Abstract
Long non-coding RNAs (lncRNAs) are emerging as important regulators in different biological processes through various ways. Because the related data, especially mutations in cancers, increased sharply, we updated the lncRNASNP to version 2 (http://bioinfo.life.hust.edu.cn/lncRNASNP2). lncRNASNP2 provides comprehensive information of SNPs and mutations in lncRNAs, as well as their impacts on lncRNA structure and function. lncRNASNP2 contains 7260238 SNPs on 141353 human lncRNA transcripts and 3921448 SNPs on 117405 mouse lncRNA transcripts. Besides the SNP information in the first version, the following new features were developed to improve the lncRNASNP2. (i) noncoding variants from COSMIC cancer data (859534) in lncRNAs and their effects on lncRNA structure and function; (ii) TCGA cancer mutations (315234) in lncRNAs and their impacts; (iii) lncRNA expression profiling of 20 cancer types in both tumor and its adjacent samples; (iv) expanded lncRNA-associated diseases; (v) optimized the results about lncRNAs structure change induced by variants; (vi) reduced false positives in miRNA and lncRNA interaction results. Furthermore, we developed online tools for users to analyze new variants in lncRNA. We aim to maintain the lncRNASNP as a useful resource for lncRNAs and their variants.
INTRODUCTION
The transcriptional landscape analysis revealed the complexity of human transcriptomes and showed human genome is pervasively transcribed to produce large amount of noncoding transcripts (1). Long non-coding RNAs (lncRNAs) are ncRNAs extensively found in all kinds of eukaryotes, and the organismal complexity is better correlated with the diversity and size of non-coding RNA expression repertoires than with that of protein-coding genes (2). lncRNAs involve in gene expression regulation by various mechanisms, such as regulation of transcription, translation, protein modification and interaction with other molecules (3). Thus they play critical roles in diverse biological processes impacting cell differentiation, senescence and individual development (4). Moreover, lncRNAs were also proved to serve as tumor suppressive or oncogenic factors in different cancers (5).
Genome variants including SNPs and mutations contribute to changes of lncRNA structure and function, thus increase the susceptibility to cancers and other diseases (6). SNPs have been reported to affect the structure, expression, and function of lncRNAs (7), such as SNP rs920778 in HOTAIR contributes to the risk of gastric cancer (8) and SNP rs11655237 in LINC00673 confers susceptibility to pancreatic cancer by creating a miR-1231 binding site (9). Two high frequency mutations in lncRNA GAS8-AS1 were associated with papillary thyroid carcinoma (10). A study of whole-genome mutational landscape of liver cancer discovered recurrent mutations in lncRNA NEAT1 and MALAT1 (11). It was reported that mutations in lncRNA NEAT1 were associated with increased expression and unfavorable outcome in papillary renal-cell carcinoma (12). These studies indicate that it is very necessary to study the variants on lncRNAs in cancers to identify biomarkers for carcinogenesis and prognosis.
Till now, there are several databases describing the genomic variants on lncRNA genes, including lincSNP, LncVar and our lncRNASNP. lincSNP (13) focused on disease associated SNPs on lncRNAs and their transcription factor binding sites; LncVar (14) identified SNPs and structural variants on lncRNAs as well as their effects on biological function of lncRNAs. While, lncRNASNP (15), the previous version of lncRNASNP2, provides comprehensive information about lncRNA related SNPs in human and mouse and explores their effects on lncRNA structure and potential function on miRNA binding.
As the increasing of identified lncRNAs and SNPs in human genome, especially mutations in cancers, we updated the lncRNASNP with the latest data and developed new functions to improve it. In lncRNASNP2, the number of human lncRNA transcripts and SNPs on them increased 3–15-fold in human and mouse. Besides, mutations on lncRNA and lncRNA expression in cancers were newly added. Furthermore, web-based tools were developed for new data analysis. With the abundant data and new features, lncRNASNP2 database will serve as a useful resource for functional studies of lncRNA, especially studies in cancer.
DATA SOURCE AND SUMMARY
We obtained 258 758 lncRNA transcripts (141 353 in human and 117 405 in mouse) of 170 002 lncRNA genes (90 062 in human and 79 940 in mouse) from NONCODE2016 (16). Next, 7 260 238 and 3 921 448 SNPs on human and mouse lncRNA transcripts were identified, respectively. Furthermore, resources associated with cancer mutations as well as other diseases were integrated. Compared with the previous version, the data amount and types in lncRNASNP2 were more comprehensive (Table 1).
