Abstract
There are many diseases and biological processes that involve circulating cells in the bloodstream, such as cancer metastasis, immune reaction/inflammation, reproductive medicine, and stem cell therapies. This has driven significant interest in new technologies for the study of circulating cells in small animal research models and clinically. Most currently used methods require drawing and enriching blood samples from the body, but these suffer from a number of limitations. In contrast, “in vivo flow cytometry” (IVFC) refers to set of technologies that allow study of cells directly in the bloodstream of the organism in vivo. In recent years the IVFC field has grown significantly and new techniques have been developed, including fluorescence microscopy, multi-photon, photo-acoustic, and diffuse fluorescence IVFC. In this paper we review recent technical advances in IVFC, with emphasis on instrumentation, contrast mechanisms, and detection sensitivity. We also describe key applications in biomedical research, including cancer research and immunology. Last, we discuss future directions for IVFC, as well as prospects for broader adoption by the biomedical research community and translation to humans clinically.
Keywords: In vivo flow cytometry, fluorescence, cell labeling, circulating tumor cells, immunology
1. Introduction and Motivation for In Vivo Flow Cytometry (IVFC)
Many areas of basic biology and pre-clinical research involve circulating cells in the bloodstream, including immunology, oncology, organ transplant biology, reproductive medicine, and stem cell therapies (Hoshimoto et al., 2012; Mathiesen et al., 2012; Danila et al., 2011; Steeg and Theodorescu, 2008; Liu et al., 2009; Wen et al., 2012; Di Marco et al., 2011; He et al., 2011; Egan et al., 2011; Costiniuk et al., 2013; Lopez et al., 2012; Park et al., 2012; Calcaterra et al., 2014). It is common practice amongst researchers to study circulating cells by removing blood samples from the body, which are then analyzed with secondary assays. For non-terminal experiments involving live animals, this is usually limited to 10% of the circulating blood volume (~2 mL in a mouse) for a single withdrawal without fluid replacement (Hoff, 2000). Most commonly, flow cytometry (FC) is used to analyze the forward scatter, side scatter, and laser-induced fluorescence properties of cells (after labeling with a dye) (Shapiro, 1995). Hemocytometry and fluorescence microscopy of isolated cell populations are also commonly used methods.
Whole blood is a complex mixture of billions of cells per mL, and the cell population of interest usually represents a small fraction of the total population. As such, purification and isolation of target cells are necessary. For example, if leukocytes or circulating tumor cells (CTCs) are of interest, red blood cells may be preferentially lysed with a buffer and then removed by centrifugation (Yu et al., 2011). The target cell population can be further isolated and characterized with fluorescent dyes that label specific bio-molecular targets. Other methods for sorting and enrichment include size based sorting, e.g. (Gossett et al., 2010), immuno-magnetic separation (Zborowski and Chalmers, 2011), or isolation using a microfluidic device (Shields et al., 2015; Karabacak et al., 2014).
Despite their wide usage, all methods that rely on extraction of blood samples may present substantial problems for researchers (Tuchin et al., 2011), including the following:
The process of drawing, storing, handling, and purifying cells can affect the underlying biology of the system. Cells may undergo morphological changes, changes in gene expression or simply lose viability. The process of drawing blood from the host organism has been shown to trigger a stress response (Pitsillides et al., 2011),. The rapid degradation of blood after it has been removed from the host is also a major problem (Wong et al., 2016).
In addition to cell death, cells may simply be lost at different stages of handling and purification, which may be significant in experiments where accurate quantification of cell populations is important (Wagar et al., 2008; Valenstein and Sirota, 2004). For example, loss of cells during centrifugation is a well-documented issue (Delahaye et al., 2015). Preparation of samples is also a time consuming process.
Due to infrequency of sampling (1–2 times per day with fluid replacement), changes in circulating cell populations that occur over the course of minutes or hours may not be readily measurable.
In the case of rare circulating cells, the small sampling volume relative to the total peripheral blood may prevent detection or accurate enumeration (Rack et al., 2014; Miller et al., 2010; Andreopoulou et al., 2012). For example, for a circulating tumor cell population of 100 cells/mL, the expected number of cells in a 50 μL sample is only 5 cells. Ignoring losses to sample handling, this would yield a measurement uncertainty of ±2.2 cells (±44%), assuming Poisson count statistics. In practice, a mouse may need to be euthanized and the entire blood volume analyzed (after euthanizing) to obtain acceptable count accuracy (Azab et al., 2012), thereby eliminating the possibility of following the same animal(s) serially over time.
