Abstract
Background and objectives
Literature on the prognosis of patients with cirrhosis who require RRT for AKI is sparse and is confounded by liver transplant eligibility. An update on outcomes in the nonlisted subgroup is needed. Our objective was to compare outcomes in this group between those diagnosed with hepatorenal syndrome and acute tubular necrosis, stratifying by liver transplant listing status.
Design, setting, participants, & measurements
Retrospective cohort study of patients with cirrhosis acutely initiated on hemodialysis or continuous RRT at five hospitals, including one liver transplant center. Multivariable regression and survival analysis were performed.
Results
Four hundred seventy-two subjects were analyzed (341 not listed and 131 listed for liver transplant). Among nonlisted subjects, 15% (51 of 341) were alive at 6 months after initiating RRT. Median survival was 21 (interquartile range [IQR], 8, 70) days for those diagnosed with hepatorenal syndrome and 12 (IQR, 3, 43) days for those diagnosed with acute tubular necrosis (P=0.25). Among listed subjects, 48% (63 of 131) received a liver transplant. Median transplant-free survival was 15 (IQR, 5, 37) days for those diagnosed with hepatorenal syndrome and 14 (IQR, 4, 31) days for those diagnosed with acute tubular necrosis (P=0.60). When stratified by transplant listing, with adjusted Cox models we did not detect a difference in the risk of death between hepatorenal syndrome and acute tubular necrosis (hazard ratio [HR], 0.81; 95% confidence interval [95% CI], 0.59 to 1.11, among those not listed; HR, 0.73; 95% CI, 0.44 to 1.19, among those listed).
Conclusions
Cause of AKI was not significantly associated with mortality in patients with cirrhosis who required RRT. Among those not listed for liver transplant, mortality rates were extremely high in patients both with hepatorenal syndrome and acute tubular necrosis.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2017_11_09_CJASNPodcast_18_1_A.mp3
Keywords: dialysis; hemodialysis; hepatorenal syndrome; continuous renal replacement therapy; acute tubular necrosis; transplant-free survival; futility; cirrhosis; liver transplantation; acute kidney injury; renal replacement therapy; Humans; Proportional Hazards Models; Retrospective Studies; Kidney Tubular Necrosis, Acute; Survival Analysis; Liver Cirrhosis; renal dialysis; Prognosis
Introduction
AKI is a common and morbid complication of cirrhosis, occurring in up to 50% of those with decompensated cirrhosis (1,2). About one-third of this AKI improves with administration of albumin or crystalloid, leaving two-thirds of these patients with persistent AKI, such as hepatorenal syndrome (HRS) or acute tubular necrosis (ATN) (1–4). These AKI subtypes carry a dismal prognosis, with 40% requiring RRT and 60% dying by 90 days (3,4). Not unexpectedly, health care costs in this setting are high (5–7).
Those who require RRT represent the most morbid subgroup of the cirrhotic population (8). In addition to the typical acute complications of RRT, such as intradialytic hypotension (9–12), increased risk of cardiac events (13–15), and complications related to venous access (16,17), there are additional physiologic challenges inherent in those with cirrhosis (18). Portal hypertension and splanchnic vasodilation result in decreased effective circulating volume, ascites formation, and low mean arterial pressure (19–21), all of which are barriers to adequate volume management and often necessitate transfer to the intensive care unit for support with intravenous vasopressors and continuous RRT (CRRT) (18).
Currently, there are few studies to guide clinical judgment of which patients with severe cirrhosis are optimal candidates for RRT. The available literature is limited by small sample sizes (22–24), older studies that do not reflect modern practice patterns (25–27), or inclusion of only liver transplantation candidates/recipients (23,24,28–35). Although RRT usually serves as a bridge to liver transplantation among those listed, literature is sparse regarding the use of RRT in those not listed for transplantation, particularly in HRS. Over time, advancements in the medical management of cirrhosis have led to allograft allocation at higher Model for End-Stage Liver Disease (MELD) scores, thus creating a larger population of noncandidates with indications for RRT (36). To provide an update in this growing group, we aimed to compare outcomes after initiation of RRT in cirrhosis, while stratifying by transplant listing status and adjusting for cause of AKI.
Materials and Methods
Patient Population and Data Collection
We performed a retrospective cohort study of all inpatients with cirrhosis and AKI requiring RRT for either HRS or ATN between 2005 and 2015 in a network of five acute care hospitals (Partners Healthcare, Massachusetts), including one liver transplant center (Massachusetts General Hospital, Boston, MA), where all transplant listing evaluation was done for this population. Data were identified using a centralized clinical data collection warehouse designed for research and quality improvement purposes (37–39). All subjects were treated using local standard of care without intervention from the study team. Follow-up data were obtained via electronic medical record and database review and, if needed, by search of online obituaries and Social Security Death Index. Data were complete for each variable, except as noted in table/figure footnotes.
Hemodialysis (HD), CRRT treatments, and clinical diagnoses were identified using the Current Procedural Terminology, 4th Edition and International Classification of Diseases-9/10 codes (see Supplemental Table 1). All potential subjects underwent review of the electronic medical record to confirm accuracy of diagnostic codes and medical history (see Definitions). MELD score and Chronic Liver Failure Consortium Acute-on-Chronic Liver Failure (CLIF-C ACLF) score were calculated at the time of RRT initiation (40,41). Subjects were excluded if they were lost to follow-up after discharge (n=3).
Definitions
Definitions and causes of cirrhosis were on the basis of clinical determination by the treating hepatologist. Subjects were classified as “not listed” if they were evaluated and rejected for liver transplant listing, or if they were felt not to be appropriate for transplant evaluation by treating clinicians (due to critical illness, ongoing alcohol use, etc.). Subjects were classified as “listed” for liver transplant if they were registered on the United Network for Organ Sharing waiting list (before or after the start of RRT) at the study’s liver transplant center.
All subjects received either intermittent HD or continuous veno-venous hemofiltration (CVVH) as their modality of RRT. CVVH was performed in lieu of intermittent HD when subjects required intravenous vasopressor support and/or were considered hemodynamically unstable by the treating nephrologist. Cause of AKI was on the basis of the clinical evaluation by the treating nephrologist at the time of initiation of RRT. HRS was diagnosed after exclusion of other potential causes of AKI on the basis of diagnostic criteria at the time (42,43). ATN was diagnosed when kidney parameters did not respond to volume administration and clinical history was consistent with ischemic or nephrotoxic AKI. One author confirmed HRS versus ATN status via chart review. If the treating nephrologist identified a mixed type of HRS and ATN, the cause was considered ATN, given HRS is a diagnosis of exclusion. If there was discordance between the treating clinician and author review, a second author provided the tie-breaking diagnosis.
