.12.7629. [DOI] [PubMed] [Google Scholar]
- 73.Mody C.H., Sims K.L., Wood C.J., Syme R.M., Spurrell J.C., Sexton M.M. Proteins in the cell wall and membrane of Cryptococcus neoformans stimulate lymphocytes from both adults and fetal cord blood to proliferate. Infect. Immun. 1996;64:4811–4819. doi: 10.1128/iai.64.11.4811-4819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mody C.H., Wood C.J., Syme R.M., Spurrell J.C. The cell wall and membrane of Cryptococcus neoformans possess a mitogen for human T lymphocytes. Infect. Immun. 1999;67:936–941. doi: 10.1128/iai.67.2.936-941.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Syme R.M., Spurrell J.C., Ma L.L., Green F.H., Mody C.H. Phagocytosis and protein processing are required for presentation of Cryptococcus neoformans mitogen to T lymphocytes. Infect. Immun. 2000;68:6147–6153. doi: 10.1128/IAI.68.11.6147-6153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huffnagle G.B., Yates J.L., Lipscomb M.F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levitz S.M., Dupont M.P. Phenotypic and functional characterization of human lymphocytes activated by interleukin-2 to directly inhibit growth of Cryptococcus neoformans in vitro. J. Clin. Investig. 1993;91:1490–1498. doi: 10.1172/JCI116354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murphy J.W., Hidore M.R., Wong S.C. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J. Clin. Investig. 1993;91:1553–1566. doi: 10.1172/JCI116361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Workman C.J., Szymczak-Workman A.L., Collison L.W., Pillai M.R., Vignali D.A.A. The Development and Function of Regulatory T Cells. Cell. Mol. Life Sci. CMLS. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corthay A. How do Regulatory T Cells Work? Scand. J. Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kroetz D.N., Deepe G.S., Jr. CCR5 dictates the equilibrium of proinflammatory IL-17+ and regulatory Foxp3+ T cells in fungal infection. J. Immunol. 2010;184:5224–5231. doi: 10.4049/jimmunol.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Netea M.G., Sutmuller R., Hermann C., van der Graaf C.A., van der Meer J.W., van Krieken J.H., Hartung T., Adema G., Kullberg B.J. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 83.Pandiyan P., Conti H.R., Zheng L., Peterson A.C., Mathern D.R., Hernández-Santos N., Edgerton M., Gaffen S.L., Lenardo M.J. CD4(+) CD25(+) Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McKinley L., Logar A.J., McAllister F., Zheng M., Steele C., Kolls J.K. Regulatory T Cells Dampen Pulmonary Inflammation and Lung Injury in an Animal Model of Pneumocystis Pneumonia. J. Immunol. 2006;177:6215–6226. doi: 10.4049/jimmunol.177.9.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen L.C., Goldman D.L., Doering T.L., Pirofski L., Casadevall A. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect. Immun. 1999;67:2218–2224. doi: 10.1128/iai.67.5.2218-2224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deshaw M., Pirofski L.A. Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV− individuals. Clin. Exp. Immunol. 1995;99:425–432. doi: 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldman D.L., Khine H., Abadi J., Lindenberg D.J., Pirofski L., Niang R., Casadevall A. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107:E66. doi: 10.1542/peds.107.5.e66. [DOI] [PubMed] [Google Scholar]
- 88.Subramaniam K., Metzger B., Hanau L.H., Guh A., Rucker L., Badri S., Pirofski L.-A. IgM(+) Memory B Cell Expression Predicts HIV-Associated Cryptococcosis Status. J. Infect. Dis. 2009;200:244–251. doi: 10.1086/599318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta S., Ellis M., Cesario T., Ruhling M., Vayuvegula B. Disseminated cryptococcal infection in a patient with hypogammaglobulinemia and normal T cell functions. Am. J. Med. 1987;82:129–131. doi: 10.1016/0002-9343(87)90388-3. [DOI] [PubMed] [Google Scholar]
