Abstract
Background and objectives
Improved knowledge about factors that influence patient choices when considering dialysis modality could facilitate health care interventions to increase rates of home dialysis. We aimed to quantify the attributes of dialysis care and the tradeoffs that patients consider when making decisions about dialysis modalities.
Design, setting, participants, & measurements
We conducted a prospective, discrete choice experiment survey with random parameter logit analysis to quantify preferences and tradeoffs for attributes of dialysis treatment in 143 adult patients with CKD expected to require RRT within 12 months (predialysis). The attributes included schedule flexibility, patient out of pocket costs, subsidized transport services, level of nursing support, life expectancy, dialysis training time, wellbeing on dialysis, and dialysis schedule (frequency and duration). We reported outcomes using β-coefficients with corresponding odds ratios and 95% confidence intervals for choosing home-based dialysis (peritoneal dialysis or hemodialysis) compared with facility hemodialysis.
Results
Home-based therapies were significantly preferred with the following attributes: longer survival (odds ratio per year, 1.63; 95% confidence interval, 1.25 to 2.12), increased treatment flexibility (odds ratio, 9.22; 95% confidence interval, 2.71 to 31.3), improved wellbeing (odds ratio, 210; 95% confidence interval, 15 to 2489), and more nursing support (odds ratio, 87.3; 95% confidence interval, 3.8 to 2014). Respondents were willing to accept additional out of pocket costs of approximately New Zealand $400 (United States $271) per month (95% confidence interval, New Zealand $333 to $465) to receive increased nursing support. Patients were willing to accept out of pocket costs of New Zealand $223 (United States $151) per month (95% confidence interval, New Zealand $195 to $251) for more treatment flexibility.
Conclusions
Patients preferred home dialysis over facility-based care when increased nursing support was available and when longer survival, wellbeing, and flexibility were expected. Sociodemographics, such as age, ethnicity, and income, influenced patient choice.
Keywords: chronic dialysis; chronic kidney disease; chronic kidney failure; dialysis; end stage kidney disease; hemodialysis; peritoneal dialysis; Adult; Humans; Patient Preference; renal dialysis; Life Expectancy; Odds Ratio; Confidence Intervals; Health Expenditures; Prospective Studies; Choice Behavior; Renal Insufficiency, Chronic; Surveys and Questionnaires
Introduction
The increasing global burden of dialysis has led to a renewed interest in home dialysis due to its cost-effectiveness (1–3) compared with facility treatment. Home dialysis may offer a number of advantages, including longer life expectancy (4,5), quality of life (6), flexibility (7), and the ability to maintain employment (8–10). However, patients and families may choose facility-based dialysis to avoid out of pocket costs (power, water, and heating) or because of a perception of less clinical support, social isolation, or a fear of having to self-manage catastrophic events (9,11–13). Insufficient understanding of patient preferences when choosing a dialysis modality may lead to lower rates of home dialysis.
Although predialysis education increases the uptake of home dialysis (14,15), there is limited information about the relative value that patients place on specific treatment attributes and the other factors that influence decision making when considering home-based dialysis care. Greater knowledge of the relative importance of sociodemographic factors, reimbursement, transport assistance, nursing support, and dialysis treatment characteristics that patients consider when making choices about dialysis modality could assist health care service and policy development to increase home-based dialysis.
In this study, we used a discrete choice experiment (DCE) design to quantify the attributes of dialysis care and the tradeoffs that patients consider when making decisions about dialysis modality.
Materials and Methods
DCEs
We used a DCE to quantify patient preferences for characteristics (or attributes) of dialysis modality. DCEs are on the basis of the concept that health care interventions (such as dialysis modality) can be described by a set of attributes or characteristics and that the value an individual places on these attributes when weighing up competing treatment choices depends on the levels of these treatment-specific attributes (16). DCEs are commonly used in health services research to quantify competing health care attributes that might influence patient preferences for treatment to inform health care policy and program development (17,18).
In a DCE, participants are presented with a series of questions describing treatment alternatives and asked to choose their preferred option in each question. Treatment alternatives are described by a set of attributes (for example, out of pocket costs) with varying levels (for example, $0, $50, $100, etc.) (Table 1). Using experimental design principles, the levels of attributes that are presented in each question are varied systematically. Respondents choose the treatment alternative (dialysis option) that they prefer, which is assumed to have the highest value to them. On the basis of the choices made, the relative importance of attributes and their levels can be calculated (19).
Table 1.