Outcomes and Statistical Analyses
The primary outcome for this study was 6-month survival. Transplant-free survival was also assessed among those listed for liver transplant. Secondary outcomes assessed included rates of solitary liver or dual liver-kidney transplantation, and dialysis dependence at 6 months. A multivariable Cox model was created using a univariate screen and stepwise selection algorithm to identify significant factors associated with 6-month mortality (covariates were entered into the model if P<0.1 and were retained in the final model if P<0.05). Differences in the primary outcome between HRS and ATN subgroups were visualized using a Kaplan–Meier curve and compared using a log-rank test. Prespecified Cox proportional hazard models were performed to determine the effect of HRS versus ATN on mortality after adjusting for age, MELD score, and initial RRT modality. Results of Cox proportional hazard models were summarized with hazard ratios (HRs) and Wald asymptotic 95% confidence intervals (95% CIs) and given a normal distribution of deviance residuals; continuous variables were presented as means and 95% CIs, except for median survival time, which was presented as median and interquartile range (IQR). SAS version 9.4 (Gary, NC) and R version 3.2.2 were used for analysis (44). Two tailed P values <0.05 were considered statistically significant.
Ethics Statement
The Partners Institutional Review Board approved this study. All procedures and practices abide by the guidelines set forth by the Declarations of Helsinki and Istanbul. The need for informed consent was waived for this study.
Results
General Demographics
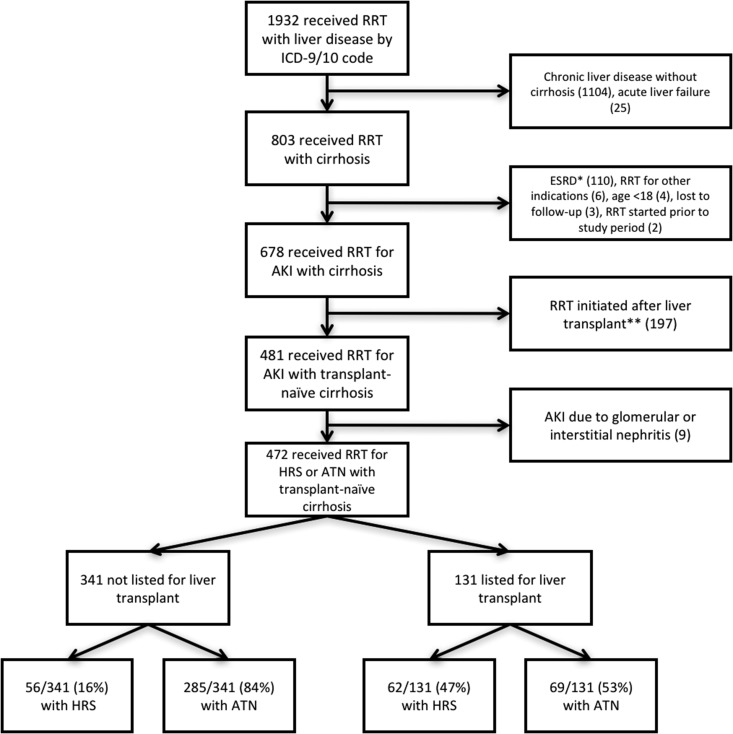
Figure 1 describes the selection of the cohort used in final analysis. Four hundred seventy-two subjects were included in the final analysis. Three hundred forty-one were not listed for liver transplant and 131 were listed for liver transplant. Eighty-five percent of subjects were either initially evaluated at Massachusetts General Hospital or were transferred there after initiating RRT for expedited transplant evaluation. Of those not listed, 16% (56 of 341) were diagnosed with HRS and 84% (285 of 341) were diagnosed with ATN. Of those listed, 47% (62 of 131) were diagnosed with HRS and 53% (69 of 131) were diagnosed with ATN. Sixty-four percent (84 of 131) were listed before initiating RRT. Subjects were less likely to be initiated on RRT for HRS if they were not listed for liver transplant (relative risk, 0.59; 95% CI, 0.48 to 0.72; P<0.001).
Figure 1.
Flow diagram of patient selection. *Includes subjects with a prior kidney transplant or on chronic dialysis. **Includes subjects initiated on RRT immediately postoperatively and subjects with a prior history of liver transplant. ATN, acute tubular necrosis; HRS, hepatorenal syndrome; ICD-9/10, International Classification of Diseases 9/10.
Demographics and baseline characteristics of subjects stratified by transplant listing status and cause of AKI are presented in Table 1. Age, sex, race, and ethnicity were similar between those with HRS and ATN in both nonlisted and listed subgroups. Among nonlisted subjects, those with HRS had higher MELD and CLIF-C ACLF scores at the start of RRT compared with those with ATN. Sepsis, admission to the intensive care unit, use of intravenous vasopressors, and mechanical ventilation were more common among those with ATN compared with HRS in both subgroups. Fifty-seven percent (271 of 472) of all subjects initially started on HD. Among these 271 subjects, 128 later transitioned to CVVH for a total usage rate of 70% (329 of 472) for CVVH.
Table 1.