- 90.De Gorgolas M., Erice A., Gil A., Gutierrez J., Rivas P., Hernando C., Rodriguez M.C. Cryptococcal meningitis in a patient with X-linked hyper-IgM1 syndrome. Scand. J. Infect. Dis. 2005;37:526–528. doi: 10.1080/00365540510036570. [DOI] [PubMed] [Google Scholar]
- 91.Iseki M., Anzo M., Yamashita N., Matsuo N. Hyper-IgM immunodeficiency with disseminated cryptococcosis. Acta Paediatr. 1994;83:780–782. doi: 10.1111/j.1651-2227.1994.tb13140.x. [DOI] [PubMed] [Google Scholar]
- 92.Verma A., Wuthrich M., Deepe G., Klein B. Adaptive immunity to fungi. Cold Spring Harb. Perspect. Med. 2014;5:a019612. doi: 10.1101/cshperspect.a019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Notarangelo L.D., Hayward A.R. X-linked immunodeficiency with hyper-IgM (XHIM) Clin. Exp. Immunol. 2000;120:399–405. doi: 10.1046/j.1365-2249.2000.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wahab J.A., Hanifah M.J., Choo K.E. Bruton’s agammaglobulinaemia in a child presenting with cryptococcal empyema thoracis and periauricular pyogenic abscess. Singapore Med. J. 1995;36:686–689. [PubMed] [Google Scholar]
- 95.Guo L.-Y., Liu L.-L., Liu Y., Chen T.-M., Li S.-Y., Yang Y.-H., Liu G. Characteristics and outcomes of cryptococcal meningitis in HIV seronegative children in Beijing, China, 2002–2013. BMC Infect. Dis. 2016;16:635. doi: 10.1186/s12879-016-1964-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Browne S.K., Burbelo P.D., Chetchotisakd P., Suputtamongkol Y., Kiertiburanakul S., Shaw P.A., Kirk J.L., Jutivorakool K., Zaman R., Ding L., et al. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 2012;367:725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monga D.P., Kumar R., Mohapatra L.N., Malaviya A.N. Experimental cryptococcosis in normal and B-cell-deficient mice. Infect. Immun. 1979;26:1–3. doi: 10.1128/iai.26.1.1-3.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rivera J., Zaragoza O., Casadevall A. Antibody-mediated protection against Cryptococcus neoformans pulmonary infection is dependent on B cells. Infect. Immun. 2005;73:1141–1150. doi: 10.1128/IAI.73.2.1141-1150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szymczak W.A., Davis M.J., Lundy S.K., Dufaud C., Olszewski M., Pirofski L.A. X-linked immunodeficient mice exhibit enhanced susceptibility to Cryptococcus neoformans Infection. MBio. 2013;4 doi: 10.1128/mBio.00265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zaragoza O., Alvarez M., Telzak A., Rivera J., Casadevall A. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect. Immun. 2007;75:2729–2739. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nabavi N., Murphy J.W. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neoformans. Infect. Immun. 1986;51:556–562. doi: 10.1128/iai.51.2.556-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aguirre K.M., Johnson L.L. A role for B cells in resistance to Cryptococcus neoformans in mice. Infect. Immun. 1997;65:525–530. doi: 10.1128/iai.65.2.525-530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelly R.M., Chen J., Yauch L.E., Levitz S.M. Opsonic requirements for dendritic cell-mediated responses to Cryptococcus neoformans. Infect. Immun. 2005;73:592–598. doi: 10.1128/IAI.73.1.592-598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kozel T.R., Follette J.L. Opsonization of encapsulated Cryptococcus neoformans by specific anticapsular antibody. Infect. Immun. 1981;31:978–984. doi: 10.1128/iai.31.3.978-984.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Netski D., Kozel T.R. Fc-dependent and Fc-independent opsonization of Cryptococcus neoformans by anticapsular monoclonal antibodies: Importance of epitope specificity. Infect. Immun. 2002;70:2812–2819. doi: 10.1128/IAI.70.6.2812-2819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taborda C.P., Casadevall A. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity. 2002;16:791–802. doi: 10.1016/S1074-7613(02)00328-X. [DOI] [PubMed] [Google Scholar]
