Abstract
Donor-specific antibodies have become an established biomarker predicting antibody-mediated rejection. Antibody-mediated rejection is the leading cause of graft loss after kidney transplant. There are several phenotypes of antibody-mediated rejection along post-transplant course that are determined by the timing and extent of humoral response and the various characteristics of donor-specific antibodies, such as antigen classes, specificity, antibody strength, IgG subclasses, and complement binding capacity. Preformed donor-specific antibodies in sensitized patients can trigger hyperacute rejection, accelerated acute rejection, and early acute antibody-mediated rejection. De novo donor-specific antibodies are associated with late acute antibody-mediated rejection, chronic antibody-mediated rejection, and transplant glomerulopathy. The pathogeneses of antibody-mediated rejection include not only complement-dependent cytotoxicity, but also complement-independent pathways of antibody-mediated cellular cytotoxicity and direct endothelial activation and proliferation. The novel assay for complement binding capacity has improved our ability to predict antibody-mediated rejection phenotypes. C1q binding donor-specific antibodies are closely associated with acute antibody-mediated rejection, more severe graft injuries, and early graft failure, whereas C1q nonbinding donor-specific antibodies correlate with subclinical or chronic antibody-mediated rejection and late graft loss. IgG subclasses have various abilities to activate complement and recruit effector cells through the Fc receptor. Complement binding IgG3 donor-specific antibodies are frequently associated with acute antibody-mediated rejection and severe graft injury, whereas noncomplement binding IgG4 donor-specific antibodies are more correlated with subclinical or chronic antibody-mediated rejection and transplant glomerulopathy. Our in-depth knowledge of complex characteristics of donor-specific antibodies can stratify the patient’s immunologic risk, can predict distinct phenotypes of antibody-mediated rejection, and hopefully, will guide our clinical practice to improve the transplant outcomes.
Keywords: donor specific antibody; antibody-mediated rejection; C1q-binding DSA; IgG subclasses; Biomarkers; Complement System Proteins; Humans; Immunoglobulin G; kidney transplantation; Phenotype; Receptors, Fc; Tissue Donors
Introduction
Antibody-mediated rejection has been recognized as the leading cause of graft dysfunction and graft loss after kidney transplant (1–4). Donor-specific antibodies (DSAs) identified before kidney transplant (preformed DSAs) can cause early rejection, such as hyperacute rejection, accelerated acute rejection, early acute antibody-mediated rejection, and graft loss (1–6). Alternatively, de novo developed DSAs after transplant are associated with late acute antibody-mediated rejection, chronic antibody-mediated rejection, and transplant glomerulopathy (3,4,7,8). However, there are also “benign” DSAs that may not be clinically relevant, because they are not associated with antibody-mediated rejection or graft failure (8–11). This paper reviews the identification of DSAs and discusses their complex characteristics, including antibody classes, specificity, strength, IgG subclasses, and complement binding capacity, as well as the different phenotypes of antibody-mediated rejection after kidney transplant. The advance in our understanding of DSA pathogenicity can help clinicians to stratify patient’s immunologic risk, predict the phenotypes of antibody-mediated rejection, and guide our clinical management.
Sensitization and DSA Identification
Sensitization is defined by the presence of antibodies in the recipient’s blood against a panel of selected HLAs representing donor population. It is reported as the percentage panel reactive antibody. Panel reactive antibody estimates the likelihood of positive crossmatch to potential donors (11). Sensitization is caused by previous exposure to HLA antigens, usually through organ transplant, pregnancy, or blood transfusion (5,6). Particularly relevant is the exposure of a woman to her partner’s HLA during pregnancy. This results in direct sensitization against the partner, potentially making the partner and/or her child an unsuitable donor (11). The technology of screening antibodies has been advanced from the complement-dependent cytotoxicity assay, the enzyme-linked immunoabsorption, to multiplexed particle-based flow cytometry (Luminex). Single antigen beads are used to characterize the preformed DSAs before transplant as well as any de novo development of DSAs after transplant (4,11).
Luminex assay can characterize the preformed HLA antibodies in sensitized patients awaiting transplant. The recurrent antibodies or highly expressed antibodies are considered clinically significant. The corresponding antigens are regarded as unacceptable for that patient, and in the United States, they are listed into the United Network of Organ Sharing database. A patient will not be offered a kidney from the deceased donor who expresses an unacceptable HLA antigen (positive virtual crossmatch). Only those patients whose HLA antibodies are not donor directed will appear on the match run (negative virtual crossmatch). Such “virtual crossmatch” improves efficiency of organ allocation (12). When a potential donor is identified, a final crossmatch with fresh serum from recipient and lymphocytes from donor is performed to rule out preformed DSAs before transplant surgery. The commonly used tests are complement-dependent cytotoxicity crossmatch and flow cytometry crossmatch (11,12).
DSA Pathogenesis
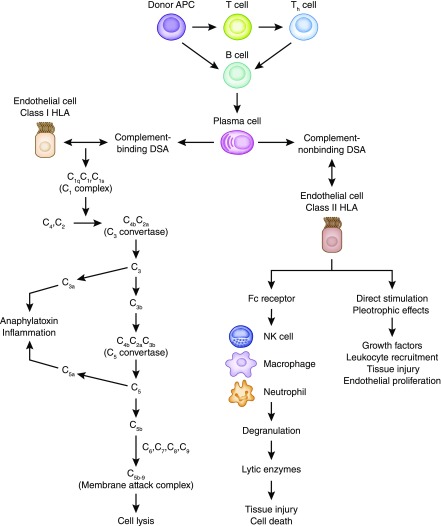
Presence of DSA, either preformed or de novo, has become a well established biomarker predicting poor transplant outcomes, including high incidence of antibody-mediated rejection, graft dysfunction, and inferior graft survival. The development of de novo DSAs after kidney transplant was reported in 13%–30% of previously nonsensitized patients (13–20). The risk factors for de novo DSA include the following: (1) high HLA mismatches (especially DQ mismatches), (2) inadequate immunosuppression and nonadherence, and (3) graft inflammation, such as viral infection, cellular rejection, or ischemia injury, which can increase graft immunogenicity (18–20). De novo DSAs are predominantly directed to donor HLA class 2 mismatches and usually occur during the first year of kidney transplant, but they can appear anytime, even several years later (18–20). Binding of DSA to antigen expressed on allograft endothelial cells can activate classic complement pathway, a key pathologic process of acute antibody-mediated rejection phenotypes (4,11). Even in absence of complement activation, some DSAs can cause graft damage through antibody-dependent cellular cytotoxicity. The innate immune cells, including neutrophils, macrophages, and natural killer cells, can bind to Fc fragments of DSAs, trigger degranulation, and release lytic enzymes, which cause tissue injury and cell death. This process can mediate smoldering damages to the endothelial cells and is proposed as an important pathogenesis in subclinical and chronic antibody-mediated rejection phenotypes (21–23). Furthermore, DSAs can cause graft injury by direct activation of endothelial proliferation through increasing vascular endothelial growth factor production, upregulating fibroblast growth factor receptor, and increasing its ligand binding as well as other signaling pathways for cellular recruitment (8,23–25). This pathogenesis may contribute to transplant glomerulopathy and vasculopathy that feature vascular intima thickness with smooth muscle cell invasion. The latter two complement-independent mechanisms can explain the clinical phenotypes of antibody-mediated rejection with negative C4d staining in peritubular capillaries. Figure 1 illustrates the three proposed pathogeneses of DSA in antibody-mediated rejection (4,8,11,21–24).
