Introduction
A 55-year-old man with a history of ESRD due to type 2 diabetes mellitus and hypertension presents with a plasma K+ of 5.5 mEq/L 1 month after a deceased donor kidney transplant. Ten years earlier, he developed sepsis-induced AKI complicated by severe hyperkalemia requiring emergent hemodialysis. Over the next several years, he developed progressive CKD, during which time he was intolerant of renin-angiotensin-aldosterone (RAAS) blockers due to hyperkalemia. While being treated with peritoneal dialysis, the plasma K+ concentration ranged from 3.6 to 4.2 mEq/L. He later transferred to hemodialysis, and the predialysis K+ concentration averaged 5.5–5.8 mEq/L.
Normal K+ Homeostasis
This patient illustrates that hyperkalemia is a common occurrence when kidney function is either acutely or chronically reduced and in the setting of some forms of dialysis. Here, we discuss how impaired kidney K+ excretion gives rise to hyperkalemia across the spectrum of kidney disease. In addition, we discuss features unique to management of ESRD and their contribution to hyperkalemia.
The normal kidney has a large capacity to excrete K+. Accumulation of K+ in the interstitium after increased intake exerts an inhibitory effect on the thick ascending limb and to a lesser extent, proximal tubular NaCl reabsorption, resulting in increased flow and Na+ delivery to the distal nephron (1). High K+ intake modulates flow and Na+ delivery through direct effects in the distal convoluted tubule. Elevations in plasma K+ are registered by cells in the initial portion of this segment (DCT1), decreasing activity of the thiazide-sensitive Na+-Cl− cotransporter (2). In consequence, flow and Na+ are delivered increasingly to the adjacent aldosterone sensitive distal nephron (DCT2 and collecting duct), where electrogenic and flow-mediated K+ secretion is enhanced. There is also a gastric kidney reflex, providing an inhibitory effect on the Na+-Cl− cotransporter, which is initiated when K+ enters into the stomach. Lastly, there is also a circadian rhythm facilitating K+ secretion during the day when K+ intake is highest.
These mechanisms underlie the prodigious capacity of the normal kidney to excrete K+ and evolved in response to the diet of prehistoric man, which contained a fourfold higher K+ content. High K+ intake has health benefits, suggesting that the evolutionary design of the kidney was to maintain K+ homeostasis in the setting of high K+ intake (3).
Acute Kidney Injury
Hyperkalemia is a common complication of AKI when the injury involves the late distal nephron and extends into the collecting duct, causing direct injury of cells responsible for K+ secretion. Such injury can result from acute tubular necrosis due to ischemia or toxins or from inflammation as in acute tubulointerstitial nephritis. Hyperkalemia is an early finding in acute urinary obstruction, because increased tubular pressure disrupts the high resistance nature of the distal nephron, leading to loss of the electrical driving force for K+ secretion. Sudden reductions in the GFR become a limiting factor for K+ secretion in patients with AKI. In patients with oligoanuria, reduced distal delivery of salt and water further contributes to decreased distal K+ secretion.
The toxicity of hyperkalemia in patients with AKI develops with modest rises in the plasma K+ concentration, because the increase is rapid. Unlike what occurs in CKD, there is inadequate time to develop adaptive mechanisms at the cellular level to mitigate toxicity. Endogenous release of K+ into the extracellular space due to tissue breakdown as in rhabdomyolysis or in settings of increased catabolism or cell shift due to acidemia further exacerbates hyperkalemia.
Chronic Kidney Disease
CKD is characterized by a loss of nephron mass and a reduction in the number of collecting ducts to secrete K+. The chronic nature of this process allows an adaptive response to occur in the remaining nephrons, allowing the amount of K+ excreted per unit GFR (fractional excretion of K+) to increase (4), which is due to increased K+ secretory capacity resulting from structural changes that occur in the distal nephron and principal cells of the collecting duct. These changes are similar to those that occur in response to chronic K+ loading in normal subjects and include proliferation and in folding of the basolateral membrane, cellular hypertrophy, and increased mitochondrial number. Amplification of the basolateral surface is accompanied by increased density and activity of the Na+- K+-ATPase pump. These structural changes are the result of increased plasma K+ and/or mineralocorticoid activity. Loss of kidney mass also increases flow and Na+ delivery to the distal nephron in the remaining nephrons.