Choice set attributes and levels
| Description of Attributes | Home HD Attribute Levels | PD Attribute Levels | In-Center Attribute Levels |
|---|---|---|---|
| Ability to change dialysis schedule | Easy to change | Easy to change | Hard to change |
| Sometimes possible to change | Sometimes possible to change | ||
| Hard to change | Hard to change | ||
| Average cost per month (New Zealand dollars) | $0 | $0 | $0 |
| $50 | $50 | ||
| $250 | $250 | ||
| $500 | $500 | ||
| Availability of transport service or reimbursement | A shuttle or taxi is always provided, or if you use your own car, all costs are covered | A shuttle or taxi is always provided, or if you use your own car, all costs are covered | A shuttle or taxi is always provided, or if you use your own car, all costs are covered |
| Sometimes a shuttle or taxi is provided, or if you use your own car, some costs are covered | Sometimes a shuttle or taxi is provided, or if you use your own car, some costs are covered | ||
| No shuttle or taxi is provided, and you have to pay all your own transport costs | No shuttle or taxi is provided, and you have to pay all your own transport costs | ||
| Staff support if needed | Nurse support on the phone is available Monday to Friday 8 a.m. to 4 p.m. | Nurse support on the phone is available Monday to Friday 8 a.m. to 4 p.m. | You have a nurse present during all dialysis treatments |
| Nurse support on the phone is available 24 h/d | Nurse support on the phone is available 24 h/d | ||
| Nurse support is available 24 h/d, and you can have a nurse home visit once per week for as long as you like | Nurse support is available 24 h/d, and you can have a nurse home visit once per week for as long as you like | ||
| You have a nurse present during all dialysis treatments for as long as needed, and telephone support is available 24 h/d | You have a nurse present during all dialysis treatments for as long as needed, and telephone support is available 24 h/d | ||
| Life expectancy, yr | 3 | 3 | 3 |
| 5 | 5 | 5 | |
| 10 | 10 | 10 | |
| 15 | 15 | 15 | |
| Dialysis training time | 6 wk (3–5/wk) | 5 d | None |
| 12 wk (3–5/wk) | 7 d | ||
| 18 wk (3–5/wk) | 10 d | ||
| How well you feel on dialysis | Worse than now | Worse than now | Worse than now |
| Better than now | Better than now | Better than now | |
| Same as now | Same as now | Same as now | |
| Dialysis time of day | During the day or evening | During the day (CAPD) | ≅During the day |
| ≅During the day | Overnight while you sleep (APD) | ||
| ≅Overnight while you sleep | |||
| Dialysis days per week | ≅3 | ≅7 | ≅3 |
| ≅5 | |||
| ≅6 | |||
| ≅7 |
HD, hemodialysis; PD, peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; APD, automated peritoneal dialysis.
DCE methodology is underpinned by random utility theory, consumer theory, and experimental design theory (20). On the basis of the choices within the DCEs, a mathematical function is estimated to numerically describe the value that respondents attach to different attributes (19). Sociodemographic data can also enter into the value functions as explanatory variables. DCEs provide rich data used to quantify the attributes that drive patient preferences, the tradeoffs between attributes that people are willing to accept, and how changes in the levels of these attributes can lead to potential changes in patient preferences (17,18,21).
Participant Selection and Recruitment
The study was conducted in two centers in New Zealand. Patients were considered for participation if they were ages 18 years old or older, were anticipated to require RRT within a year (predialysis), and had received formal education about options for RRT. Patients were eligible to participate in the study if they were deemed clinically suitable for either facility or home dialysis in the opinion of their treating clinical team and able to read English. Patients were invited to participate by a nephrologist, CKD coordinator, or predialysis nurse specialist from a list of all eligible patients. The study was approved by relevant hospital ethics committees (Counties Manukau Institutional Review Board [reference no. 1771] and Hawke’s Bay Institutional Review Board [reference no. 14/06/160]). All respondents provided written informed consent.
Survey Design
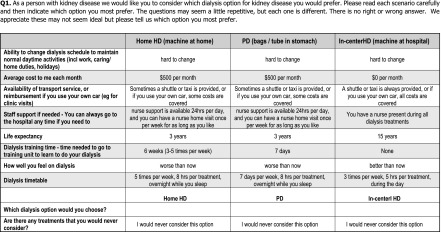
The survey was presented to participants as follows: (1) introduction and explanation of the study, (2) description and explanation of attributes, and (3) an example choice set. Respondents were shown 12 choice scenarios and asked to choose the treatment that they most preferred in each scenario (Figure 1). Sociodemographic data were also collected, including self-identified ethnicity, number of people living in household, educational attainment, and employment.
Figure 1.
Choice set example presented to patients. Example of choice set presented to each patient before dialysis in the discrete choice survey. HD, hemodialysis; PD, peritoneal dialysis.
We piloted the initial survey design in face to face consultation with 13 patients to ensure an adequate understanding and interpretation of the attributes and levels. We also asked for feedback on the length and number of scenarios in the survey and the demographics questionnaire. Respondent feedback was used to inform the final efficient design, which was generated in NGENE software (www.choice-metrics.com). The final design had a d error of 0.12 and consisted of 12 choice sets of three alternative dialysis modalities (home hemodialysis [HD], peritoneal dialysis [PD], and facility HD) (22).
Data Collection
Patient recruitment and data collection were performed between November of 2015 and July of 2016. After verbal consent was obtained, participants were given the survey by either the investigator (R.C.W.) or the predialysis nurse, who explained how to complete survey and showed this with the example scenario. The respondents then either completed the survey at that time or took it home to complete and returned the completed survey via mail. There were no missing data in the completed surveys.