Figure 1.
The three proposed pathogeneses of donor-specific antibodies (DSAs) in antibody-mediated rejection. Donor antigen-presenting cells include macrophages, dendritic cells, and B cells. Complement binding DSAs target the class 1 HLA on endothelial cells, activate the classic complement cascade, and deliver complement-dependent cytotoxicity in acute antibody-mediated rejection. Complement nonbinding DSAs recruit innate immune cells (NK cells, macrophages, and neutrophils) through Fc receptors and lead to antibody-dependent cellular toxicity. In addition, complement nonbinding DSAs have direct stimulation and pleotrophic effects that cause tissue injury, cellular recruitment, and endothelial proliferation. The latter two mechanisms play an important role in acute antibody-mediated rejection with negative C4d deposit in peritubular capillaries as well as chronic antibody-mediated rejection, transplant glomerulopathy, and vasculopathy (4,8,11,21–24). APC, antigen-presenting cells; NK, natural killer cells.
DSA Classes and Specificity
DSAs target specific epitopes in the polymorphic regions of HLA antigens. HLA class 1 antigens (A, B, and C) are expressed on all nucleated cells, and each antigen consists of one a-chain and one β2-microglobulin. The epitopes reside only in the polymorphic a-chain (6,7,11). HLA class 2 antigens (DR, DQ, and DP) are normally restricted to antigen-presenting cells (dendritic cells, B cells, and macrophages), but they can be upregulated and expressed after inflammatory insults, such as ischemia-reperfusion injury, infection, and rejection (6–10). Each class 2 antigen consists of one a-chain and one β-chain, and both chains are polymorphic. The β-chain of DQ is particularly polymorphic, which adds clinical complexity of DQ antibodies (25). Preformed DSAs in sensitized patients can be class 1, class 2, or both, and they may target either private or public epitopes (26,27). Presence of preformed DSAs directly affects the transplant decision making. Kidney transplant should not proceed if there is a positive T cell crossmatch secondary to cytotoxic IgG antibody, which is usually complement binding IgG1 or IgG3 subclass (6,8,27,28). The majority of de novo DSAs after kidney transplant are class 2 antibodies, especially DQ. Class 1 de novo DSAs are usually detected sooner after transplant and more likely IgG1 and IgG3 subclasses. They are associated with acute antibody-mediated rejection and early graft loss (7,8). Class 2 de novo DSAs appear later and are commonly noncomplement binding IgG2 or IgG4 subclass. They tend to be persistent and are associated with chronic antibody-mediated rejection and transplant glomerulopathy (6,8,29,30). Trying aggressively to eliminate class 2 DSA, especially the DQ, may not be successful, and it can put patients at great risk of excessive immunosuppression without much benefit (27). Table 1 compares the predominant characteristics of classes 1 and 2 DSAs, although certain overlaps do exist between the two classes (6–10,25–30).
Table 1.
Comparison of the dominant characteristics of classes 1 and 2 donor-specific antibodies (6–10,25–30)
| Class 1 Donor-Specific Antibodies | Class 2 Donor-Specific Antibodies | |
|---|---|---|
| HLA | ||
| Antigens | A, B, and C | DR, DQ, and DP |
| Epitopes location | α-chain | α- and β-chains |
| Expression | All nucleated cells | Antigen-presenting cells |
| Preformed donor-specific antibodies | ||
| Important | Very | Less |
| Positive crossmatch | T cells | B cells |
| Transplant decision | No transplant | Permissible |
| De novo donor-specific antibodies | ||
| Detection | Sooner | Later |
| IgG subclasses | IgG1, IgG3 | IgG2, IgG4 |
| Complement binding | Strong | Weak/no |
| Frequency | Fewer | Common, especially DQ |
| Antibody-mediated rejection | ||
| Phenotypes | Acute | Chronic, subclinical |
| Presentation | Early | Later |
| Graft dysfunction | Rapidly | Slowly |
| C4d deposit | Positive | Negative |
| Treatment | More responsive | Less responsive |
| Graft loss | Early | Later |
DSA Strength
The DSA strength (or titer) is usually expressed as the mean fluorescence intensity by Luminex solid-phase assay. Early studies have shown the clinical correlation between mean fluorescence intensity and risk of antibody-mediated rejection. High titer of DSA has been correlated with complement binding capability and more severe tissue injuries (28–34). Theoretically, high DSA titer provides a sufficient number of IgG molecules that can bind to antigens close together to form hexametric complexes that activate complement more effectively (28,31). However, the correlation between DSA strength and clinical outcome is far from perfect. DSAs with similar mean fluorescence intensity do not always activate the complement cascade. There are patients with transplants with high levels of circulating DSAs who escape rejection or graft dysfunction (6,8,11). The ability of DSAs to bind on beads may not be the same as that to bind on HLA antigens of endothelial cells. Currently, there is no standardization and normalization of the solid-phase bead assays. The thresholds reported for clinically significant mean fluorescence intensity vary widely between studies from 1000 to 10,000 depending on the antigen specificities (6,8,27,28,35). Our HLA laboratory uses the common cutoffs of 3000 for class 1 DSAs and 5000 for class 2 DSAs as clinically significant mean fluorescence intensity (1,6). Several technical limitations of solid-phase bead assays have been captured. False positive or high titers may be reported due to the presence of antibodies to denatured HLA molecules. DSAs targeting one of the shared epitopes may be diluted across the beads. False negative or low titers can also occur in the presence of inhibitors or “prozone effects,” affecting the assay of very high levels of DSAs (26–28,35). Tambur et al. (28) noted that the “prozone effect” was very common (71%) in patients with multiple DSAs, and serial dilution of sera before assay provided more accurate measure of DSA strength.