Demographics and baseline characteristics of 472 patients with cirrhosis initiated on RRT for AKI
| Characteristic | Not Listed for Liver Transplant | Listed for Liver Transplant | ||||
|---|---|---|---|---|---|---|
| All Not Listed | HRS | ATN | All Listed | HRS | ATN | |
| n=341 | n=56 | n=285 | n=131 | n=62 | n=69 | |
| Age, yr | 58 (57 to 59) | 57 (53 to 60) | 58 (57 to 60) | 55 (54 to 57) | 56 (54 to 59) | 54 (52 to 57) |
| Female sex | 111 (33) | 17 (30) | 94 (33) | 51 (39) | 26 (42) | 25 (36) |
| White race | 257 (75) | 40 (71) | 217 (76) | 112 (86) | 55 (89) | 57 (83) |
| Non-Hispanic ethnicity | 317 (93) | 53 (95) | 264 (93) | 124 (95) | 59 (95) | 65 (94) |
| Comorbidities | ||||||
| Diabetes mellitus | 126 (37) | 20 (36) | 106 (37) | 44 (34) | 22 (36) | 22 (32) |
| Coronary artery disease | 98 (29) | 17 (30) | 81 (28) | 28 (21) | 14 (23) | 14 (20) |
| CKD | 54 (16) | 18 (32) | 36 (13) | 25 (19) | 12 (19) | 13 (19) |
| Hypertension | 187 (55) | 32 (57) | 155 (54) | 76 (58) | 41 (66) | 35 (51) |
| Reason for admission | ||||||
| Complications of cirrhosis | 96 (28) | 28 (50) | 68 (24) | 75 (57) | 41 (66) | 34 (49) |
| AKI | 43 (13) | 15 (27) | 28 (10) | 23 (18) | 15 (24) | 8 (12) |
| Infection | 126 (37) | 8 (14) | 118 (41) | 23 (18) | 4 (6) | 19 (28) |
| Other | 76 (22) | 5 (9) | 71 (25) | 10 (8) | 2 (3) | 8 (12) |
| Other hospitalization characteristics | ||||||
| Sepsis | 206 (60) | 17 (30) | 189 (66) | 73 (56) | 27 (44) | 46 (67) |
| Admission/transfer to intensive care unit | 287 (85) | 34 (61) | 253 (89) | 99 (76) | 41 (66) | 58 (84) |
| Intravenous vasopressor use | 260 (76) | 28 (50) | 232 (81) | 91 (69) | 35 (56) | 56 (81) |
| Mechanical ventilation | 197 (58) | 9 (16) | 188 (66) | 54 (41) | 19 (31) | 35 (51) |
| Length of stay, d | 21 (19 to 23) | 18 (14 to 23) | 21 (18 to 24) | 35 (30 to 39) | 33 (26 to 39) | 36 (29 to 43) |
| Initial renal replacement modality | ||||||
| Intermittent hemodialysis | 195 (57) | 41 (73) | 154 (54) | 76 (58) | 39 (63) | 37 (54) |
| Continuous veno-venous hemofiltration | 146 (43) | 15 (27) | 131 (46) | 55 (42) | 23 (37) | 32 (46) |
| Cause of cirrhosisa | ||||||
| Alcohol | 135 (40) | 24 (45) | 111 (39) | 40 (31) | 23 (37) | 17 (25) |
| Hepatitis C | 59 (17) | 7 (13) | 52 (18) | 27 (21) | 12 (19) | 15 (22) |
| Nonalcoholic steatohepatitis | 21 (6) | 5 (9) | 16 (6) | 20 (15) | 10 (16) | 10 (14) |
| Multifactorial | 45 (13) | 9 (16) | 36 (13) | 14 (11) | 6 (10) | 8 (12) |
| Other | 79 (23) | 10 (18) | 69 (24) | 30 (23) | 11 (17) | 19 (28) |
| Prior complications of liver disease | ||||||
| Ascites | 263 (77) | 53 (95) | 210 (74) | 124 (95) | 62 (100) | 62 (90) |
| Encephalopathy | 186 (55) | 36 (64) | 150 (53) | 93 (71) | 49 (79) | 44 (64) |
| Gastrointestinal bleeding | 215 (63) | 39 (70) | 176 (62) | 86 (66) | 44 (71) | 42 (61) |
| Spontaneous bacterial peritonitis | 61 (18) | 9 (16) | 52 (18) | 53 (40) | 30 (48) | 23 (33) |
| Hepatocellular carcinoma | 29 (9) | 8 (14) | 21 (7) | 12 (9) | 4 (6) | 8 (12) |
| MELD score | 34 (33 to 34) | 36 (34 to 38) | 33 (32 to 34) | 36 (35 to 37) | 37 (35 to 38) | 35 (33 to 37) |
| CLIF-C ACLF score | 60 (59 to 62) | 50 (47 to 53) | 62 (60 to 63) | 58 (56 to 60) | 55 (52 to 58) | 59 (56 to 63) |
| Laboratory values | ||||||
| Sodium, meq/L | 136 (135 to 136) | 134 (133 to 136) | 136 (135 to 137) | 135 (134 to 136) | 135 (133 to 136) | 135 (134 to 137) |
| BUN, mg/dl | 65 (61 to 69) | 61 (53 to 68) | 66 (61 to 70) | 65 (60 to 70) | 70 (63 to 77) | 61 (54 to 68) |
| Creatinine, mg/dl | 4.3 (4.1 to 4.5) | 5.3 (4.9 to 5.8) | 4.1 (3.9 to 4.4) | 4.1 (3.9 to 4.4) | 4.6 (4.2 to 4.9) | 3.8 (3.4 to 4.2) |
| Urine sodium, mmol/Lb | 36 (29 to 42) | 33 (1 to 66) | 36 (32 to 40) | 24 (19 to 29) | 20 (14 to 25) | 29 (21 to 36) |
| White blood count, K/μl | 14.8 (13.7 to 15.9) | 10.5 (8.7 to 12.3) | 15.6 (14.4 to 16.9) | 10.4 (9.2 to 11.6) | 9.6 (8.3 to 10.9) | 11.1 (9.1 to 13.2) |
| Hemoglobin, g/dl | 9.3 (9.2 to 9.5) | 9.4 (8.9 to 9.9) | 9.3 (9.1 to 9.5) | 9.0 (8.7 to 9.2) | 9.0 (8.6 to 9.3) | 9.0 (8.6 to 9.3) |
| Platelets, K/μl | 102 (95 to 110) | 88 (72 to 104) | 105 (97 to 113) | 76 (68 to 85) | 72 (61 to 83) | 80 (67 to 93) |
| Albumin, g/dlc | 2.9 (2.8 to 3.0) | 3.2 (3.1 to 3.4) | 2.8 (2.8 to 2.9) | 3.3 (3.2 to 3.4) | 3.4 (3.2 to 3.5) | 3.2 (3.0 to 3.4) |
| International normalized ratio | 2.1 (2.0 to 2.2) | 2.0 (1.8 to 2.3) | 2.1 (2.0 to 2.2) | 2.2 (2.1 to 2.3) | 2.1 (2.0 to 2.3) | 2.2 (2.0 to 2.4) |
| Total bilirubin, mg/dl | 12 (10 to 13) | 14 (10 to 19) | 11 (10 to 13) | 16 (13 to 18) | 16 (12 to 20) | 16 (13 to 19) |
| Aspartate aminotransferase, U/Ld | 577 (390 to 764) | 452 (−20 to 924) | 602 (397 to 807) | 254 (−20 to 528) | 101 (46 to 156) | 391 (−130 to 913) |
| Alanine aminotransferase, U/Ld | 189 (137 to 241) | 177 (11 to 344) | 192 (139 to 244) | 120 (−8 to 250) | 40 (29 to 52) | 191 (−54 to 436) |
| Alkaline phosphatase, U/Ld | 162 (145 to 178) | 146 (112 to 181) | 165 (146 to 183) | 114 (102 to 126) | 113 (97 to 130) | 116 (98 to 133) |
Cells represent N (percent) for categoric variables and mean (95% confidence interval) for continuous variables. HRS, hepatorenal syndrome; ATN, acute tubular necrosis; MELD, Model for End Stage Liver Disease; CLIF-C ACLF, Chronic Liver Failure Consortium Acute-on-Chronic Liver Failure Score.
n=470 available.
n=357 available.
n=471 available.
n=468 available.
