With the progression of CKD, increasingly severe disturbances of mineral and bone metabolism are observed, which are reflected by the term CKD-related mineral and bone disorder. The clinician’s goal is to prevent or correct these complications and many other CKD-associated complications as early and as completely as deemed adequate, taking into consideration both efficacy and safety.
Hyperphosphatemia represents one among several modifiable risk factors, and the avoidance of hyperphosphatemia is a well-established goal. This is on the basis of a large body of preclinical, clinical, and epidemiologic evidence indicating harmful effects of phosphate excess in CKD. Hyperphosphatemia may contribute to the development of vascular calcification, cardiovascular events, and increased mortality risk either directly or indirectly via the induction of various endocrine and metabolic abnormalities. However, many unsolved questions remain, including the following: (1) which type of hyperphosphatemia control should be preferred?, (2) should action be taken already early in the course of CKD progression to prevent hyperphosphatemia?, and (3) how intensively should established hyperphosphatemia be corrected?
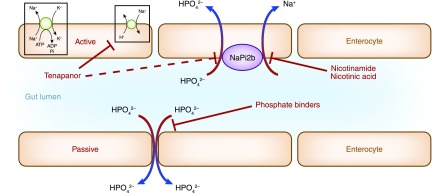
Because the optimal control of hyperphosphatemia remains challenging, the most recent approach chosen is pharmacologic interference with active intestinal phosphate transport using either already available drugs or developing novel inhibitors. Already available drugs include nicotinamide and nicotinic acid (also called niacin, which is transformed to nicotinamide in the body) (1,2). They inhibit the intestinal sodium-dependent phosphate transporter NaPi2b (also called Ntp2b). Nicotinic acid and nicotinamide have slightly different mechanisms of action. Nicotinic acid alone causes flushing due to stimulation of prostaglandin D2 and E2 secretion by subcutaneous Langerhans cells via the G protein–coupled receptor 109A niacin receptor (3). Nicotinamide does not bind to this receptor (4). The second approach is the synthesis of tenapanor, an inhibitor of the sodium/hydrogen exchanger isoform 3, which reduces sodium and phosphate absorption in the gut (5). Figure 1 shows a schematic representation of the action of these inhibitors compared with that of intestinal phosphate binders. Here, we focus on nicotinamide and niacin.
Figure 1.
Schematic view of the action of oral inhibitors of active intestinal phosphate absorption compared with the chelation of phosphate in the gut lumen by oral phosphate binders. Modified from reference 15, with permission.
In the last decade, several clinical studies have shown that the administration of niacin or nicotinamide reduced hyperphosphatemia in patients on dialysis together with a lower pill burden. However, the majority of these studies were limited by short treatment periods, small sample size, and uncontrolled study design (i.e., failure to include a phosphate binder as a comparator in patients with CKD on dialysis or patients with CKD not on dialysis or a placebo in patients with CKD not yet on dialysis). Attempts to overcome these limitations were made in two recent studies.
In this issue of the Clinical Journal of the American Society of Nephrology, Malhotra et al. (6) report the effects of extended release niacin, 1500 or 2000 mg/d, compared with placebo on plasma phosphate in 352 individuals with CKD stages 3 and 4 (eGFR<60 ml/min per 1.73 m2). This was a subgroup analysis of the randomized, controlled AIM-HIGH Trial that enrolled 3414 individuals with prevalent cardiovascular disease, low serum HDL cholesterol, and high triglyceride levels who were all on statin therapy (7). Because of flushing effects of niacin, the placebo was designed to contain a small dose (50 mg) of immediate release niacin to mask the identity of blinded treatment. The parent trial failed to show a reduction by niacin of recurrent cardiovascular disease over 3 years. In this subgroup analysis, 297 patients (84%) had plasma samples available for measurement of mineral markers at year 1, and 140 (40%) had plasma samples available for measurement of mineral markers at year 3. Randomization to niacin led to a decrease of serum phosphate from 3.4 to 3.3 mg/dl compared with an increase from 3.4 to 3.6 mg/dl in placebo group. In intent to treat analysis, a summary estimate showed that active treatment led to 0.08-mg/dl lower plasma phosphate per year of treatment compared with placebo. Although statistically significant (P<0.01), the clinical relevance of the observed effect seems to be poor, at least when considered solely on the basis of changes in essentially normal plasma levels at baseline. Information on changes in urinary phosphate excretion might have helped to obtain a more favorable view of niacin’s effect on phosphate control. Deceivingly, however, randomization to niacin was also not associated with significant changes in plasma intact fibroblast growth factor, parathyroid hormone, calcium, or vitamin D metabolites over 3 years. Finally, patients on niacin treatment had higher rates of flushing than those receiving placebo.