Complement Binding DSA
The recent advance of Luminex-based assay for complement binding DSAs has significantly improved our ability to predict antibody-mediated rejection phenotypes and graft failure (13–17,29–37). Several studies have shown that, compared with C1q nonbinding DSAs, C1q binding DSAs are associated with significantly higher risk of antibody-mediated rejection, severe tissue injury, and graft loss (13–18,33–38). In the large cohort study from Loupy et al. (33), C1q binding DSA detected at 1 year after kidney transplant or during antibody-mediated rejection was an independent factor of graft loss. It increased the risk of graft loss by 4.78-fold (95% confidence interval [95% CI], 2.69 to 8.49). C1q binding DSA remained independently associated with the risk of loss of the kidney allograft after adjustment for the mean fluorescence intensity (hazard ratio [HR], 4.5; 95% CI, 2.2 to 9.0). Compared with patients with C1q nonbinding DSAs, C1q binding DSAs were associated with higher incidence of antibody-mediated rejection with more severe graft injuries that included more extensive inflammation, microvasculitis, endarteritis, transplant glomerulopathy, and C4d deposits (33). Recently, Guidicelli et al. (18) compared the long-term effects of C1q binding versus C1q nonbinding DSAs on graft survivals. They found that both are associated with graft loss. However, C1q binding DSAs were associated with graft loss occurring quickly after their appearances, and C1q nonbinding DSAs led to graft loss in the long term. Calp-Inal et al. (36) studied C1q binding DSA in 284 patients prospectively (group 1) as well as 405 patients retrospectively (group 2). The incidences of acute antibody-mediated rejection were significantly higher in patients with C1q-positive DSAs in groups 1 (45%) and 2 (15%) compared with patients with C1q-negative DSAs (5% and 2%, respectively) and patients who were DSA negative (1% and 3%, respectively; P<0.001 and P=0.001, respectively). The incidences of chronic antibody-mediated rejection were also significantly higher in patients with C1q-positive DSAs in groups 1 (36%) and 2 (51%) compared with patients with C1q-negative DSAs (5% and 25%, respectively) and patients who were DSA negative (2% and 6%, respectively; P<0.001). Graft survival in group 1 was numerically lower in patients with C1q-positive DSAs (73%) compared with patients with C1q-negative DSAs (95%) and patients who were DSA negative (94%; P=0.21). Presence of C1q binding DSAs was associated with both acute and chronic phenotypes of antibody-mediated rejection in the study (36). Yabu et al. (37) showed that, although testing DSA by IgG level was very sensitive for positive C4d deposit, positive C1q binding DSA was more specific for transplant glomerulopathy and graft loss. In conclusion, these studies indicate that C1q binding DSAs are more detrimental than C1q nonbinding DSAs. Positive C1q binding DSA is an independent risk of antibody-mediated rejection and graft loss beyond the traditional DSA mean fluorescence intensity.
There are preliminary data suggesting C3d or C4d binding DSA as a predictor of antibody-mediated rejection. Sicard et al. (34) compared both C1q and C3d binding DSA assays at the time of rejection diagnosis. They found that presence of C3d binding DSA was associated with a higher risk of graft loss independent of mean fluorescence intensity. C3d binding DSA was a better predictor of graft loss than C1q binding DSA (34). Comoli et al. (38) reported similar results in the pediatric population: C3d binding DSA was better than C1q binding DSA in stratifying the risk of antibody-mediated rejection and graft loss. Theoretically, these two assays analyze different steps of the complement activation. C1q is the first step of the classic pathway, and it may not be necessarily indicative of full activation of the complement cascade. C3d is more downstream and more likely indicates complete complement activation. In another study, preformed DSAs able to bind C4d were reported to predict antibody-mediated rejection and graft loss, although the crossmatch was negative before transplant (5). More rigid and large studies are needed to elucidate the role of C3d and C4d binding DSAs in predicting antibody-mediated rejection and graft loss. Table 2 summarizes the recent studies of complement binding DSAs in patients with kidney transplants.
Table 2.
Recent studies of complement binding donor-specific antibodies in patients with kidney transplants
| Ref. | Design | Patient/DSA | Sera Collecting | C Binding DSA | Follow-Up | Positive Complement Binding DSA-Associated Outcomes |
|---|---|---|---|---|---|---|
| 37 | Retrospective | 274/31 | Pre, biopsy | 12 C1q | 10 | Predict transplant glomerulopathy and graft loss |
| 36 | Prospective | 284/31 | De novo | 11 C1q | 4 | Predict acute and chronic antibody-mediated rejection; low graft survival |
| 36 | Retrospective | 405/77 | De novo | 33 C1q | 4 | Predict acute and chronic antibody-mediated rejection; low graft survival |
| 29 | Retrospective | 284/54 | De novo | 34 C1q | 5 | DQ-DSA studied; predict acute antibody-mediated rejection and graft loss; more likely IgG1/IgG3 subclasses |
| 31 | Prospective | 34/34 | Biopsy | 12 C1q | 8 | Reflect high DSA strength; predict antibody-mediated rejection |
| 33 | Prospective | 1016/316 | Pre, biopsy, 1 yr | 77 C1q | 7 | With lowest 4-yr graft survival; predict acute antibody-mediated rejection and more severe graft injury |
| 18 | Retrospective | 246/47 | De novo | 12 C1q | 10 | Predict both short- and long-term graft loss; C1q nonbinding DSAs predict long-term graft loss only |
| 15 | Retrospective | 83/52 | De novo | 13 C1q | N/A | Increase risk of antibody-mediated rejection and graft loss |
| 13 | Retrospective | 517/60 | Preformed | 13 C1q | 8 | Predict graft loss and antibody-mediated rejection better than DSA strength |
| 14 | Retrospective | 30/30 | Rejection | 30 C1q | 3 | Reduction of C1q binding DSA after treatment predicts better graft survival than the reduction of DSA titer |
| 16 | Retrospective | 193/35 | De novo | 15 C1q | 7 | Predict antibody-mediated rejection, positive C4d deposit, and graft loss |
| 17 | Retrospective | 62/26 | Biopsy | 9 C1q | 4 | Predict active chronic antibody-mediated rejection, transplant glomerulopathy, and graft loss |
| 42 | Prospective | 851/Pre: 110; post: 186 | Pre and 1 and 2 yr, biopsy | C1q; pre: 35; post: 57 | 5 | IgG3 subclass or C1q binding DSA detected pre- and post-transplant predicted graft loss in >60% of patients; also predicted acute and chronic antibody-mediated rejection |
| 34 | Retrospective | 938/69 | Biopsy | 30 C1q, 40 C3d | 6 | C3d binding DSAs predict graft loss; C1q binding DSAs are less predictive |
| 38 | Prospective | 114/39 | De novo | 25 C1q, 9 C3d | 10 | C3d binding DSAs predict graft loss; C1q binding DSAs are less predictive |
| 5 | Retrospective | 52/52 | Preformed | 10 C4d | 5 | Predict acute antibody-mediated rejection and graft loss despite of negative pretransplant crossmatch |
Patient/DSA, total numbers of study patients/number of patients with positive donor-specific antibody; C binding DSA, number of patients with positive complement (C1q, C3d, or C4d) binding donor-specific antibody; Follow-up, follow-up years; DSA, donor-specific antibody; pre, pretransplant; biopsy, at the time of kidney biopsy; N/A, not applicable; post, post-transplant.