Table 1. Data summary in lncRNASNP2 database.
| Data content | Version 1.0 | Version 2.0 | ||
|---|---|---|---|---|
| Human | Mouse | Human | Mouse | |
| lncRNA genes/transcripts | 17 436/32 108 | 25 512/36 471 | 90 062/141 353 | 79 940/117 405 |
| All SNPs | 495 729 | 777 095 | 7 260 238 | 3 921 149 |
| lncRNA SNP in GWASa | 142/197 827 | NA/NA | 602/2 859 147 | NA/NA |
| SNP affected MLPb | 262 154/280 012 | 366 731/357 246 | 4 524 236/4 559 403 | 2 644 936/1 313 063 |
| All Predicted MLPb | 6 413 273 | 7 448 200 | 8 842 103 | 8 100 887 |
| Conserved/validated MLPc | 69 837/8091 | 13 780/NA | 42 787/18 595 | 13 972/NA |
| TCGA cancer mutations | NA | NA | 315 234 | NA |
| TCGA mutations affected MLPb | NA | NA | 83 633/80 114 | NA |
| CosmicNCVs | NA | NA | 859 534 | NA |
| CosmicNCVs affected MLPb | NA | NA | 362 940/35 0827 | NA |
| lncRNA expressions | NA | NA | 11 857 | NA |
| lncRNA-associated diseasesd | NA | NA | 12 2871/697 | NA |
alncRNA SNPs are GWAS TagSNPs/lncRNA SNPs in GWAS LD regions
bMLP represents miRNA-lncRNA target pair, variants (SNPs, TCGA mutations, CosmicNCVs) in lncRNAs induce the potential MLP loss/gain.
cThe miRNA target sites conserved among human, mouse, rat and dog/miRNA-lncRNA target pairs supported by CLIP experiment results from starbase.
dThe number of lncRNA transcripts associated with diseases (predicted) /the number of experimentally supported lncRNA-associated disease pairs.
IMPROVED CONTENT AND NEW FEATURES
CosmicNCVs in lncRNA transcripts
To provide mutation information on lncRNA in cancers, we collected the CosmicNCVs (Cosmic NonCoding Variants) from COSMIC (Catalogue Of Somatic Mutations In Cancer) database (17). We identified 859 534 CosmicNCVs in human lncRNA transcripts. Furthermore, we integrated the mutation impact scores calculated by FATHMM from COSMIC to evaluate their functional effects. According to the suggestion of COSMIC, score >0.7 is considered as ‘Pathogenic’ and score <0.5 is considered to be ‘Neutral’. For example, COSN4621983 (score 0.9955) in genes was reported to provide a potential treatment target for mantle cell lymphoma (18). Totally, we identified 71 410 (account for 8%) pathogenic variants on lncRNA transcripts.
TCGA Cancer Mutations in lncRNA transcripts
Except for CosmicNCVs, we also collected cancer mutations from the TCGA (19) project. Genomic coordinates of those mutations were converted from genome assembly GRCh37 to GRCh38 using CrossMap (20). Finally, we identified 315 234 mutations in lncRNA transcripts among 34 cancer types. The top three cancer types with the highest mutation numbers in lncRNAs were SKCM (Skin Cutaneous Melanoma), COAD (Colon adenocarcinoma) and STAD (Stomach adenocarcinoma). Inspired by COSMIC, FATHMM was used to assess mutation impacts on lncRNA transcripts, as the TCGA cancer mutations we collected were filtered by MutSig (http://archive.broadinstitute.org/cancer/cga/mutsig), 78.35% of them tend to be ‘Pathogenic’.
lncRNA expression in TCGA cancers
In recent years, the dysregulation of lncRNAs was found related to tumor progression and survival. For example, lncRNA GAS5 was downregulated in cervical cancer tissues and significantly associated with advanced tumor progression (21). The upregulation of lncRNA DANCR was associated with aggressive progression and poor prognosis in colorectal cancer (22). Here, we collected expression data of 11 857 lncRNA genes in 20 cancer types from TANRIC database (23). Expressions of lncRNA genes (RPKM) ranged from 0 to 360.19 in tumor samples and from 0 to 318.71 in tumor adjacent samples among 20 cancers, but 93% lncRNA genes were lowly expressed with RPKM <10.