Attributes
We presented patients with three dialysis modalities in each question: home HD, PD, and in-center HD (Figure 1). The attributes and levels used to describe each dialysis alternative were on the basis of our prior research, including qualitative interviews with patients (13,23) and thematic syntheses of patient and caregiver values, beliefs, and experiences when considering home dialysis (8,9). Levels for patient survival were obtained from the Australia New Zealand Dialysis and Transplant Registry (24) to include a range of plausible values. Dialysis alternatives were described using the following attributes: flexibility of dialysis schedule (easy to change, sometimes possible to change, and hard to change), out of pocket costs to patient, availability of transport services (or reimbursement of travel costs), life expectancy, dialysis training time, levels of nursing support, wellbeing on dialysis, and dialysis schedule (time of day, frequency, and duration) (Table 1).
Data Analyses
We used NLOGIT, version 5.0 (Econometric Software, Castle Hill, NSW, Australia; www.limdep.com/products/nlogit/) to analyze the data. Internal validity (that is, the extent to which results were consistent with the researchers’ prior expectations) was assessed by examining the signs and significance of parameter estimates.
We hypothesized a priori that respondents would prefer home dialysis when out of pocket costs were lower, transportation was not reimbursed, patients felt better on dialysis, and the dialysis schedule had greater flexibility. We built more complex models to increase the goodness of fit and enable greater exploration of observed heterogeneity. Different attribute-level specifications were examined, including using different distributions and coding as continuous or categorical (effects coded) variables and as random and nonrandom parameters. We also examined the influence of sociodemographic characteristics as interactions with both attributes and the constant.
The final model was a mixed multinomial logit model within a panel specification (also termed a random parameters logit model). The final model specification was determined on the basis of the P value of specified parameters, log likelihood tests, McFadden pseudo-R2, and Akaike Information Criteria. A panel specification was used to account for correlated choices within an individual, because each respondent completed 12 choice tasks. In the final model, cost, life expectancy, flexibility, wellness on dialysis, and time of treatment were treated as continuous linear variables; all other categorical attributes were effects coded. We included an interaction between distance from the dialysis unit (<100 or >100 km) and availability of transport. The attributes were specified as random with normal distributions, with 5000 Halton draws. Some attributes were specified with generic levels across home HD and PD, whereas others had levels that were specific to either home HD or PD. Interactions between treatment characteristics (attributes) and other sociodemographic variables that did not improve the model fit were not included in the final model. We also examined the influence of sociodemographic characteristics (age, education, ethnicity, home ownership, household size, distance from dialysis unit, preference for access type, income, and employment) as interactions with the alternative specific constants for home HD and PD. Model results were expressed as β-parameters and the odds ratios (ORs; 95% confidence intervals [95% CIs]) of choosing home HD or PD compared with facility HD. Marginal rates of substitution (tradeoffs) between the out of pocket cost attribute and other attributes were also examined.
Results
Of the 170 eligible respondents, 143 (84%) completed the survey (Supplemental Figure 1). The participant characteristics are shown in Table 2. The mean age was 56 years old (range, 18–85). Seventy-eight (55%) were men, and 85 (59%) were married or living with a partner. Seventy-six (53%) owned their own home, 50 (34%) were in full- or part-time employment, and 53 (37%) had less than high school–level qualifications.
Table 2.
Respondent characteristics
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Men | 78 (55) |
| Women | 65 (45) |
| Age, yr | |
| <65 | 88 (62) |
| >65 | 55 (38) |
| Employment status | |
| Pensioner | 52 (36) |
| Part time | 15 (10) |
| Full time | 35 (24) |
| Not employed/beneficiary | 41 (29) |
| Education | |
| Primary | 14 (10) |
| Some high school | 39 (27) |
| Completed high school | 26 (18) |
| Trade certificate | 21 (15) |
| Diploma/certificate | 23 (16) |
| Postgraduate | 20 (14) |
| Ethnicity | |
| European | 61 (43) |
| Māori | 49 (34) |
| Pacific Islander | 21 (15) |
| Asian | 7 (5) |
| Indian | 5 (3) |
| Marital status | |
| Married/de facto | 85 (59) |
| Partnered but not living together | 9 (6) |
| Single | 21 (15) |
| Widowed | 24 (17) |
| Divorced | 3 (2) |
| Other | 1 (1) |
| Housing | |
| Own | 76 (53) |
| Government housing | 35 (24) |
| Private rental | 31 (22) |
| Supported housing | 1 (1) |
| No. of people living in household | |
| 1 | 20 (14) |
| 2 | 47 (33) |
| 3 | 13 (9)) |
| 4 | 22 (15) |
| 5+ | 41 (29) |
| Distance from dialysis unit, km (miles) | |
| 0–10 (0–6.2) | 45 (31) |
| 11–50 (6.8–31) | 71 (50) |
| 51–100 (32–62) | 8 (6) |
| 101+ (63+) | 19 (13) |
| Preferred dialysis access | |
| Fistula | 46 (32) |
| Peritoneal dialysis catheter | 59 (41) |
| Central venous catheter | 8 (6) |
| Either | 30 (21) |
| Gross annual household income (New Zealand dollars) | |
| $0–19,999 | 16 (11) |
| $20,000–39,999 | 34 (24) |
| $40,000–59,999 | 42 (29) |
| $60,000–79,999 | 24 (17) |
| $80,000–99,999 | 15 (10) |
| $100,000+ | 12 (8) |
Preferences
Influence of Treatment Characteristics on Choice.