These limitations have motivated the development of optical technologies where individual circulating cells can be detected, enumerated, and characterized directly in vivo without having to draw blood samples. The general term for this technology is “in vivo flow cytometry” (IVFC) or “intravital flow cytometry” (IFC), which was first developed and reported by Lin et. al. in 2004 (Novak et al., 2004; Georgakoudi et al., 2004) and Zharov et. al. in 2005 (Zharov et al., 2005a, b). Since then, the field has grown significantly. Many technical innovations have been developed, and IVFC has been used in a wide range of biomedical research applications.
The purpose of this paper is to review the main categories of fluorescence IVFC instrumentation (section 2), and describe the key technical considerations regarding fluorescence labeling and detection of circulating cells (section 3). We also discuss important example applications for IVFC, and prospects for broader adoption of the technology by the biomedical research community in the future (section 4). Last, we note that this review is focused primarily on IVFC technologies that use fluorescence contrast in the blood, as apposed to non-fluorescence methods (Le et al., 2009; Biris et al., 2009), and most notably photo-acoustic flow cytometry for which there are already a number of excellent recent reviews (Galanzha and Zharov, 2012; Tuchin et al., 2011).
2. Overview of IVFC instrumentation
2.1 Microscopy-based approaches
2.1.1 Fluorescence microscopy IVFC
In ‘conventional’ flow cytometry (FC), an artificial flow stream is used to pass individual cells single-file through a set of lasers and filtered optical detectors (photomultiplier tubes; PMTs), which allows measurement of their scatter and laser-induced fluorescence properties (Shapiro, 1995). The basic principle of fluorescence IVFC is to use flowing blood vessels in the body in lieu of the flow stream, so that fluorescently-labeled cells can be detected and enumerated directly in vivo (methods of cell labeling and contrast are discussed in section 3, below). However, measurement of fluorescence from individual moving cells in vivo is significantly more challenging, since there is substantial non-specific autofluorescence background, and because visible and near-infrared light is highly scattered and absorbed in biological tissues.
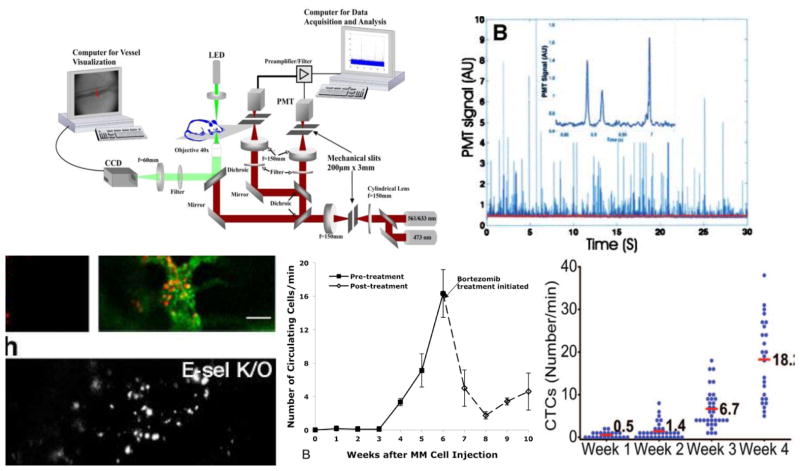
The first fluorescence IVFC design (figure 1a) used a microscope objective to focus excitation laser light into a slit across an accessible small blood vessel (Novak et al., 2004; Georgakoudi et al., 2004) in an arteriole in the ear of a mouse. As fluorescently-labeled cells pass through the excitation light, a small burst of fluorescence light is emitted, allowing confocal detection of transient ‘spikes’ with a filtered photomultiplier tube (PMT) while rejecting the static autofluorescence background (fig. 1b). Mice are held under anesthesia and placed on a warming pad, so that data can be acquired continuously for several hours. Fluorescence spikes are counted, either in real-time, or off-line using automated software. In combination with targeted fluorescent probes and fluorescent proteins, this allows optical enumeration of specific cell populations over time.
Figure 1.
(a) Microscopy IVFC design, reprinted with permission from (Novak et al., 2004; Georgakoudi et al., 2004). A laser is focused across a blood vessel in the ear of a mouse. (b) As fluorescently-labeled cells pass through the slit, transient fluorescence pulses are generated and detected. Example applications of IVFC, including, (c) the effect of treatment on homing of leukemic cells, figure reprinted with permission from (Sipkins et al., 2005), (d) the number of circulating MM cells, figure reprinted with permission from (Runnels et al., 2011), and, (e) circulating lung tumor cells during disease progression and response to treatment, figure reprinted with permission from (He et al., 2007).
