Abstract
Morphological changes are a very common and effective strategy for pathogens to survive in the mammalian host. During interactions with their host, human pathogenic fungi undergo an array of morphological changes that are tightly associated with virulence. Candida albicans switches between yeast cells and hyphae during infection. Thermally dimorphic pathogens, such as Histoplasma capsulatum and Blastomyces species transform from hyphal growth to yeast cells in response to host stimuli. Coccidioides and Pneumocystis species produce spherules and cysts, respectively, which allow for the production of offspring in a protected environment. Finally, Cryptococcus species suppress hyphal growth and instead produce an array of yeast cells—from large polyploid titan cells to micro cells. While the morphology changes produced by human fungal pathogens are diverse, they all allow for the pathogens to evade, manipulate, and overcome host immune defenses to cause disease. In this review, we summarize the morphology changes in human fungal pathogens—focusing on morphological features, stimuli, and mechanisms of formation in the host.
Keywords: human fungal pathogen, morphology change, host-pathogen interaction, titan cell, spherules, hyphae
1. Introduction
Fungi are both ubiquitous and highly diversified. A “conservative” estimate for the number of fungal species is 1.5 million [1], with a recent estimate suggesting there may be over 5 million fungal species on earth [2]. About 100,000 species of fungi have been formally described [2]. Over 300 fungi have been shown to infect humans [3]. Most individuals in their lifetimes will contract superficial fungal infections. Fungal infections of the skin, hair, and nails are a common global problem. 20–25% of the world’s population have skin mycoses that are primarily caused by dermatophytes, or dermatophytosis [4]. Fungal infections of oral and genital tracts are also common. Mucocutaneous C. albicans infections are frequent in babies, immunocompromised individuals, diabetics, and obese individuals. In their childbearing years, 70–75% of women suffer from vulvovaginal candidiasis [5]. Invasive fungal infections have a much lower disease incidence than superficial infections, but invasive fungal infections cause unacceptably high mortality rates. About one and half million people die from invasive fungal infections annually [6], making them a significant global public health problem. While fungal diseases can affect healthy individuals, they pose a serious threat to immunocompromised individuals. Morphology switching is frequently associated with fungal pathogens and are tightly linked with virulence.
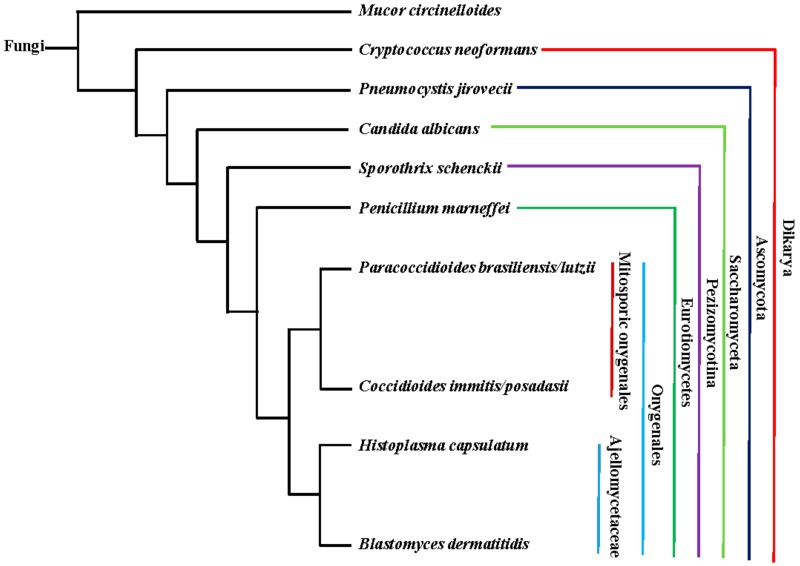
This review will discuss 12 common fungal pathogens that undergo morphology transitions upon interaction with the human host (Figure 1). The commensal fungus Candida albicans switches between yeast, pseudohyphae, and hyphae during infection and disease. Thermally dimorphic pathogens (Histoplasma capsulatum, Blastomyces spp., Talaromyces marneffei, Coccidioides spp., Paracoccidioides spp., Sporothrix schenckii, and others) transform from hyphal growth to yeast cells in response to elevated temperature. Mucor circinelloides changes from hyphae to multi-budded yeasts in anaerobic/high CO2 conditions. Coccidioides spp. and Pneumocystis spp. produce spherules and cysts, respectively, which allow for the production of offspring in a protected environment. Finally, Cryptococcus spp. suppress hyphal growth and instead produces an array of yeast cells—from large polyploid titan cells to small micro cells. These morphology changes dramatically influence the host-pathogen interaction and allow for these fungi to cause disease.
Figure 1.
Evolutionary relationship between common human fungal pathogens that exhibit morphology changes. The tree was generated using Common Tree from the National Center for Biotechnology Information. M. circinelloides belongs to “Fungi incertae sedis”. C. neoformans is in the phylum Basidiomycota. The rest are in the phylum Ascomycota.
2. C. albicans—A Commensal Fungal Pathogen
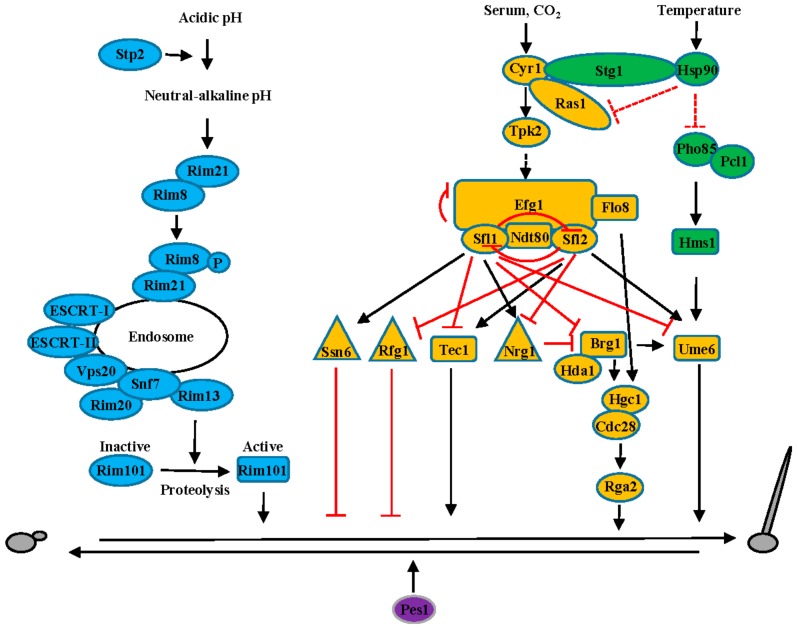
C. albicans is a commensal fungal pathogen of humans that colonizes the skin and mucosal surfaces of most healthy individuals. C. albicans causes life-threatening infections in individuals with compromised immune systems. C. albicans exists as yeast, psuedophyphae, and hyphae, all of which are important for virulence. Yeast cells are essential for dissemination [7], while hyphal forms may be essential for invading mucosal surfaces. The morphology switch between budding yeast and hyphal growth is triggered by diverse host environmental cues, including temperature, pH, serum, and CO2 (Figure 2).
