Abstract
Background and objectives
Large studies on long-term kidney outcome in patients with anti-glomerular basement membrane (anti-GBM) GN are lacking. This study aimed to identify clinical and histopathologic parameters that predict kidney outcome in these patients.
Design, setting, participants, & measurements
This retrospective analysis included a total of 123 patients with anti-GBM GN between 1986 and 2015 from six centers worldwide. Their kidney biopsy samples were classified according to the histopathologic classification for ANCA-associated GN. Clinical data such as details of treatment were retrieved from clinical records. The primary outcome parameter was the occurrence of ESRD. Kidney survival was analyzed using the log-rank test and Cox regression analyses.
Results
The 5-year kidney survival rate was 34%, with an improved rate observed among patients diagnosed after 2007 (P=0.01). In patients with anti-GBM GN, histopathologic class and kidney survival were associated (P<0.001). Only one of 15 patients with a focal class biopsy sample (≥50% normal glomeruli) developed ESRD. Patients with a sclerotic class biopsy sample (≥50% globally sclerotic glomeruli) and patients with 100% cellular crescents did not recover from dialysis dependency at presentation. In multivariable analysis, dialysis dependency at presentation (hazard ratio [HR], 3.17; 95% confidence interval [95% CI], 1.59 to 6.32), percentage of normal glomeruli (HR, 0.97; 95% CI, 0.95 to 0.99), and extent of interstitial infiltrate (HR, 2.02; 95% CI, 1.17 to 3.50) were predictors of ESRD during follow-up.
Conclusions
Dialysis dependency, low percentage of normal glomeruli, and large extent of interstitial infiltrate are associated with poor kidney outcome in anti-GBM GN. Kidney outcome has improved during recent years; the success rate doubled after 2007.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2017_11_21_CJASNPodcast_18_1_v.mp3
Keywords: anti-GBM disease; Goodpasture-s syndrome; kidney biopsy; ANCA; Antibodies, Antineutrophil Cytoplasmic; Retrospective Studies; Multivariate Analysis; Confidence Intervals; Survival Rate; Follow-Up Studies; Glomerular Basement Membrane; glomerulonephritis; antiglomerular basement membrane antibody; kidney; Autoantibodies; Biopsy; Regression Analysis; Kidney Failure, Chronic; renal dialysis
Introduction
Anti-glomerular basement membrane (anti-GBM) disease is an aggressive autoimmune disease (1), with an estimated incidence of 1–2 cases per million population per year (2). The disease affects glomerular capillaries, which usually leads to rapidly progressive GN, and pulmonary capillaries, possibly manifesting as alveolar hemorrhage. Kidney disease predominates in the majority of patients and is characterized by glomerular fibrinoid necrosis and crescent formation (3). To distinguish anti-GBM GN from other kidney diseases, immunofluorescence appearances are decisive, showing linear Ig deposits along the glomerular basement membrane (GBM) (4). In most cases, these deposits signify binding of circulating IgG autoantibodies with the NC1 domain of the α-3 chain of type IV collagen in the GBM (5,6). Anti-GBM autoantibodies can be detected in the serum of most patients, serving as a diagnostic tool. Besides anti-GBM antibody positivity, approximately one third of patients have ANCA (3,7).
The discovery of anti-GBM antibodies and their pathogenicity provide the rationale for treatment with plasma exchange and immunosuppressive agents, particularly cyclophosphamide and corticosteroids (8). This therapy combined with better supportive care and earlier diagnosis has improved the extremely poor outcome of patients with anti-GBM GN over the past decades (9–12). Despite these improvements, the rapid deterioration caused by the disease still causes ESRD, requiring dialysis at presentation in approximately 55% of patients (9). At 1-year follow-up, reported kidney survival rates range between 20% and 40% (10,12–15). Kidney outcome has been correlated with the severity of kidney failure at presentation and the percentage of crescents on kidney biopsy (9,11–16).
In a landmark paper from 2001, patients with anti-GBM GN who were dialysis dependent at presentation and had 100% crescents on their kidney biopsy did not recover kidney function (9). On the basis of this study, the Kidney Disease: Improving Global Outcomes guidelines currently recommend that patients who present with dialysis dependency and have 100% cellular crescents in an adequate biopsy sample, and who are without lung hemorrhage, can refrain from intensive treatment with plasma exchange, corticosteroids, and cyclophosphamide (17). However, a recent study suggested that all patients with anti-GBM GN should receive intensive therapy because the combination of plasma exchange, corticosteroids, and cyclophosphamide had a favorable effect even on patients who presented with clinical and/or histologic parameters indicative of a poor prognosis (11). In contrast, another recent study found that oligo-anuric patients who received intensive treatment and those who received minimal treatment had similarly poor patient and kidney survival (16). Because none of these patients recovered kidney function, a kidney biopsy may not be essential for predicting the prognosis of oligo-anuric patients (16).
Given the controversies regarding the predictive value of the kidney biopsy and the utility of intensive therapy in patients with anti-GBM GN, large studies on long-term kidney outcome are highly warranted to further improve patient-tailored therapy. Therefore, we investigated the clinical and histopathologic predictors of long-term kidney outcome in a large, international cohort of patients with anti-GBM GN.
Materials and Methods
Study Cohort
Patients were eligible for this study if they had a clinical presentation compatible with anti-GBM GN in combination with positive (>1+) glomerular linear IgG staining on immunofluorescence and/or positive serum anti-GBM antibodies. Entry criterion for this study was the availability of a kidney biopsy sample, which was collected from a pathology department at one of the following centers: the University of North Carolina at Chapel Hill (n=34); Imperial College London (n=26); Addenbrooke’s Hospital, Cambridge (n=25); LabPlus, Auckland (n=16); Leiden University Medical Center (n=15); and University Medical Center Utrecht (n=7). The cohort was divided according to year of diagnosis from which we created three groups of similar sizes in advance, which led to the following categorization: 1986–2000, 2001–2006, and 2007–2015. Patients with double positivity for anti-GBM antibodies and ANCA were included in the study, whereas patients with any other coexisting kidney disease were excluded. This study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki.
