Abstract
Background and objectives
Niacin downregulates intestinal sodium-dependent phosphate transporter 2b expression and reduces intestinal phosphate transport. Short-term studies have suggested that niacin lowers serum phosphate concentrations in patients with CKD and ESRD. However, the long-term effects of niacin on serum phosphate and other mineral markers are unknown.
Design, setting, participants, & measurements
The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Trial was a randomized, double-blind, placebo-controlled trial testing extended release niacin in persons with prevalent cardiovascular disease. We examined the effect of randomized treatment with niacin (1500 or 2000 mg) or placebo on temporal changes in markers of mineral metabolism in 352 participants with eGFR<60 ml/min per 1.73 m2 over 3 years. Changes in each marker were compared over time between the niacin and placebo arms using linear mixed effects models.
Results
Randomization to niacin led to 0.08 mg/dl lower plasma phosphate concentrations per year of treatment compared with placebo (P<0.01) and 0.25 mg/dl lower mean phosphate 3 years after baseline (3.32 versus 3.57 mg/dl; P=0.03). In contrast, randomization to niacin was not associated with statistically significant changes in plasma intact fibroblast growth factor 23, parathyroid hormone, calcium, or vitamin D metabolites over 3 years.
Conclusions
The use of niacin over 3 years lowered serum phosphorous concentrations but did not affect other markers of mineral metabolism in participants with CKD.
Keywords: mineral metabolism; chronic kidney disease; hyperphosphatemia; Humans; Niacin; fibroblast growth factor 23; parathyroid hormone; Metabolic Syndrome X; calcium; Sodium; Double-Blind Method; glomerular filtration rate; Global Health; Random Allocation; Phosphates; Renal Insufficiency, Chronic; Phosphorus; Minerals; Triglycerides; Cardiovascular Diseases; Kidney Failure, Chronic; Phosphate Transport Proteins; Vitamin D; Fibroblast Growth Factors
Introduction
Over 26 million Americans have CKD (1). Beyond heightened risk for developing ESRD, these individuals are at substantially higher risk of cardiovascular disease and mortality (2). Mechanisms responsible for these links are uncertain. However, higher phosphate concentrations are a leading potential explanatory factor.
In vitro, higher serum phosphate concentrations induce vascular smooth muscle cells to transform into osteoblast-like cells and promote calcification in the arterial wall (3,4). In humans, higher phosphate concentrations are associated with arterial calcification, arterial stiffness, and cardiovascular events, independent of traditional cardiovascular disease risk factors or the severity of CKD (5,6). Higher phosphate may also stimulate increased concentrations of counter-regulatory hormones, including fibroblast growth factor 23 (FGF23) and parathyroid hormone (PTH) (7,8). FGF23, in turn, induces cardiac myocyte hypertrophy in animal models and is associated with left ventricular hypertrophy and higher heart failure and mortality risk in patients with CKD (9). Thus, higher phosphate concentrations may directly or indirectly promote cardiovascular disease risk in patients with CKD. This hypothesis has led to considerable interest in lower serum phosphate concentrations in patients with CKD in an effort to minimize development of cardiovascular disease (10).
In patients with ESRD, intestinal phosphate binders are frequently used to lower phosphate. These drugs bind phosphate within the intestinal lumen and decrease the amount systemically absorbed. Multiple studies have tested the efficacy of phosphate binders in persons with stages 3–5 CKD (11–14). These studies show modest or, in many instances, no effect on serum phosphate concentrations (8,15). The lipid drug niacin may provide an alternative strategy for phosphate lowering, because it is structurally similar to nicotinamide (vitamin B3) and is converted to nicotinamide as part of its metabolism (16,17). Rather than functioning as a binder, nicotinamide reduces expression of the sodium phosphate cotransporter 2b (Npt2b) in the small bowel (18). Nicotinamide may also stimulate greater urinary phosphate excretion by decreasing sodium phosphate cotransporters in the proximal tubule of the kidney, such that more of the filtered phosphate is excreted in the urine (19). In a pilot study conducted over 6 months, we found reductions of serum phosphate concentrations in patients with CKD randomized to niacin versus placebo (20). However, the long-term effects of niacin on phosphate concentration are unknown. Moreover, the effects of niacin on other markers of mineral metabolism, including FGF23 and PTH, are uncertain.
The Atherothrombosis Intervention in Metabolic syndrome with low HDL/High triglycerides: Impact on Global Health (AIM-HIGH) Trial is a randomized, double-blind, placebo-controlled study that enrolled 3414 individuals with prevalent cardiovascular disease, low HDL, and high triglycerides who were already on 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (“statins”). Individuals were randomized to extended release niacin 1500–2000 mg versus matched placebo and followed for the primary end point of recurrent cardiovascular disease events over 3 years (21). There was no significant reduction in the primary outcome between the niacin and placebo groups (with niacin: hazard ratio, 1.02; 95% confidence interval, 0.87 to 1.21; P=0.80). Among the trial participants, 352 had eGFR<60 ml/min per 1.73 m2 at baseline. Here, we investigate the effect of niacin on serum phosphate concentrations and other markers of mineral metabolism over 3 years in the 352 CKD participants. A priori, we hypothesized that, relative to placebo, participants randomized to niacin would have sustained reductions in phosphate over 3 years and associated lowering of FGF23 and PTH concentrations.