Figure 2.
Regulation of the dimorphic transition in C. albicans via multiple signaling pathways. Signaling pathways are illustrated in different colors: cyclic AMP (cAMP)- protein kinase A (PKA) pathway (yellow), Hsp90 pathway (green), Rim101 pathway (blue) and Pes1 pathway (purple). Solid lines or arrows represent direct regulation. Dotted lines or arrows represent indirect or unknown regulation. Positive and negative relationships between components are illustrated in black and red, respectively. Transcription factors are illustrated with a rectangle, transcriptional co-repressors with a triangle, and other proteins with an oval.
Signal transduction pathways that trigger morphogenetic changes in response to the host environment have been extensively studied in C. albicans (Figure 2). The cyclic AMP (cAMP)—protein kinase A (PKA) pathway is the major pathway controlling hyphal growth induced by serum and CO2 in C. albicans [8,9]. Serum and CO2 act directly on the adenylate cyclase Cyr1 to activate the catalytic subunit of protein kinase A, Tpk2 [8,9,10]. Tpk2 then directly or indirectly phosphorylates the key transcription factor Efg1 [11,12,13]. Efg1 is a negatively auto-regulated transcription factor, which controls morphogenesis via interaction with heat shock factor-type transcriptional regulators Sfl1 and Sfl2, as well as transcription factors Ndt80 and Flo8 [11,12,13,14,15,16]. Sfl1 and Sfl2 antagonistically regulate morphogenesis in C. albicans [14]. Sfl1 acts via interaction with Efg1 and Ndt80 to repress expression of morphogenesis activators, such as transcription factors Ume6, Tec1, and Brg1, and Sfl2, while upregulating morphogenesis repressors, such as transcriptional co-repressors Ssn6 and Nrg1 [14,17,18,19,20,21,22]. In contrast, Sfl2 functions via Efg1 and Ndt80 to upregulate activators of hyphal growth, such as Ume6 and Tec1, while downregulating repressors of morphogenesis, such as the transcriptional co-repressor Nrg1 and Rfg1, as well as Sfl1 [14,18,19,21,22,23,24]. Nrg1 represses expression of its downstream target Brg1, which directly upregulates Ume6 and the hypha-specific G1 cyclin Hgc1 via interaction with the histone deacetylase Hda1 [24]. Hgc1 then activates the cyclin-dependent kinase Cdc28 to phosphorylate Rga2, a GTPase-activating protein of the central polarity regulator Cdc42 [25,26]. In addition, expression of Hgc1 is also controlled by Flo8, which functions via interaction with Efg1 [15,16].
In C. albicans, the molecular chaperone Hsp90 is a global regulator of morphogenesis in response to elevated temperature and acts by recruiting the co-chaperone Sgt1 to repress activity of Cyr1 [27]. Hsp90 also inhibits the activity of Cyr1 via direct or indirect interactions with Ras1 [28,29,30]. Finally, Hsp90 also controls morphogenesis by directly or indirectly repressing activity of Pho85Pcl1, which phosphorylates the transcription factor Hms1 to activate expression of Ume6 [18,31].
C. albicans senses alkaline pH via the receptor Rim21, which leads to the phosphorylation of the β-arrestin protein Rim8 [32]. This phosphorylated Rim8-Rim21 complex is taken up by endocytosis and recruits ESCRT-I, ESCRT-II, and Vps20-Snf7 [32,33]. Snf7 oligomerizes and recruits the scaffold protein Rim20 and the protease Rim13 to process the transcription factor Rim101 [34,35,36,37]. This processing activates Rim101 to promote transcriptional changes in genes that are required for the hyphal switch at alkaline pH [36,38]. C. albicans is also able to alters environmental pH, such as within the phagosome, to promote hyphal morphogenesis via the transcription factor Stp2. Stp2 upregulates expression of multiple amino acid permeases that are required for alkalinization of the phagosome [39].
While the transition from yeast to hyphae has been extensively studied in C. albicans, the switch from hyphae to yeast still remains poorly understood. Pes1, a pescadillo homolog in C. albicans, is involved in the hyphae to yeast switch, especially in the budding of yeast from lateral filamentous cells [40].
These detailed signal transduction pathway studies were recently expanded using genomic approaches. A genome-scale C. albicans mutant library was screened to identify additional regulators of morphogenesis in response to host-relevant environmental cues [41]. 872 mutants were identified with varying filamentation defects in response to serum [41]. Gene Ontology analysis found that many genes were involved in vesicle and intracellular protein transport, ergosterol biosynthesis, and N-linked glycosylation [41]. Many known morphogenetic regulators were identified, including Ras1, Hsp90, Cyr1, and Flo8 [41]. 102 genes encoding repressors of filamentation were identified, including 50 genes associated with cell cycle, such as the mitotic cyclin Clb2, the cyclin-dependent kinase Cdc28, members of the structural maintenance of chromosomes complex, as well as regulators of DNA replication [41].
Detailed analysis of signal transduction pathways in C. albicans have identified an intricate network that coordinates signals from diverse environmental cues to modify basic cellular functions, such as cell cycle, membrane and cell wall synthesis, and transport. Ultimately, these modifications allow for C. albicans to seamlessly transition between yeast and hyphal growth throughout its interaction with the host.
3. Thermally Dimorphic Fungal Pathogens
Thermally dimorphic human pathogenic fungi represent a group of fungi that grow in hyphal form in the environment but shift to yeast form within the host (Figure 3 and Figure 4). For many of these organisms, elevated temperature is one of the main host signals that initiate the hyphal to yeast conversion (Figure 3).