There was no overall preference for either home HD or PD compared with in-center dialysis.
Respondent preferences for dialysis modality were significantly influenced by the attributes of the respective services. Both home HD and PD were preferred to in-center dialysis when treatment options offered a longer life expectancy (per 1 year of additional life expectancy: OR, 1.63; 95% CI, 1.25 to 2.1); more treatment flexibility (easy to change or sometimes possible to change versus hard to change: OR, 9.22; 95% CI, 2.71 to 31.3); wellbeing on dialysis (worse than now to same as now and same as now to better than now: OR, 210; 95% CI, 15.0 to 2489); more staff support (high versus lower level of support: OR, 87.3; 95% CI, 3.78 to 2014); and ability to dialyze during the day or evening (day or evening versus day only: OR, 89.0; 95% CI, 6.51 to 1217).
Higher patient preferences for home HD were associated with a training time of 6 weeks (OR, 15.7; 95% CI, 1.95 to 127) or 18 weeks (OR, 237; 95% CI, 15.8 to 3548) versus 12 weeks (usual training time in New Zealand) and when nocturnal HD was available, for 8 hours compared with 5 hours (OR, 82.7; 95% CI, 8.21 to 832). Patient preferences were not associated with dialysis frequency. Patients preferred PD with a longer training time (7 versus 5 days: OR, 7.77; 95% CI, 1.31 to 45.9). The availability of overnight PD (automated PD) did not significantly influence patient preferences for PD (OR, 5.05; 95% CI, 0.16 to 164.2).
Patients who lived farther than 100 km from the dialysis unit were less likely to prefer home modalities if there was no subsidized transportation (OR, 0.02; 95% CI, <0.01 to 0.48). Patient preference for home dialysis was markedly lower when treatment was only available overnight compared with daytime treatment only (OR, <0.01; 95% CI, <0.01 to 0.90) and when treatment resulted in higher out of pocket costs (per $100 increase: OR, 98.0; 95% CI, 70.0 to 99.0) (Table 3).
Table 3.
Patient preferences for home dialysis (HD and PD) compared with in-center dialysis
| Attributes for Home Dialysis (HD and PD) | β | OR (95% CI) | P Value |
|---|---|---|---|
| Treatment attributes (random parameters) | |||
| Out of pocket cost (per extra $) | −0.02 | 0.98 (0.97 to 0.99) | 0.003 |
| Life expectancy (per extra year) | 0.49 | 1.63 (1.25 to 2.12) | <0.001 |
| Flexibility of treatments (per unit increase in flexibility: hard to change, sometimes possible to change, or easy to change) | 2.22 | 9.22 (2.71 to 31.3) | <0.001 |
| How well you feel on dialysis (per unit improvement)a | 5.35 | 210 (15.0 to 2489) | <0.001 |
| Availability of transport (versus all transport provided) | |||
| Respondents living <100 km from nearest dialysis center | |||
| Transport sometimes provided | −0.86 | 0.42 (0.05 to 3.24) | 0.40 |
| Transport never provided | −1.61 | 0.20 (0.02 to 1.59) | 0.10 |
| Respondents living >100 km from nearest dialysis center | |||
| Transport sometimes provided | 3.20 | 24.5 (0.85 to 706) | 0.06 |
| Transport never provided | −3.82 | 0.02 (0.001 to 0.48) | 0.02 |
| Availability of nurse support (versus nurse present during each dialysis session) | |||
| Minimal nurse support (telephone only 8 a.m. to 4 p.m.) | 1.64 | 5.18 (0.42 to 63.3) | 0.20 |
| Limited nurse support (24-h phone support and scheduled home visits) | −4.27 | 0.01 (<0.01 to 3.28) | 0.10 |
| Unlimited nurse support (24-h phone support and as many home visits as requested) | 4.47 | 87.30 (3.78 to 2014) | <0.01 |
| Treatment time (versus during day only) | |||
| During day/evening | 4.49 | 89.01 (6.51 to 1217) | <0.001 |
| Only overnight | −5.83 | <0.01 (<0.01 to 0.09) | <0.001 |
OR>1 favors home hemodialysis or peritoneal dialysis. Mixed logit model (n=143). Log likelihood of −936.77768. Akaike Information Criterion n=1.188. McFadden pseudo-R2 =0.496. HD, hemodialysis; PD, peritoneal dialysis; OR, odds ratio; 95% CI, 95% confidence interval.