- 107.Vecchiarelli A., Pietrella D., Lupo P., Bistoni F., McFadden D.C., Casadevall A. The polysaccharide capsule of Cryptococcus neoformans interferes with human dendritic cell maturation and activation. J. Leukoc. Biol. 2003;74:370–378. doi: 10.1189/jlb.1002476. [DOI] [PubMed] [Google Scholar]
- 108.Mukherjee S., Feldmesser M., Casadevall A. J774 murine macrophage-like cell interactions with Cryptococcus neoformans in the presence and absence of opsonins. J. Infect. Dis. 1996;173:1222–1231. doi: 10.1093/infdis/173.5.1222. [DOI] [PubMed] [Google Scholar]
- 109.Mukherjee J., Scharff M.D., Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mukherjee S., Lee S.C., Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect. Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rachini A., Pietrella D., Lupo P., Torosantucci A., Chiani P., Bromuro C., Proietti C., Bistoni F., Cassone A., Vecchiarelli A. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect. Immun. 2007;75:5085–5094. doi: 10.1128/IAI.00278-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muller U., Stenzel W., Kohler G., Werner C., Polte T., Hansen G., Schutze N., Straubinger R.K., Blessing M., McKenzie A.N., et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 2007;179:5367–5377. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 113.Qiu Y., Dayrit J.K., Davis M.J., Carolan J.F., Osterholzer J.J., Curtis J.L., Olszewski M.A. Scavenger Receptor A Modulates the Immune Response to Pulmonary Cryptococcus neoformans Infection. J. Immunol. 2013;191:238–248. doi: 10.4049/jimmunol.1203435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arora S., Hernandez Y., Erb-Downward J.R., McDonald R.A., Toews G.B., Huffnagle G.B. Role of IFN- γ in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 2005;174:6346–6356. doi: 10.4049/jimmunol.174.10.6346. [DOI] [PubMed] [Google Scholar]
- 115.Lovchik J.A., Wilder J.A., Huffnagle G.B., Riblet R., Lyons C.R., Lipscomb M.F. Ig heavy chain complex-linked genes influence the immune response in a murine cryptococcal infection. J. Immunol. 1999;163:3907–3913. [PubMed] [Google Scholar]
- 116.Kawakami K., Tohyama M., Xie Q., Saito A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin. Exp. Immunol. 1996;104:208–214. doi: 10.1046/j.1365-2249.1996.14723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beenhouwer D.O., Shapiro S., Feldmesser M., Casadevall A., Scharff M.D. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect. Immun. 2001;69:6445–6455. doi: 10.1128/IAI.69.10.6445-6455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buchanan K.L., Doyle H.A. Requirement for CD4(+) T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infect. Immun. 2000;68:456–462. doi: 10.1128/IAI.68.2.456-462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou Q., Gault R.A., Kozel T.R., Murphy W.J. Immunomodulation with CD40 stimulation and interleukin-2 protects mice from disseminated cryptococcosis. Infect. Immun. 2006;74:2161–2168. doi: 10.1128/IAI.74.4.2161-2168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou Q., Gault R.A., Kozel T.R., Murphy W.J. Protection from Direct Cerebral Cryptococcus Infection by Interferon-γ-Dependent Activation of Microglial Cells. J. Immunol. 2007;178:5753–5761. doi: 10.4049/jimmunol.178.9.5753. [DOI] [PubMed] [Google Scholar]
- 121.Netea M.G., Brouwer A.E., Hoogendoorn E.H., van der Meer J.W., Koolen M., Verweij P.E., Kullberg B.J. Two patients with cryptococcal meningitis and idiopathic CD4 lymphopenia: Defective cytokine production and reversal by recombinant interferon-γ therapy. Clin. Infect. Dis. 2004;39:e83–e87. doi: 10.1086/425121. [DOI] [PubMed] [Google Scholar]
- 122.Pappas P.G., Bustamante B., Ticona E., Hamill R.J., Johnson P.C., Reboli A., Aberg J., Hasbun R., Hsu H.H. Recombinant interferon-γ 1b as adjunctive therapy for AIDS-related acute cryptococcal meningitis. J. Infect. Dis. 2004;189:2185–2191. doi: 10.1086/420829. [DOI] [PubMed] [Google Scholar]