Materials and Methods
Study Participants
The AIM-HIGH Trial design has been described in detail previously (21). Briefly, participants were recruited from 92 centers in the United States and Canada. The key inclusion criteria required age ≥45 years old, prevalent cardiovascular disease (defined as coronary heart disease, cerebrovascular disease, or peripheral arterial disease), and an atherogenic lipid profile. Exclusion criteria included serum creatinine concentration >2.5 mg/dl. A total of 3414 participants were enrolled; 505 patients had eGFR<60 ml/min per 1.73 m2 by serum creatinine–based Chronic Kidney Disease Epidemiology Collaboration equation. Among them, 352 had eGFR<60 ml/min per 1.73 m2 by the creatinine and cystatin combined equation and constituted the analytic sample for this study.
After informed consent, participants entered a 4- to 8-week open label run-in phase, during which they received simvastatin 40 mg daily plus niacin at doses increased weekly from 500 to 2000 mg/d. Those who achieved a dose ≥1500 mg/d with acceptable side effect profiles were randomized in a 1:1 ratio to niacin (1500 or 2000 mg) plus simvastatin or matched placebo plus simvastatin. In both arms, simvastatin was titrated using a prespecified algorithm to maintain LDL cholesterol concentrations between 40 and 80 mg/dl, and ezetimibe could be added at a dose of 10 mg/d if needed to achieve the LDL target. Because of flushing effects of niacin, the placebo was designed to contain a small dose (50 mg) of immediate release niacin to mask the identity of blinded treatment. Fasting venous EDTA plasma specimens were obtained at the baseline, year 1, and year 3 visits and stored at −80°C for use in ancillary studies.
Mineral Metabolism and Kidney Function Measurements
Blood samples from baseline, year 1, and year 3 were used for mineral metabolism measurements in 2014. Specimens were obtained in the morning after an overnight fast. All measurements were made centrally at the Department of Laboratory Medicine at the University of Washington. We completed measurements en bloc, such that each participant had their baseline, year 1, and year 3 mineral metabolism measurements made at the same time to minimize analytic drift.
Phosphate was measured using a time reaction colorimetric reaction with ammonium molybdate at acidic pH on a Beckman DxC Synchron clinical analyzer. The analytic measurement range was from 0.5 to 12.0 mg/dl. The interassay coefficient of variation (CV) in our hands was <4.3% across the analytic range.
The AIM-HIGH Trial only stored EDTA plasma samples. EDTA binds calcium, preventing use of a standard clinical chemistry analyzer. Thus, we measured calcium using atomic absorption on a Perkin Elmer Analyst 200 Atomic Absorption Spectrometer, which is considered the gold standard method for calcium measurement. In our hands, the CVs across the analytic range were <4.5%.
We measured FGF23 in first thawed specimens using an intact FGF23 ELISA (Kainos). Interassay CVs were <9.5% across the analytic range. Intact PTH levels were also measured on the first thaw using a two-site immunoassay on a Beckman Unicell DxI clinical analyzer, which comprises amino acid residues 1–84 on the PTH molecule. CVs across the analytic range were <8.9%.
We used immunoaffinity enrichment-liquid chromatography-tandem mass spectrometry to measure vitamin D metabolites (25-hydroxyvitamin D2 and D3 and 1,25-dihydroxyvitamin D2 and D3) using methods described elsewhere (22). The CVs were <18% for 25-hydroxyvitamin D2 and <7% for 25-hydroxyvitamin D3. Corresponding CVs for 1,25-dihydroxyvitamin D2 and D3 were <13% and <14%, respectively. Concentrations of vitamin D2 and D3 were added to provide a summary estimate of each analyte.
Plasma creatinine was measured on the Beckman DxC Synchron clinical analyzer using the rate Jaffe method and calibrated to the isotope dilution-mass spectrometry standard. The interassay CV was <4.5%. We also measured plasma cystatin C using a nephelometric immunoassay on a Siemens BN-II nephelometer. The data were calibrated using a certified reference material ERM-DA471/IFCC (23). The interassay CVs were <4.5%. We used plasma creatinine and cystatin C concentrations along with age, sex, and race to estimate GFR using the Chronic Kidney Disease Epidemiology Collaboration equation (24). Urine albumin or protein assessment was not available in the AIM-HIGH Trial.