From worse than now to same as now and from same as now to better than now.
Influence of Patient Characteristics on Preferences.
Several sociodemographic characteristics were associated with dialysis modality choice. Pacific Island ethnicity was associated with a lower preference for home-based dialysis (home HD versus facility: OR, <0.01; 95% CI, <0.01 to 0.03; peritoneal versus facility: OR, <0.01; 95% CI, <0.01 to <0.01). Identifying as New Zealand Māori was associated with a higher preference for PD (versus in-center HD: OR, 132; 95% CI, 5.79 to 3023). Older age (>65 years old) was associated with a lower preference for home-based dialysis (home HD: OR, 0.82; 95% CI, 0.75 to 0.90; PD: OR, 0.84; 95% CI, 0.76 to 0.92).
Annual household income below New Zealand $60,000 (United States $42,000) was associated with lower preference for home HD (OR, 0.02; 95% CI, <0.01 to 0.18) and PD (OR, 0.11; 95% CI, 0.02 to 0.54). A higher household density (more than five people) was associated with a preference for home dialysis (PD: OR, 1958; 95% CI, 77.7 to 49,450; home HD: OR, 56.8; 95% CI, 6.49 to 497) as was living farther than 100 km from the nearest dialysis unit (PD: OR, 31.5; 95% CI, 4.95 to 201; home HD: OR, 521; 95% CI, 34.4 to 7881) (Table 4).
Table 4.
Patient preferences for home HD and PD compared with in-center dialysis
| Home HD | PD | |||||
|---|---|---|---|---|---|---|
| Attributes for Home HD and PD | β | OR (95% CI) | P Value | β | OR (95% CI) | P Value |
| Constant | 7.98 | 2926 (0.04 to 208,746,259) | 0.16 | 5.62 | 276 (0.001 to 141,306,239) | 0.40 |
| Training duration | ||||||
| Home HD training time 6 wk (versus 12 wk) | 2.76 | 15.7 (1.95 to 127) | <0.01 | |||
| Home HD training time 18 wk (versus 12 wk) | 5.47 | 237 (15.8 to 3548) | <0.001 | |||
| PD training time 7 d (versus 5 d) | 2.05 | 7.77 (1.31 to 45.92) | 0.02 | |||
| PD training 10 d (versus 5 d) | −1.24 | 0.29 (0.06 to 1.35) | 0.01 | |||
| Length of treatment | ||||||
| Short daily dialysis (3 versus 5 h) | −3.54 | 0.03 (<0.01 to 1.10) | 0.06 | |||
| Overnight dialysis (8 versus 5 h) | 4.41 | 82.7 (8.21 to 832) | <0.001 | |||
| Overnight dialysis (8 h versus four exchanges per day) | 1.62 | 5.05 (0.16 to 164.2) | 0.40 | |||
| No. of treatments per week (per extra treatment from three per week) | 1.79 | 5.96 (0.95 to 37.45) | 0.06 | |||
| Sociodemographic factors (fixed parameters) | ||||||
| Age group (<65 versus >65 yr) | −0.19 | 0.82 (0.75 to 0.90) | <0.001 | −0.18 | 0.84 (0.76 to 0.92) | <0.001 |
| Less than high school education (versus more than high school education) | −1.41 | 0.24 (0.08 to 0.73) | 0.01 | −1.61 | 0.20 (0.07 to 0.59) | 0.004 |
| Ethnicity (versus New Zealand European) | ||||||
| New Zealand Maori | 0.53 | 1.69 (0.24 to 11.9) | 0.60 | 4.89 | 132 (5.79 to 3023) | 0.002 |
| Pacific Islander | −7.96 | <0.01 (<0.01 to 0.03) | <0.001 | −14.16 | <0.01 (<0.01 to <0.01) | <0.001 |
| Other ethnicity | 3.01 | 20.3 (0.14 to 2926) | 0.20 | 0.52 | 1.68 (0.01 to 436) | 0.90 |
| Not own home owner | 0.23 | 1.26 (0.44 to 3.60) | 0.70 | 1.19 | 3.29 (0.96 to 11.3) | 0.06 |
| Household no. >5 | 4.04 | 56.8 (6.49 to 497) | <0.001 | 7.58 | 1958 (77.7 to 49,450) | <0.001 |
| Distance to nearest dialysis unit >100 km | 6.26 | 521 (34.4 to 7881) | <0.001 | 3.45 | 31.5 (4.95 to 201) | <0.001 |
| No preferred dialysis access choice | −2.85 | 0.06 (0.01 to 0.32) | 0.001 | −7.31 | <0.01 (<0.01 to 0.02) | <0.001 |
| Annual household income <$60,000/yr | −3.73 | 0.02 (0.003 to 0.18) | <0.001 | −2.23 | 0.11 (0.02 to 0.54) | <0.01 |
| Employed full time | 2.13 | 8.42 (1.67 to 42.4) | <0.01 | 2.37 | 10.73 (2.33 to 49.4) | 0.002 |
OR>1 favors home HD or PD. Mixed logit model (n=143). Log likelihood of −936.77768. Akaike Information Criterion n=1.188. McFadden pseudo-R2 =0.496. HD, hemodialysis; PD, peritoneal dialysis; OR, odds ratio; 95% CI, 95% confidence interval.