DSA IgG Subclasses
IgG has several subclasses (IgG1, -2, -3, and -4), and they have various abilities to activate complement and recruit effector cells through the Fc receptor. Over the course of immune response and antibody production, there is a sequential IgG subclass switching, typically from IgG3 to IgG1 to IgG2 to IgG4 (39–41). IgG1 and IgG3 are usually complement binding subclasses. IgG1 is the most abundant subclass and mirrors the total IgG level, but IgG3 has the greater binding efficiency to C1q and activate complement cascade. Although IgG2 can activate complement weakly, IgG4 does not activate complement at all, but both can recruit effector cells through the Fc receptor. IgG4 is also considered as a biomarker of matured alloresponse and associated with advanced stage of rejection (8,35,39). Therefore, the pathogenicities of DSA are dynamic and vary according to IgG subclass profile. Lefaucheur et al. (41) recently studied 125 patients with DSAs detected in the first year after kidney transplants: 51 of them had acute antibody-mediated rejection, 36 patients had subclinical antibody-mediated rejection, and 38 were antibody-mediated rejection free. Patients with antibody-mediated rejection had multiple DSA IgG subclasses and C1q binding immune-dominant donor-specific antibody (iDSA). Antibody-mediated rejection–free patients did not have C1q binding iDSAs or IgG3 or IgG4 iDSAs. Interestingly, they found that acute antibody-mediated rejection was mainly driven by IgG3 iDSA (91%), whereas subclinical antibody-mediated rejection was driven by IgG4 iDSA (76%). IgG3 iDSA was associated with a shorter time to rejection, increased microcirculation injury, and positive C4d deposits, which indicate the complement-dependent cytotoxicity. IgG4 iDSA was associated with later graft injury with increased transplant glomerulopathy and interstitial fibrosis/tubular atrophy. Further analysis indicated that IgG3 and C1q binding iDSAs were strongly and independently associated with graft loss (HR, 4.8; 95% CI, 1.7 to 13.3 and HR, 3.6; 95% CI, 1.1 to 11.7, respectively). IgG4 iDSA–associated subacute and chronic phenotypes of antibody-mediated rejection were consistent with complement-independent pathogeneses (21–24,35). Viglietti et al. (42) recently published a prospective study of 851 kidney recipients. Systemic DSA screening and characterization (titer, C1q binding capacity, and IgG subclasses) were performed at transplant, 1 and 2 years post-transplant, and the time of clinical events. They found that the addition of IgG3 subclass or C1q binding capacity to the conventional approach of DSA strength improved the performance in assessing the individual risk for allograft loss in >60% of patients. The advances in complement binding DSA assay and IgG subclass analysis have improved our ability to assess patient’s immunologic risk. They can help clinicians to identify more harmful DSAs, predict the distinct phenotypes of antibody-mediated rejection, and guide our clinical decision for kidney biopsy as well as immunosuppressive management.
DSA and C4d Deposit
C4d is a degradation product of the classic complement pathway. A unique feature of C4d is that it binds covalently to the endothelial basement membrane, thereby avoiding removal during tissue processing. Positive C4d deposit in peritubular capillaries serves as an immunologic footprint of antibody-mediated rejection. It is in a linear pattern and best shown by immunofluorescence in frozen tissue section (43,44). Some patients with antibody-mediated rejection may not have positive C4d staining. Cases with positive DSA but negative C4d staining may result from either technique error (false negative) or noncomplement-activating DSA (45). Also, antibody-mediated rejection can be caused by non-HLA antibodies that may result in positive C4d deposit without DSA against HLA (46–52). Furthermore, Kikić et al. (53) reported that patients who were C4d positive, with or without antibody-mediated rejection histologic features, had worse death-censored 8-year graft survival than patients who were C4d negative. Positive C4d deposit was independently associated with a steeper decline of kidney function and an independent risk factor for graft failure. Recently, Orandi et al. (54) compared the patient outcomes of C4d-positive versus -negative antibody-mediated rejection. They found that both phenotypes were associated with significantly lower graft survival compared with in patients who were rejection free, and C4d-positive antibody-mediated rejection had the worst graft survival. C4d-positive antibody-mediated rejection was significantly more likely to have a clinical presentation (85.3% versus 54.9%) and happen earlier (median =14 versus 46 days), and it was three times more common (7.8% versus 2.5%). This supports the hypothesis that C4d-positive antibody-mediated rejection is directly caused by complement-dependent cytotoxicity and associated with more acute and severe clinical presentation, whereas C4d-negative antibody-mediated rejection may be mediated by complement-independent mechanisms, which are subclinical or chronic in nature and lead to late graft dysfunction and graft loss.
Non-HLA Antibodies
Graft rejection can occur in HLA-identical sibling transplants, indicating an immune response to non-HLA antigens (47,48). Non-HLA antibodies can cause antibody-mediated rejection and graft loss. ABO blood antigens are expressed on not only red blood cells but also, vascular endothelium and other cells. ABO-incompatible organ transplant can cause hyperacute rejection due to presence of the preformed hemagglutinin A and/or B antibody. Rhesus factor and other red cell antigens are not that relevant to organ transplant, because they are not expressed on endothelial cells (11). H-Y minor histocompatibility antigens are encoded by the Y chromosome in men and can induce alloimmune response when an organ from a man is transplanted into a woman (49). The MHC1-related chains A and B are minor histocompatibility molecules expressed on endothelial cells, and their antibodies can trigger antibody-mediated rejection (46,51). Several other antibodies have been also reported, such as antiendothelial antibodies, antiglutathione S-transferase T1, and antiangiotensin-2 receptor (47,50,52). Interestingly, angiotensin-2 receptor antibody has an agonistic effect that causes severe vascular constriction and hypertension. It responds well to the treatment with angiotensin receptor blocker (6,52). As our knowledge in transplant immunology advances, there will likely be more alloreactive and autoreactive non-HLA antibodies to be discovered.