Statistical Analyses
We divided participants by randomized treatment assignment and evaluated the distribution of demographics, cardiovascular disease risk factors, eGFR, and mineral metabolism measures at the baseline examination. We next evaluated the mean level of serum phosphate and other mineral metabolism measures at baseline, year 1, and year 3 separately, because the number of individuals with available data differed by follow-up visit. Randomized treatment assignment groups were compared by t tests, with the exception of FGF23 and PTH, where we used the Wilcoxon rank sum because of skewed distributions. We used linear mixed models to incorporate data from baseline, year 1, and year 3 and provide a summary estimate of the treatment effect of niacin for each marker. We used an unstructured covariance matrix, because it has the benefit of improved model fit and little chance of losing too many degrees of freedom. The P value for the linear mixed model analysis represents the treatment × time effect, and time was modeled as a continuous variable. Analyses were repeated and stratified by baseline eGFR <45 versus >45 ml/min per 1.73 m2.
In secondary analysis, we evaluated patients “as treated” rather than by the intent to treat to evaluate the magnitude of possible efficacy of niacin on phosphate and FGF23 in patients who complied with therapy. Statistical analyses were conducted using Stata SE, version 11 (Stata Corp, College Station, TX), and P values <0.05 were considered statistically significant for all analyses.
Results
Among the 352 AIM-HIGH Trial participants with eGFR<60 ml/min per 1.73 m2 by the combined creatinine and cystatin C estimating equation, the mean age was 71±7 years old, 80% were men, 93% were white, 81% had a history of hypertension, and 40% had diabetes. At baseline, the mean eGFR was 46±8 ml/min per 1.73 m2, and mean plasma phosphate concentration was 3.4±0.6 mg/dl; 174 were randomized to placebo, and 178 were randomized to niacin. Table 1 shows baseline characteristics by randomized treatment arm; distributions were similar for all variables except for plasma calcium levels, which were lower in the placebo than in the niacin arm at baseline (9.6 versus 9.7 mg/dl; P=0.03).
Table 1.
Baseline characteristics of CKD participants in the AIM-HIGH Trial by randomized treatment arm
| Variables | Placebo, n=174 | Niacin, n=178 |
|---|---|---|
| Demographics | ||
| Age, yr, ±SD | 71±7 | 71±7 |
| Men, n (%) | 138 (79) | 144 (81) |
| Nonwhite, n (%) | 14 (8) | 10 (6) |
| Vascular history, n (%) | ||
| Myocardial infarction | 89 (51) | 85 (48) |
| Coronary artery bypass grafting | 89 (51) | 75 (42) |
| Percutaneous coronary intervention | 100 (58) | 96 (54) |
| Stroke or cerebrovascular disease | 71 (41) | 60 (34) |
| Peripheral artery disease | 39 (22) | 40 (23) |
| Risk factors | ||
| Systolic BP, mm Hg, ±SD | 129±16 | 132±18 |
| Diastolic BP, mm Hg, ±SD | 70±11 | 72±9 |
| BP medication use, n (%) | 171 (98) | 176 (99) |
| History of hypertension, n (%) | 139 (80) | 142 (82) |
| Fasting glucose, mg/dl, ±SD | 110±24 | 108±24 |
| HbA1c, %, ±SD | 6.2±0.9 | 6.2±0.9 |
| Hypoglycemic medication use, n (%) | 48 (28) | 54 (30) |
| History of diabetes, n (%) | 68 (39) | 71 (40) |
| Weight, kg, ±SD | 90±19 | 91±17 |
| Height, cm, ±SD | 172±9 | 172±9 |
| Body mass index, kg/m2, ±SD | 30±6 | 31±5 |
| Smoking | ||
| Never, n (%) | 43 (25) | 43 (24) |
| Current, n (%) | 26 (15) | 17 (10) |
| Smokeless, n (%) | 0 (0) | 3 (2) |
| Former, n (%) | 103 (60) | 114 (64) |
| Pack-years, median (IQR) | 3.8 (2.0–5.3) | 4.0 (2.0–4.7) |
| Kidney-related medication use, n (%) | ||
| ACE/ARB use | 132 (76) | 139 (78) |
| β-Blocker use | 147 (85) | 138 (78) |
| Calcium channel blocker use | 56 (32) | 61 (34) |
| Diuretic use | 110 (63) | 100 (56) |
| Aspirin use | 157 (90) | 158 (89) |
| Clopidogrel use | 64 (37) | 56 (32) |
| Warfarin use | 19 (11) | 16 (9) |
| Insulin use | 30 (17) | 26 (15) |
| Oral hypoglycemic use | 48 (28) | 54 (30) |
| Other laboratory values | ||
| Uric acid, mg/dl, ±SD | 8.0±1.7 | 7.8±1.7 |
| AST, IU/L, ±SD | 26±8 | 25±7 |
| Creatinine kinase, IU/L, ±SD | 120±84 | 116±113 |
| Total cholesterol, mg/dl, ±SD | 146±25 | 145±24 |
| LDL cholesterol, mg/dl, ±SD | 74±21 | 73±20 |
| HDL cholesterol, mg/dl, ±SD | 35±6 | 35±6 |
| Triglycerides, mg/dl, median (IQR) | 158 (133–229) | 170 (131–215) |
| Mineral and kidney function laboratory values | ||
| eGFR, ml/min per 1.73 m2, ±SD | 47±8 | 45±9 |
| eGFR<45 ml/min per 1.73 m2, n (%) | 69 (40) | 72 (40) |
| Phosphate, mg/dl, ±SD | 3.4±0.6 | 3.4±0.6 |
| Phosphate >4.5 mg/dl, n (%) | 6 (3) | 12 (7) |
| Calcium, mg/dl, ±SD | 9.6±0.5 | 9.7±0.5 |
| Intact PTH, pg/ml, median (IQR) | 53 (38–76) | 51 (36–71) |
| 25(OH) vitamin D, ng/ml, ±SD | 25±9 | 25±10 |
| 25(OH) vitamin D <20 ng/ml, n (%) | 38 (23) | 55 (32) |
| 1,25(OH)2 vitamin D, ng/ml, ±SD | 32±11 | 31±11 |
| 24,25(OH)2 vitamin D, ng/ml, ±SD | 2.8±1.7 | 2.7±1.6 |
| FGF23, pg/ml, median (IQR) | 74 (60–97) | 72 (54–101) |
AIM-HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health; HbA1c, glycosylated hemoglobin; IQR, interquartile range; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; PTH, parathyroid hormone; FGF23, fibroblast growth factor 23.