Tradeoffs
Respondents were willing to accept additional costs of approximately New Zealand $400 (United States $271) per month (95% CI, New Zealand $333 to $465) to have unlimited nursing support. Patients were willing to accept costs of New Zealand $223 (United States $151) per month (95% CI, New Zealand $195 to $251) for greater treatment flexibility (Table 5).
Table 5.
Tradeoff between out of pocket cost and dialysis characteristics
| Attribute Tradeoff | Mean (New Zealand Dollars) | Upper 95% CI | Lower 95% CI |
|---|---|---|---|
| Unlimited nurse support | 399.21 | 333.39 | 465.02 |
| Increased flexibility | 223.03 | 195.20 | 250.85 |
New Zealand dollars have been exchanged to United States dollars in the text using the Organisation for Economic Co-operation and Development (OECD) calculator (https://eppi.ioe.ac.uk/costconversion/default.aspx).
Discussion
This study highlights several findings regarding the preferences of patients predialysis when choosing a dialysis modality. Respondents preferred home-based dialysis when unlimited nursing support was available (including phone support and home visits), with respondents willing to pay New Zealand $400 (United States $271) per month to have increased nursing support. Respondents preferred home dialysis treatments when their out of pocket costs were lower and were more likely to choose home HD when nocturnal dialysis was available. Age, ethnicity, and household income also influenced patient choice of home dialysis. In addition, respondents were willing to pay New Zealand $223 (United States $151) per month for increased treatment flexibility.
These findings suggest that additional nurse support may increase the likelihood that patients would choose home-based dialysis. This is consistent with qualitative data suggesting that the preference for increased nursing support is important during the period of transition to home-based dialysis, when patients are at their most apprehensive and least confident with the treatment (23). Patient preferences for increased nursing support are also consistent with our finding that patients preferred longer training times. Extended, rather than shorter, training times were also preferred in a previous study (25). It has been established that patients contemplating and performing home dialysis have concerns around safety, isolation, and support (8,9,12,23) and that addressing perceived need for increased support and longer training may help to allay these concerns and support home dialysis.
These findings also suggest that patients are more likely to choose home dialysis when out of pocket costs are low. This tenet is supported by our findings that patients with lower incomes were less likely to prefer home dialysis and has important implications for services and policy development. Qualitative research involving patients training for home HD has shown that patients incur higher out of pocket expenses. These costs deplete personal financial reserves and were considered during decision making (26). In our previous qualitative study, both patients and caregivers believed that it was unfair and inequitable that patients treated with home dialysis subsidized the cost of their treatment, whereas patients treated with facility dialysis did not incur these additional out of pocket costs (13). The financial barriers to home HD may be substantial and have been acknowledged previously as a barrier to a nephrologist’s recommendation of home dialysis (27). However, in some countries, these additional out of pocket costs are already covered, and despite this, home dialysis rates remain low. Because facility HD is more expensive than home treatments to the payer, reimbursement for out of pocket expenses may be offset by increased home dialysis uptake and may allow reimbursement of home dialysis treatments to remain cost effective. Further analysis may help to inform and ultimately motivate future policy change.
Socioeconomic disadvantage has already been established as a barrier to patients choosing home dialysis (13), and in this study, those with lower income and those in minority ethnic groups or indigenous populations were less likely to prefer home dialysis. This observation is not specific to New Zealand. International research has found that minority populations and indigenous groups have significantly lower rates of home HD for reasons that are poorly understood (24,28). These findings raise the need for policies that recognize patients who are financially disadvantaged by home dialysis and address the situation to achieve equitable access to home dialysis across all socioeconomic levels.
Our study suggested that older patients were less likely to choose home dialysis, despite emerging evidence that home dialysis is associated with a lower risk of perceived worsening health in this population (29). As a consequence, these results taken together suggest there is a need for policymakers and providers to explore ways to support older patients in their choice of home dialysis, including assisted care models for PD that have shown positive results in France and Norway (30). Specific phased support for patients transitioning to home HD has proven to be successful in supporting patients with home HD (31).
Several findings are supported by previous studies, including patient preference for home-based therapies when a longer survival and improved wellbeing were expected and preference of treatment schedules being more flexible, important for those in paid employment (8,9,25,32). Despite increasing flexibility in home dialysis scheduling, global rates of home-based dialysis remain perhaps lower than expected. Enhanced focus on treatment flexibility in predialysis education may encourage eligible patients to consider this treatment option. Further research, including consideration of cost-effectiveness, is needed to explore models of increased nursing support for patients transitioning and established on home dialysis, including the role of remote patient monitoring. Future research should also test the generalizability of more novel models of supported home dialysis, such as the Australia and New Zealand concept of community houses (33,34), that help to alleviate barriers often associated with sociodemographic disadvantage.