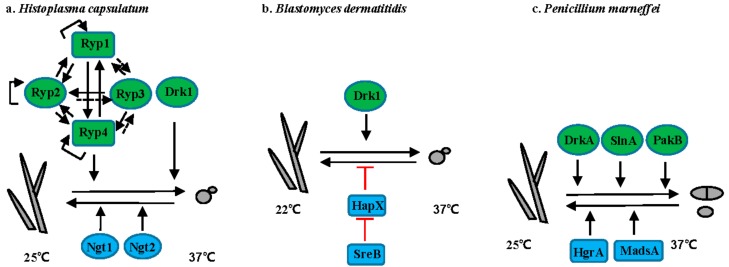
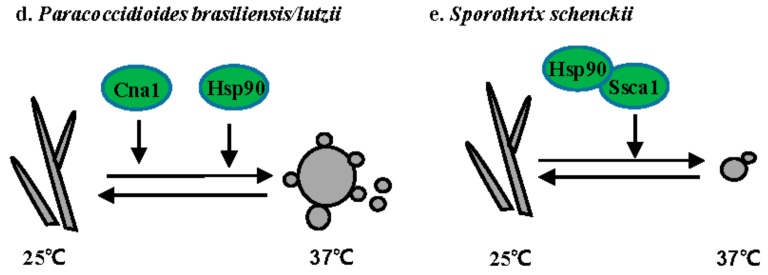
Figure 3.
Regulation of the morphogenetic transition in thermally dimorphic fungal pathogens. (a) H. capsulatum; (b) B. dermatitidis; (c) T. marneffei; (d) P. brasiliensis; and, (e) S. schenckii. Solid lines or arrows represent direct regulation. Dotted lines or arrows represent indirect or unknown regulation. Positive and negative relationships between components are illustrated in black and red, respectively. Transcription factors are illustrated as rectangles, and other proteins as ovals. Proteins controlling hyphal to yeast transitions are indicated in green. Proteins controlling yeast to hyphal transitions are indicated in blue.
Figure 4.
Dimorphic transition of M. circinelloides in response to O2/CO2. Positive and negative relationships between components are illustrated in black and red, respectively.
3.1. Histoplasma Species
Histoplasma spp. cause life-threatening histoplasmosis, predominantly in immunocompromised individuals. Histoplasmosis occurs worldwide, with H. duboissii endemic in Africa, whereas H. capsulatum is mainly endemic in the Ohio and Mississippi river valleys in the United States, South America, Southeast Asia, and Africa [42]. H. capsulatum grows as filamentous cells at 25 °C, while at 37 °C it converts into budding yeast (Figure 3a). Transition from hyphae to yeast is positively controlled by the hybrid histidine kinase Drk1 [43]. In addition, three transcriptional regulators—Ryp1, Ryp2, Ryp3, and Ryp4—are required for the switch from hyphae to yeast at 37 °C [44,45,46]. All four Ryp proteins require each of the others for their expression and directly interact with RYP1, RYP2, and RYP4 promoters in response to elevated temperature [46]. Although none of the Ryp proteins directly associate with the RYP3 promoter, RYP3 expression level still depends on other Ryp proteins. Acting opposite to temperature, N-acetylglucosamine (GlcNAc) promotes the transition from yeast to hyphae via the GlcNAc transporters Ngt1 and Ngt2 in H. capsulatum at 25 °C [47].
3.2. Blastomyces Species
Blastomycosis is caused by B. dermatitidis, B. gilcristii, and B. percursus. These species are endemic in the Ohio-Mississippi and St. Lawerence river valleys, southeastern US, Great Lakes region, and Canada [48]. B. dermatitidis grows as a mold at 22 °C and as yeast at 37 °C (Figure 3b). Like in H. capsulatum, the hybrid histidine kinase Drk1 is required for transition from mold to yeast and strains with reduced DRK1 expression are avirulent [43]. Transition from yeast to mold in B. dermatitidis is controlled by two transcription factors SreB and HapX [49,50]. Mutation of SREB or overexpression of HAPX inhibit the yeast-to-mold transition [50]. SreB negatively controls the expression of HAPX via direct interaction with the promoter of HAPX, although the defect in SreB null mutants is thought to be related to impaired biosynthesis of neutral lipids and not directly to HAPX expression [50].
3.3. T. marneffei
T. marneffei, an emerging human-pathogenic fungus endemic to Southeast Asia, causes severe, and often deadly, infection in immunocompromised patients. At 25 °C, T. marneffei grows as filamentous hyphae and undergoes asexual development to produce infectious conidia. At 37 °C, T. marneffei grows as yeast cells that replicate by fission (Figure 3c). The two-component histidine kinases DrkA and SlnA are involved in morphology switching, as overexpression of DrkA or SlnA promotes transition from hyphal to yeast growth even at 25 °C, whereas the deletion of DrkA or SlnA results in yeast growth at 37 °C in vitro [51]. Interestingly, PakB, encoding a p21-activated kinase is required for yeast morphogenesis within macrophages, but not during yeast growth in vitro at 37 °C, suggesting PakB is involved in the signaling pathway that controls the dimorphic switch in response to host stimuli, not temperature [52]. Additionally, two transcription factors MadsA and HgrA are required for the dimorphic switch from yeast to hyphae [53,54]. Overexpression of MADSA can induce hyphal growth at 37 °C [53]. Similarly, deletion of HGRA causes a defect in the dimorphic transition from yeast to hyphae [54].
3.4. Paracoccidioides Species
P. brasiliensis and P. lutzii are temperature-dependent dimorphic fungi that cause paracoccidioidomycosis (PCM), the most prevalent human deep mycosis in Latin America [55]. P. brasiliensis grows as hyphae at 25 °C and as yeast with multiple buds at 37 °C or in the host (Figure 3d). Both the calcineurin catalytic subunit Cna1 and Hsp90 are involved in the hyphal to yeast transition. Treatment with either the calcineurin inhibitor cyclosporine A or the Hsp90 inhibitor geldanamycin prevents the hyphal to yeast transition [56,57].
3.5. S. schenckii
S. schenckii is a dimorphic fungus that causes sporothrichosis worldwide [58]. Sporotrichosis is usually acquired via traumatic inoculation with contaminated plant debris, thorns, and soil [58]. S. schenckii grows as hyphae at 25 °C and as yeast at 37 °C (Figure 3e). The calcium/calmodulin kinase 1 Sscm1 is involved in the hyphal to yeast transition via direct interaction with Hsp90 [59]. A DRK1 homolog has also been identified in S. schenckii. While the role of Drk1 has not been directly dissected in S. schenckii, it is upregulated 24-fold in yeast when compared to hyphae.