A reference group of 159 hospitalized subjects with cirrhosis, severe AKI (serum creatinine >4 mg/dl), and documentation that RRT was not offered due to patient/provider decision was compared with the 341 nonlisted subjects who did receive RRT (see Supplemental Table 2). Subjects treated with RRT were similar to those not treated with RRT in sex, race, ethnicity, comorbidities, and cause of cirrhosis. Subjects not treated with RRT were slightly older (61 [95% CI, 59 to 63] versus 58 [95% CI, 57 to 59] years; P=0.02), had higher MELD scores (40 [95% CI, 39 to 42] versus 34 [95% CI, 33 to 35]; P<0.001), were more likely to be diagnosed with HRS versus ATN (43% versus 16%; P<0.001), were more likely to have hepatocellular carcinoma (25% versus 9%; P<0.001), were less likely to use intensive care unit resources, had shorter median hospital length of stay (10 [IQR, 5, 16] versus 17 [IQR, 8, 28] days; P<0.001), and had shorter median survival (2 [IQR, 1, 7] versus 14 [IQR, 4, 50] days; P<0.001).
Six-Month Survival: All Subjects
Twenty-four percent (114 of 472) of all subjects were alive at 6 months. Table 2 summarizes demographics and characteristics of all subjects by vital status at 6 months. Age, sex, and ethnicity were similar between those who were alive versus died by 6 months. Those alive at 6 months were more likely to be of white race, were more commonly initiated on HD compared with CVVH, and had lower CLIF-C ACLF scores. Figure 2 summarizes raw outcomes at 6 months for all subjects, stratified by transplant listing status.
Table 2.
Characteristics of 472 patients with cirrhosis initiated on RRT for AKI, by vital status at 6 mo
| Characteristic | Alive at 6 mo (n=114) | Died by 6 mo (n=358) | P Value |
|---|---|---|---|
| Age, yr | 56 (54 to 58) | 58 (56 to 59) | 0.28 |
| Female sex | 39 (34) | 123 (34) | 0.97 |
| White race | 97 (85) | 272 (76) | 0.04 |
| Non-Hispanic ethnicity | 109 (96) | 332 (93) | 0.28 |
| Comorbidities | |||
| Diabetes mellitus | 51 (45) | 119 (33) | 0.03 |
| Coronary artery disease | 32 (28) | 94 (26) | 0.70 |
| CKD | 24 (21) | 55 (15) | 0.15 |
| Hypertension | 74 (65) | 189 (53) | 0.02 |
| Reason for admission | <0.001 | ||
| Complications of cirrhosis | 53 (46) | 118 (33) | |
| AKI | 25 (22) | 41 (11) | |
| Infection | 20 (18) | 129 (36) | |
| Other | 16 (14) | 70 (20) | |
| Other hospitalization characteristics | |||
| Sepsis | 57 (50) | 222 (62) | 0.02 |
| Admission/transfer to intensive care unit | 71 (63) | 315 (88) | <0.001 |
| Intravenous vasopressor use | 65 (57) | 286 (80) | <0.001 |
| Mechanical ventilation | 41 (36) | 210 (59) | <0.001 |
| Length of stay, d | 38 (32 to 44) | 20 (18 to 22) | <0.001 |
| Initial renal replacement modality | 0.01 | ||
| Intermittent hemodialysis | 77 (68) | 194 (54) | |
| Continuous veno-venous hemofiltration | 37 (32) | 164 (46) | |
| Cause of cirrhosisa | 0.10 | ||
| Alcohol | 36 (32) | 139 (39) | |
| Hepatitis C | 17 (15) | 69 (19) | |
| Nonalcoholic steatohepatitis | 16 (14) | 25 (7) | |
| Multifactorial | 16 (14) | 43 (12) | |
| Other | 29 (25) | 80 (22) | |
| Prior complications of liver disease | |||
| Ascites | 95 (83) | 292 (82) | 0.67 |
| Encephalopathy | 78 (68) | 201 (56) | 0.02 |
| Gastrointestinal bleeding | 74 (65) | 227 (63) | 0.77 |
| Spontaneous bacterial peritonitis | 35 (31) | 79 (22) | 0.06 |
| Hepatocellular carcinoma | 10 (9) | 31 (9) | 0.97 |
| MELD score | 33 (32 to 35) | 35 (34 to 35) | 0.12 |
| CLIF-C ACLF score | 55 (52 to 57) | 61 (60 to 63) | <0.001 |
| Cause of AKI | <0.001 | ||
| Hepatorenal syndrome | 43 (38) | 75 (21) | |
| Acute tubular necrosis | 71 (62) | 283 (79) | |
| Laboratory values | |||
| Sodium, meq/L | 135 (134 to 136) | 136 (135 to 136) | 0.27 |
| BUN, mg/dl | 62 (56 to 68) | 66 (62 to 69) | 0.26 |
| Creatinine, mg/dl | 4.6 (4.2 to 5.0) | 4.2 (4.0 to 4.4) | 0.02 |
| Urine sodium, mmol/Lb | 31 (25 to 37) | 33 (27 to 38) | 0.66 |
| White blood count, K/μl | 11.5 (10.0 to 13.1) | 14.2 (13.2 to 15.2) | 0.01 |
| Hemoglobin, g/dl | 9.0 (8.7 to 9.2) | 9.3 (9.2 to 9.5) | 0.04 |
| Platelets, K/μl | 96 (83 to 108) | 95 (88 to 102) | 0.92 |
| Albumin, g/dlc | 3.1 (3.0 to 3.2) | 3.0 (2.9 to 3.0) | 0.03 |
| International normalized ratio | 1.9 (1.8 to 2.0) | 2.2 (2.1 to 2.3) | <0.001 |
| Total bilirubin, mg/dl | 12 (9 to 14) | 13 (12 to 15) | 0.32 |
| Aspartate aminotransferase, U/Ld | 306 (−17 to 629) | 544 (366 to 721) | 0.20 |
| Alanine aminotransferase, U/Ld | 89 (40 to 137) | 195 (129 to 261) | 0.01 |
| Alkaline phosphatase, U/Ld | 128 (113 to 143) | 155 (139 to 171) | 0.01 |
Cells represent N (percent) for categoric variables and mean (95% confidence interval) for continuous variables. MELD, Model for End Stage Liver Disease; CLIF-C ACLF, Chronic Liver Failure Consortium Acute-on-Chronic Liver Failure Score.
n=470 available.
n=357 available.
n=471 available.
n=468 available.