Biopsy Specimen Evaluation
A diagnostic kidney specimen was available for light microscopic evaluation in 118 patients. Biopsy specimens were evaluated independently by one of two experienced nephropathologists (I.M.B. or J.C.J.) who were blinded to the clinical data. The Berden classification for ANCA-associated GN was used to score the biopsy samples; focal class contained biopsy samples with ≥50% normal glomeruli, crescentic class contained biopsy samples with ≥50% glomeruli with cellular crescents, sclerotic class contained biopsy samples with ≥50% glomeruli with global sclerosis, and the remaining biopsy samples were designated as mixed class (18). Moreover, tubulointerstitial parameters were evaluated; interstitial fibrosis and tubular atrophy and interstitial infiltrate were scored semiquantitatively (scale from 0 to 3), and tubulitis was scored as absent or present (0 or 1). Kidney biopsy samples from five patients could not be retrieved, but pathology reports had sufficient information to classify the biopsy samples according to the Berden classification, and medical files comprised complete clinical data. Results from immunofluorescence were assessed from the pathology report in all patients.
Clinical Data
Clinical data were retrieved from medical records at the participating centers. Kidney function at baseline (before start of dialysis) was expressed as serum creatinine (milligrams per deciliter) and was classified as serum creatinine <5.7 or ≥5.7 mg/dl as reported in previous studies (9,12,16). Dialysis dependency at presentation was defined as need for acute RRT during the first hospital admission. The outcome of ESRD was defined as dialysis dependency during follow-up or kidney transplantation. Kidney survival was expressed as time to ESRD, and patient survival as time to death. Standard therapy was similar between centers and consisted of plasma exchange (minimum of seven exchanges or until anti-GBM antibodies were negative), oral and/or intravenous cyclophosphamide for 3–6 months, and oral and/or intravenous corticosteroids. Missing data are reported in the legends of tables, and percentages of the total of nonmissing data are presented. Each analysis included patients with complete data on the variable of interest.
Statistical Analyses
Continuous variables are expressed as means±SD or medians (interquartile range), and were compared between groups using the t test, one-way ANOVA, Mann–Whitney U test, or Kruskal–Wallis H test, as appropriate. Categoric variables are expressed as numbers (%), and differences were assessed with the chi-squared test or chi-squared trend test. Kidney and patient survival was analyzed using the Kaplan–Meier method and log-rank test. Univariable and multivariable Cox regression analyses were performed to identify predictors of kidney survival. Results are expressed as hazard ratio (HR) with 95% confidence interval (95% CI). SPSS version 23 (IBM Corp., Armonk, NY) was used for all analyses, and P values below 0.05 were considered statistically significant.
Results
Patient Characteristics
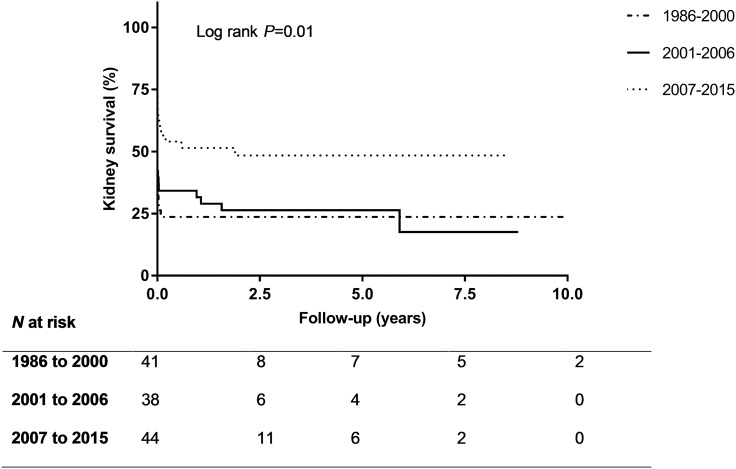
The characteristics of the total 123 patients are depicted in Table 1. Males in the cohort (54%) were significantly younger than females (47±20 versus 55±18 years; P=0.02). Data on serology were available for 108 patients; 49% were positive for anti-GBM antibodies only (“single positive”); 32% were positive for anti-GBM antibodies and ANCA (“double positive”); 6% had detectable ANCA only; and 13% had no detectable anti-GBM antibodies or ANCA. Double-positive patients were older than single-positive patients (P=0.003; Table 2). Patients who had negative serology test results were significantly younger compared with patients with positive serology test results (i.e., anti-GBM antibody and/or ANCA positivity) (P=0.02; Supplemental Table 1). Median duration of follow-up was 3.9 (1.3–6.1) years. Five patients were lost to follow-up within 1 year. The 5-year kidney survival rate was 34%, and the 5-year patient survival rate was 83%. Kidney survival at 5 years was 50% in patients who were diagnosed after 2007 (Figure 1).
Table 1.