3.6. Summary
Recent studies show that multiple signaling pathways control morphogenetic switches in thermally dimorphic fungal pathogens, including two-component signaling pathways, Hsp90 and calcium signaling. In addition, an array of novel transcription factors are critical for hyphal to yeast transitions. For many of the thermally dimorphic fungal pathogens, the upstream and downstream components of these signaling pathways and their link to the transcription factors remains unknown. Finally, temperature is not the only stimulus for morphology change during these host-pathogen interactions. Future research on the mechanisms by which the thermally dimorphic fungal pathogens sense and respond to the host environment will provide invaluable insight into aspects of the dimorphic switch specific to interactions with the host.
4. Aerobically/Anaerobically Dimorphic Fungal Pathogen
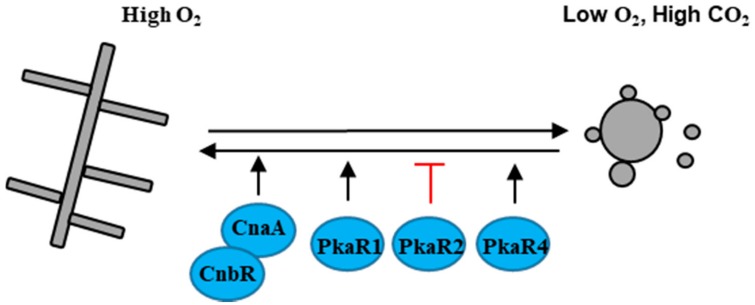
M. circinelloides is the causal agent of the fungal infection mucormycosis, an uncommon but frequently lethal fungal infection of humans. M. circinelloides usually grows as hyphae aerobically, and as multi-budded yeast in anaerobic conditions [60] (Figure 4). M. circinelloides encodes three protein kinase A regulatory subunits PkaR1, PkaR2, and PkaR4 that play different roles in dimorphic transition [61,62,63]. Over-expression of PKAR1 promotes filamentation and branching, whereas the mutation of PKAR1 leads to a defect in the yeast to hyphal transition [61,62]. PkaR4 is essential in M. circinelloides, but the hererokaryon mutant pkaR4 shows a defect in germ tube emergence upon shift from anaerobic to aerobic growth, indicating that PkaR4 also promotes the yeast to hyphal transition [63]. Unlike PkaR1 and PkaR4, mutation of PKAR2 promotes the transition from yeast to hyphae [63]. Additionally, inhibition of the calcineurin catalytic A subunit CnaA or mutation of the calcineurin regulatory B subunit CNAR results in cells locked in the yeast phase, indicating that calcinurin governs the dimorphic transition in M. circinelloides [64].
M. circinelloides is an emerging zygomycete fungal pathogen, and the current status of research on dimorphic switches in M. circinelloides is in its infancy. However, the genetic and molecular tools have been well developed in M. circinelloides, which will promote the advancement in understanding the dimorphic transition and its roles in the pathogenesis of M. circinelloides.
5. Fungal Pathogens That Exhibit Cell Size Variation
Pneumocystis spp., Coccidioides spp., and Cryptococcus spp. all exhibit changes in cell size during infection (Figure 5). However, each has its own unique morphological characteristics. Pneumocystis spp. form small trophic cells and larger cysts. Coccidioides spp. are not only thermally and aerobically dimorphic, but also generate large spherules. Finally, while Cryptococcus spp. can grow both as hyphae (during sexual development) and yeast in the environment, only yeast are observed in vivo. These Cryptococcus yeast can differentiate into both small micro cells and large polyploid titan cells.
Figure 5.
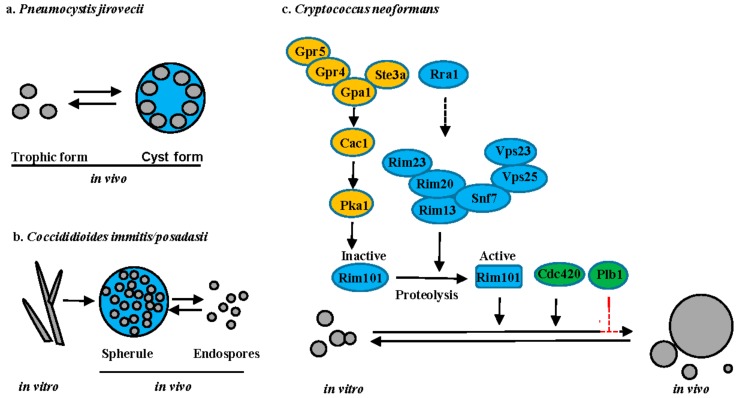
Changes in cell size upon interaction with host. (a) P. jirovecii; (b) C. immitis; and, (c) C. neoformans. Signaling pathways are illustrated with different colors: cAMP-PKA (yellow), Rim101 (blue) and others (green). Solid lines or arrows represent direct regulation. Dotted lines or arrows represent indirect or unknown regulation. Positive and negative relationships between components are illustrated in black and red, respectively. Transcription factors are illustrated as rectangles and other proteins illustrated as ovals.
5.1. Pneumocystis Species
Pneumocystis spp. are host organism specific with P. carinii infecting rats, P. murina infecting mice and P. jirovecii infecting humans. P. jirovecii causes a severe pneumonia (Pneumocystis pneumonia or PCP) in immunocompromised individuals worldwide. Microscopic analysis of tissue sections has revealed that Pneumocystis has two predominant life-cycle forms: the trophic form and the cyst form (Figure 5a). The trophic form can replicate either sexually or asexually. During sexual reproduction, two haploid trophic forms conjugate to produce an early cyst with two nuclei. The nuclei undergo meiosis to form intermediate cyst with four nuclei. The nuclei in the intermediate cyst undergo mitosis to produce the mature cyst containing eight haploid trophic cells. The cyst ruptures to release the trophic cells that either reproduce asexually via mitosis or re-enter the sexual cycle. [65]. A functional beta-1,3-d-glucanase is exclusively expressed in cysts [66]. Interestingly, cysts are thought to be the infected form of Pneumocystis, based on the rodent model of PCP [67]. Unfortunately, the molecular mechanisms underlining the troph to cyst morphology change and the role of these two morphologies during the host-pathogen interaction remain largely unknown. A reproducible genetic system in either P. carinii or P. murina is needed to allow for the identification of the molecular signals underlying these morphology changes and their unique role in both virulence and transmission.