This study uses well established discrete choice methods to assess the characteristics of dialysis important from the perspective of patients predialysis. We used extensive qualitative research and systematic review of literature to inform dialysis attributes and their levels. Our model had a good fit, indicating that we identified the important and relevant treatment attributes in dialysis treatment decision making. Our study, however, has several potential limitations that might be considered. First, we had a relatively small sample size that may have limited the ability to test for significance in all interaction effects, particularly for some of the sociodemographic effects (particularly ethnicity), as evidenced by the wide 95% CIs for some results. Second, we collected data on patients’ stated preferences rather than their actual choices made in a clinical setting, although in health, actual uptake will be strongly influenced by the real availability of services within a given health system. Third, our respondents were all patients predialysis, and therefore, some attributes may not have had the same value or importance compared with those in patients who had already commenced dialysis treatment. Fourth, we did not collect data on patient comorbidities, cause of kidney disease, or transplant candidacy, which may have provided more detailed explanations about patient preference, Fifth, our research was undertaken in New Zealand, and although we surveyed respondents across two contrasting units, the geographic, cultural, ethnic, and New Zealand–specific policies (for example, transportation and nursing support) may not be applicable outside the New Zealand context.
In conclusion, patients preferred home dialysis when increased nursing support was available and when improved survival, wellbeing, and flexibility were probable. Sociodemographics, such as age, ethnicity, and income, influenced patient choices about home dialysis. Payers and policymakers should explore ways to enable equitable access to home dialysis for socially disadvantaged groups and older patients.
Disclosures
M.R.M. is a full-time employee of Baxter Healthcare (Asia-Pacific; Shanghai, China), a part-time employee of the University of Auckland (Auckland, New Zealand) as an adjunct associate professor, and a part-time employee of Counties Manukau Health (Auckland, New Zealand) as a clinical nephrologist. Baxter Healthcare had no input into study conception, design, or execution. The other authors have no potential or actual conflicts of interest related to this study.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the participation of patients involved in the study. We also thank the clinicians involved for assistance with recruitment.
R.C.W. is supported by a University of Sydney Australian Postgraduate Award Scholarship, the Baxter Clinical Evidence Council research program, and a New Zealand Lotteries Health Research Grant. R.L.M. is supported by Australian National Health and Medical Research Council Early Career Researcher fellowship ID1054216. S.C.P. is supported by a Rutherford Discovery Fellowship from the Royal Society of New Zealand. M.R.M. is supported by a Jacquot Research Establishment Fellowship of the Royal Australasian College of Physicians. A.T. is supported by National Health and Medical Research Council fellowship ID1106716.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice editorial, “Trust Patient Insights at Both the Individual and National Level,” on pages 1–2.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06830617/-/DCSupplemental.
References
- 1.Walker R, Marshall MR, Morton RL, McFarlane P, Howard K: The cost-effectiveness of contemporary home haemodialysis modalities compared with facility haemodialysis: A systematic review of full economic evaluations. Nephrology (Carlton) 19: 459–470, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Karopadi AN, Mason G, Rettore E, Ronco C: Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant 28: 2553–2569, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Treharne C, Liu FX, Arici M, Crowe L, Farooqui U: Peritoneal dialysis and in-centre haemodialysis: A cost-utility analysis from a UK payer perspective. Appl Health Econ Health Policy 12: 409–420, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall MR, Walker RC, Polkinghorne KR, Lynn KL: Survival on home dialysis in New Zealand. PLoS One 9: e96847, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall MR, Polkinghorne KR, Kerr PG, Agar JW, Hawley CM, McDonald SP: Temporal changes in mortality risk by dialysis modality in the Australian and New Zealand dialysis population. Am J Kidney Dis 66: 489–498, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Wyld M, Morton RL, Hayen A, Howard K, Webster AC: A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med 9: e1001307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morton RL, Tong A, Webster AC, Snelling P, Howard K: Characteristics of dialysis important to patients and family caregivers: A mixed methods approach. Nephrol Dial Transplant 26: 4038–4046, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Tong A, Lesmana B, Johnson DW, Wong G, Campbell D, Craig JC: The perspectives of adults living with peritoneal dialysis: Thematic synthesis of qualitative studies. Am J Kidney Dis 61: 873–888, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Walker RC, Hanson CS, Palmer SC, Howard K, Morton RL, Marshall MR, Tong A: Patient and caregiver perspectives on home hemodialysis: A systematic review. Am J Kidney Dis 65: 451–463, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Nakayama