Characteristics of the study population according to the year of diagnosis
| Characteristic | Total (n=123) | 1986–2000 (n=41) | 2001–2006 (n=38) | 2007–2015 (n=44) |
|---|---|---|---|---|
| Baseline (n=123) | ||||
| Age, yr | 51±19 | 54±19 | 46±21 | 53±17 |
| Males | 66 (54) | 22 (54) | 24 (63) | 20 (46) |
| Anti-GBM antibodies positivea | 92 (82) | 31 (87) | 28 (78) | 33 (81) |
| Anti-GBM antibodies, ANCA negative | 53 (60) | 12 (44) | 20 (71) | 21 (64) |
| Anti-GBM antibodies, ANCA positive | 35 (40) | 15 (56) | 8 (27) | 12 (36) |
| ANCA typeb | ||||
| Anti-MPO | 23 (85) | 9 (90) | 7 (100) | 7 (70) |
| Anti-PR3 | 4 (15) | 1 (10) | 0 (0) | 3 (30) |
| Lung involvementc | 41 (35) | 14 (37) | 12 (33) | 15 (35) |
| Serum creatinine, mg/dld | 7.0 (3.5–10.3) | 9.0 (5.8–13.9) | 7.3 (4.5–11.4) | 4.6 (1.9–7.0) |
| Dialysis dependentd | 69 (56) | 28 (68) | 27 (71) | 14 (32) |
| Follow-up (n=123) | ||||
| Duration of follow-up, yrd | 4.6±4.0 | 5.3±5.1 | 5.9±3.5 | 2.8±2.2 |
| ESRD at end of follow-upe | 82 (67) | 31 (76) | 29 (76) | 22 (50) |
| Transplantation | 32 (26) | 9 (22) | 15 (40) | 8 (18) |
| Deathe | 34 (28) | 17 (42) | 11 (29) | 6 (14) |
| Treatment (n=110) | ||||
| Plasma exchange | 91 (83) | 24 (71) | 31 (89) | 38 (88) |
| Median number of exchanges | 7 (6–12) | 6 (5–10) | 10 (7–10) | 7 (6–14) |
| Cyclophosphamidee | 88 (80) | 21 (62) | 29 (88) | 38 (88) |
| Oralf | 43 (60) | 13 (68) | 14 (67) | 16 (50) |
| Intravenousf | 25 (35) | 6 (32) | 6 (28) | 13 (41) |
| Both | 4 (5) | 0 (0) | 1 (5) | 3 (9) |
| Corticosteroids | 106 (96) | 33 (94) | 32 (97) | 42 (98) |
| Azathioprine | 21 (19) | 5 (15) | 6 (18) | 10 (23) |
| Mycophenolate mofetil | 12 (11) | 4 (12) | 4 (12) | 4 (9) |
| Rituximabd | 10 (9) | 0 (0) | 0 (0) | 10 (23) |
Data are presented as numbers (%), means±SD, or medians (interquartile range). GBM, glomerular basement membrane; MPO, myeloperoxidase; PR3, proteinase 3.
Results from anti-GBM antibody ELISA were available in 112 patients. Results from ANCA ELISA were available in 114 patients. Both tests were performed in 108 patients; four patients with positivity for anti-GBM antibodies had no available results from ANCA ELISA.
Thirty-five patients were anti-GBM antibodies and ANCA positive. Of these, ANCA type (anti-MPO or anti-PR3) was known in 27 patients.
Total, n=117 patients.
Different between subgroups with P value <0.001.
Different between subgroups with P value <0.05.
Route of administration of cyclophosphamide was known for 72 patients. The mean oral cyclophosphamide dose at the start of therapy was 118±49 g (n=30), and the mean intravenous dose was 1113±460 g (n=8).
Table 2.
Differences between single- and double-positive patients
| Variable | Single Positivea (n=53) | Double Positivea (n=35) | P Value |
|---|---|---|---|
| Age, yr | 47±19 | 59±17 | 0.003 |
| Males | 27 (51) | 19 (54) | 0.76 |
| Lung involvementb | 15 (28) | 13 (39) | 0.29 |
| Serum creatinine at presentation, mg/dl | 7.2 (3.5–10.0) | 7.2 (3.9–10.1) | 0.92 |
| Dialysis dependent at presentation | 32 (60) | 20 (57) | 0.76 |
| Year of diagnosis | 0.11 | ||
| 1986–2000 | 12 (23) | 15 (43) | |
| 2001–2006 | 20 (38) | 8 (23) | |
| 2007–2015 | 21 (39) | 12 (34) | |
| ESRD at end of follow-up | 36 (68) | 25 (71) | 0.73 |
| Histopathologic class | 0.03 | ||
| Focal | 7 (13) | 4 (11) | |
| Crescentic | 37 (70) | 18 (51) | |
| Mixed | 9 (17) | 8 (23) | |
| Sclerotic | 0 (0) | 5 (14) | |
| Normal glomeruli, percentagec | 0 (0–26) | 0 (0–13) | 0.40 |
| Cellular crescents, percentagec | 61±32 | 58±32 | 0.63 |
| Sclerotic glomeruli, percentagec | 2 (0–16) | 13 (0–28) | 0.07 |
| Interstitial fibrosis and tubular atrophyc | 0.07 | ||
| 0–1 | 36 (72) | 18 (53) | |
| 2–3 | 14 (28) | 16 (47) | |
| Interstitial infiltratec | 0.10 | ||
| 0–1 | 22 (44) | 9 (27) | |
| 2–3 | 28 (56) | 25 (73) | |
| Tubulitisc | 0.20 | ||
| 0 | 18 (36) | 17 (50) | |
| 1 | 32 (64) | 17 (50) | |
| Intensive treatmentd | 34 (71) | 14 (45) | 0.02 |
| Maintenance therapy with azathioprine or mycophenolate mofetile | 7 (14) | 13 (38) | 0.01 |
Data are presented as numbers (%), means±SD, or medians (interquartile range).
Single-positive patients were positive for serum anti-glomerular basement membrane (anti-GBM) antibodies and negative for ANCA. Double-positive patients were positive for anti-GBM antibodies and positive for ANCA.