Figure 2.
Summary of unadjusted outcomes at 6 months among 472 patients with cirrhosis initiated on RRT for AKI. *Includes subjects who died within 6 months after liver or liver/kidney transplant (n=8). ATN, acute tubular necrosis; HRS, hepatorenal syndrome.
Twenty candidate variables were entered into a stepwise algorithm to evaluate 6-month mortality in multivariable Cox regression. MELD score was included over CLIF-C ACLF score due to colinearity and its wider current clinical use. Seven variables were significantly associated with mortality in the final model (see Table 3), including nonlisted transplant status, MELD score, age, admission to the intensive care unit serum alanine aminotransferase, mechanical ventilation, and initiation with CVVH.
Table 3.
Multivariable Cox regression model for 6-mo mortality among 472 patients with cirrhosis initiated on RRT for AKI
| Variable | Wald Chi Square Score | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|---|
| Not listed for liver transplant | 48.4 | 2.67 | (2.02 to 3.51) | <0.001 |
| MELD score (per five points) | 28.7 | 1.20 | (1.12 to 1.29) | <0.001 |
| Age (per 10 yr) | 17.4 | 1.21 | (1.11 to 1.33) | <0.001 |
| Admission to the intensive care unit | 13.1 | 1.99 | (1.37 to 1.68) | <0.001 |
| Serum ALT (per 100 U/L) | 12.8 | 1.03 | (1.02 to 1.05) | <0.001 |
| Mechanical ventilation | 5.1 | 1.32 | (1.04 to 1.67) | 0.02 |
| Initial renal replacement: CVVH | 3.9 | 1.25 | (1.00 to 1.57) | 0.05 |
Twenty candidate variables from key demographics and a univariate screen of significant univariate associations with 6-mo mortality were entered into a stepwise selection algorithm for this Cox regression. Variables were entered in the model if P<0.10 and were retained in the final model if P<0.05. 95% CI, 95% confidence interval; MELD, Model for End-Stage Liver Disease; ALT, alanine aminotransferase; CVVH, continuous veno-venous hemofiltration.
Fifty-nine subjects who were alive at 6 months and did not receive a liver transplant were analyzed for factors associated with kidney recovery. Thirty-four of 59 (58%) recovered off dialysis by 6 months. Those who recovered were similar in sex, race, ethnicity, comorbidities, and MELD/CLIF-C ACLF scores compared with those who did not recover. Those who recovered were younger (54 [95% CI, 50 to 59] versus 62 [95% CI, 56 to 67] years; P=0.05), were less likely to have preadmission CKD (33% versus 67%; P=0.02), were less likely to have a prior history of ascites (49% versus 82%; P=0.02), and were more likely to have alcoholic cirrhosis (41% versus 21%; P=0.01). During their admission, those who recovered were more likely to be diagnosed with ATN versus HRS (69% versus 29%; P<0.01), have sepsis (74% versus 45%; P=0.02), have higher white blood cell count (13.3 [95% CI, 9.8 to 16.9] versus 9.3 [95% CI, 7.1 to 11.5]; P=0.05), and require mechanical ventilation (77% versus 47%; P=0.02) and CVVH (81% versus 50%; P=0.03).
Outcomes for Nonlisted Subjects
Among nonlisted subjects, 15% (51 of 341) were alive at 6 months after initiating RRT. Survival was similar between HRS and ATN subgroups, with an unadjusted median survival of 21 (IQR, 8, 70) days for HRS, 12 (IQR, 3, 43) days for ATN (P=0.25; Figure 3A), and 14 (IQR, 4, 50) days for all nonlisted subjects. In a model adjusting for age, MELD score, and initial RRT modality, subjects diagnosed with HRS had similar risk of death as ATN (HR, 0.81; 95% CI, 0.59 to 1.11; P=0.19; Table 4). Sensitivity analyses showed similar results when (1) substituting CLIF-C ACLF score for MELD score and age, (2) substituting other indicators of critical illness for initial RRT modality (admission to the intensive care unit, use of intravenous vasopressors, use of mechanical ventilation), and (3) including cause of cirrhosis as an additional covariate. Seventy-eight percent (40 of 51) who were alive at 6 months recovered kidney function and were off dialysis (2 of 9 for HRS and 38 of 42 for ATN).
Figure 3.
Survival of 472 patients with cirrhosis acutely initiated on RRT, according to clinical diagnosis at dialysis initiation. (A) Survival among subjects not listed for liver transplant. (B) Survival among subjects listed for liver transplant. (C) Transplant-free survival among subjects listed for liver transplant.
Table 4.
Multivariable Cox regression models for 6-mo outcomes among 472 patients with cirrhosis initiated on RRT for AKI
| Model | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| A. Not listed for liver transplant, death by 6 mo | |||
| Hepatorenal syndromea | 0.81 | (0.59 to 1.11) | 0.19 |
| Age (per 10 yr) | 1.17 | (1.06 to 1.28) | 0.001 |
| MELD score (per 5 points) | 1.28 | (1.19 to 1.37) | <0.001 |
| Initial renal replacement: CVVHb | 1.63 | (1.28 to 2.08) | <0.001 |
| B. Listed for liver transplant, death by 6 mo | |||
| Hepatorenal syndromea | 0.73 | (0.44 to 1.19) | 0.21 |
| Age (per 10 yr) | 1.05 | (0.81 to 1.36) | 0.71 |
| MELD score (per 5 points) | 0.88 | (0.73 to 1.07) | 0.21 |
| Initial renal replacement: CVVHb | 1.95 | (1.19 to 3.19) | <0.01 |
| C. Listed for liver transplant, death or transplant by 6 mo | |||
| Hepatorenal syndromea | 0.93 | (0.64 to 1.35) | 0.70 |
| Age (per 10 yr) | 1.22 | (1.00 to 1.50) | 0.05 |
| MELD score (per 5 points) | 1.25 | (1.07 to 1.46) | <0.01 |
| Initial renal replacement: CVVHb | 2.58 | (1.72 to 3.87) | <0.001 |
95% CI, 95% confidence interval; MELD, Model for End-Stage Liver Disease; CVVH, continuous veno-venous hemofiltration.