Acute Phenotypes of Antibody-Mediated Rejection
There are several phenotypes of antibody-mediated rejection along the post-transplant course that seem to be determined by the extent, timing, and various characteristics of DSA during humoral response. Hyperacute rejection occurs immediately after transplant. It is mediated by high titer of preformed antidonor antibodies: anti-HLA, anti-ABO, or other non-HLA antibodies (4,8,11). Hyperacute rejection results in an irreversible vascular rejection, intravascular thrombosis, and graft necrosis, and graft nephrectomy is usually indicated. The routine pretransplant crossmatch and verification of ABO compatibility should prevent the majority of hyperacute rejection.
Accelerated acute rejection (or delayed hyperacute rejection) can occur within 24 hours to several days after transplant. It represents an anamnestic response by memory B and plasma cells from prior sensitization (4,11,55,56). Even a negative crossmatch before transplant may not prevent it, because the preformed DSA titer may be too low to be detected before transplant, but alloresponse from memory cells can trigger a surge of DSA titer after transplant. Indeed, with a novel HLA B cell enzyme-linked immunospot assay, Lúcia et al. (55) reported that circulating memory B cells against donor HLA antigens can be identified in the sensitized patients on waiting list but cannot be identified in the nonsensitized patients. Furthermore, memory B cells can be depleted by rituximab, a chimeric anti-CD20 mAb; therefore, anamnestic response may be abrogated by rituximab treatment before kidney transplant (56). Presence of long-lived plasma cells remains a challenge, because they are usually resistant to current available therapeutics (4,55,56).
Acute antibody-mediated rejection is more common than hyperacute rejection and accelerated acute rejection, and it may be further divided into early and late subtypes. Early acute antibody-mediated rejection occurs sooner after transplant from rising titer of preformed DSA. Late acute antibody-mediated rejection develops due to the emergence of de novo DSA after kidney transplant (4). Antibody-mediated rejection can also develop in a graft already suffering from delayed graft function. This can be difficult to be recognized if the patient remains anuric or oliguric (57). Therefore, any new kidney transplant with delayed graft function should have serial DSA monitoring and protocol biopsies to detect covert rejection and be treated properly (57). Early acute rejection is usually more responsive to treatment, whereas late acute rejection may not respond to treatment well and is associated with inferior graft function and poorer long-term outcome.
Treatment of Acute Antibody-Mediated Rejection
The optimal therapy for acute antibody-mediated rejection and accelerated acute rejection remains to be defined but usually consists of a combination of several modalities, such as plasmapheresis or immunoadsorption, intravenous Igs, rituximab, bortezomib, antithymocyte globulin, and corticosteroids (58–64). Plasmapheresis or immunoadsorption with protein A removes the circulating DSA, but neither can suppress antibody production (58,59). Intravenous Igs are often administrated after plasmapheresis. Possible mechanisms of intravenous Igs include anti-idiotypic antibodies neutralizing DSAs and immune-modulatory molecules inhibiting complement binding or activation and suppressing DSA production (58,60–62). Rituximab depletes both pre-B and mature B cells, thus reducing the transformation of plasma cell from B cells (61–63). Bortezomib inhibits the proteasome and activates apoptosis of antibody-producing plasma cells; therefore, it can directly decrease DSA production. There are several studies showing the benefit of bortezomib in treating antibody-mediated rejection (63–66). Antithymocyte globulin remains beneficial, because it can control the B cell response directly by inducing B cell apoptosis as well as indirectly by depleting helper T cells (2,4). In addition, there are several unconventional approaches that can be considered, especially for difficult patients unresponsive to the traditional therapies. Splenectomy was reported as a rescue therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplant, and the combination of splenectomy with eculizumab may be more effective than either modality alone (67). Eculizumab is a humanized mAb to C5, and it inhibits its cleavage to C5a and C5b. Stegall et al. (68) studied it as part of a desensitization protocol in patients with positive crossmatch. They found that eculizumab treatment significantly decreased the incidence of early acute antibody-mediated rejection compared with a historical control group (6.7% versus 43.8%, respectively). However, after a 2-year follow-up, eculizumab did not prevent the development of chronic antibody-mediated rejection (69). This supports the complement-dependent pathogenesis in acute antibody-mediated rejection and complement-independent mechanisms in chronic antibody-mediated rejection. Recently, Viglietti et al. (70) reported a pilot study of a C1 inhibitor (berinert, a human plasma–derived C1 esterase inhibitor) added to high-dose intravenous Igs for treating acute antibody-mediated rejection that initially failed to respond to conventional therapy. Berinert appeared to be well tolerated. After a 6-month treatment, they found an improvement in kidney function, a decrease in C4d deposition rate, and a decrease in C1q binding DSA status. Theoretically, C1 inhibitor should offer some benefits over C5 inhibitor, because its block is proximal to C5, preventing the production of C3a and C5a, which are anaphylatoxins leading to further inflammatory responses. Formal clinical trials of complement inhibitors will be needed to elucidate the efficacy in acute antibody-mediated rejection.
Chronic Antibody-Mediated Rejection
Acute antibody-mediated rejection is a predictor of developing chronic antibody-mediated rejection and transplant glomerulopathy (2,4,7,11,71). Developing or worsening proteinuria after acute rejection episode suggests the development of chronic rejection or transplant glomerulopathy (4,11). Subclinical antibody-mediated rejection is defined as immunohistologic evidence of antibody-mediated rejection by protocol biopsy with normal graft function, and it is also a predictor of chronic antibody-mediated rejection if not treated (3,4,10,11). Chronic antibody-mediated rejection is diagnosed by a combination of the typical chronic changes from biopsy (glomerular double counters, multilayering of peritubular capillary basement membrane, interstitial fibrosis/tubular atrophy, and fibrous intimal thickening in arteries) and ongoing humoral activity (positive DSA, non-HLA antibody, or C4d staining). Chronic rejection is usually irreversible and predominantly caused by class 2 DSAs that tend to be persistent and difficult to treat (68–70). Bortezomib and large doses of intravenous Igs were shown to reduce class 2 DSA level less effectively than class 1 DSA level (72,73). Attempts to aggressively eliminate class 2 DSAs (especially the DQ) may not be achievable, because they can put the patient at great risk of overimmunosuppression (27). The treatment with rituximab and/or intravenous Igs can provide partial control of humoral activity and was reported to improve graft survival (62,73,74). The maintenance immunosuppressive drugs may also be increased or adjusted with more potent ones with the hope to control humoral activity and stabilize the graft function. Kulkarni et al. (75) recently reported a pilot trial of 6-month eculizumab therapy for chronic antibody-mediated rejection. The treatment group had a stabilization of graft function compared with the slowly deteriorating kidney function noted in the control group. It suggests a complement-dependent pathogenesis in chronic antibody-mediated rejection, although complement-independent pathways are commonly proposed. The combination of complement-dependent and -independent mechanisms may also exist in chronic antibody-mediated rejection.