miRNA-lncRNA interaction prediction
lncRNA may interact with miRNA as a miRNA sponge to regulate gene expression (24), thus, the identification of miRNA target sites on lncRNA will provide a clue on lncRNA functional research. We collected mature miRNA sequences from miRBase (release 21) (25). To reduce false positives, we intersected the results of MiRanda, TargetScan and Pita as the final miRNA targets in lncRNASNP2. Finally, 8 842 103 and 8 100 887 pairs of miRNA and lncRNA interactions in human and mouse were predicted, respectively. Meanwhile, we identified 548,195 target sites conserved among human, mouse, rat and dog. Furthermore, variants on lncRNA could induce miRNA target sites gain/loss. Among the variants we identified, 80.94% (5 876 208) SNPs, 33.53% (105,683) TCGA cancer mutations and 54.12% (465 178) CosmicNCVs in human were attributed to induce the potential miRNA target sites gain/loss, respectively, and for mouse, the number of SNPs was 79.76% (3 127 650). In addition, experimentally validated miRNA-lncRNA interactions collected from StarbaseV2.0 (26) were increased from 8091 to 18 595.
Prediction of lncRNA-associated diseases
Given the large number of lncRNAs, the relations with human diseases remain unknown for most of them. Here, we predicted the lncRNA-associated diseases using software TAM (27), which was designed for identifying meaningful categories for given miRNAs. In our work, we chose the disease-associated miRNA set from HMDD database (28) as the disease miRNA set. For each lncRNA, we integrated the high probability targeted miRNAs predicted by three tools described above as the miRNA set of the lncRNA, then possible associated diseases of each lncRNA were predicted by enrichment analysis with the disease miRNA set and the targeted miRNAs. The result was measured by P-value, which was calculated by hypergeometric test and adjusted by Bonferroni correction. Meanwhile, we collected 697 pairs of experimentally supported lncRNA-disease entries from LncRNADisease (29).
SNPs in GWAS-trait associated regions
We collected 34 398 GWAS tagSNPs from NHGRI GWAS Catalog (30) and identified 602 GWAS tagSNPs in human lncRNA transcripts. In addition, for each GWAS SNP, we obtained GWAS-trait associated LD regions using SNAP (SNP Annotation and Proxy Search) (31) and identified 2 859 147 (account for 39.4%) lncRNA SNPs in those regions.
lncRNA structure changes induced by variants
In lncRNASNP2, we used RNAsnp (32) to assess variant effects on lncRNA secondary structure. Compared with MFE (Minimum Free Energy) in previous version, P-value of RNAsnp calculated from Boltzmann ensemble are more stable and reliable (32). There were three modes in RNAsnp, in our work, the mode 1 was used for lncRNA <1000 nt, while the mode 2 for lncRNA >1000 nt. Empirical P-value in the result <0.2 means the variant has effect on lncRNA structure. Totally, 1 425 449 (19.63%) and 395 443 (10.08%) SNPs in human and mouse lncRNA transcripts were identified to impact on lncRNA structure, respectively. Except for SNPs, we also predicted lncRNA structure changes caused by cancer mutations, and results showed that 16.67% TCGA cancer mutations and 17.3% CosmicNCVs may lead to the change of lncRNA secondary structure.
Web-based analysis tools
With the prevalence of sequencing technologies, new SNPs and lncRNAs are being or will be characterized. To make it convenient for users to explore those new data, we developed two web-based tools: one for predicting miRNA target sites on lncRNA or miRNA target sites gain/loss caused by variants; the other for users to analyze effect of SNP on lncRNA secondary structure.
DATABASE ORGANIZATION AND WEB INTERFACE
The lncRNASNP2 database was built with the Flask open source framework (http://flask.pocoo.org/), all data mentioned above were organized into MongoDB. The lncRNASNP2 database is freely available at http://bioinfo.life.hust.edu.cn/lncRNASNP2.
lncRNASNP2 was composed of five sections: lncRNA, SNP, Mutation, miRNA and Tool sections. A quick search box was designed on the head of home page to search by keywords including SNP ID, lncRNA ID, miRNA ID, abbreviation of TCGA cancer types, CosmicNCV ID and genomic regions. Fuzzy query was allowed for users to get results by searching part of a keyword. The ‘Tool’ menu in the navigation bar includes search tools and prediction tools. Search tools were designed for users to query data by specific IDs and prediction tools can help users to analyze their own data.