Reference group: acute tubular necrosis.
Reference group: initial renal replacement: hemodialysis.
Outcomes for Listed Subjects
Among listed subjects, survival was similar between HRS and ATN subgroups (P=0.12; Figure 3B). In a model adjusting for age, MELD score, and initial RRT modality, subjects with HRS had similar risk of death as ATN (HR, 0.73; 95% CI, 0.44 to 1.19; P=0.21; Table 4). This was also true for transplant-free survival in unadjusted analysis (median transplant-free survival of 15 [IQR, 5, 37] days for HRS and 14 [IQR, 4, 31] days for ATN; P=0.60; Figure 3C) and adjusted analysis (HR, 0.93; 95% CI, 0.64 to 1.35; P=0.70; Table 4). Median transplant-free survival was 14 (IQR, 4, 34) days for all listed subjects. As with the nonlisted subgroup, sensitivity analyses substituting other critical illness indicators, CLIF-C ACLF score, and including cause of cirrhosis did not change results.
Once listed, 48% (63 of 131) of subjects later received a liver transplant. Twenty-nine percent (18 of 63) of those who received a liver transplant also received dual kidney transplant. Liver transplantation rates were similar between HRS and ATN subgroups (52% versus 45%; P=0.44). Among listed subjects who did not receive a liver transplant, 38% (3 of 8) of those who were alive at 6 months recovered kidney function and were off dialysis (2 of 6 with HRS and 1 of 2 with ATN).
Discussion
Those with AKI and cirrhosis represent an extremely challenging and highly morbid group of patients. Current therapies for AKI are largely supportive, with no drug therapies approved for ATN. Although terlipressin is an effective therapy for HRS, it is not available in North America (45,46). Thus, it is essential that we carefully employ existing therapies, such as RRT. Given a paucity of evidence around nonlisted cirrhotic patients who require RRT, we provide an important prognostic update for this growing population. We confirmed a high mortality overall, especially in the nonlisted subgroup; however, we found no significant differences in outcomes between those diagnosed with HRS and those diagnosed with ATN within listed or nonlisted groups.
As context, there are only three studies examining survival after acute initiation of RRT among nonlisted subjects with cirrhosis (25–27). Two were published in the 1970s and had 100% short-term mortality in small cohorts of 14 and 25 cirrhotic patients (25,26). In a third 2004 study of 30 patients, mortality was 100% among those treated with CRRT and mechanical ventilation (27). This literature has guided our approach to RRT in cirrhosis, suggesting that offering RRT to nonlisted patients with HRS may not be of benefit, but may not reflect the effect of recent advances in the general care of the cirrhotic patient (43,47).
Our observation that mortality was similar between HRS and ATN warrants further discussion. Several prior studies, including work at our center, have shown a mortality difference when comparing HRS, ATN, and prerenal injury across a wide spectrum of AKI severity (3,4). However, when isolating those with severe AKI requiring RRT, we did not observe a statistically significant difference between HRS and ATN subgroups. Although we should acknowledge that sample size and site-specific practice patterns raise the possibility of a false-negative statistical result, the short overall median survival of 14 days in the nonlisted population suggests that AKI cause may not translate into a clinically meaningful factor in the dialysis-requiring cirrhotic population. Complications of dialysis are not AKI-cause–specific and their clinical effect may supersede that of underlying HRS versus ATN in this group (9–19). Alternatively, AKI requiring RRT in severe liver failure may be a marker of the likelihood of further deterioration or other organ dysfunction that may not necessarily be improved by the provision of RRT. Our stepwise multivariable model showed that several known predictors of survival in cirrhosis (such as MELD score, age, and transplant listing status) and indicators of critical illness (such as initiation with CVVH and mechanical ventilation) were more closely associated with mortality and thus should be emphasized more than cause of AKI when deciding to employ RRT. Of note, our results likely apply best to patients in North America, where first-line vasoconstrictor therapy is limited to midodrine/octreotide (an ineffective, off-label therapy) (48), and therefore does not capture terlipressin’s effect on the natural history of HRS (49).
In addition, it is worth considering the potential for confounding-by-indication bias in this population. RRT was offered less frequently to those with HRS who were not listed for transplant. Our data show that those treated with RRT with ATN represented a sicker population compared with HRS. This suggests that the decision to employ RRT was dependent on underlying AKI diagnosis. However, given 6-month mortality after initiation of RRT was similar among nonlisted patients with ATN and a more selected group with HRS (approximately 85%), a wider appreciation of the extremely high mortality associated with ATN in cirrhosis is needed, as are further studies that improve understanding of best care practices regarding the intensity of care and the role of supportive services in this setting.
Overall, we would encourage clinicians to continue a thoughtful, evidence-based approach when employing RRT in the nonlisted cirrhotic population, acknowledging the guarded prognosis in this group. Although mortality is high in both HRS and ATN, there may be a select group of patients where a time-limited trial of RRT is appropriate, particularly those who exhibit fewer signs of critical illness. Chance of kidney recovery may also factor into this clinical decision making. Still, this population utilizes tremendous medical resources and has long hospital lengths of stay, with a majority requiring intensive care unit level of care. Our data confirms that use of RRT as a bridge to transplant in the listed population should be a straightforward treatment decision. In this group, we included 47 patients who were listed after initiation of RRT, which adds generalizability to those where transplant candidacy is unknown when RRT is required. Although the use of time-limited trials of RRT was not easily quantifiable in this study, it is reasonable to incorporate this approach in these high-risk groups.
This study should be interpreted in the context of its limitations. This was a retrospective analysis; thus, all findings should be viewed as associations rather than causal relationships. However, it is unlikely that a prospective or randomized trial will be conducted in this population; thus, this may be the optimal practical study design. Although data were obtained from five acute care hospitals, only one was a liver transplant center, which evaluated a majority of patients in this study. Thus, results may be colored by local practice patterns and these findings should be confirmed at other liver transplant centers. Potential for confounding-by-indication bias in patient selection was outlined in detail above. In the listed subgroup, competing risks between death, transplant listing, and receipt of transplant should be acknowledged. There was no significant difference between time from RRT initiation to transplant listing date between subjects with HRS versus ATN, and results were similar when analyzing survival versus transplant-free survival; thus, we feel this was unlikely to have a major statistical effect on our overall conclusions. Several clinical diagnoses were made by a range of treating physicians; thus, there may be variability in interpretation of clinical guidelines. Overall, this study reflects actual practice patterns without influence of the study team and we believe this adds interpretability to our results.