5.2. Coccidioides Species
C. immitis and C. posadasii are dimorphic pathogenic fungi that cause coccidioidomycosis, also known as Valley fever in the southwestern United States, parts of Mexico, and South America [68]. Coccidioides spp. are thermally dimorphic and evolutionarily closely related to other thermal dimorphs [69]. However, Coccidioides can produce large spherules upon inhalation, in which yeast-like endospores develop (Figure 5b). Mature spherules are too large (30–80 µm) to be phagocytosed, resulting in the hypothesis that they provide a protected environment for cellular replication [69]. Upon rupture, endospores that are produced within the spherule are released and can survive within phagocytes [69]. Dissemination of Coccidioides from the lungs to secondary tissues is thought to be via the endospores. Interestingly, aerobic/anaerobic conditions (CO2 tension) and estradiol are important for spherule maturation, but the underlying molecular pathways involved in spherule production are still largely unknown.
5.3. Cryptococcus Species
Disease that is due to C. neoformans is predominantly observed in immunocompromised individuals, whereas C. gattii can cause disease in both immune-compromised and apparently healthy individuals. In vitro, Cryptococcus cells typically grow as round yeast cells, ranging in size from 4–7 µm. However, C. neoformans exhibits a vast diversity of cell sizes in vivo, particularly in the lungs, with cell sizes ranging from 1 to over 100 µm in cell body diameter [70,71,72,73,74] (Figure 5c). Recent studies have shown that cells with different sizes have distinct traits, resulting in classification of some of the cell types, such as micro cells and titan cells [70,71,72,73,74,75]. The most well studied are the titan cells that are generally larger than 10 µm in cell body diameter. Beyond their large size, titan cells have several unique characteristics when compared to typical cells that are grown in vitro, such as polyploidy, thicker cell wall with high chitin content, and a dense highly cross-linked capsule [73,74,76]. These novel traits promote survival and dissemination of C. neoformans during infection by limiting phagocytosis by host macrophages, increasing resistance to oxidative, and nitrosative stresses, triggering a detrimental Th2 immune response, and producing daughter cell progeny with genomic diversity [74,76,77,78]. Titan cell formation is regulated by the pheromone signaling pathway, the cAMP/PKA pathway, and the Rim101 pathway [73,74,79,80,81,82]. Both the pheromone receptor Ste3a and the G-protein coupled receptor Gpr5 can independently stimulate titan cell production via interaction with the G-protein Gpa1 to activate the adenylate cyclase Cac1, which then activates Pka1 to phosphorylate the transcription factor Rim101 [73,74,80,81,82]. Activation of Rim101 requires both phosphorylation by Pka1 and proteolysis by the proteolysis complex [81,82]. As in C. albicans, activation of the proteolysis complex requires assembly of ESCRT-I Vps23, ESCRT-II Vps25, and ESCRT-III Snf7 as a scaffolding platform [82]. Recently, a novel transmembrane protein Rra1 was reported to act upstream of Rim23, likely functioning as a sensor similar to the Rim21 sensor in C. albicans. The predictive structure of Rra1 contains 7-transmembrane domains, similar to Rim21 [82]. Additionally, both Cdc420 and phospholipase B (Plb1) have been implicated in titan cell formation, but their mode of action remains unclear [80,83].
5.4. Summary
In addition to traditional hyphal to yeast dimorphic transitions, some of the human pathogenic fungi also exhibit unique morphology changes that impact pathogenesis. Some of these morphology changes are associated with cell size and ploidy changes. The large size of Cryptococcus titan cells and Coccidioides spherules both protect from phagocytosis, and thus may serve as protective structures to reduce impact of innate immune responses. Cryptococcus titan cells, Pneumocystis cysts, and Coccidioides spherules are also all polypoid. Polyploid cells are very common in nature and are found in plants, animals, and arthropods [84]. Polyploid cells are usually formed through endoreplicative cycles [84], thus it is likely that endoreplicative cycles generate titan cells and spherules. Pneumocystis has differential activity of a Cdc2/Cdc13 cyclin-dependent kinase complex over the life cycle, with greater activity in cysts when compared with the trophic forms, also supporting a link between cell cycle and ploidy variation [85]. Yet the exact nature of the cycle cycle alterations in Cryptococcus, Pneumocystis, and Coccidioides that generate the large polyploidy structures remain unknown. Further, how differences in these cell cycles lead to the different structures produced by these human pathogenic fungi and their subsequent daughter cells also remain to be explored.
6. Conclusions
As described above, the human fungal pathogens undergo diverse morphology changes in response to the host environment. These morphology changes enable the human fungal pathogens to rapidly adapt to and survive not only the high temperature and low oxygen environment of the host, but also protect against the host immune response. With recent developments in genetic, genomic, and post-genomic tools, the signaling pathways that are involved in these morphological transitions have begun to be elucidated using gene knockout or knockdown, transcriptomic, and proteomic studies. While the human fungal pathogens exhibit diverse morphologies, many commonalities in the underlying mechanisms that are controlling these morphological transitions have been uncovered. For example, elevated temperature induces morphology transitions in C. albicans and the thermally dimorphic human fungal pathogens. Similarly, CO2 triggers morphological switchs in C. albicans, M. circinelloides, and Coccidioides immitis. The cAMP/PKA and Hsp90 pathways are used to control morphological responses in many of the human pathogenic fungi.
Given the importance of morphological transitions in the pathogenesis of the human fungal pathogens, more extensive and more detailed studies are needed to gain deeper insights into the commonalities between the morphologies and their function during infection. Ultimately, future studies addressing how human fungal pathogens control morphology and virulence in response to host environmental cues will provide information that could lead to the development of novel treatment strategies, which are aimed at blocking these important morphological changes.
Acknowledgments
The writing of this review was supported by National Institutes of Health grants AI080275 and AI22352 to Kirsten Nielsen.