Total, n=86 patients.
Total, n=84 patients.
Intensive treatment consisted of at least seven plasma exchanges, corticosteroids, and cyclophosphamide, mycophenolate mofetil, or rituximab. Excluding patients without intensive treatment and relatively preserved renal function at presentation. Total, n=79 patients.
The minimal duration of maintenance therapy was 3 months. Total, n=84 patients.
Figure 1.
The kidney survival is highest in patients who were diagnosed between 2007 and 2015.
Evaluation of Diagnostic Renal Biopsy Specimens
Immunofluorescence was performed successfully in 117 patients and was positive for linear IgG in all of them; six specimens for immunofluorescence did not contain assessable glomeruli. Ten biopsy samples were classified as sclerotic class, 15 biopsy samples as focal class, 72 biopsy samples as crescentic class, and the remaining 26 biopsy samples as mixed class (Table 3). The focal group had a younger mean age than the other three classes together (41±19 versus 52±19 years; P=0.03). Serum anti-GBM antibodies were more frequently detected in patients from the crescentic class than in those from the other classes. All patients from the sclerotic class who were positive for anti-GBM antibodies and who were tested for ANCA showed double positivity for both antibodies. The amount of interstitial fibrosis and tubular atrophy differed significantly between classes, but the extent of interstitial infiltrate did not (Table 4). Double-positive patients tended to have a higher percentage of sclerotic glomeruli and a higher interstitial fibrosis and tubular atrophy score compared with single-positive patients (P=0.07 for both parameters; Table 2). The minimum number of glomeruli was set at six in this study, whereas the Berden classification set a minimum of ten glomeruli. The predictive value of glomerular lesions was greater in biopsy samples with ten or more glomeruli (n=101) compared with biopsy samples with 6–10 glomeruli (n=22). However, including biopsy samples with 6–10 glomeruli did not change the predictive value of the glomerular lesions (Supplemental Table 2); therefore, they were included. No differences in treatment were observed between histopathologic classes, except for cyclophosphamide; patients from the focal and mixed class received cyclophosphamide more frequently than patients from the crescentic and sclerotic class (Table 5).
Table 3.
Characteristics stratified by histopathologic class: Baseline and follow-up characteristics
| Characteristic | Focal (n=15) | Crescentic (n=72) | Mixed (n=26) | Sclerotic (n=10) | P Value |
|---|---|---|---|---|---|
| Age, yr | 41±19 | 53±19 | 53±16 | 50±25 | 0.17 |
| Males | 9 (60) | 41 (57) | 10 (39) | 6 (60) | 0.37 |
| Anti-GBM antibodies positivea | 11 (79) | 58 (91) | 17 (71) | 6 (60) | 0.02 |
| Anti-GBM antibodies, ANCA negative | 7 (64) | 37 (67) | 9 (53) | 0 (0) | 0.03 |
| Anti-GBM antibodies, ANCA positive | 4 (36) | 18 (33) | 8 (47) | 5 (100) | |
| ANCA typeb | 0.001 | ||||
| Anti-MPO | 0 (0) | 14 (100) | 7 (87) | 2 (100) | |
| Anti-PR3 | 3 (100) | 0 (0) | 1 (13) | 0 (0) | |
| Lung involvementc | 7 (50) | 26 (37) | 4 (16) | 4 (57) | 0.06 |
| Serum creatinine at presentation, mg/dl | 1.4 (1.0–1.7) | 7.8 (6.4–12.6) | 4.2 (2.6–7.2) | 6.6 (4.5–15.2) | <0.001 |
| Dialysis dependent at presentation | 0 (0) | 51 (71) | 10 (39) | 8 (80) | <0.001 |
| Year of diagnosis | |||||
| 1986–2000 | 3 (20) | 28 (39) | 4 (15) | 6 (60) | <0.01 |
| 2001–2006 | 2 (13) | 26 (36) | 8 (31) | 2 (20) | |
| 2007–2015 | 10 (67) | 18 (25) | 14 (54) | 2 (20) | |
| Duration of follow-up, yr | 4.7±3.7 | 4.9±4.2 | 4.3±3.8 | 3.4±3.6 | 0.72 |
| ESRD at end of follow-up | 1 (7) | 57 (79) | 15 (58) | 9 (90) | <0.001 |
| Transplantation | 0 (0) | 25 (35) | 4 (15) | 3 (30) | 0.01 |
| Death | 2 (13) | 24 (33) | 5 (19) | 3 (30) | 0.32 |
Data are number (%), mean±SD, or median (interquartile range). GBM, glomerular basement membrane; MPO, myeloperoxidase; PR3, proteinase 3.
Results from anti-GBM antibody ELISA were available in 112 patients. Results from ANCA ELISA were available in 114 patients. Both tests were performed in 108 patients.
Thirty-five patients were anti-GBM antibodies and ANCA positive. Of these, ANCA type (anti-MPO or anti-PR3) was known in 27 patients.
Total, n=117 patients.
Table 4.