- 123.Yilmaz-Demirdag Y., Wilson B., Lowery-Nordberg M., Bocchini J.A., Jr., Bahna S.L. Interleukin-2 treatment for persistent cryptococcal meningitis in a child with idiopathic CD4(+) T lymphocytopenia. Allergy Asthma Proc. 2008;29:421–424. doi: 10.2500/aap.2008.29.3143. [DOI] [PubMed] [Google Scholar]
- 124.Mora D.J., Fortunato L.R., Andrade-Silva L.E., Ferreira-Paim K., Rocha I.H., Vasconcelos R.R., Silva-Teixeira D.N., Nascentes G.A., Silva-Vergara M.L. Cytokine profiles at admission can be related to outcome in AIDS patients with cryptococcal meningitis. PLoS ONE. 2015;10:e0120297. doi: 10.1371/journal.pone.0120297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murphy K.M., Reiner S.L. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 126.Szabo S.J., Sullivan B.M., Peng S.L., Glimcher L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 127.Chang C.C., Lim A., Omarjee S., Levitz S.M., Gosnell B.I., Spelman T., Elliott J.H., Carr W.H., Moosa M.Y., Ndung’u T., et al. Cryptococcosis-IRIS is associated with lower Cryptococcus-specific IFN-γ responses before antiretroviral therapy but not higher T-cell responses during therapy. J. Infect. Dis. 2013;208:898–906. doi: 10.1093/infdis/jit271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siddiqui A.A., Brouwer A.E., Wuthiekanun V., Jaffar S., Shattock R., Irving D., Sheldon J., Chierakul W., Peacock S., Day N., et al. IFN-γ at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J. Immunol. 2005;174:1746–1750. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 129.Lortholary O., Dromer F., Mathoulin-Pelissier S., Fitting C., Improvisi L., Cavaillon J.M., Dupont B. Immune mediators in cerebrospinal fluid during cryptococcosis are influenced by meningeal involvement and human immunodeficiency virus serostatus. J. Infect. Dis. 2001;183:294–302. doi: 10.1086/317937. [DOI] [PubMed] [Google Scholar]
- 130.Boulware D.R., Bonham S.C., Meya D.B., Wiesner D.L., Park G.S., Kambugu A., Janoff E.N., Bohjanen P.R. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J. Infect. Dis. 2010;202:962–970. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jarvis J.N., Meintjes G., Bicanic T., Buffa V., Hogan L., Mo S., Tomlinson G., Kropf P., Noursadeghi M., Harrison T.S. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog. 2015;11:e1004754. doi: 10.1371/journal.ppat.1004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Blasi E., Barluzzi R., Mazzolla R., Pitzurra L., Puliti M., Saleppico S., Bistoni F. Biomolecular events involved in anticryptococcal resistance in the brain. Infect. Immun. 1995;63:1218–1222. doi: 10.1128/iai.63.4.1218-1222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kleinschek M.A., Muller U., Brodie S.J., Stenzel W., Kohler G., Blumenschein W.M., Straubinger R.K., McClanahan T., Kastelein R.A., Alber G. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J. Immunol. 2006;176:1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 134.Kawakami K., Qureshi M.H., Zhang T., Okamura H., Kurimoto M., Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-γ production. J. Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 135.Kawakami K., Koguchi Y., Qureshi M.H., Kinjo Y., Yara S., Miyazato A., Kurimoto M., Takeda K., Akira S., Saito A. Reduced host resistance and Th1 response to Cryptococcus neoformans in interleukin-18 deficient mice. FEMS Microbiol. Lett. 2000;186:121–126. doi: 10.1111/j.1574-6968.2000.tb09092.x. [DOI] [PubMed] [Google Scholar]
- 136.Uicker W.C., Doyle H.A., McCracken J.P., Langlois M., Buchanan K.L. Cytokine and chemokine expression in the central nervous system associated with protective cell-mediated immunity against Cryptococcus neoformans. Med. Mycol. 2005;43:27–38. doi: 10.1080/13693780410001731510. [DOI] [PubMed] [Google Scholar]