Plasma K+ concentration in CKD remains below 5.5 mEq/L until the GFR falls below 15 ml/min, except in the presence of oliguria, consumption of a high-K+ diet, increased tissue breakdown, or reduced aldosterone secretion or responsiveness. Additional loss of functioning kidney mass requires a progressively steeper rise in the steady-state plasma K+ concentration to maintain K+ homeostasis.
Despite these adaptive changes, patients with CKD are at risk for hyperkalemia; this is because the ability to further increase K+ secretion is extremely limited and because the rise in plasma K+ is greater and persists longer after an exogenous load compared with normal subjects. Hyperkalemia is an early finding in patients with diabetes, in whom decreased mineralocorticoid activity is often an early manifestation of hyporeninemic hypoaldosteronism, or advanced stages of heart failure with accompanying reductions in distal delivery of Na+ combined with concurrent use of drugs, which interfere with the RAAS system.
Hyperkalemia in patients with CKD is managed by restricting K+ intake, use of effective diuretic therapy, correction of metabolic acidosis, and reducing the dose or discontinuing medications impairing K+ excretion (5). Withholding RAAS blockers creates a therapeutic dilemma, because these drugs provide cardiovascular protection in patients with disease states (CKD, diabetes mellitus, and congestive heart failure) who are most susceptible to the development of hyperkalemia. New K+ binding agents are available that are well tolerated and effective in maintaining the plasma K+ concentration in the normal range without reducing the dose or discontinuing RAAS inhibitors.
Hemodialysis and Peritoneal Dialysis
Dialysis is required to maintain normal or near-normal plasma K+ concentrations after a patient reaches ESRD. A concentration gradient is established favoring removal of K+ by using a dialysate K+ concentration less than plasma. Because K+ is freely permeable across the dialysis membrane, most K+ removal occurs in the first 2 hours of the procedure when the concentration gradient is highest. As the plasma K+ concentration falls, movement of K+ from the intracellular space to the extracellular space becomes limiting, causing K+ removal to become less efficient during the latter half of the procedure. Factors influencing the distribution of K+ between these two spaces, and therefore, the total amount of K+ removed include changes in tonicity, glucose, and insulin concentration; catecholamine activity; and acid-base status (6).
In a typical hemodialysis session, approximately 80–100 mEq K+ is removed from the body per treatment (300 mEq/wk). An adaptive increase in colonic K+ secretion to as much as 35% of daily intake may play a critical role in maintaining total body K+ content, because dietary K+ intake exceeds weekly dialytic removal by 100–200 mEq (7). This adaptation involves increased expression of a high conductance K+ channel and increased expression of Na+- K+-ATPase pump sites on the apical and basolateral surfaces, respectively, of colonic epithelial cells. Colonic K+ secretion is stimulated by mineralocorticoids, potentially explaining the occasional development of hyperkalemia in anuric patients on dialysis given RAAS blockers. Constipation on maintenance dialysis is a concern, because the amount of K+ excreted in stool correlates with the wet stool weight. Other causes of hyperkalemia include dietary indiscretion, inadequate dialysis due to missed treatments or shortened treatment times, and unrecognized recirculation in the vascular access. Additionally, drugs impairing K+ secretion in patients with CKD can facilitate predisposition to hyperkalemia in patients on hemodialysis with residual kidney function. Patients on dialysis who are unable to eat before procedures can develop hyperkalemia secondary to decreased insulin levels. This complication can be avoided by administering a dextrose-containing solution and insulin or dextrose alone in patients who are not diabetic during the fasting period.
Peritoneal dialysis is not often associated with hyperkalemia. The absence of hyperkalemia might seem paradoxical when one considers K+ balance with this treatment modality. In patients undergoing 10 L of drainage per day, approximately 35–46 mEq K+ is removed. Despite daily K+ intake exceeding this amount, significant hyperkalemia is uncommon, and up to 30% of patients may have hypokalemia (8). K+ balance is maintained by the continuous nature of the treatment, increased colonic secretion of K+, preserved residual kidney excretion, decreased intake of K+-rich foods, and transcellular shift driven by insulin release in response to the obligatory glucose absorption.