Characteristics stratified by histopathologic class: Biopsy specimen characteristics
| Characteristic | Focal (n=15) | Crescentic (n=68) | Mixed (n=25) | Sclerotic (n=10) | P Value |
|---|---|---|---|---|---|
| Total number of glomeruli | 22±12 | 17±9 | 19±9 | 19±9 | 0.35 |
| Normal glomeruli, percentage | 71 (62–90) | 0 (0–8) | 9 (0–31) | 0 (0–0) | <0.001 |
| Cellular crescents, percentage | 12±10 | 81±16 | 32±15 | 19±16 | <0.001 |
| Sclerotic glomeruli, percentage | 0 (0–5) | 0 (0–14) | 17 (8–30) | 63 (58–70) | <0.001 |
| Interstitial fibrosis and tubular atrophy | <0.001 | ||||
| 0 | 8 (53) | 16 (24) | 1 (4) | 0 (0) | |
| 1 | 7 (47) | 30 (44) | 11 (44) | 1 (10) | |
| 2 | 0 (0) | 19 (28) | 10 (40) | 3 (30) | |
| 3 | 0 (0) | 3 (4) | 3 (12) | 6 (60) | |
| Interstitial infiltrate | 0.16 | ||||
| 0 | 5 (33) | 5 (8) | 1 (4) | 1 (10) | |
| 1 | 9 (60) | 17 (25) | 12 (48) | 2 (20) | |
| 2 | 1 (7) | 22 (32) | 6 (24) | 7 (70) | |
| 3 | 0 (0) | 24 (35) | 6 (24) | 0 (0) | |
| Tubulitis | 0.02 | ||||
| 0 | 12 (80) | 27 (40) | 15 (60) | 6 (60) | |
| 1 | 3 (20) | 41 (60) | 10 (40) | 4 (40) |
Data are number (%), mean±SD, or median (interquartile range). Excluding five patients without kidney biopsy samples available for re-evaluation. Total, n=118 patients.
Table 5.
Characteristics stratified by histopathologic class: Treatment
| Treatment | Focal (n=14) | Crescentic (n=65) | Mixed (n=23) | Sclerotic (n=8) | P Value |
|---|---|---|---|---|---|
| Intensive treatmenta | 8 (57) | 33 (51) | 16 (70) | 4 (50) | 0.46 |
| Plasma exchange | 12 (86) | 53 (82) | 21 (91) | 5 (63) | 0.32 |
| Cyclophosphamide | 13 (93) | 46 (71) | 23 (100) | 6 (75) | <0.01 |
| Corticosteroids | 14 (100) | 62 (95) | 23 (100) | 7 (88) | 0.33 |
| Azathioprine | 4 (29) | 8 (12) | 6 (26) | 3 (38) | 0.11 |
| Mycophenolate mofetil | 2 (14) | 7 (11) | 2 (9) | 1 (13) | 0.92 |
| Rituximab | 3 (21) | 3 (5) | 4 (17) | 0 (0) | 0.06 |
Data are number (%), mean±SD, or median (interquartile range). Total, n=110 patients.
Intensive treatment was defined as treatment consisting of at least seven plasma exchanges, corticosteroids, and cyclophosphamide, mycophenolate mofetil or rituximab.
Kidney Function According to Histopathologic Class
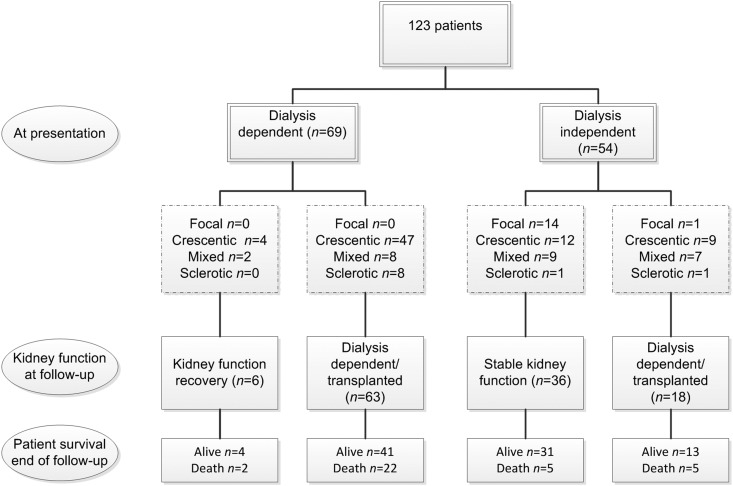
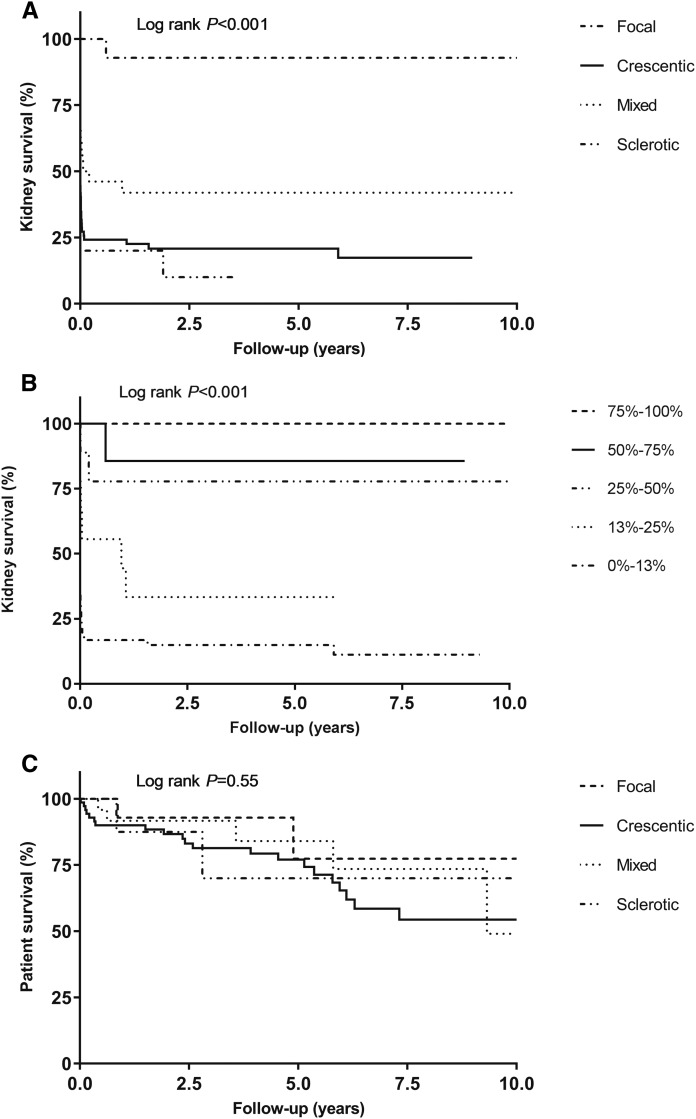
Kidney function at baseline in terms of serum creatinine was significantly better in the focal class compared with the other three classes (Table 3). No patient from the focal class required dialysis at presentation, and only one developed ESRD during follow-up (Figure 2). Most patients (90%) from the sclerotic class developed ESRD at some time point, whereas the outcome in crescentic and mixed classes was more variable (Figures 2 and 3A). When patients were categorized according to the percentage of normal glomeruli, kidney survival decreased with each lower percentage category of normal glomeruli (Figure 3B). Patient survival did not differ between histopathologic classes (Figure 3C).