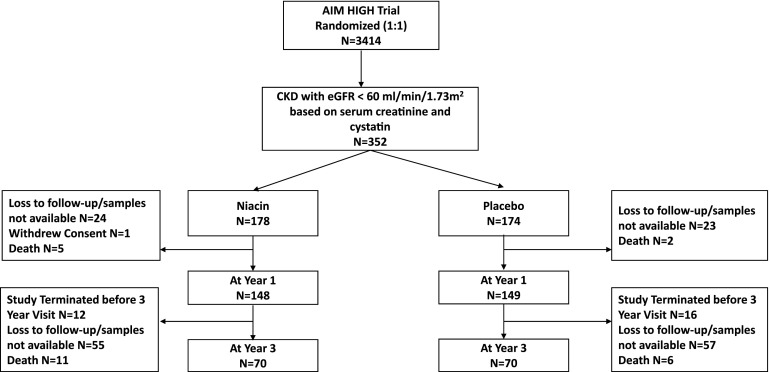
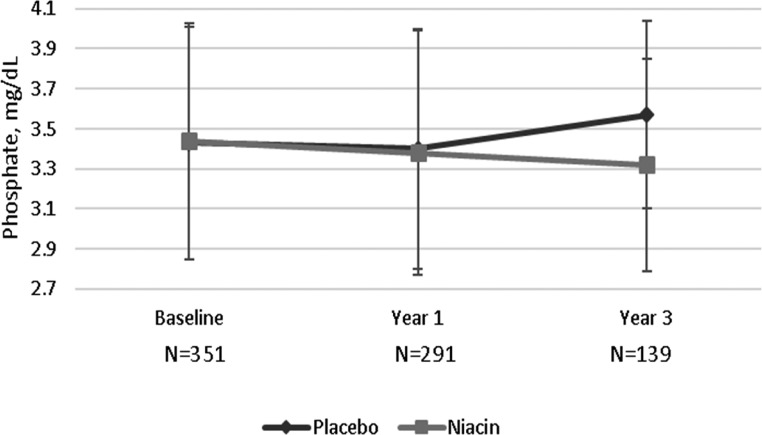
Among the 352 participants, 297 (84%) had plasma samples available for measurement of mineral markers at year 1, and 140 (40%) had plasma samples available at year 3 (Figure 1). Table 2 shows the change in markers of mineral metabolism by randomized treatment arm in intent to treat analysis. Plasma phosphate levels were similar by randomized treatment arm at year 1, but by year 3, phosphate levels were significantly lower in the niacin arm relative to placebo (3.32 versus 3.57 mg/dl; P=0.03) (Figure 2). The linear mixed model provided a summary estimate and showed that niacin treatment led to 0.08 mg/dl lower plasma phosphate per year of treatment compared with placebo (P<0.01). When we stratified the sample on the basis of eGFR<45 versus >45 ml/min per 1.73 m2, the point estimate for annual change in slope was similar in both strata (−0.08 mg/dl per year) (Supplemental Table 1).
Figure 1.
Patient selection flow diagram. AIM-HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health.
Table 2.