- 137.Arora S., Olszewski M.A., Tsang T.M., McDonald R.A., Toews G.B., Huffnagle G.B. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect. Immun. 2011;79:1915–1926. doi: 10.1128/IAI.01270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boulware D.R., Meya D.B., Bergemann T.L., Wiesner D.L., Rhein J., Musubire A., Lee S.J., Kambugu A., Janoff E.N., Bohjanen P.R. Clinical Features and Serum Biomarkers in HIV Immune Reconstitution Inflammatory Syndrome after Cryptococcal Meningitis: A Prospective Cohort Study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wozniak K.L., Hardison S.E., Kolls J.K., Wormley F.L. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS ONE. 2011;6:e17204. doi: 10.1371/journal.pone.0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huffnagle G.B., Boyd M.B., Street N.E., Lipscomb M.F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6) J. Immunol. 1998;160:2393–2400. [PubMed] [Google Scholar]
- 141.Scriven J.E., Rhein J., Hullsiek K.H., von Hohenberg M., Linder G., Rolfes M.A., Williams D.A., Taseera K., Meya D.B., Meintjes G., et al. Early ART After Cryptococcal Meningitis Is Associated With Cerebrospinal Fluid Pleocytosis and Macrophage Activation in a Multisite Randomized Trial. J. Infect. Dis. 2015;212:769–778. doi: 10.1093/infdis/jiv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chaka W., Heyderman R., Gangaidzo I., Robertson V., Mason P., Verhoef J., Verheul A., Hoepelman A.I. Cytokine profiles in cerebrospinal fluid of human immunodeficiency virus-infected patients with cryptococcal meningitis: No leukocytosis despite high interleukin-8 levels. University of Zimbabwe Meningitis Group. J. Infect. Dis. 1997;176:1633–1636. doi: 10.1086/517344. [DOI] [PubMed] [Google Scholar]
- 143.Schoffelen T., Illnait-Zaragozi M.-T., Joosten L.A.B., Netea M.G., Boekhout T., Meis J.F., Sprong T. Cryptococcus gattii Induces a Cytokine Pattern That Is Distinct from Other Cryptococcal Species. PLoS ONE. 2013;8:e55579. doi: 10.1371/journal.pone.0055579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abe K., Kadota J., Ishimatsu Y., Iwashita T., Tomono K., Kawakami K., Kohno S. Th1–Th2 cytokine kinetics in the bronchoalveolar lavage fluid of mice infected with Cryptococcus neoformans of different virulences. Microbiol. Immunol. 2000;44:849–855. doi: 10.1111/j.1348-0421.2000.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 145.Wang J.L., Li S.Y., Luo Y.F., Zhai Y.P., Wei X.Q. Changes of Th1/Th2 cytokines in immunocompetent patients with pulmonary cryptococcosis. Genet. Mol. Res. 2013;12:5733–5742. doi: 10.4238/2013.November.18.22. [DOI] [PubMed] [Google Scholar]
- 146.Lortholary O., Sitbon K., Dromer F. Evidence for human immunodeficiency virus and Cryptococcus neoformans interactions in the pro-inflammatory and anti-inflammatory responses in blood during AIDS-associated cryptococcosis. Clin. Microbiol. Infect. 2005;11:296–300. doi: 10.1111/j.1469-0691.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- 147.Huffnagle G.B., Strieter R.M., Standiford T.J., McDonald R.A., Burdick M.D., Kunkel S.L., Toews G.B. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- 148.Olszewski M.A., Huffnagle G.B., Traynor T.R., McDonald R.A., Cook D.N., Toews G.B. Regulatory effects of macrophage inflammatory protein 1α/CCL3 on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infect. Immun. 2001;69:6256–6263. doi: 10.1128/IAI.69.10.6256-6263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gyetko M.R., Sud S., Chen G.H., Fuller J.A., Chensue S.W., Toews G.B. Urokinase-type plasminogen activator is required for the generation of a type 1 immune response to pulmonary Cryptococcus neoformans infection. J. Immunol. 2002;168:801–809. doi: 10.4049/jimmunol.168.2.801. [DOI] [PubMed] [Google Scholar]