Figure 2.
The flowchart shows a favorable outcome in the focal class, a poor outcome in the sclerotic class, and variable outcomes in the mixed and crescentic class.
Figure 3.
Kidney survival is highest in patients with a focal class biopsy, and patient survival is similar in all histopathological classes. (A) Kidney survival according to histopathologic class. (B) Kidney survival according to percentage of normal glomeruli. (C) Patient survival according to histopathologic class.
Predictors of ESRD
In Kaplan–Meier analysis, double-positive patients had a similar kidney survival to single-positive patients (P=0.75; Supplemental Figure 1A). Kidney survival was also similar in patients with negative and positive serology (P=0.69; Supplemental Figure 1B). Age, serum creatinine level, dialysis dependency at presentation, histopathologic classification, percentage of normal glomeruli, percentage of cellular crescents, extent of interstitial fibrosis and tubular atrophy, and extent of interstitial infiltrate were associated with ESRD in univariable Cox regression analyses (Table 6). Dialysis dependency at presentation was a discontinuous variable that predicted ESRD most significantly. Therefore, each parameter that was significant in univariable analysis was evaluated separately in a multivariable Cox regression analysis that included dialysis dependency at presentation. The percentage of normal glomeruli and the extent of interstitial infiltrate remained significant predictors of ESRD in multivariable analysis. A multivariable analysis, including dialysis dependency, percentage of normal glomeruli, and extent of interstitial infiltrate, showed that these parameters predicted ESRD independently (Table 6).
Table 6.
Prognostic significance of clinical and histopathologic parameters on ESRD
| Variable | Univariable Analysis | Multivariable Analysisa | Multivariable Analysisb | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, yr | 1.02 (1.01 to 1.03) | <0.01 | 1.01 (1.00 to 1.03) | 0.06 | ||
| Males | 1.19 (0.77 to 1.84) | 0.44 | ||||
| Lung involvement | 1.19 (0.75 to 1.88) | 0.47 | ||||
| Double positivity | 1.07 (0.64 to 1.78) | 0.80 | ||||
| Serum creatinine at presentation ≥5.7 mg/dl | 4.83 (2.75 to 8.47) | <0.001 | 1.98 (0.96 to 4.10) | 0.07 | ||
| Dialysis dependency at presentation | 6.50 (3.68 to 11.47) | <0.001 | a | a | 3.2 (1.6 to 6.3) | 0.001 |
| Histopathologic classification | <0.01 | 0.15 | ||||
| Focal (reference) | ||||||
| Crescentic | 20.29 (2.80 to 147.26) | 8.38 (1.10 to 63.87) | ||||
| Mixed | 11.67 (1.54 to 88.47) | 6.77 (0.87 to 52.65) | ||||
| Sclerotic | 25.72 (3.24 to 204.51) | 11.17 (1.34 to 3.13) | ||||
| Percentage normal glomeruli | 0.95 (0.93 to 0.98) | <0.001 | 0.97 (0.95 to 0.99) | <0.01 | 0.97 (0.95 to 0.99) | 0.01 |
| Percentage cellular crescents | 1.02 (1.01 to 1.02) | <0.001 | 1.02 (1.00 to 1.05) | 0.09 | ||
| Percentage globally Sclerotic glomeruli | 1.01 (1.00 to 1.02) | 0.20 | ||||
| Interstitial fibrosis and tubular atrophy, score 2–3 | 1.88 (1.20 to 2.95) | <0.01 | 1.38 (0.87 to 2.18) | 0.17 | ||
| Interstitial infiltrate, score 2–3 | 2.35 (1.44 to 3.83) | 0.001 | 1.90 (1.16 to 3.13) | 0.01 | 2.02 (1.2 to 3.5) | 0.01 |
| Tubulitis, present | 1.51 (0.96 to 2.35) | 0.07 | ||||
HR, hazard ratio; 95% CI, 95% confidence interval.
Each parameter that was significant in univariable analysis, was analyzed in a multivariable analysis including dialysis dependency at presentation. Dialysis dependency at presentation remained significant in each multivariable analysis with P values ≤0.002.
Parameters that were significant in the multivariable analysisa were included in this multivariate analysis.