Summary
DSAs, either preformed or de novo, have become a well established biomarker predicting antibody-mediated rejection and graft loss. Preformed DSAs can cause instant hyperacute rejection and graft necrosis, whereas memory B and plasma cell response may trigger an accelerated acute rejection within hours or days of kidney transplant. Preformed DSAs are also responsible for early acute antibody-mediated rejection after transplant. The development of de novo DSAs in previously nonsensitized patients is associated with late acute antibody-mediated rejection, chronic antibody-mediated rejection, and transplant glomerulopathy. Clinical phenotypes of antibody-mediated rejection are determined by the complex characteristics of DSAs, including HLA classes, specificity, strength, IgG subclasses, and complement binding capacity. IgG subclasses have various abilities to activate complement and recruit effector cells through the Fc receptor. C1q binding IgG3 DSA is associated with acute antibody-mediated rejection, more severe graft injury, and early graft loss. This indicates a predominant role of complement-dependent pathogenesis. C1q nonbinding IgG4 DSA is more related with subclinical or chronic antibody-mediated rejection and transplant glomerulopathy, which suggests complement-independent pathways as the underline pathogeneses.
In our practice, complement binding DSA and IgG subclass assays are not routinely performed. They should be considered and can be helpful in several clinical situations. HLA-incompatible kidney transplant is associated with high incidence of antibody-mediated rejection and inferior graft survival. The current practice is to reduce the preformed DSA to an acceptable level by various desensitization protocols before proceeding with the transplant. If we integrate the complement binding and IgG subclass assays into risk assessment and add disappearance of C1q binding IgG3 DSA as a goal of desensitization protocols, then the risk of antibody-mediated rejection and graft loss may be significantly reduced. In the post-transplant setting, de novo DSAs are frequently detected, even in stable transplant recipients. C1q binding and IgG subclass assays can provide noninvasive tools to identify those at high risk of antibody-mediated rejection, so that close monitoring and early biopsy are indicated. These assays can also guide our clinical treatment of different phenotypes of antibody-mediated rejection. Complement inhibitors may be considered as part of treatment for acute rejection mediated by C1q binding IgG3 DSA, and they are unlikely to be beneficial in treating rejection caused by C1q nonbinding IgG4 DSA. These should also be taken into consideration when future clinical trials are designed to evaluate the efficacy of complement inhibitors on antibody-mediated rejection. The advances in transplant immunology will provide us with more insights in the complex pathogeneses of antibody-mediated rejection. Our in-depth knowledge of DSA characteristics can stratify the patient’s immunologic risk, distinguish harmful DSAs from benign DSAs, and predict the phenotypes of antibody-mediated rejection, and hopefully, they will guide our clinical practice to improve the transplant outcomes.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Halloran PF, Reeve JP, Pereira AB, Hidalgo LG, Famulski KS: Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: New insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int 85: 258–264, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Thaunat O, Koenig A, Leibler C, Grimbert P: Effect of immunosuppressive drugs on humoral allosensitization after kidney transplant. J Am Soc Nephrol 27: 1890–1900, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M: Diagnosis and management of antibody-mediated rejection: Current status and novel approaches. Am J Transplant 14: 255–271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence C, Willicombe M, Brookes PA, Santos-Nunez E, Bajaj R, Cook T, Roufosse C, Taube D, Warrens AN: Preformed complement-activating low-level donor-specific antibody predicts early antibody-mediated rejection in renal allografts. Transplantation 95: 341–346, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Filippone EJ, Farber JL: Humoral immune response and allograft function in kidney transplantation. Am J Kidney Dis 66: 337–347, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Lee PC, Zhu L, Terasaki PI, Everly MJ: HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation 88: 568–574, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Valenzuela NM, Reed EF: Antibodies in transplantation: The effects of HLA and non-HLA antibody binding and mechanisms of injury. Methods Mol Biol 1034: 41–70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosmoliaptsis V, Sharples LD, Chaudhry AN, Halsall DJ, Bradley JA, Taylor CJ: Predicting HLA class II alloantigen immunogenicity from the number and physiochemical properties of amino acid polymorphisms. Transplantation 91: 183–190, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Loupy A, Vernerey D, Tinel C, Aubert O, Duong van Huyen JP, Rabant M, Verine J, Nochy D, Empana JP, Martinez F, Glotz D, Jouven X, Legendre C, Lefaucheur C: Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. J Am Soc Nephrol 26: 1721–1731, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumbala D, Zhang R: Essential concept of transplant immunology for clinical practice. World J Transplant 3: 113–118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren Q, Paramesh AS, Yau CL, Florman S, Killackey M, Slakey DP, Alper B, Simon E, Hamm LL, Zhang R: Kidney transplantation in highly sensitized African Americans. Transpl Int 24: 259–265, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Malheiro J, Tafulo S, Dias L, Martins S, Fonseca I, Beirão I, Castro-Henriques A, Cabrita A: Determining donor-specific antibody C1q-binding ability improves the prediction of antibody-mediated rejection in human leucocyte antigen-incompatible kidney transplantation. Transpl Int 30: 347–359, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Ramon DS, Huang Y, Zhao L, Rendulic T, Park JM, Sung RS, Samaniego M: Use of complement binding assays to assess the efficacy of antibody mediated rejection therapy and prediction of graft survival in kidney transplantation. Hum Immunol 78: 57–63, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Süsal C, Wettstein D, Döhler B, Morath C, Ruhenstroth A, Scherer S, Tran TH, Gombos P, Schemmer P, Wagner E, Fehr T, Živčić-Ćosić S, Balen S, Weimer R, Slavcev A, Bösmüller C, Norman DJ, Zeier M, Opelz G; Collaborative Transplant Study Report : Association of kidney graft loss with de novo produced donor-specific and non-donor-specific HLA antibodies detected by single antigen testing. Transplantation 99: 1976–1980, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB: Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant 16: 12–17, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Fichtner A, Süsal C, Höcker B, Rieger S, Waldherr R, Westhoff JH, Sander A, Opelz G, Tönshoff B: Association of C1q-fixing DSA with late graft failure in pediatric renal transplant recipients. Pediatr