Effect of niacin on mineral metabolism parameters in CKD participants in the AIM-HIGH Trial (intent to treat analysis)
| Variables | Placebo | Niacin | P Value |
|---|---|---|---|
| Phosphate, mg/dl | |||
| Baseline, n=351, mean±SD | 3.4±0.6 | 3.4±0.6 | 0.85 |
| Year 1, n=291, mean±SD | 3.4±0.6 | 3.4±0.6 | 0.76 |
| Year 3, n=139, mean±SD | 3.6±0.5 | 3.3±0.5 | 0.003 |
| Difference in slopes (95% CI) | −0.08 (−0.13 to −0.02) | <0.01 | |
| Intact FGF23, pg/ml | |||
| Baseline, n=352, median (IQR) | 74 (60–97) | 72 (54–101) | 0.75 |
| Year 1, n=297, median (IQR) | 80 (60–102) | 73 (60–102) | 0.09 |
| Year 3, n=140, median (IQR) | 71 (54–94) | 75 (57–102) | 0.87 |
| Difference in slopes (95% CI) | −0.47 (−5.49 to 4.53) | 0.86 | |
| Calcium, mg/dl | |||
| Baseline, n=344, mean±SD | 9.5±0.6 | 9.7±0.5 | 0.03 |
| Year 1, n=289, mean±SD | 9.6±0.5 | 9.6±0.6 | 0.16 |
| Year 3, n=135, mean±SD | 9.6±0.5 | 9.7±0.6 | 0.77 |
| Difference in slopes (95% CI) | −0.03 (−0.08 to 0.03) | 0.31 | |
| Intact PTH, pg/ml | |||
| Baseline, n=349, median (IQR) | 53 (38–76) | 51 (36–71) | 0.57 |
| Year 1, n=295, median (IQR) | 54 (34–75) | 44 (29–65) | 0.003 |
| Year 3, n=140, median (IQR) | 51 (34–79) | 45 (36–67) | 0.87 |
| Difference in slopes (95% CI) | −2.11 (−4.94 to 0.71) | 0.14 | |
| 25-Hydroxyvitamin D, ng/ml | |||
| Baseline, n=339, mean±SD | 25±9 | 25±10 | 0.67 |
| Year 1, n=280, mean±SD | 26±9 | 26±10 | 0.89 |
| Year 3, n=135, mean±SD | 28±10 | 26±11 | 0.29 |
| Difference in slopes (95% CI) | −0.48 (−1.32 to 0.35) | 0.26 | |
| 1,25-Dihydroxyvitamin D, ng/ml | |||
| Baseline, n=339, mean±SD | 32±11 | 32±11 | 0.32 |
| Year 1, n=280, mean±SD | 31±11 | 31±10 | 0.78 |
| Year 3, n=135, mean±SD | 33±12 | 32±13 | 0.59 |
| Difference in slopes (95% CI) | −0.17 (−1.33 to 0.98) | 0.77 | |
| 24,25-Dihydroxyvitamin D, ng/ml | |||
| Baseline, n=339, mean±SD | 2.8±1.7 | 2.7±1.6 | 0.57 |
| Year 1, n=280, mean±SD | 2.9±1.6 | 2.8±1.6 | 0.94 |
| Year 3, n=135, mean±SD | 2.9±1.0 | 2.8±1.0 | 0.51 |
| Difference in slopes (95% CI) | <0.01 (−0.10 to 0.11) | 0.95 | |
AIM-HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health; 95% CI, 95% confidence interval; FGF23, fibroblast growth factor 23; IQR, interquartile range; PTH, parathyroid hormone.
Figure 2.
Niacin in comparison to placebo led to 0.25 mg/dl lower mean phosphate level 3 years after baseline.
In linear mixed models, none of the other measures of mineral metabolism show significant changes over 3 years. The median FGF23 levels were nominally lower in the niacin versus placebo arm at year 1 (73 versus 80 pg/ml; P=0.09), but this difference was no longer apparent at year 3. Similarly, the median PTH levels were lower in the niacin versus placebo arm at year 1 (44 versus 54 pg/ml; P=0.03), but this association was also no longer apparent by year 3. A priori, we defined the linear mixed models as the primary end point, because it provides a summary estimate of change in biomarkers across years. Niacin treatment was not associated with lower FGF23 or PTH in the linear mixed models. We observed no significant differences in the calcium or calcitriol levels across treatment arms.
In a secondary analysis, we investigated the changes associated with niacin versus placebo treatment in an “as treated” analysis to preliminarily evaluate the efficacy of niacin in persons who were compliant with therapy. Results were generally similar in the as treated analysis as in the intent to treat analysis (Table 3). Reasons for discontinuation are shown in Table 4. There was a significantly higher rate of discontinuation of niacin compared with placebo (38% versus 22%; P=0.001). We also observed higher rates of flushing with niacin compared with the placebo arm (Table 4).
Table 3.