Treatment
Data on therapy were complete for 110 patients. Intensive treatment consisting of at least seven plasma exchanges (19), corticosteroids, and cyclophosphamide, mycophenolate mofetil, or rituximab was given to 61 patients. Of the 49 remaining patients, 25 patients were treated with plasma exchange but not with cyclophosphamide and 24 with cyclophosphamide but no or less than seven plasma exchanges. Five of these 49 patients had relatively preserved kidney function at presentation (serum creatinine ≤1.4 mg/dl); therefore, they were not included in the analysis comparing intensive versus mild treatment (Supplemental Table 3). Older patients, double-positive patients, and patients with severe kidney failure at presentation were less likely to receive intensive treatment. Moreover, patients with a lower percentage of normal glomeruli and a higher percentage of crescentic glomeruli were treated less intensively. Over time, there was a significant trend toward increased use of an intensive treatment regimen (Supplemental Table 3). Twenty-one patients received azathioprine, and 12 patients received mycophenolate mofetil for induction and/or remission therapy. Double-positive patients received maintenance therapy with azathioprine or mycophenolate mofetil more frequently than single-positive patients (Table 2). Ten patients were treated with rituximab; nine of these patients also received cyclophosphamide concurrently or before treatment with rituximab.
Discussion
In one of the largest studies to date, we investigated the long-term outcome of 123 patients with anti-GBM GN from six centers worldwide. We analyzed the predictive value of the kidney biopsy in anti-GBM GN by applying the histopathologic classification for ANCA-associated GN (18). The histopathologic classification was a significant predictor of kidney survival in univariable analysis, but not in multivariable analysis including dialysis dependency at presentation. However, the percentage of normal glomeruli and the extent of interstitial infiltrate remained significant predictors in multivariable analysis. We also found that patients with ≥50% globally sclerotic glomeruli did not recover from the need for acute dialysis. The crescentic and mixed classes, as defined for ANCA-associated GN, seemed less important in predicting the outcome of anti-GBM GN because their kidney outcome was variable. In line with the study by Levy et al. (9), we found that patients who were dialysis dependent at presentation and had 100% cellular crescents at biopsy did not recover kidney function.
Age seemed to be an important denominator in determining the therapeutic strategy and in outcome. Younger patients more often had a focal class biopsy specimen, were more likely to stay dialysis independent, and received intensive treatment more frequently. However, when we performed multivariable analyses, age was no longer significantly associated with ESRD. From our data, we can conclude that histopathologic parameters, i.e., percentage of normal glomeruli and extent of interstitial infiltrate, are more predictive of outcome. Nevertheless, we feel that it is important to take age into account when defining the optimal individual treatment strategy. Interestingly, older age and double positivity were significantly associated, but double positivity was not associated with a worse outcome compared with single positivity.
The 5-year kidney survival rate in this study was 34%, which is higher than reported by Cui et al. (11), but in line with results from Levy et al. (9). Patients who were diagnosed after 2007 had a two-fold higher kidney survival rate compared with patients who were diagnosed before 2007. Interestingly, most patients with a focal class biopsy sample were diagnosed recently (2007–2015), suggesting earlier detection of the disease or increased awareness of the heterogeneity of the disease (20,21). The favorable outcome of this focal group can partially explain the observation of improved kidney outcome since 2007. The other contributing factor to this improvement is possibly the significant trend toward increased use of intensive therapy. From 1986 to 2000, 36% of the patients received intensive therapy, whereas 64% of the patients diagnosed after 2007 were treated intensively.
The heterogeneity of anti-GBM disease has recently been described by Nasr et al. (21) They described an atypic variant of anti-GBM disease, characterized by linear GBM staining for Igs, negative serum anti-GBM antibodies, absence of a crescentic phenotype, mild renal insufficiency, and absence of pulmonary hemorrhage. In our study, 20 patients had linear IgG deposits, but no detectable serum anti-GBM antibodies; three of them fulfilled the description of atypic anti-GBM disease. Therefore, the group of patients without detectable serum anti-GBM antibodies, but with linear IgG deposits, is rather heterogeneous.
Forty-five percent of our patients did not receive intensive treatment and the reason for refraining from intensive therapy was not always reported. However, some medical reports stated that treatment was withdrawn because of the results of kidney biopsy (i.e., a high percentage of affected glomeruli), therapy-related complications such as serious infections, or lack of improvement under therapy. Five patients who did not receive intensive treatment presented with relatively preserved kidney function, which was probably the reason for not initiating the full treatment regimen. Our data suggest that patients who are dialysis dependent at presentation and have either ≥50% globally sclerotic glomeruli or 100% cellular crescents do not benefit from intensive therapy and could therefore avoid the risk of immunosuppression and follow a conservative regimen. Other patients, including those with alveolar hemorrhage, should be treated intensively.
In our cohort, ten patients were treated with rituximab. In one center, rituximab along with cyclophosphamide has become standard therapy in patients who are double positive for anti-GBM antibodies and ANCA. Other indications for rituximab were found in three patients. In one patient, the kidney biopsy specimen showed a large infiltrate of CD20 positive B cells, providing a rationale to start anti-CD20 therapy (i.e., rituximab). Another patient did not tolerate azathioprine; therefore, rituximab was given instead. A third patient received rituximab, because the side-effects of cyclophosphamide were likely to cause problems. Because only nine patients were treated with rituximab and cyclophosphamide, conclusions on the response to rituximab could not be drawn.
This study included 123 patients with anti-GBM GN and is therefore the largest study evaluating kidney biopsy samples in relation to clinical outcome in this rare disease. Another strength of the study is the relatively long duration of follow-up (median 3.9 years). We consider the possibility of the development of ESRD after the last date of follow-up unlikely, because progression to ESRD usually occurs in the initial phase of this disease (9). Because of the rapid onset of anti-GBM disease, patient or referral delay have probably not influenced our findings. A limitation of the study is the possibility of selection bias; we only included patients with a diagnostic kidney biopsy performed, thereby excluding patients with a contraindication for a kidney biopsy (e.g., frail patients). Moreover, treatment was a potential confounder in our study; therefore, we have investigated treatment in detail. Future studies on histopathology in anti-GBM GN with patients who are treated similarly are highly warranted.