The cause of AKI (HRS versus ATN) was not significantly associated with mortality in patients with cirrhosis who required RRT. Transplant listing status, MELD score, and indicators of critical illness were more closely associated with survival. Among those not listed for liver transplant, mortality rates were extremely high in patients both with HRS and ATN. These findings should be validated at other high-volume liver transplant referral centers to improve generalizability of the results.
Disclosures
None.
Supplementary Material
Acknowledgments
A.S.A. is supported by a grant from the American College of Gastroenterology and has consulted for Ferring Pharmaceuticals. R.T.C. is supported by National Institutes of Health (NIH) grant K24DK078772. R.I.T. is supported by NIH grants R01DK094486 and K24DK094872. N.D.E. is supported by NIH grant K23DK114526.
All authors meet International Committee of Medical Journal Editors criteria and have approved the final manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03610417/-/DCSupplemental.
References
- 1.Garcia-Tsao G, Parikh CR, Viola A: Acute kidney injury in cirrhosis. Hepatology 48: 2064–2077, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Huelin P, Piano S, Solà E, Stanco M, Solé C, Moreira R, Pose E, Fasolato S, Fabrellas N, de Prada G, Pilutti C, Graupera I, Ariza X, Romano A, Elia C, Cárdenas A, Fernández J, Angeli P, Ginès P: Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute on chronic liver failure. Clin Gastroenterol Hepatol 15: 438–445, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Martín-Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, Solá E, Pereira G, Marinelli M, Pavesi M, Fernández J, Rodés J, Arroyo V, Ginès P: Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 140: 488–496.e4, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Allegretti AS, Ortiz G, Wenger J, Deferio JJ, Wibecan J, Kalim S, Tamez H, Chung RT, Karumanchi SA, Thadhani RI: Prognosis of acute kidney injury and hepatorenal syndrome in patients with cirrhosis: A prospective cohort study. Int J Nephrol 2015: 108139, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suneja M, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM: Population based trends in the incidence of hospital admission for the diagnosis of hepatorenal syndrome: 1998-2011. Int J Nephrol 2016: 8419719, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, Dellavalle R, Danaei G, Ezzati M, Fahimi A, Flaxman D, Foreman, Gabriel S, Gakidou E, Kassebaum N, Khatibzadeh S, Lim S, Lipshultz SE, London S, Lopez, MacIntyre MF, Mokdad AH, Moran A, Moran AE, Mozaffarian D, Murphy T, Naghavi M, Pope C, Roberts T, Salomon J, Schwebel DC, Shahraz S, Sleet DA, Murray, Abraham J, Ali MK, Atkinson C, Bartels DH, Bhalla K, Birbeck G, Burstein R, Chen H, Criqui MH, Dahodwala, Jarlais, Ding EL, Dorsey ER, Ebel BE, Ezzati M, Fahami, Flaxman S, Flaxman AD, Gonzalez-Medina D, Grant B, Hagan H, Hoffman H, Kassebaum N, Khatibzadeh S, Leasher JL, Lin J, Lipshultz SE, Lozano R, Lu Y, Mallinger L, McDermott MM, Micha R, Miller TR, Mokdad AA, Mokdad AH, Mozaffarian D, Naghavi M, Narayan KM, Omer SB, Pelizzari PM, Phillips D, Ranganathan D, Rivara FP, Roberts T, Sampson U, Sanman E, Sapkota A, Schwebel DC, Sharaz S, Shivakoti R, Singh GM, Singh D, Tavakkoli M, Towbin JA, Wilkinson JD, Zabetian A, Murray, Abraham J, Ali MK, Alvardo M, Atkinson C, Baddour LM, Benjamin EJ, Bhalla K, Birbeck G, Bolliger I, Burstein R, Carnahan E, Chou D, Chugh SS, Cohen A, Colson KE, Cooper LT, Couser W, Criqui MH, Dabhadkar KC, Dellavalle RP, Jarlais, Dicker D, Dorsey ER, Duber H, Ebel BE, Engell RE, Ezzati M, Felson DT, Finucane MM, Flaxman S, Flaxman AD, Fleming T, Foreman, Forouzanfar MH, Freedman G, Freeman MK, Gakidou E, Gillum RF, Gonzalez-Medina D, Gosselin R, Gutierrez HR, Hagan H, Havmoeller R, Hoffman H, Jacobsen KH, James SL, Jasrasaria R, Jayarman S, Johns N, Kassebaum N, Khatibzadeh S, Lan Q, Leasher JL, Lim S, Lipshultz SE, London S, Lopez, Lozano R, Lu Y, Mallinger L, Meltzer M, Mensah GA, Michaud C, Miller TR, Mock C, Moffitt TE, Mokdad AA, Mokdad AH, Moran A, Naghavi M, Narayan KM, Nelson RG, Olives C, Omer SB, Ortblad K, Ostro B, Pelizzari PM, Phillips D, Raju M, Razavi H, Ritz B, Roberts T, Sacco RL, Salomon J, Sampson U, Schwebel DC, Shahraz S, Shibuya K, Silberberg D, Singh JA, Steenland K, Taylor JA, Thurston GD, Vavilala MS, Vos T, Wagner GR, Weinstock MA, Weisskopf MG, Wulf S, Murray; U.S. Burden of Disease Collaborators : The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA 310: 591–608, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR: Underestimation of liver-related mortality in the United States. Gastroenterology 145: 375–382.e1–2, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadkarni GN, Simoes PK, Patel A, Patel S, Yacoub R, Konstantinidis I, Kamat S, Annapureddy N, Parikh CR, Coca SG: National trends of acute kidney injury requiring dialysis in decompensated cirrhosis hospitalizations in the United States. Hepatol Int 10: 525–531, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM: Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 26: 724–734, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TI, Paik J, Greene T, Desai M, Bech F, Cheung AK, Chertow GM: Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol 22: 1526–1533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoji T, Tsubakihara Y, Fujii M, Imai E: Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 66: 1212–1220, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Tislér A, Akócsi K, Borbás B, Fazakas L, Ferenczi S, Görögh S, Kulcsár I, Nagy L, Sámik J, Szegedi J, Tóth E, Wágner G, Kiss I: The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant 18: 2601–2605, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Rombolà G, Colussi G, De Ferrari ME, Frontini A, Minetti L: Cardiac arrhythmias and electrolyte changes during haemodialysis. Nephrol Dial Transplant 7: 318–322, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Buemi M, Coppolino G, Bolignano D, Sturiale A, Campo S, Buemi A, Crascì E, Romeo A: Arrhythmias and hemodialysis: Role of potassium and new diagnostic tools. Ren Fail 31: 75–80, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, Chertow GM: Cardiac arrest and sudden death in dialysis units. Kidney Int 60: 350–357, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Van Der Meersch H, De Bacquer D, Vandecasteele SJ, Van den Bergh B, Vermeiren P, De Letter J, De Vriese AS: Hemodialysis catheter design and catheter performance: A randomized controlled trial. Am J Kidney Dis 64: 902–908, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Parienti JJ, Mégarbane B, Fischer MO, Lautrette A, Gazui N, Marin N, Hanouz JL, Ramakers M, Daubin C, Mira