Effect of niacin on mineral metabolism parameters in CKD participants in the AIM-HIGH Trial (as treated analysis)
| Variables | Placebo | Niacin | P Value |
|---|---|---|---|
| Phosphate, mg/dl | |||
| Baseline, n=132, mean±SD | 3.5±0.5 | 3.4±0.6 | 0.52 |
| Year 1, n=132, mean±SD | 3.4±0.6 | 3.4±0.6 | 0.60 |
| Year 3, n=132, mean±SD | 3.6±0.5 | 3.3±0.5 | <0.01 |
| Difference in slopes (95% CI) | −0.07 (−0.13 to −0.01) | 0.02 | |
| Intact FGF23, pg/ml | |||
| Baseline, n=136, median (IQR) | 58 (55–90) | 66 (51–88) | 0.61 |
| Year 1, n=136, median (IQR) | 72 (54–97) | 68 (55–89) | 0.39 |
| Year 3, n=136, median (IQR) | 73 (54–96) | 75 (57–103) | 0.86 |
| Difference in slopes (95% CI) | 0.55 (−5.17 to 6.27) | 0.85 | |
| Calcium, mg/dl | |||
| Baseline, n=127, mean±SD | 9.6±0.5 | 9.7±0.6 | 0.36 |
| Year 1, n=127, mean±SD | 9.6±0.5 | 9.6±0.5 | 0.55 |
| Year 3, n=127, mean±SD | 9.6±0.5 | 9.7±0.6 | 0.67 |
| Difference in slopes (95% CI) | −0.02 (−0.07 to 0.05) | 0.64 | |
| Intact PTH, pg/ml | |||
| Baseline, n=135, median (IQR) | 53 (39–80) | 52 (40–78) | 0.86 |
| Year 1, n=135, median (IQR) | 52 (31–75) | 44 (31–66) | 0.20 |
| Year 3, n=135, median (IQR) | 51 (34–79) | 44 (35–71) | 0.80 |
| Difference in slopes (95% CI) | −1.74 (−4.93 to 1.44) | 0.28 | |
| 25-Hydroxyvitamin D, ng/ml | |||
| Baseline, n=122, mean±SD | 25±9 | 25±9 | 0.70 |
| Year 1, n=122, mean±SD | 25±9 | 24±9 | 0.50 |
| Year 3, n=122, mean±SD | 29±10 | 29±10 | 0.34 |
| Difference in slopes (95% CI) | −0.73 (−1.73 to 0.27) | 0.15 | |
| 1,25-Dihydroxyvitamin D, ng/ml | |||
| Baseline, n=122, mean±SD | 32±11 | 34±12 | 0.25 |
| Year 1, n=122, mean±SD | 33±11 | 32±10 | 0.64 |
| Year 3, n=122, mean±SD | 33±12 | 33±13 | 0.90 |
| Difference in slopes (95% CI) | −0.46 (−1.84 to 0.91) | 0.51 | |
| 24,25-Dihydroxyvitamin D, ng/ml | |||
| Baseline, n=122, mean±SD | 2.7±1.7 | 2.6±1.4 | 0.84 |
| Year 1, n=122, mean±SD | 2.7±1.7 | 2.6±1.4 | 0.60 |
| Year 3, n=122, mean±SD | 3.1±1.6 | 2.9±1.6 | 0.53 |
| Difference in slopes (95% CI) | −0.05 (−0.20 to 0.11) | 0.57 | |
AIM-HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health; 95% CI, 95% confidence interval; FGF23, fibroblast growth factor 23; IQR, interquartile range; PTH, parathyroid hormone.
Table 4.
Reasons for drug discontinuation in CKD participants in the AIM-HIGH Trial by randomized treatment arm
| Variables | Placebo, n=174 | Niacin, n=178 | P Value |
|---|---|---|---|
| No. (%) discontinuing | 39 (22) | 68 (38) | 0.001 |
| Reason niacin/placebo discontinued, no. (%) | |||
| Flushing, itching | 6 (3) | 19 (11) | |
| Liver function test abnormality | 0 (0) | 1 (0.6) | |
| Patient request | 18 (10) | 21 (12) | |
| Nonstudy physician request | 5 (3) | 7 (4) | |
| Other clinical reason to discontinue | 7 (4) | 11 (6) | |
| Increased glucose | 2 (1) | 4 (2) | |
| GI symptoms | 1 (0.6) | 4 (2) | |
| Reason not specified | 0 (0) | 1 (0.6) |
AIM-HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health; GI, gastrointestinal.
Discussion
Among the subgroup of individuals with eGFR<60 ml/min per 1.73 m2 who participated in the AIM-HIGH Trial, randomization to niacin resulted in lower plasma phosphate concentrations at the end of 3 years of therapy. The magnitude of effect was relatively modest; those randomized to niacin had 0.27 mg/dl lower phosphate concentrations than those randomized to placebo at 3 years. Results were generally similar in “as treated” analyses and in the subset of participants with eGFR<45 ml/min per 1.73 m2 at baseline. We found no significant effects of randomization to niacin on any of the other mineral markers evaluated, including FGF23 and PTH.
In vitro and animal experiments show that niacin decreases expression of Npt2b (17), and several small, short-term studies in humans have shown that niacin therapy lowered serum phosphate concentrations in CKD and ESRD (16,20,22,23). However, these studies were limited by small sample size and varied study designs. Moreover, the effects on other markers of mineral metabolism are largely unexplored. We designed this study to determine the effects of niacin over 3-years duration (21). Our findings confirm that niacin lowers phosphate concentrations in patients with CKD and show that the effect is evident over 3 years of treatment.