Author Contributions
Zhongming Li and Kirsten Nielsen jointly wrote and edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hawksworth D.L. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycol. Res. 2001;105:1422–1432. doi: 10.1017/S0953756201004725. [DOI] [Google Scholar]
- 2.Blackwell M. The fungi: 1, 2, 3... 5.1 million species? Am. J. Bot. 2011;98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- 3.Taylor L.H., Latham S.M., Woolhouse M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:983–998. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havlickova B., Czaika V.A., Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;4:2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 5.Sobel J.D. Vulvovaginal candidosis. Lancet. 2007;369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 6.Brown G.D., Denning D.W., Gow N.A., Levitz S.M., Netea M.G., White T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 7.Saville S.P., Lazzell A.L., Monteagudo C., Lopez-Ribot J.L. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X.L., Lee R.T., Fang H.M., Wang Y.M., Li R., Zou H., Zhu Y., Wang Y. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4:28–39. doi: 10.1016/j.chom.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Klengel T., Liang W.J., Chaloupka J., Ruoff C., Schröppel K., Naglik J.R., Eckert S.E., Mogensen E.G., Haynes K., Tuite M.F., et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr. Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonneborn A., Bockmühl D.P., Gerads M., Kurpanek K., Sanglard D., Ernst J.F. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol. Microbiol. 2000;35:386–396. doi: 10.1046/j.1365-2958.2000.01705.x. [DOI] [PubMed] [Google Scholar]
- 11.Stoldt V.R., Sonneborn A., Leuker C.E., Ernst J.F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bockmühl D.P., Ernst J.F. A potential phosphorylation site for an A-type kinase in the Efg1 regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics. 2001;157:1523–1530. doi: 10.1093/genetics/157.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lassak T., Schneider E., Bussmann M., Kurtz D., Manak J.R., Srikantha T., Soll D.R., Ernst J.F. Target specificity of the Candida albicans Efg1 regulator. Mol. Microbiol. 2011;82:602–618. doi: 10.1111/j.1365-2958.2011.07837.x. [DOI] [PubMed] [Google Scholar]
- 14.Znaidi S., Nesseir A., Chauvel M., Rossignol T., d’Enfert C. A comprehensive functional portrait of two heat shock factor-type transcriptional regulators involved in Candida albicans morphogenesis and virulence. PLoS Pathog. 2013;9:e1003519. doi: 10.1371/journal.ppat.1003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao F., Lane S., Raniga P.P., Lu Y., Zhou Z., Ramon K., Chen J., Liu H. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol. Biol. Cell. 2006;17:295–307. doi: 10.1091/mbc.E05-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du H., Guan G., Xie J., Cottier F., Sun Y., Jia W., Mühlschlegel F.A., Huang G. The transcription factor Flo8 mediates CO2 sensing in the human fungal pathogen Candida albicans. Mol. Biol. Cell. 2012;23:2692–2701. doi: 10.1091/mbc.E12-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleary I.A., Lazzell A.L., Monteagudo C., Thomas D.P., Saville S.P. BRG1 and NRG1 form a novel feedback circuit regulating Candida albicans hypha formation and virulence. Mol. Microbiol. 2012;85:557–573. doi: 10.1111/j.1365-2958.2012.08127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee M., Thompson D.S., Lazzell A., Carlisle P.L., Pierce C., Monteagudo C., López-Ribot J.L., Kadosh D. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol. Biol. Cell. 2008;19:1354–1365. doi: 10.1091/mbc.E07-11-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweizer A., Rupp S., Taylor B.N., Röllinghoff M., Schröppel K. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol. Microbiol. 2000;38:435–445. doi: 10.1046/j.1365-2958.2000.02132.x. [DOI] [PubMed] [Google Scholar]
- 20.Hwang C.S., Oh J.H., Huh W.K., Yim H.S., Kang S.O. Ssn6, an important factor of morphological conversion and virulence in Candida albicans. Mol. Microbiol. 2003;47:1029–1043. doi: 10.1046/j.1365-2958.2003.03353.x. [DOI] [PubMed] [Google Scholar]
- 21.Murad A.M., Leng P., Straffon M., Wishart J., Macaskill S., MacCallum D., Schnell N., Talibi D., Marechal D., Tekaia F., et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001;20:4742–4752. doi: 10.1093/emboj/20.17.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun B.R., Kadosh D., Johnson A.D. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. EMBO J. 2001;20:4753–4761. doi: 10.1093/emboj/20.17.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadosh D., Johnson A.D. Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans. Mol. Cell. Biol. 2001;21:2496–2505. doi: 10.1128/MCB.21.7.2496-2505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y., Su C., Liu H. A GATA transcription factor recruits Hda1 in response to reduced Tor1 signaling to establish a hyphal chromatin state in Candida albicans. PLoS Pathog. 2012;8:e1002663. doi: 10.1371/journal.ppat.1002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X., Wang Y., Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng X.D., Lee R.T., Wang Y.M., Lin Q.S., Wang Y. Phosphorylation of Rga2, a Cdc42 GAP, by CDK/Hgc1 is crucial for Candida albicans hyphal growth. EMBO J. 2007;26:3760–3769. doi: 10.1038/sj.emboj.7601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro R.S., Zaas A.K., Betancourt-Quiroz M., Perfect J.R., Cowen L.E. The Hsp90 co-chaperone Sgt1 governs Candida albicans morphogenesis and drug resistance. PLoS ONE. 2012;7:e44734. doi: 10.1371/journal.pone.0044734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro R.S., Uppuluri P., Zaas A.K., Collins C., Senn H., Perfect J.R., Heitman J., Cowen L.E. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr. Biol. 2009;19:621–629. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Q., Summers E., Guo B., Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 1999;181:6339–6346. doi: 10.1128/jb.181.20.6339-6346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang H.M., Wang Y. RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol. Microbiol. 2006;61:484–496. doi: 10.1111/j.1365-2958.2006.05248.x. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro R.S., Sellam A., Tebbji F., Whiteway M., Nantel A., Cowen L.E. Pho85, Pcl1, and Hms1 signaling governs Candida albicans morphogenesis induced by high temperature or Hsp90 compromise. Curr. Biol. 2012;22:461–470. doi: 10.1016/j.cub.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Raja J., Davis D.A. The β-arrestin-like protein Rim8 is hyperphosphorylated and complexes with Rim21 and Rim101 to promote adaptation to neutral-alkaline pH. Eukaryot Cell. 2012;11:683–693. doi: 10.1128/EC.05211-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu W., Smith F.J., Jr., Subaran R., Mitchell A.P. Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol. Biol. Cell. 2004;15:5528–5537. doi: 10.1091/mbc.E04-08-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kullas A.L., Li M., Davis D.A. Snf7p, a component of the ESCRT-III protein complex, is an upstream member of the RIM101 pathway in Candida albicans. Eukaryot. Cell. 2004;3:1609–1618. doi: 10.1128/EC.3.6.1609-1618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf J.M., Davis D.A. Mutational analysis of Candida albicans SNF7 reveals genetically separable Rim101 and ESCRT functions and demonstrates divergence in bro1-domain protein interactions. Genetics. 2010;184:673–694. doi: 10.1534/genetics.109.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis D., Wilson R.B., Mitchell A.P. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 2000;20:971–978. doi: 10.1128/MCB.20.3.971-978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M., Martin S.J., Bruno V.M., Mitchell A.P., Davis D.A. Candida albicans Rim13p, a protease required for Rim101p processing at acidic and alkaline pHs. Eukaryot. Cell. 2004;3:741–751. doi: 10.1128/EC.3.3.741-751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bensen E.S., Martin S.J., Li M., Berman J., Davis D.A. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol. Microbiol. 2004;54:1335–1351. doi: 10.1111/j.1365-2958.2004.04350.x. [DOI] [PubMed] [Google Scholar]
- 39.Vylkova S., Lorenz M.C. Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport. PLoS Pathog. 2014;10:e1003995. doi: 10.1371/journal.ppat.1003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen J., Cowen L.E., Griffin A.M., Chan L., Köhler J.R. The Candida albicans pescadillo homolog is required for normal hypha-to-yeast morphogenesis and yeast proliferation. Proc. Natl. Acad. Sci. USA. 2008;105:20918–22023. doi: 10.1073/pnas.0809147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Meara T.R., Veri A.O., Ketela T., Jiang B., Roemer T., Cowen L.E. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat. Commun. 2015;6 doi: 10.1038/ncomms7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Retallack D.M., Woods J.P. Molecular epidemiology, pathogenesis, and genetics of the dimorphic fungus Histoplasma capsulatum. Microbes Infect. 1999;1:817–825. doi: 10.1016/S1286-4579(99)80084-7. [DOI] [PubMed] [Google Scholar]
- 43.Nemecek J.C., Wüthrich M., Klein B.S. Global control of dimorphism and virulence in fungi. Science. 2006;312:583–588. doi: 10.1126/science.1124105. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen V.Q., Sil A. Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc. Natl. Acad. Sci. USA. 2008;105:4880–4885. doi: 10.1073/pnas.0710448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webster R.H., Sil A. Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum. Proc. Natl. Acad. Sci. USA. 2008;105:14573–14578. doi: 10.1073/pnas.0806221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beyhan S., Gutierrez M., Voorhies M., Sil A. A temperature-responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol. 2013;11:e1001614. doi: 10.1371/journal.pbio.1001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilmore S.A., Naseem S., Konopka J.B., Sil A. N-acetylglucosamine (GlcNAc) triggers a rapid, temperature-responsive morphogenetic program in thermally dimorphic fungi. PLoS Genet. 2013;9:e1003799. doi: 10.1371/journal.pgen.1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.López-Martínez R., Méndéz-Tovar L.J. Blastomycosis. Clin. Dermatol. 2012;30:565–572. doi: 10.1016/j.clindermatol.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Gauthier G.M., Sullivan T.D., Gallardo S.S., Brandhorst T.T., Vanden Wymelenberg A.J., Cuomo C.A., Suen G., Currie C.R., Klein B.S. SREB, a GATA transcription factor that directs disparate fates in Blastomyces dermatitidis including morphogenesis and siderophore biosynthesis. PLoS Pathog. 2010;6:e1000846. doi: 10.1371/journal.ppat.1000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marty A.J., Broman A.T., Zarnowski R., Dwyer T.G., Bond L.M., Lounes-Hadj Sahraoui A., Fontaine J., Ntambi J.M., Keleş S., Kendziorski C., et al. Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in Blastomyces dermatitidis. PLoS Pathog. 2015;11:e1004959. doi: 10.1371/journal.ppat.1004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyce K.J., Schreider L., Kirszenblat L., Andrianopoulos A. The two-component histidine kinases DrkA and SlnA are required for in vivo growth in the human pathogen Penicillium marneffei. Mol. Microbiol. 2011;82:1164–1184. doi: 10.1111/j.1365-2958.2011.07878.x. [DOI] [PubMed] [Google Scholar]
- 52.Boyce K.J., Schreider L., Andrianopoulos A. In vivo yeast cell morphogenesis is regulated by a p21-activated kinase in the human pathogen Penicillium marneffei. PLoS Pathog. 2009;5:e1000678. doi: 10.1371/journal.ppat.1000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang E., Chow W.N., Wang G., Woo P.C., Lau S.K., Yuen K.Y., Lin X., Cai J.J. Signature gene expression reveals novel clues to the molecular mechanisms of dimorphic transition in Penicillium marneffei. PLoS Genet. 2014;10:e1004662. doi: 10.1371/journal.pgen.1004662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bugeja H.E., Hynes M.J., Andrianopoulos A. HgrA is necessary and sufficient to drive hyphal growth in the dimorphic pathogen Penicillium marneffei. Mol. Microbiol. 2013;88:998–1014. doi: 10.1111/mmi.12239. [DOI] [PubMed] [Google Scholar]
- 55.Teixeira M.M., Theodoro R.C., Nino-Vega G., Bagagli E., Felipe M.S. Paracoccidioides Species Complex: Ecology, Phylogeny, Sexual Reproduction, and Virulence. PLoS Pathog. 2014;10:e1004397. doi: 10.1371/journal.ppat.1004397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campos C.B., Di Benedette J.P., Morais F.V., Ovalle R., Nobrega M.P. Evidence for the role of calcineurin in morphogenesis and calcium homeostasis during mycelium-to-yeast dimorphism of Paracoccidioides brasiliensis. Eukaryot. Cell. 2008;7:1856–1864. doi: 10.1128/EC.00110-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matos T.G., Morais F.V., Campos C.B. Hsp90 regulates Paracoccidioides brasiliensis proliferation and ROS levels under thermal stress and cooperates with calcineurin to control yeast to mycelium dimorphism. Med. Mycol. 2013;51:413–421. doi: 10.3109/13693786.2012.725481. [DOI] [PubMed] [Google Scholar]