In summary, dialysis independency at presentation, a high percentage of normal glomeruli, and a lower extent of interstitial infiltrate are associated with a favorable outcome in patients with anti-GBM GN. Dialysis-dependent patients with 100% cellular crescents or ≥50% sclerotic glomeruli are very unlikely to recover, and may be refrained from intensive therapy. Kidney survival has improved during recent years, doubling the success rate after 2007. This is possibly the result of earlier detection accompanied by the increased use of intensive therapy in anti-GBM GN.
Disclosures
None.
Supplementary Material
Acknowledgments
E.E.v.D. is a student supported by the Kolff Student Researcher Grant from the Dutch Kidney Foundation. M.A.A. was supported by Consejo Nacional de Ciencia y Tecnología, Mexico. S.P.M. and C.D.P. were supported by the National Institute for Health Research Imperial Biomedical Research Centre.
Part of this study was presented at the American Society of Nephrology Kidney Week 2015 Annual Meeting, November 5–8, in San Diego, CA (abstract TH-PO681, J Am Soc Nephrol 26: 245A, 2015), and at the 18th International Vasculitis and ANCA Workshop, March 25–28, 2017 in Tokyo, Japan (abstract P1–129, Rheumatology [Oxford] 56: Suppl 3, 2017).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice editorial, “Trust Patient Insights at Both the Individual and National Level,” on pages 1–2.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04290417/-/DCSupplemental.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Canney M, O’Hara PV, McEvoy CM, Medani S, Connaughton DM, Abdalla AA, Doyle R, Stack AG, O’Seaghdha CM, Clarkson MR, Griffin MD, Holian J, Dorman AM, Niland A, Keogan M, Wallace EM, Conlon NP, Walsh C, Kelly A, Little MA: Spatial and temporal clustering of anti-glomerular basement membrane disease. Clin J Am Soc Nephrol 11: 1392–1399, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennette JC: Rapidly progressive crescentic glomerulonephritis. Kidney Int 63: 1164–1177, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chang A: Anti-GBM glomerulonephritis. In: Diagnostic Pathology: Kidney Diseases, 2nd Ed., Philadelphia, PA, Elsevier Health Sciences, 2015 [Google Scholar]
- 5.Turner N, Mason PJ, Brown R, Fox M, Povey S, Rees A, Pusey CD: Molecular cloning of the human Goodpasture antigen demonstrates it to be the alpha 3 chain of type IV collagen. J Clin Invest 89: 592–601, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmark T, Segelmark M, Unger C, Burkhardt H, Saus J, Wieslander J: Identification of a clinically relevant immunodominant region of collagen IV in Goodpasture disease. Kidney Int 55: 936–944, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD: Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int 66: 1535–1540, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Lerner RA, Glassock RJ, Dixon FJ: The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med 126: 989–1004, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy JB, Turner AN, Rees AJ, Pusey CD: Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med 134: 1033–1042, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Savage CO, Pusey CD, Bowman C, Rees AJ, Lockwood CM: Antiglomerular basement membrane antibody mediated disease in the British Isles 1980-4. Br Med J (Clin Res Ed) 292: 301–304, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Z, Zhao J, Jia XY, Zhu SN, Jin QZ, Cheng XY, Zhao MH: Anti-glomerular basement membrane disease: Outcomes of different therapeutic regimens in a large single-center Chinese cohort study. Medicine (Baltimore) 90: 303–311, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Huart A, Josse AG, Chauveau D, Korach JM, Heshmati F, Bauvin E, Cointault O, Kamar N, Ribes D, Pourrat J, Faguer S; French Society of Hemapheresis : Outcomes of patients with Goodpasture syndrome: A nationwide cohort-based study from the French Society of Hemapheresis. J Autoimmun 73: 24–29, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Herody M, Bobrie G, Gouarin C, Grünfeld JP, Noel LH: Anti-GBM disease: Predictive value of clinical, histological and serological data. Clin Nephrol 40: 249–255, 1993 [PubMed] [Google Scholar]
- 14.Merkel F, Pullig O, Marx M, Netzer KO, Weber M: Course and prognosis of anti-basement membrane antibody (anti-BM-Ab)-mediated disease: Report of 35 cases. Nephrol Dial Transplant 9: 372–376, 1994 [PubMed] [Google Scholar]
- 15.Daly C, Conlon PJ, Medwar W, Walshe JJ: Characteristics and outcome of anti-glomerular basement membrane disease: A single-center experience. Ren Fail 18: 105–112, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Alchi B, Griffiths M, Sivalingam M, Jayne D, Farrington K: Predictors of renal and patient outcomes in anti-GBM disease: Clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant 30: 814–821, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group : Clinical practice guideline for glomerulonephritis. Anti-glomerular basement membrane antibody glomerulonephritis. Kidney Int Suppl (2011) 2: 240–242, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Lockwood CM, Rees AJ, Pearson TA, Evans DJ, Peters DK, Wilson CB: Immunosuppression and plasma-exchange in the treatment of Goodpasture’s syndrome. Lancet 1: 711–715, 1976 [DOI] [PubMed] [Google Scholar]
- 20.Glassock RJ: Atypical anti-glomerular basement membrane disease: Lessons learned. Clin Kidney J 9: 653–656, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasr SH, Collins AB, Alexander MP, Schraith DF, Herrera Hernandez L, Fidler ME, Sethi S, Leung N, Fervenza FC, Cornell LD: The clinicopathologic characteristics and outcome of atypical anti-glomerular basement membrane nephritis. Kidney Int 89: 897–908, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.