Our secondary objective was to determine the effects of niacin on other markers of mineral metabolism: in particular, FGF23 and PTH. These hormones were lower at year 1 but not at year 3, and no significant differences were observed in linear effects models. Little is known about the effects of nicotinamide or niacin on these hormones. An open label randomized trial in 100 patients with ESRD compared nicotinamide 0.5–2.0 g/d (mean dose 1.3 g/d) with sevelamer 3.2–9.6 g/d (mean dose 8.6 g/d) for 24 weeks. Both groups had similar decreases in serum phosphorous concentrations after baseline. Nicotinamide therapy resulted in no changes in FGF23 or PTH relative to baseline (25). We had previously conducted a pilot study to assess the effects of niacin on FGF23 in patients with eGFR between 30 and 74 ml/min per 1.73 m2 (20). Participants were randomized to niacin, niacin plus laropiprant (an antiflushing agent), and placebo (26). We observed FGF23 lowering in the niacin-only group (n=97) but not in the niacin plus laropiprant group. We had no a priori hypotheses about laropiprant’s effects on FGF23. Moreover, by chance, FGF23 concentrations were statistically significantly higher at baseline in the niacin-only arm relative to the other two arms; thus, regression to the mean may explain our prior findings rather than a true biologic effect. This AIM-HIGH Trial is larger, has longer follow-up, and included patients with lower eGFR. Here, although FGF23 was lower in niacin-treated patients at 1 year, we could not confirm effects of niacin on FGF23 over 3 years.
Beyond phosphate levels, other FGF23 regulators have been identified, including α-Klotho, inflammation, iron deficiency, and others (8,27–30). These factors may be more potent regulators of FGF23 than sodium phosphate cotransporter 2b phosphate transport per se. Alternatively, more substantial reductions in serum phosphate that were observed in AIM-HIGH may be required to alter FGF23 (31,32). Recent studies evaluating tenapanor, a sodium hydrogen exchange inhibitor, resulted in reductions of serum phosphate larger than observed here and commensurate decreases in FGF23 (least squares mean ratio end point/baseline: 0.73–0.91) in patients with ESRD (31). Similarly, randomization to the calcimemetic drug etelcalcetide resulted in more marked reductions in serum phosphate and concurrent reductions in FGF23 (32).
The magnitude of phosphate lowering (0.25 mg/dl) observed here was relatively modest. Niacin did not significantly reduce cardiovascular events in the AIM-HIGH Trial, and there was an unexpected higher rate of strokes in the niacin arm (21). Results were similar in the subgroup with CKD (33). Thus, we do not advocate use of niacin for cardiovascular disease risk reduction in patients with CKD. Rather than changes in clinical care, our findings support approaches to novel mechanisms of phosphate lowering in patients with CKD. Previous clinical trials suggest that phosphate binders are only minimally effective for phosphate lowering in patients with CKD (11,17). By binding phosphate and rendering it less available for intestinal absorption, binders are known to upregulate Npt2b expression in the small intestine, which may paradoxically stimulate greater absorption of phosphate at times when binders are not present in the intestinal lumen (17). Niacin and nicotinamide, in turn, reduce Npt2b expression. Thus, concurrent use of an intestinal phosphate binder and niacin or nicotinamide may synergistically induce greater reductions in serum phosphate concentrations. The ongoing CKD Optimal Management With BInders and NicotinamidE (COMBINE) Trial is testing nicotinamide alone, lanthanum carbonate alone, and both compounds in combination versus dual placebo among patients with moderate to severe CKD, and it may soon provide additional insights (34).
This study has important limitations. Subjects were trial participants with prevalent cardiovascular disease and hyperlipidemia, and serum creatinine >2.5 mg/dl was an exclusion criterion. In addition, the majority of patients were elderly, only 40% had an eGFR<45 ml/min per 1.73 m2, and urine specimens were not available to assess proteinuria. Whether results generalize to other populations is uncertain. We evaluated fasting morning serum phosphate and other mineral metabolism concentrations. Diet is known to influence circadian rhythms in serum phosphate concentrations in patients with CKD (35), and whether niacin may have influenced phosphate concentrations more dramatically at other times of the day remains unknown. Lastly, urine specimens were not obtained in the AIM-HIGH Trial, limiting the ability to evaluate and measure urine phosphate excretion.
In conclusion, niacin treatment lowers phosphate concentrations, an effect that is evident over 3 years in patients with mild to moderate CKD. However, the observed effects were relatively modest in magnitude, and we found no definitive evidence for changes in FGF23, PTH, or other mineral metabolism parameters. These findings, in the context of prior studies, do not support use of niacin in isolation for the purposes of cardiovascular disease prevention in CKD. However, future trials combining niacin or nicotinamide with phosphate binders or other phosphate-lowering therapies may provide new synergistic opportunities for attacking mineral bone disorders in patients with CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Trial was funded by the National Heart, Lung, and Blood Institute with study drug donation from Abbott and Merck. This ancillary study was supported by grants R01DK101720 and K24DK11042 from the National Institutes of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Lowering Expectations with Niacin Treatment for CKD-MBD,” on pages 6–8.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05440517/-/DCSupplemental.