M, Ishida M, Ogihara M, Hanaoka K, Tamura M, Kanai H, Tonozuka Y, Marshall MR: Social functioning and socioeconomic changes after introduction of regular dialysis treatment and impact of dialysis modality: A multi-centre survey of Japanese patients. Nephrology (Carlton) 20: 523–530, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Walker RC, Marshall MR: Increasing the uptake of peritoneal dialysis in New Zealand: A national survey. J Ren Care 40: 40–48, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Tong A, Palmer S, Manns B, Craig JC, Ruospo M, Gargano L, Johnson DW, Hegbrant J, Olsson M, Fishbane S, Strippoli GF: The beliefs and expectations of patients and caregivers about home haemodialysis: An interview study. BMJ Open 3: e002148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker RC, Howard K, Tong A, Palmer SC, Marshall MR, Morton RL: The economic considerations of patients and caregivers in choice of dialysis modality. Hemodial Int 20: 634–642, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin K, Manns B, Mortis G, Hons R, Taub K: Why patients with ESRD do not select self-care dialysis as a treatment option. Am J Kidney Dis 41: 380–385, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Goovaerts T, Jadoul M, Goffin E: Influence of a pre-dialysis education programme (PDEP) on the mode of renal replacement therapy. Nephrol Dial Transplant 20: 1842–1847, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Lancaster JK: A new approach to a consumer theory. J Polit Econ 74: 132–157, 1966 [Google Scholar]
- 17.de Bekker-Grob EW, Ryan M, Gerard K: Discrete choice experiments in health economics: A review of the literature. Health Econ 21: 145–172, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J: Conjoint analysis applications in health–a checklist: A report of the ISPOR good research practices for conjoint analysis task force. Value Health 14: 403–413, 2011 [DOI] [PubMed]
- 19.Ryan MGK, Amaya-Amaya M: Using Discrete Choice Experiments to Value Health and Health Care, Doredrecht, The Netherlands, Springer, 2008 [Google Scholar]
- 20.Ryan M, Farrar S: Using conjoint analysis to elicit preferences for health care. BMJ 320: 1530–1533, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancsar E, Louviere J: Conducting discrete choice experiments to inform healthcare decision making: A user’s guide. Pharmacoeconomics 26: 661–677, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Rose J, Bliemer MJ: Sample size requirements for stated choice experiments. Transportation 40: 1021–1041, 2013 [Google Scholar]
- 23.Walker RC, Howard K, Morton RL, Palmer SC, Marshall MR, Tong A: Patient and caregiver values, beliefs and experiences when considering home dialysis as a treatment option: A semi-structured interview study. Nephrol Dial Transplant 31: 133–141, 2016 [DOI] [PubMed] [Google Scholar]
- 24.ANZDATA Registry: 39th Report: Summary of Dialysis and Transplant in Australia and New Zealand. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia, 2016. Available at: www.anzdata.org.au/v1/report_2016.html. Accessed March 6, 2017
- 25.Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, Howard K: Dialysis modality preference of patients with CKD and family caregivers: A discrete-choice study. Am J Kidney Dis 60: 102–111, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Hanson CS, Chapman JR, Craig JC, Harris DC, Kairaitis LK, Nicdao M, Mikaheal M, Tong A: Patient experiences of training and transition to home haemodialysis: A mixed‐methods study. Nephrology (Carlton) 22: 631–641, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Ludlow MJ, George CR, Hawley CM, Mathew TH, Agar JW, Kerr PG, Lauder LA: How Australian nephrologists view home dialysis: Results of a national survey. Nephrology (Carlton) 16: 446–452, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Avalere Health: Distribution of Dialysis Patients Utilizing Home Modalities in 2012 by State, 2013. Available at: http://homedialysisalliance.org/userfiles/2014%20edition_Distribution%20of%20Dialysis%20Patients%20Utilizing%20Home%20Modalities%20by%20State%20in%202012.pdf. Accessed August 26, 2016
- 29.Derrett S, Samaranayaka A, Schollum JBW, McNoe B, Marshall MR, Williams S, Wyeth EH, Walker RJ: Predictors of health deterioration among older adults after 12 months of dialysis therapy: A longitudinal cohort study from New Zealand [published online ahead of print August 17, 2017]. Am J Kidney Dis doi:10.1053/j.ajkd.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 30.Olsen J, Bonnevie B, Palmhøj-Nielsen C, Povlsen JV: Economic consequences of an increased number of patients on outgoing dialysis. Scand J Urol Nephrol 44: 452–458, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Pierratos A, Tremblay M, Kandasamy G, Woodward G, Blake P, Graham J, et al. : Personal Support Worker (PSW)‐supported home hemodialysis: A paradigm shift. Hemodial Int 21: 173–179, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Morton RL, Devitt J, Howard K, Anderson K, Snelling P, Cass A: Patient views about treatment of stage 5 CKD: A qualitative analysis of semistructured interviews. Am J Kidney Dis 55: 431–440, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Marshall MR, van der Schrieck N, Lilley D, Supershad SK, Ng A, Walker RC, Dunlop JL: Independent community house hemodialysis as a novel dialysis setting: An observational cohort study. Am J Kidney Dis 61: 598–607, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Villarba A, Warr K: Home haemodialysis in remote Australia. Nephrology (Carlton) 9[Suppl 4]: S134–S137, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.