Lenglet et al. (8) randomized 100 patients on chronic hemodialysis to either oral nicotinamide or sevelamer hydrochloride treatment for 24 weeks to examine noninferiority and safety; they observed a comparable decrease in serum phosphate from 6.5 to 5.6 mg/dl and from 7.1 to 5.3 mg/dl, respectively. The criterion for noninferiority was, however, not met due to a more limited number of patients being included than planned. It is worth mentioning that serum C-terminal FGF23 levels decreased by nearly 50% in the sevelamer group, whereas they increased by nearly 30% in the nicotinamide group, and serum α-Klotho levels decreased in the nicotinamide group but increased in the sevelamer group. These differences were highly significant. Treatment discontinuation due to adverse events was 1.6 times higher in the nicotinamide group than in the sevelamer group, with only 55% of study completers in the nicotinamide arm versus 90% in the sevelamer arm. Thrombocytopenia was observed in four nicotinamide-treated patients. Of further concern is the observation of a large increase of nicotinamide metabolite N-methyl-2-pyridone-5-carboxamide (2PY), especially in patients on dialysis treated with nicotinamide. Its derivatives may be uremic toxins, enhancing oxidative stress and disturbing cellular repair processes via an inhibition of poly(ADP-ribose) polymerase-1 activity. Inhibitors of poly(ADP-ribose) polymerase are under development in cancer treatment, and the first studies show that they frequently induce hematologic disorders, including thrombocytopenia, in a dose-dependent manner (9).
Classic, more widely used means of controlling phosphate retention in CKD in the clinic are briefly mentioned here.
Phosphate excess in CKD can be avoided by restricting protein intake and other foods and beverages rich in phosphate. However, this approach is generally of limited efficacy. Moreover, overzealous protein restriction may favor malnutrition.
In patients with CKD stage G5, the use of optimal renal replacement modalities is an established, although generally insufficient means to control hyperphosphatemia.
The most efficacious therapeutic approach in advanced stages of CKD is the prescription of oral phosphate binders. When effectively taken, they reduce elevated serum phosphate levels, although normalization is generally difficult if not impossible to achieve. One of the major issues is failing long-term compliance owing to high pill burden and gastrointestinal intolerance. Lack of compliance is difficult to assess. It certainly is highly variable depending on each patient’s acceptance of ingesting large amounts of bulky drugs and specific gastrointestinal side effects. Another issue is that of potentially harmful effects on organs and tissues other than the digestive tract. Prescription of highly efficacious aluminum-containing phosphate binders has been largely abandoned because of serious aluminum toxicity. The prescription of high doses of calcium-based phosphate binders should be avoided because of recent evidence from three randomized, controlled trials showing that excess exposure to calcium through diet, medications, or dialysate may be harmful in patients with CKD (10).
Given the above limitations of available means to control serum phosphate in CKD, several new compounds have been developed more recently with the goal to increase efficacy and compliance. Novel magnesium-based binders include iron/magnesium hydroxycarbonate and calcium acetate/magnesium carbonate, novel iron-based phosphate binders include sucroferric oxyhydroxide and ferric citrate, and iron-magnesium hydroxycarbonate contains both magnesium and iron. Although these new compounds may present advantages over the classic phosphate binders, none of them have provided definitive evidence of superiority in terms of hyperphosphatemia control, not to speak of intermediate or hard patient outcomes (11,12).
Although starting phosphate binder use in early CKD stages can slightly reduce phosphate retention, it remains uncertain whether this translates into clinical benefit (13,14). The 2017 Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline Update states the following: “There is an absence of data supporting that efforts to maintain phosphate in the normal range are of benefit to CKD G3a–G4 patients, including some safety concerns” (10). The work group, therefore, suggested that treatment should be aimed at overt hyperphosphatemia and that decisions about phosphate-lowering treatment should be on the basis of progressively or persistently elevated serum phosphate (10).