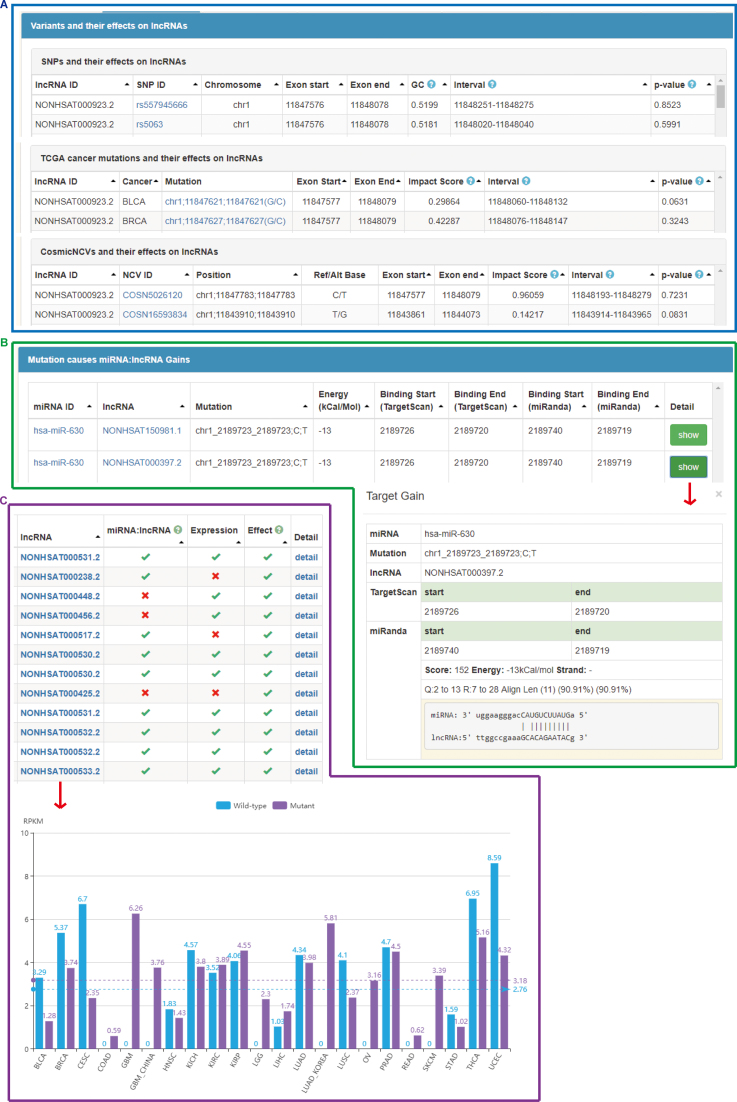
In lncRNA section, there were two ways to browse lncRNAs in our database: browse by disease or by chromosome. In the lncRNA detail page of each lncRNA, there are several tags, including lncRNA detail, variants in lncRNA, lncRNA diseases, miRNA target sites on lncRNA, miRNA target gain, miRNA target loss and expression. Under the ‘Variants in lncRNA’ tag, we integrated SNPs, TCGA cancer mutations and CosmicNCVs in lncRNA (Figure 1A). Under the ‘miRNA target sites on lncRNA’ tag, we presented a miRNA targeting lncRNA graph to display miRNA targets on the lncRNA, by clicking the miRNA name, users can browse detail about this interaction, including binding regions predicted by Pita, TargetScan and MiRanda as well as binding score, energy and nucleotide pairs.
Figure 1.
Overview of lncRNASNP2 database. (A) Variants (SNPs, TCGA cancer mutations and CosmicNCVs) in lncRNA and their impacts on lncRNA structure or function. (B) An example of miRNA target gain induced by a variant. (C) An example of lncRNA expressions in cancers displayed in histogram.