Nephrol 31: 1157–1166, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Guidicelli G, Guerville F, Lepreux S, Wiebe C, Thaunat O, Dubois V, Visentin J, Bachelet T, Morelon E, Nickerson P, Merville P, Taupin JL, Couzi L: Non-complement-binding de novo donor-specific anti-HLA antibodies and kidney allograft survival. J Am Soc Nephrol 27: 615–625, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ntokou I-SA, Iniotaki AG, Kontou EN, Darema MN, Apostolaki MD, Kostakis AG, Boletis JN: Long-term follow up for anti-HLA donor specific antibodies postrenal transplantation: High immunogenicity of HLA class II graft molecules. Transpl Int 24: 1084–1093, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Anthony RM, Nimmerjahn F: The role of differential IgG glycosylation in the interaction of antibodies with FcγRs in vivo. Curr Opin Organ Transplant 16: 7–14, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, Russell PS, Colvin RB: A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant 12: 313–321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hidalgo LG, Sis B, Sellares J, Campbell PM, Mengel M, Einecke G, Chang J, Halloran PF: NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: Evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant 10: 1812–1822, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Reed EF: Effect of antibodies on endothelium. Am J Transplant 9: 2459–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haarberg KM, Tambur AR: Detection of donor-specific antibodies in kidney transplantation. Br Med Bull 110: 23–34, 2014 [DOI] [PubMed] [Google Scholar]
- 26.El-Awar N, Terasaki PI, Nguyen A, Sasaki N, Morales-Buenrostro LE, Saji H, Maruya E, Poli F: Epitopes of human leukocyte antigen class I antibodies found in sera of normal healthy males and cord blood. Hum Immunol 70: 844–853, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Patel A, Tinckam K: Donor-specific antibody monitoring: Where is the beef? Adv Chronic Kidney Dis 23: 317–325, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Tambur AR, Herrera ND, Haarberg KM, Cusick MF, Gordon RA, Leventhal JR, Friedewald JJ, Glotz D: Assessing antibody strength: Comparison of MFI, C1q, and titer information. Am J Transplant 15: 2421–2430, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Freitas MC, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, Briley KP, Haisch CE, Bolin P, Parker K, Kendrick WT, Kendrick SA, Harland RC, Terasaki PI: The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation 95: 1113–1119, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, Catrou PG, Bolin P, Kendrick WT, Kendrick SA, Harland RC, Terasaki PI: Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation 95: 410–417, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Yell M, Muth BL, Kaufman DB, Djamali A, Ellis TM: C1q binding activity of de novo donor-specific HLA antibodies in renal transplant recipients with and without antibody-mediated rejection. Transplantation 99: 1151–1155, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Loupy A, Vernerey D, Viglietti D, Aubert O, Duong Van Huyen JP, Empana JP, Bruneval P, Glotz D, Legendre C, Jouven X, Lefaucheur C: Determinants and outcomes of accelerated arteriosclerosis: Major impact of circulating antibodies. Circ Res 117: 470–482, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen J-P, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana J-P, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, Scoazec JY, Bachelet T, Lepreux S, Visentin J, Merville P, Fremeaux-Bacchi V, Morelon E, Taupin J-L, Dubois V, Thaunat O: Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol 26: 457–467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefaucheur C, Viglietti D, Mangiola M, Loupy A, Zeevi A: From humoral theory to performant risk stratification in kidney transplantation. J Immunol Res 2017: 5201098, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calp-Inal S, Ajaimy M, Melamed ML, Savchik C, Masiakos P, Colovai A, Akalin E: The prevalence and clinical significance of C1q-binding donor-specific anti-HLA antibodies early and late after kidney transplantation. Kidney Int 89: 209–216, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB: C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation 91: 342–347, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Comoli P, Cioni M, Tagliamacco A, Quartuccio G, Innocente A, Fontana I, Trivelli A, Magnasco A, Nocco A, Klersy C, Rubert L, Ramondetta M, Zecca M, Garibotto G, Ghiggeri GM, Cardillo M, Nocera A, Ginevri F: Acquisition of C3d-binding activity by de novo donor-specific HLA antibodies correlates with graft loss in nonsensitized pediatric kidney recipients. Am J Transplant 16: 2106–2116, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Khovanova N, Daga S, Shaikhina T, Krishnan N, Jones J, Zehnder D, Mitchell D, Higgins R, Briggs D, Lowe D: Subclass analysis of donor HLA-specific IgG in antibody-incompatible renal transplantation reveals a significant association of IgG4 with rejection and graft failure. Transpl Int 28: 1405–1415, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Zelm MC: B cells take their time: Sequential IgG class switching over the course of an immune response? Immunol Cell Biol 92: 645–646, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Lefaucheur C, Viglietti D, Bentlejewski C, Duong van Huyen JP, Vernerey D, Aubert O, Verine J, Jouven X, Legendre C, Glotz D, Loupy A, Zeevi A: IgG donor-specific anti-human HLA antibody subclasses and kidney allograft antibody-mediated injury. J Am Soc Nephrol 27: 293–304, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viglietti D, Loupy A, Vernerey D, Bentlejewski C, Gosset C, Aubert O, Duong van Huyen JP, Jouven X, Legendre C, Glotz D, Zeevi A, Lefaucheur C: Value of donor-specific anti-HLA antibody monitoring and characterization for risk stratification of kidney allograft loss. J Am Soc Nephrol 28: 702–715, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worthington JE, McEwen A, McWilliam LJ, Picton ML, Martin S: Association between C4d staining in renal transplant biopsies, production of donor-specific HLA antibodies, and graft outcome. Transplantation 83: 398–403, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Troxell ML, Weintraub LA, Higgins JP, Kambham N: Comparison of C4d immunostaining methods in renal allograft biopsies. Clin J Am Soc Nephrol 1: 583–591, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M; Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Hankey KG, Drachenberg CB, Papadimitriou JC, Klassen DK, Philosophe B, Bartlett ST, Groh V, Spies T, Mann DL: MIC expression in renal and pancreatic allografts. Transplantation 73: 304–306, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Sun Q, Cheng Z, Cheng D, Chen J, Ji S, Wen J, Zheng C, Liu Z: De novo development of circulating anti-endothelial cell antibodies rather than pre-existing antibodies is associated with post-transplant allograft rejection. Kidney Int 79: 655–662, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Dinavahi R, George A, Tretin A, Akalin E, Ames S, Bromberg JS, Deboccardo G, Dipaola N, Lerner SM, Mehrotra A, Murphy BT, Nadasdy