To date, no prospective randomized trial has convincingly shown improved survival for patients with CKD as a consequence of hyperphosphatemia correction or prevention. Therefore, the work group of the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of CKD-Related Mineral and Bone Disorder suggested lowering elevated phosphate levels toward the normal range (10), meaning that they must not necessarily be completely normalized. This formulation expresses persisting uncertainty as to which degree of hyperphosphatemia should be corrected without creating more harm than benefit.
In conclusion, the results of the trials with oral inhibitors of active phosphate transport do not support the use of niacin or nicotinamide alone in the control of serum phosphate in CKD. It remains to be seen whether low-dose nicotinamide treatments, such as the ones used in the ongoing trials COMBINE (https://clinicaltrials.gov/ct2/show/NCT02258074) (15) and NOPHOS (https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-000488-95/AT), will show clinically meaningful efficacy together with fewer side effects. These trials examine the effect of nicotinamide as add-on therapy to classic phosphate binders in patients with moderate to severe CKD and patients on dialysis. As to nicotinic acid alone, it probably has lost the battle.
Disclosures
T.B.D. reports personal fees from Amgen, FMC, Genentech-Roche, Kyowa Hakko Kirin, Sanofi, and Vifor. Z.A.M. reports grants for CKD Réseau Epidémiologie et Information en Néphrologie (REIN) and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp and Dohme-Chibret, Sanofi-Genzyme, Lilly, Otsuka, and the French government as well as fees and grants to charities from Amgen, Bayer, and Sanofi-Genzyme.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “The Effect of Extended Release Niacin on Markers of Mineral Metabolism in CKD,” on pages 36–44.
References
- 1.Ginsberg C, Ix JH: Nicotinamide and phosphate homeostasis in chronic kidney disease. Curr Opin Nephrol Hypertens 25: 285–291, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenglet A, Liabeuf S, Guffroy P, Fournier A, Brazier M, Massy ZA: Use of nicotinamide to treat hyperphosphatemia in dialysis patients. Drugs R D 13: 165–173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamanna VS, Kashyap ML: Mechanism of action of niacin. Am J Cardiol 101: 20B–26B, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bodor ET, Offermanns S: Nicotinic acid: An old drug with a promising future. Br J Pharmacol 153[Suppl 1]: S68–S75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block GA, Rosenbaum DP, Leonsson-Zachrisson M, Åstrand M, Johansson S, Knutsson M, Langkilde AM, Chertow GM: Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol 28: 1933–1942, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malhotra R, Katz R, Hoofnagle A, Bostom A, Rifkin DE, Mcbride R, Probstfield JL, Block GA, Ix JH: The effect of extended release niacin on markers of mineral metabolism in chronic kidney disease. Clin J Am Soc Nephrol 13: 36–44, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W; AIM-HIGH Investigators : Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 365: 2255–2267, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Lenglet A, Liabeuf S, El Esper N, Brisset S, Mansour J, Lemaire-Hurtel AS, Mary A, Brazier M, Kamel S, Mentaverri R, Choukroun G, Fournier A, Massy ZA: Efficacy and safety of nicotinamide in haemodialysis patients: The NICOREN study. Nephrol Dial Transplant 32: 870–879, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Park SR, Chen A: Poly(Adenosine diphosphate-ribose) polymerase inhibitors in cancer treatment. Hematol Oncol Clin North Am 26: 649–670, ix, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB: Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update: What’s changed and why it matters. Kidney Int 92: 26–36, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Floege J: Phosphate binders in chronic kidney disease: A systematic review of recent data. J Nephrol 29: 329–340, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Peter WLS, Wazny LD, Weinhandl E, Cardone KE, Hudson JQ: A review of phosphate binders in chronic kidney disease: Incremental progress or just higher costs? Drugs 77: 1155–1186, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drüeke TB, Massy ZA: Phosphate binders in CKD: Bad news or good news? J Am Soc Nephrol 23: 1277–1280, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Isakova T, Ix JH, Sprague SM, Raphael KL, Fried L, Gassman JJ, Raj D, Cheung AK, Kusek JW, Flessner MF, Wolf M, Block GA: Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 26: 2328–2339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]