JP, Charbonneau P, du Cheyron D; Cathedia Study Group : Catheter dysfunction and dialysis performance according to vascular access among 736 critically ill adults requiring renal replacement therapy: A randomized controlled study. Crit Care Med 38: 1118–1125, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Belcher JM: Is there a role for dialysis in patients with hepatorenal syndrome who are not liver transplant candidates? Semin Dial 27: 288–291, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J: Peripheral arterial vasodilation hypothesis: A proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 8: 1151–1157, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Ginès P, Schrier RW: Renal failure in cirrhosis. N Engl J Med 361: 1279–1290, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Wadei HM, Mai ML, Ahsan N, Gonwa TA: Hepatorenal syndrome: Pathophysiology and management. Clin J Am Soc Nephrol 1: 1066–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kreuzer M, Gähler D, Rakenius AC, Prüfe J, Jack T, Pfister ED, Pape L: Dialysis-dependent acute kidney injury in children with end-stage liver disease: Prevalence, dialysis modalities and outcome. Pediatr Nephrol 30: 2199–2206, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Wong F, Leung W, Al Beshir M, Marquez M, Renner EL: Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transpl 21: 300–307, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Capling RK, Bastani B: The clinical course of patients with type 1 hepatorenal syndrome maintained on hemodialysis. Ren Fail 26: 563–568, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Parsons V, Wilkinson SP, Weston MJ: Use of dialysis in the treatment of renal failure in liver disease. Postgrad Med J 51: 515–520, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson SP, Weston MJ, Parsons V, Williams R: Dialysis in the treatment of renal failure in patients with liver disease. Clin Nephrol 8: 287–292, 1977 [PubMed] [Google Scholar]
- 27.Witzke O, Baumann M, Patschan D, Patschan S, Mitchell A, Treichel U, Gerken G, Philipp T, Kribben A: Which patients benefit from hemodialysis therapy in hepatorenal syndrome? J Gastroenterol Hepatol 19: 1369–1373, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Wong LP, Blackley MP, Andreoni KA, Chin H, Falk RJ, Klemmer PJ: Survival of liver transplant candidates with acute renal failure receiving renal replacement therapy. Kidney Int 68: 362–370, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Gonwa TA, Wadei HM: The challenges of providing renal replacement therapy in decompensated liver cirrhosis. Blood Purif 33: 144–148, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB: Renal replacement therapy and orthotopic liver transplantation: The role of continuous veno-venous hemodialysis. Transplantation 71: 1424–1428, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Fraley DS, Burr R, Bernardini J, Angus D, Kramer DJ, Johnson JP: Impact of acute renal failure on mortality in end-stage liver disease with or without transplantation. Kidney Int 54: 518–524, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Cholongitas E, Senzolo M, Patch D, Shaw S, O’Beirne J, Burroughs AK: Cirrhotics admitted to intensive care unit: The impact of acute renal failure on mortality. Eur J Gastroenterol Hepatol 21: 744–750, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Davis CL, Feng S, Sung R, Wong F, Goodrich NP, Melton LB, Reddy KR, Guidinger MK, Wilkinson A, Lake J: Simultaneous liver-kidney transplantation: Evaluation to decision making. Am J Transplant 7: 1702–1709, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Keller F, Heinze H, Jochimsen F, Passfall J, Schuppan D, Büttner P: Risk factors and outcome of 107 patients with decompensated liver disease and acute renal failure (including 26 patients with hepatorenal syndrome): The role of hemodialysis. Ren Fail 17: 135–146, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Goodrich NP, Zhang M, Guidinger MK, Schaubel DE, Merion RM: Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol 8: 1135–1142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Axelrod DA, Vagefi PA, Roberts JP: The evolution of organ allocation for liver transplantation: Tackling geographic disparity through broader sharing. Ann Surg 262: 224–227, 2015 [DOI] [PubMed] [Google Scholar]
- 37.McMahon GM, Zeng X, Waikar SS: A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med 173: 1821–1828, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhee CM, Bhan I, Alexander EK, Brunelli SM: Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med 172: 153–159, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Allegretti AS, Ortiz G, Cui J, Wenger J, Bhan I, Chung RT, Thadhani RI, Irani Z: Changes in kidney function after transjugular intrahepatic portosystemic shunts versus large-volume paracentesis in cirrhosis: A matched cohort analysis. Am J Kidney Dis 68: 381–391, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R; United Network for Organ Sharing Liver Disease Severity Score Committee : Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 124: 91–96, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium : Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 61: 1038–1047, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V: Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 56: 1310–1318, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G; International Club of Ascites : Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. Gut 64: 531–537, 2015 [DOI] [PubMed] [Google Scholar]
- 44.R Development Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org. Accessed October 1, 2016
- 45.Israelsen M, Krag A, Allegretti AS, Jovani M, Goldin AH, Winter RW, Gluud LL: Terlipressin versus other vasoactive drugs for hepatorenal syndrome. Cochrane Database Syst Rev 9: CD011532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gluud LL, Christensen K, Christensen E, Krag A: Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev 9: CD005162, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J: Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 341: 403–409, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, Romanelli RG, Colletta C, Salinas F, Di Giacomo A, Ridola L, Fornasiere E, Caraceni P, Morando F, Piano S, Gatta A, Angeli P; Italian Association for the Study of the Liver Study Group on Hepatorenal Syndrome : Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology 62: 567–574, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, Soriano G, Terra C, Fábrega E, Arroyo V, Rodés J, Ginès P; TAHRS Investigators : Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: A randomized study. Gastroenterology 134: 1352–1359, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.