The newly added TCGA mutation section was displayed by cancer types. Users can browse mutations on lncRNAs and their effects of the selected cancer. Taking the BLCA (Bladder urothelial carcinoma) as an example, on the page of BLCA mutations in lncRNA, the first line is ‘chr1:2189723 C>T on lncRNA NONHSAT000397.2’. Results under ‘Mutation in lncRNAs’ tag in the mutation detail page showing the functional impact score (predicted by FATHMM) of this mutation is 0.93374, which means this is a pathogenic mutation. The content under ‘miRNA target gain’ tag indicates that the mutation creates a target site of miR-630 and the ‘show’ button will present the detailed interaction pair (Figure 1B). It was reported that the expression of miR-630 was increased in BLCA and it may be a novel prognostic factor for bladder urothelial carcinoma (33). lncRNA expressions were displayed in two ways, which are boxplot showing expressions in single cancer type in TCGA mutation section and histogram showing expressions in 20 cancer types in lncRNA section (Figure 1C). In CosmicNCV section, users can browse the basic information, the impacts on lncRNA structure and function of each CosmicNCV in lncRNAs.
SUMMARY AND FUTURE PERSPECTIVES
As the increasing of identified lncRNAs and SNPs/mutations in human genomes, we updated lncRNASNP with the latest data and new features to improve it. Disease resources including cancers and other diseases will provide a comprehensive reference for lncRNA function. The web-based tools make it convenient for users to analyze new data. With the innovations in RNA-seq technologies and computational biology, lncRNAs are being identified and characterized at a rapid pace, and we believe that more function of lncRNAs will be revealed in the future. To keep pace of the lncRNA researches, we will update the lncRNASNP database regularly. We commit to make our lncRNASNP a helpful repository for functional study of lncRNAs and variants on them.
ACKNOWLEDGEMENTS
We thank colleagues in data production and database construction in groups of NONCODE, dbSNP, TCGA, TANRIC, LncRNADisease and COSMIC.
FUNDING
National Natural Science Foundation of China (NSFC) [31471247, 31771458]. Funding for open access charge: NSFC.
Conflict of interest statement. None declared.
REFERENCES
- 1. Hon C.C., Ramilowski J.A., Harshbarger J., Bertin N., Rackham O.J., Gough J., Denisenko E., Schmeier S., Poulsen T.M., Severin J. et al. An atlas of human long non-coding RNAs with accurate 5΄ ends. Nature. 2017; 543:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quinn J.J., Chang H.Y.. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016; 17:47. [DOI] [PubMed] [Google Scholar]
- 3. Peng W.X., Koirala P., Mo Y.Y.. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017; 36:5661–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bischof O., Martinez-Zamudio R.I.. MicroRNAs and lncRNAs in senescence: A re-view. IUBMB Life. 2015; 67:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prensner J.R., Chinnaiyan A.M.. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011; 1:391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wapinski O., Chang H.Y.. Long noncoding RNAs and human disease. Trends Cell Biol. 2011; 21:354–361. [DOI] [PubMed] [Google Scholar]
- 7. Xing F., Matsumiya T., Hayakari R., Yoshida H., Kawaguchi S., Takahashi I., Nakaji S., Imaizumi T.. Alteration of antiviral signalling by single nucleotide polymorphisms (SNPs) of mitochondrial antiviral signalling protein (MAVS). PLoS One. 2016; 11:e0151173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan W., Liu L., Wei J., Ge Y., Zhang J., Chen H., Zhou L., Yuan Q., Zhou C., Yang M.. A functional lncRNA HOTAIR genetic variant contributes to gastric cancer susceptibility. Mol. Carcinog. 2016; 55:90–96. [DOI] [PubMed] [Google Scholar]
- 9. Zheng J., Huang X., Tan W., Yu D., Du Z., Chang J., Wei L., Han Y., Wang C., Che X. et al. Pancreatic cancer risk variant in LINC00673 creates a miR-1231 binding site and interferes with PTPN11 degradation. Nat. Genet. 2016; 48:747–757. [DOI] [PubMed] [Google Scholar]
- 10. Pan W.T., Zhou L.Q., Ge M.H., Zhang B., Yang X.Y., Xiong X.Y., Fu G.B., Zhang J., Nie X.L., Li H.M. et al. Whole exome sequencing identifies lncRNA GAS8-AS1 and LPAR4 as novel papillary thyroid carcinoma driver alternations. Hum. Mol. Genet. 2016; 25:1875–1884. [DOI] [PubMed] [Google Scholar]
- 11. Fujimoto A., Furuta M., Totoki Y., Tsunoda T., Kato M., Shiraishi Y., Tanaka H., Taniguchi H., Kawakami Y., Ueno M. et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat. Genet. 2016; 48:500–509. [DOI] [PubMed] [Google Scholar]