T, Paz-Artal E, Salomon DR, Schröppel B, Sehgal V, Sachidanandam R, Heeger PS: Antibodies reactive to non-HLA antigens in transplant glomerulopathy. J Am Soc Nephrol 22: 1168–1178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott DM, Ehrmann IE, Ellis PS, Chandler PR, Simpson E: Why do some females reject males? The molecular basis for male-specific graft rejection. J Mol Med (Berl) 75: 103–114, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Aguilera I, Alvarez-Marquez A, Gentil MA, Fernandez-Alonso J, Fijo J, Saez C, Wichmann I, Nuñez-Roldan A: Anti-glutathione S-transferase T1 antibody-mediated rejection in C4d-positive renal allograft recipients. Nephrol Dial Transplant 23: 2393–2398, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Zou Y, Stastny P, Süsal C, Döhler B, Opelz G: Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med 357: 1293–1300, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schönemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G: Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 352: 558–569, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Kikić Ž, Kainz A, Kozakowski N, Oberbauer R, Regele H, Bond G, Böhmig GA: Capillary C4d and kidney allograft outcome in relation to morphologic lesions suggestive of antibody-mediated rejection. Clin J Am Soc Nephrol 10: 1435–1443, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orandi BJ, Alachkar N, Kraus ES, Naqvi F, Lonze BE, Lees L, Van Arendonk KJ, Wickliffe C, Bagnasco SM, Zachary AA, Segev DL, Montgomery RA: Presentation and outcomes of C4d-negative antibody-mediated rejection after kidney transplantation. Am J Transplant 16: 213–220, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lúcia M, Luque S, Crespo E, Melilli E, Cruzado JM, Martorell J, Jarque M, Gil-Vernet S, Manonelles A, Grinyó JM, Bestard O: Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int 88: 874–887, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Zachary AA, Lucas DP, Montgomery RA, Leffell MS: Rituximab prevents an anamnestic response in patients with cryptic sensitization to HLA. Transplantation 95: 701–704, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Umber A, Killackey M, Paramesh A, Liu Y, Qin H, Atiq M, Lee B, Alper AB, Simon E, Buell J, Zhang R: A comparison of three induction therapies on patients with delayed graft function after kidney transplantation. J Nephrol 30: 289–295, 2017 [DOI] [PubMed] [Google Scholar]
- 58.Rocha PN, Butterly DW, Greenberg A, Reddan DN, Tuttle-Newhall J, Collins BH, Kuo PC, Reinsmoen N, Fields T, Howell DN, Smith SR: Beneficial effect of plasmapheresis and intravenous immunoglobulin on renal allograft survival of patients with acute humoral rejection. Transplantation 75: 1490–1495, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Lorenz M, Regele H, Schillinger M, Kletzmayr J, Haidbauer B, Derfler K, Druml W, Böhmig GA: Peritransplant immunoadsorption: A strategy enabling transplantation in highly sensitized crossmatch-positive cadaveric kidney allograft recipients. Transplantation 79: 696–701, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Luke PP, Scantlebury VP, Jordan ML, Vivas CA, Hakala TR, Jain A, Somani A, Fedorek S, Randhawa P, Shapiro R: Reversal of steroid- and anti-lymphocyte antibody-resistant rejection using intravenous immunoglobulin (IVIG) in renal transplant recipients. Transplantation 72: 419–422, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Mulley WR, Hudson FJ, Tait BD, Skene AM, Dowling JP, Kerr PG, Kanellis J: A single low-fixed dose of rituximab to salvage renal transplants from refractory antibody-mediated rejection. Transplantation 87: 286–289, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Fehr T, Rüsi B, Fischer A, Hopfer H, Wüthrich RP, Gaspert A: Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation 87: 1837–1841, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Waiser J, Budde K, Schütz M, Liefeldt L, Rudolph B, Schönemann C, Neumayer HH, Lachmann N: Comparison between bortezomib and rituximab in the treatment of antibody-mediated renal allograft rejection. Nephrol Dial Transplant 27: 1246–1251, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Kizilbash S, Claes D, Ashoor I, Chen A, Jandeska S, Matar RB, Misurac J, Sherbotie J, Twombley K, Verghese P: Bortezomib in the treatment of antibody-mediated rejection in pediatric kidney transplant recipients: A multicenter Midwest Pediatric Nephrology Consortium study. Pediatr Transplant 21: e12873, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, Roy-Chaudhury P, Govil A, Mogilishetty G, Rike AH, Cardi M, Wadih G, Tevar A, Woodle ES: Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation 86: 1754–1761, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Pearl MH, Nayak AB, Ettenger RB, Puliyanda D, Palma Diaz MF, Zhang Q, Reed EF, Tsai EW: Bortezomib may stabilize pediatric renal transplant recipients with antibody-mediated rejection. Pediatr Nephrol 31: 1341–1348, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orandi BJ, Zachary AA, Dagher NN, Bagnasco SM, Garonzik-Wang JM, Van Arendonk KJ, Gupta N, Lonze BE, Alachkar N, Kraus ES, Desai NM, Locke JE, Racusen LC, Segev DL, Montgomery RA: Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 98: 857–863, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM: Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 11: 2405–2413, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD: Positive crossmatch kidney transplant recipients treated with eculizumab: Outcomes beyond 1 year. Am J Transplant 15: 1293–1302, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Viglietti D, Gosset C, Loupy A, Deville L, Verine J, Zeevi A, Glotz D, Lefaucheur C: C1 Inhibitor in acute antibody-mediated rejection nonresponsive to conventional therapy in kidney transplant recipients: A pilot study. Am J Transplant 16: 1596–1603, 2016 [DOI] [PubMed] [Google Scholar]
- 71.Opelz G, Döhler B: Collaborative Transplant Study Report: Influence of time of rejection on long-term graft survival in renal transplantation. Transplantation 85: 661–666, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Philogene MC, Sikorski P, Montgomery RA, Leffell MS, Zachary AA: Differential effect of bortezomib on HLA class I and class II antibody. Transplantation 98: 660–665, 2014 [DOI] [PubMed] [Google Scholar]
- 73.Cooper JE, Gralla J, Klem P, Chan L, Wiseman AC: High dose intravenous immunoglobulin therapy for donor-specific antibodies in kidney transplant recipients with acute and chronic graft dysfunction. Transplantation 97: 1253–1259, 2014 [DOI] [PubMed] [Google Scholar]
- 74.Smith RN, Malik F, Goes N, Farris AB, Zorn E, Saidman S, Tolkoff-Rubin N, Puri S, Wong W: Partial therapeutic response to Rituximab for the treatment of chronic alloantibody mediated rejection of kidney allografts. Transpl Immunol 27: 107–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kulkarni S, Kirkiles-Smith NC, Deng YH, Formica RN, Moeckel G, Broecker V, Bow L, Tomlin R, Pober JS: Eculizumab therapy for chronic antibody-mediated injury in kidney transplant recipients: A pilot randomized controlled trial. Am J Transplant 17: 682–691, 2017 [DOI] [PubMed] [Google Scholar]