References
- 1.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165: 473–481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo KT, Choong HL, Wong KS, Tan HB, Chan CM: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 81: 1044–1045, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM: Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: E10–E17, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Razzaque MS: Phosphate toxicity and vascular mineralization. Contrib Nephrol 180: 74–85, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR: Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20: 381–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD: Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol 18: 2116–2124, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, Fernandez Cruz L, Campistol JM, Torres A, Rodriguez M: High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol 9: 1845–1852, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Martin A, David V, Quarles LD: Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 92: 131–155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutiérrez OM, Bansal V, Rosas SE, Nigwekar S, Yee J, Kramer H: KDOQI US Commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) [published online ahead of print September 20, 2017]. Am J Kidney Dis doi:10.1053/j.ajkd.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 11.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoyama K, Hirakata H, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y, Block GA: Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol 9: 543–552, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isakova T, Gutiérrez OM, Smith K, Epstein M, Keating LK, Jüppner H, Wolf M: Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant 26: 584–591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Parra E, Gonzalez-Casaus ML, Galán A, Martinez-Calero A, Navas V, Rodriguez M, Ortiz A: Lanthanum carbonate reduces FGF23 in chronic kidney disease stage 3 patients. Nephrol Dial Transplant 26: 2567–2571, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Fishbane S, Block GA, Loram L, Neylan J, Pergola PE, Uhlig K, Chertow GM: Effects of ferric citrate in patients with nondialysis-dependent CKD and iron deficiency anemia. J Am Soc Nephrol 28: 1851–1858, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng SC, Young DO, Huang Y, Delmez JA, Coyne DW: A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol 3: 1131–1138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg C, Ix JH: Nicotinamide and phosphate homeostasis in chronic kidney disease. Curr Opin Nephrol Hypertens 25: 285–291, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katai K, Tanaka H, Tatsumi S, Fukunaga Y, Genjida K, Morita K, Kuboyama N, Suzuki T, Akiba T, Miyamoto K, Takeda E: Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant 14: 1195–1201, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Wu KI, Bacon RA, Al-Mahrouq HA, Kempson SA: Nicotinamide as a rapid-acting inhibitor of renal brush-border phosphate transport. Am J Physiol 255: F15–F21, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Ix JH, Ganjoo P, Tipping D, Tershakovec AM, Bostom AG: Sustained hypophosphatemic effect of once-daily niacin/laropiprant in dyslipidemic CKD stage 3 patients. Am J Kidney Dis 57: 963–965, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W; AIM-HIGH Investigators : Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 365: 2255–2267, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Strathmann FG, Laha TJ, Hoofnagle AN: Quantification of 1α,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem 57: 1279–1285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grubb A, Blirup-Jensen S, Lindström V, Schmidt C, Althaus H, Zegers I; IFCC Working Group on Standardisation of Cystatin C (WG-SCC) : First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med 48: 1619–1621, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenglet A, Liabeuf S, El Esper N, Brisset S, Mansour J, Lemaire-Hurtel AS, Mary A, Brazier M, Kamel S, Mentaverri R, Choukroun G, Fournier A, Massy ZA: Efficacy and safety of nicotinamide in haemodialysis patients: The NICOREN study. Nephrol Dial Transplant 32: 870–879, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Rao M, Steffes M, Bostom A, Ix JH: Effect of niacin on FGF23 concentration in chronic kidney disease. Am J Nephrol 39: 484–490, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M; Chronic Renal Insufficiency Cohort : Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 7: 1155–1162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imel EA, Gray AK, Padgett LR, Econs MJ: Iron and fibroblast growth factor 23 in X-linked hypophosphatemia. Bone 60: 87–92, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ix JH, Chonchol M, Laughlin GA, Shlipak MG, Whooley MA: Relation of sex and estrogen therapy to serum fibroblast growth factor 23, serum phosphorus, and urine phosphorus: The Heart and Soul study. Am J Kidney Dis 58: 737–745, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N: Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res 25: 1711–1723, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Block GA, Rosenbaum DP, Leonsson-Zachrisson M, Åstrand M, Johansson S, Knutsson M, Langkilde AM, Chertow GM: Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol 28: 1933–1942, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block GA, Bushinsky DA, Cunningham J, Drueke TB, Ketteler M, Kewalramani R, Martin KJ, Mix TC, Moe SM, Patel UD, Silver J, Spiegel DM, Sterling L, Walsh L, Chertow GM: Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: Two randomized clinical trials. JAMA 317: 146–155, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Kalil RS, Wang JH, de Boer IH, Mathew RO, Ix JH, Asif A, Shi X, Boden WE: Effect of extended-release niacin on cardiovascular events and kidney function in chronic kidney disease: A post hoc analysis of the AIM-HIGH trial. Kidney Int 87: 1250–1257, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isakova T, Ix JH, Sprague SM, Raphael KL, Fried L, Gassman JJ, Raj D, Cheung AK, Kusek JW, Flessner MF, Wolf M, Block GA: Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 26: 2328–2339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ix JH, Anderson CA, Smits G, Persky MS, Block GA: Effect of dietary phosphate intake on the circadian rhythm of serum phosphate concentrations in chronic kidney disease: A crossover study. Am J Clin Nutr 100: 1392–1397, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.