- 58.Téllez M.D., Batista-Duharte A., Portuondo D., Quinello C., Bonne-Hernández R., Carlos I.Z. Sporothrix schenckii complex biology: Environment and fungal pathogenicity. Microbiology. 2014;160:2352–2365. doi: 10.1099/mic.0.081794-0. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez-Caban J., Gonzalez-Velazquez W., Perez-Sanchez L., Gonzalez-Mendez R., Rodriguez-del Valle N. Calcium/calmodulin kinase1 and its relation to thermotolerance and HSP90 in Sporothrix schenckii: An RNAi and yeast two-hybrid study. BMC Microbiol. 2011;11:162. doi: 10.1186/1471-2180-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orlowski M. Mucor dimorphism. Microbiol. Rev. 1991;55:234–258. doi: 10.1128/mr.55.2.234-258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolff A.M., Appel K.F., Petersen J.B., Poulsen U., Arnau J. Identification and analysis of genes involved in the control of dimorphism in Mucor circinelloides (syn. racemosus) FEMS Yeast Res. 2002;2:203–213. doi: 10.1111/j.1567-1364.2002.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 62.Ocampo J., Nuñez L.F., Silva F., Pereyra E., Moreno S., Garre V., Rossi S. A subunit of protein kinase A regulates growth and differentiation in the fungus Mucor circinelloides. Eukaryot. Cell. 2009;8:933–944. doi: 10.1128/EC.00026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ocampo J., McCormack B., Navarro E., Moreno S., Garre V., Rossi S. Protein kinase a regulatory subunit isoforms regulate growth and differentiation in Mucor circinelloides: Essential role of PKAR4. Eukaryot. Cell. 2012;11:989–1002. doi: 10.1128/EC.00017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S.C., Li A., Calo S., Heitman J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 2013;9:e1003625. doi: 10.1371/journal.ppat.1003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas C.F., Jr., Limper A.H. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat. Rev. Microbiol. 2007;5:298–308. doi: 10.1038/nrmicro1621. [DOI] [PubMed] [Google Scholar]
- 66.Kutty G., Davis A.S., Ma L., Taubenberger J.K., Kovacs J.A. Pneumocystis encodes a functional endo-β-1,3-glucanase that is expressed exclusively in cysts. J. Infect. Dis. 2015;211:719–728. doi: 10.1093/infdis/jiu517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cushion M.T., Linke M.J., Ashbaugh A., Sesterhenn T., Collins M.S., Lynch K., Brubaker R., Walzer P.D. Echinocandin treatment of pneumocystis pneumonia in rodent models depletes cysts leaving trophic burdens that cannot transmit the infection. PLoS ONE. 2010;5:e8524. doi: 10.1371/journal.pone.0008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whiston E., Zhang Wise H., Sharpton T.J., Jui G., Cole G.T., Taylor J.W. Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PLoS ONE. 2012;7:e41034. doi: 10.1371/journal.pone.0041034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muñoz-Hernández B., Palma-Cortés G., Cabello-Gutiérrez C., Martínez-Rivera M.A. Parasitic polymorphism of Coccidioides spp. BMC Infect. Dis. 2014;14:213. doi: 10.1186/1471-2334-14-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cruickshank J.G., Cavill R., Jelbert M. Cryptococcus neoformans of Unusual Morphology. Appl. Microbiol. 1973;25:309–312. doi: 10.1128/am.25.2.309-312.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J.M., Zhou Q., Cai H.R., Zhuang Y., Zhang Y.F., Xin X.Y., Meng F.Q., Wang Y.P. Clinicopathological features of pulmonary cryptococcosis with cryptococcal titan cells: A comparative analysis of 27 cases. Int. J. Clin. Exp. Pathol. 2014;7:4837–4846. [PMC free article] [PubMed] [Google Scholar]
- 72.Zaragoza O. Multiple Disguises for the Same Party: The Concepts of Morphogenesis and Phenotypic Variations in Cryptococcus neoformans. Front. Microbiol. 2011;2 doi: 10.3389/fmicb.2011.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaragoza O., García-Rodas R., Nosanchuk J.D., Cuenca-Estrella M., Rodríguez-Tudela J.L., Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010;6:e1000945. doi: 10.1371/annotation/0675044c-d80f-456f-bb63-4f85fb1d0c33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okagaki L.H., Strain A.K., Nielsen J.N., Charlier C., Baltes N.J., Chrétien F., Heitman J., Dromer F., Nielsen K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010;6:e1000953. doi: 10.1371/annotation/1b59fd9e-9ac9-4ea8-a083-14c413c80b03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alanio A., Vernel-Pauillac F., Sturny-Leclère A., Dromer F. Cryptococcus neoformans host adaptation: Toward biological evidence of dormancy. mBio. 2015;6:e02580–14. doi: 10.1128/mBio.02580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiesner D.L., Specht C.A., Lee C.K., Smith K.D., Mukaremera L., Lee S.T., Lee C.G., Elias J.A., Nielsen J.N., Boulware D.R., et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015;11:e1004701. doi: 10.1371/journal.ppat.1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okagaki L.H., Nielsen K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot. Cell. 2012;11:820–826. doi: 10.1128/EC.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerstein A.C., Fu M.S., Mukaremera L., Li Z., Ormerod K.L., Fraser J.A., Berman J., Nielsen K. Polyploid Titan Cells Produce Haploid and Aneuploid Progeny To Promote Stress Adaptation. mBio. 2015;6:e01340–15. doi: 10.1128/mBio.01340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi J., Vogl A.W., Kronstad J.W. Regulated expression of cyclic AMP-dependent protein kinase A reveals an influence on cell size and the secretion of virulence factors in Cryptococcus neoformans. Mol. Microbiol. 2012;85:700–715. doi: 10.1111/j.1365-2958.2012.08134.x. [DOI] [PubMed] [Google Scholar]
- 80.Okagaki L.H., Wang Y., Ballou E.R., O’Meara T.R., Bahn Y.S., Alspaugh J.A., Xue C., Nielsen K. Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryot. Cell. 2011;10:1306–1316. doi: 10.1128/EC.05179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Meara T.R., Norton D., Price M.S., Hay C., Clements M.F., Nichols C.B., Alspaugh J.A. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog. 2010;6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ost K.S., O’Meara T.R., Huda N., Esher S.K., Alspaugh J.A. The Cryptococcus neoformans alkaline response pathway: Identification of a novel rim pathway activator. PLoS Genet. 2015;11:e1005159. doi: 10.1371/journal.pgen.1005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evans R.J., Li Z., Hughes W.S., Djordjevic J.T., Nielsen K., May R.C. Cryptococcal phospholipase B1 is required for intracellular proliferation and control of titan cell morphology during macrophage infection. Infect Immun. 2015;83:1296–1304. doi: 10.1128/IAI.03104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edgar B.A., Zielke N., Gutierrez C. Endocycles: A recurrent evolutionary innovation for post-mitotic cell growth. Nat. Rev. Mol. Cell. Biol. 2014;15:197–210. doi: 10.1038/nrm3756. [DOI] [PubMed] [Google Scholar]
- 85.Kottom T.J., Thomas C.F., Jr., Mubarak K.K., Leof E.B., Limper A.H. Pneumocystis carinii uses a functional cdc13 B-type cyclin complex during its life cycle. Am. J. Respir. Cell. Mol. Biol. 2000;22:722–731. doi: 10.1165/ajrcmb.22.6.3838. [DOI] [PubMed] [Google Scholar]