- 12. Li S., Shuch B.M., Gerstein M.B.. Whole-genome analysis of papillary kidney cancer finds significant noncoding alterations. PLos Genet. 2017; 13:e1006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ning S., Zhao Z., Ye J., Wang P., Zhi H., Li R., Wang T., Li X.. LincSNP: a database of linking disease-associated SNPs to human large intergenic non-coding RNAs. BMC Bioinformatics. 2014; 15:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X., Hao Y., Cui Y., Fan Z., He S., Luo J., Chen R.. LncVar: a database of genetic variation associated with long non-coding genes. Bioinformatics. 2016; 33:112–118. [DOI] [PubMed] [Google Scholar]
- 15. Gong J., Liu W., Zhang J., Miao X., Guo A.-Y.. lncRNASNP: a database of SNPs in lncRNAs and their potential functions in human and mouse. Nucleic Acids Res. 2014; 43:D181–D186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Y., Li H., Fang S., Kang Y., Wu W., Hao Y., Li Z., Bu D., Sun N., Zhang M.Q. et al. NONCODE 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Res. 2016; 44:D203–D208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forbes S.A., Bindal N., Bamford S., Cole C., Kok C.Y., Beare D., Jia M., Shepherd R., Leung K., Menzies A. et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011; 39:D945–D950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bea S., Valdes-Mas R., Navarro A., Salaverria I., Martin-Garcia D., Jares P., Gine E., Pinyol M., Royo C., Nadeu F. et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:18250–18255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomczak K., Czerwinska P., Wiznerowicz M.. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn). 2015; 19:A68–A77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao H., Sun Z., Wang J., Huang H., Kocher J.-P., Wang L.. CrossMap: a versatile tool for coordinate conversion between genome assemblies. Bioinformatics. 2013; 30:1006–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao S., Liu W., Li F., Zhao W., Qin C.. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int. J. Clin. Exp. Pathol. 2014; 7:6776–6783. [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y., Zhang M., Liang L., Li J., Chen Y.-X.. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 2015; 8:11480. [PMC free article] [PubMed] [Google Scholar]
- 23. Li J., Han L., Roebuck P., Diao L., Liu L., Yuan Y., Weinstein J.N., Liang H.. TANRIC: an interactive open platform to explore the function of lncRNAs in cancer. Cancer Res. 2015; 75:3728–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnsson P., Ackley A., Vidarsdottir L., Lui W.-O., Corcoran M., Grandér D., Morris K.V.. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat. Struct. Mol. Biol. 2013; 20:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kozomara A., Griffiths-Jones S.. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2013; 42:D68–D73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J.-H., Liu S., Zhou H., Qu L.-H., Yang J.-H.. starBase v2. 0: decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2013; 42:D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu M., Shi B., Wang J., Cao Q., Cui Q.. TAM: a method for enrichment and depletion analysis of a microRNA category in a list of microRNAs. BMC Bioinformatics. 2010; 11:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y., Qiu C., Tu J., Geng B., Yang J., Jiang T., Cui Q.. HMDD v2.0: a database for experimentally supported human microRNA and disease associations. Nucleic Acids Res. 2014; 42:D1070–D1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J., Ma R., Ma W., Chen J., Yang J., Xi Y., Cui Q.. LncDisease: a sequence based bioinformatics tool for predicting lncRNA-disease associations. Nucleic Acids Res. 2016; 44:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Welter D., MacArthur J., Morales J., Burdett T., Hall P., Junkins H., Klemm A., Flicek P., Manolio T., Hindorff L. et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014; 42:D1001–D1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson A.D., Handsaker R.E., Pulit S.L., Nizzari M.M., O’donnell C.J., De Bakker P.I.. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008; 24:2938–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sabarinathan R., Tafer H., Seemann S.E., Hofacker I.L., Stadler P.F., Gorodkin J.. RNAsnp: efficient detection of local RNA secondary structure changes induced by SNPs. Hum. Mutat. 2013; 34:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z.Y., Zhang W., Yang J.J., Song D.K., Wei J.X.. Expression of miRNA-630 in bladder urothelial carcinoma and its clinical significance. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016; 36:705–709. [DOI] [PubMed] [Google Scholar]