In patients treated with hemodialysis or peritoneal dialysis, the presence of hyperkalemia correlates poorly with total body K+ content. Quantification of K+ in skeletal muscle biopsies shows body stores that are normal, increased, or reduced. These contradictory results may be reconciled by considering the clinical setting. For example, a true K+ deficit, correctable by K+ supplements, occurs during the course of advancing kidney failure when accompanied by vomiting, high-dose diuretic therapy, and low K+ intake. In the absence of kidney function, cellular uptake of K+ becomes an important defense against the development of hyperkalemia. Activity of the Na+-K+-ATPase pump is decreased in uremia, limiting the ability to shift K+ into cells. Studies in red blood cells show that this defect can be reversed when incubated in normal plasma or after dialysis, suggesting the presence of a circulating inhibitor. This defect may account for why some patients develop hyperkalemia, despite reduced total body K+ content. Decreased intracellular stores due to this mechanism are not correctable by K+ supplements. Increased circulating insulin and catecholamine levels in patients with CKD may serve to attenuate uremic-induced alterations in cell function that limit uptake of K+ into skeletal muscle (9). Reduced body K+ content refractory to supplements also occurs with decreased muscle mass where cellular K+ concentration is normal.
Kidney Transplantation
Hyperkalemia is common after kidney transplantation. Similar to in the patient with CKD, administration of drugs, such as trimethoprim and RAAS blockers, in the setting of a reduced GFR plays a contributory role. Tubular injury leading to impaired K+ secretion is often present in these patients. Tubular dysfunction can result from interstitial inflammation as with allograft rejection or be the result of interstitial fibrosis and tubular atrophy. This later histology occurs with increasing frequency over the first year after transplantation and is due to immune-mediated damage, prior ischemic-reperfusion injury, or factors related to the donor, particularly in patients with transplants from expanded criteria donors.
In patients maintained on calcineurin inhibitors, such as tacrolimus or cyclosporin, hyperkalemia can develop through mechanisms similar to those of familial hyperkalemic hypertension (10). Calcineurin normally exerts a dephosphorylating effect on the Na+-Cl− cotransporter in cells of the initial collecting duct (DCT1). Inhibition of calcineurin leads to unopposed phosphorylation of the cotransporter by the WNK-regulated SPAK/OSR1 kinases and increased activity of the cotransporter. The reduced delivery of Na+ and flow to the aldosterone-sensitive distal nephron impairs K+ secretion, causing hyperkalemia, whereas retention of NaCl leads to hypertension and volume expansion, which secondarily suppress circulating levels of renin and aldosterone.
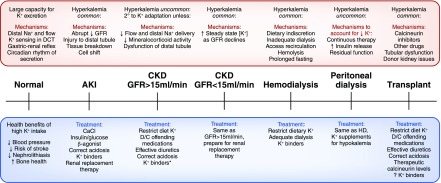
The kidney has an important role in maintaining K+ homeostasis. Figure 1 is a summary of pathophysiology and treatments of hyperkalemia across the continuum of kidney function.
Figure 1.
The causes and treatment of hyperkalemia across the continuum of kidney function and with kidney replacement therapies. The treatments listed for AKI are indicated for other settings within the continuum when there is severe hyperkalemia or evidence of cardiac toxicity. *K+ binders include sodium polystyrene sulfonate, patiromer, and sodium zirconium cyclosilicate (the latter of which is not yet approved for clinical use). Patiromer and sodium zirconium cyclosilicate have been shown effective in maintaining normokalemia, despite ongoing use of renin-angiotensin-aldosterone (RAAS) blockers. There are no published data on the use of these drugs in patients with kidney transplants. D/C, discontinue; DCT, distal convoluted tubule; HD, hemodialysis.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Palmer BF: Regulation of potassium homeostasis. Clin J Am Soc Nephrol 10: 1050–1060, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, Yang CL, Ellison DH, Wang WH: Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer BF, Clegg DJ: Achieving the benefits of a high potassium, Paleolithic diet, without the toxicity. Mayo Clin Proc 91: 496–508, 2016 [DOI] [PubMed] [Google Scholar]
- 4.van Ypersele de Strihou C: Potassium homeostasis in renal failure. Kidney Int 11: 491–504, 1977 [DOI] [PubMed] [Google Scholar]
- 5.Palmer BF: Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 351: 585–592, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Palmer BF: Individualizing the dialysate in the hemodialysis patient. Semin Dial 14: 41–49, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Hayes Jr. CP, McLeod ME, Robinson RR: An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 80: 207–216, 1967 [PubMed] [Google Scholar]
- 8.Torlén K, Kalantar-Zadeh K, Molnar MZ, Vashistha T, Mehrotra R: Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol 7: 1272–1284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allon M: Treatment and prevention of hyperkalemia in end-stage renal disease. Kidney Int 43: 1197–1209, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Hoorn EJ, Walsh SB, McCormick JA, Fürstenberg A, Yang CL, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, Unwin RJ, Ellison DH: The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17: 1304–1309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]