- 150.Haddow L.J., Colebunders R., Meintjes G., Lawn S.D., Elliott J.H., Manabe Y.C., Bohjanen P.R., Sungkanuparph S., Easterbrook P.J., French M.A., et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: Proposed clinical case definitions. Lancet Infect. Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lortholary O., Fontanet A., Memain N., Martin A., Sitbon K., Dromer F. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19:1043–1049. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 152.Shelburne S.A., Darcourt J., 3rd, White A.C., Jr., Greenberg S.B., Hamill R.J., Atmar R.L., Visnegarwala F. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 2005;40:1049–1052. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 153.Kambugu A., Meya D.B., Rhein J., O’Brien M., Janoff E.N., Ronald A.R., Kamya M.R., Mayanja-Kizza H., Sande M.A., Bohjanen P.R., et al. Outcome of cryptococcal meningitis in Uganda before and after availability of HAART. Clin. Infect. Dis. 2008;46:1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Murdoch D.M., Venter W.D., Feldman C., van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: A prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 155.Lawn S.D., Bekker L.G., Myer L., Orrell C., Wood R. Cryptococcocal immune reconstitution disease: A major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2052. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 156.Davis M.J., Tsang T.M., Qiu Y., Dayrit J.K., Freij J.B., Huffnagle G.B., Olszewski M.A. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4 doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koguchi Y., Kawakami K. Cryptococcal infection and Th1–Th2 cytokine balance. Int. Rev. Immunol. 2002;21:423–438. doi: 10.1080/08830180213274. [DOI] [PubMed] [Google Scholar]
- 158.Jarvis J.N., Casazza J., Stone H.H., Meintjes G., Lawn S.D., Levitz S.M., Harrison T.S., Koup R.A. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J. Infect. Dis. 2013;207:1817–1828. doi: 10.1093/infdis/jit099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Okagaki L.H., Strain A.K., Nielsen J.N., Charlier C., Baltes N.J., Chretien F., Heitman J., Dromer F., Nielsen K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010;6:e1000953. doi: 10.1371/annotation/1b59fd9e-9ac9-4ea8-a083-14c413c80b03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zaragoza O., Garcia-Rodas R., Nosanchuk J.D., Cuenca-Estrella M., Rodriguez-Tudela J.L., Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010;6:e1000945. doi: 10.1371/annotation/0675044c-d80f-456f-bb63-4f85fb1d0c33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Okagaki L.H., Nielsen K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot. Cell. 2012;11:820–826. doi: 10.1128/EC.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Crabtree J.N., Okagaki L.H., Wiesner D.L., Strain A.K., Nielsen J.N., Nielsen K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect. Immun. 2012;80:3776–3785. doi: 10.1128/IAI.00507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zaragoza O., Nielsen K. Titan cells in Cryptococcus neoformans: Cells with a giant impact. Curr. Opin. Microbiol. 2013;16:409–413. doi: 10.1016/j.mib.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Voelz K., Johnston S.A., Smith L.M., Hall R.A., Idnurm A., May R.C. ‘Division of labour’ in response to host oxidative burst drives a fatal Cryptococcus gattii outbreak. Nat. Commun. 2014;5:5194. doi: 10.1038/ncomms6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huston S.M., Li S.S., Stack D., Timm-McCann M., Jones G.J., Islam A., Berenger B.M., Xiang R.F., Colarusso P., Mody C.H. Cryptococcus gattii is killed by dendritic cells, but evades adaptive immunity by failing to induce dendritic cell maturation. J. Immunol. 2013;191:249–261. doi: 10.4049/jimmunol.1202707. [DOI] [PubMed] [Google Scholar]
- 166.Saijo T., Chen J., Chen S.C.A., Rosen L.B., Yi J., Sorrell T.C., Bennett J.E., Holland S.M., Browne S.K., Kwon-Chung K.J. Anti-Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies Are a Risk Factor for Central Nervous System Infection by Cryptococcus gattii in Otherwise Immunocompetent Patients. MBio. 2014;5 doi: 10.1128/mBio.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ngamskulrungroj P., Chang Y., Sionov E., Kwon-Chung K.J. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. MBio. 2012;3 doi: 10.1128/mBio.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]