As discussed in section 1, there are many advantages to microscopy IVFC over conventional methods for studying circulating cells that require drawing, handling and storing of blood samples. Example applications include changes in homing behavior of leukemic cells in response to treatment (fig. 1c) (Sipkins et al., 2005), progression of cancer cell shedding into the circulation in multiple myeloma (fig. 1d) (Runnels et al., 2011) and lung cancer (fig. 1e) (He et al., 2007)
As summarized in table 1, the microscopy-IVFC design has been used to study a wide range of cell types in mice and rats, including blood cells (Novak et al., 2004; Novak and Puoris’haag, 2007; Golan et al., 2012; Fan et al., 2012), stem cells (Boutrus et al., 2007; Suo et al., 2015), and circulating cancer cells (Fan et al., 2012; Hui et al., 2014; Nedosekin et al., 2014; Runnels et al., 2011). While the initial design used a red laser and fluorophore combination, subsequent variants have been developed that are capable of detecting green and yellow visible fluorophores (Fan et al., 2012). Multi-color versions have also been developed, which allow multiplexed study of different cell populations concurrently, or the study of multiple molecular targets (molecular profiling) in individual cells in vivo (Boutrus et al., 2007; Hwu et al., 2011).
Table 1.
Reported IVFC methods for fluorescence study of circulating cells in vivo
| IVFC Technique | Reference(s) | Animal Model | Cell Line(s) | Fluorophore(s) |
|---|---|---|---|---|
| Microscopy IVFC | (Georgakoudi et al., 2004) | SCID mice, Copenhagen rat | Leukemia (MLL), prostate cancer (LNCaP) | DiD |
| (Novak et al., 2004) | BALB/c mice | Red blood cells (RBC), white blood cells (WBC) | DiD, Cy-Chrome-CD45 | |
| (Sipkins et al., 2005) | BALB/c, SCID mice | Leukemic (NALM-6) | DiD, DiR | |
| (Wei et al., 2005) | SCID mice | Prostate cancer (MatLyLu) | AF-647 | |
| (Lee et al., 2006) | BALB/c mice | T cells (CD4+) | DiD | |
| (Boutrus et al., 2007) | NOD/SCID mice | Breast cancer (SUM149), Hematopoietic stem cells (HSC) | GFP, PE, APC | |
| (Novak and Puoris’haag, 2007) | BALB/c mice | RBC, T-Cells | DiD, CellTracker | |
| (Fan et al., 2010) | C57BL/6 mice | T-cells | GFP, DsRED | |
| (Hwu et al., 2011) | SCID mice | Breast cancer (SUM1315, DU4475) | GFP, FITC, (red) autofluorescence | |
| (Li et al., 2011) | BALB/c, Slac-nu mice | LNCaP, PC-3, HCCLM3, liver cancer (Hep62) | DiD, GFP | |
| (Runnels et al., 2011) | SCID, BALB/c, Col2.3 Mice | Multiple myeloma (MM) | GFP, DiD, DiO | |
| (Fan et al., 2012) | BALB/c mice | Hepatocellular carcinoma (HCCLM3) | GFP | |
| (Golan et al., 2012) | Human | RBC, WBC | Intrinsic scatter | |
| (Aceto et al., 2014) | SCID Mice | Breast cancer (MDA-MB-231-LM) | DiD | |
| (Hui et al., 2014) | BALB/c mice | Cervical cancer (HeLa) | Fluorescent nanodiamond (FND) | |
| (Nedosekin et al., 2014) | Nu/nu mice | Mammary adenocarcinoma (MTLn3) | Dendra2 | |
| (Suo et al., 2015) | BALB/c Mice | Bone marrow cells, mesenchymal stem cells (MSC) | DiO, IR-780 EGFP | |
| (Yan et al., 2015) | BALB/c mice | HCC | GFP | |
| (Suo et al., 2017) | BALB/c mice | HCCLM3, prostate cancer (PC-3) | GFP | |
| Multi-photon (MP-IVFC) | (He et al., 2007) | BALB/c mice | Leukemia (L1210A) | DiD, AF488, GFP, FITC |
| (Zhong et al., 2008) | Nu/nu mice | T-Lymphocytes (Jurkat), cervical cancer (KB), peripheral mononuclear blood cells (PMBC) | CFSE, DeepRed | |
| (Tkaczyk et al., 2008; | Nu/nu, CD-1 mice | RBCs, breast cancer | DeepRed, DiD | |
| Tkaczyk and Tkaczyk, 2011) | (MCF-7, MDA-MB-435) | |||
| (Chang et al., 2010) | CD1 mice | Fibrosarcoma (MCA-207) | DiD, GFP | |
| (Li et al., 2010) | BALB/c, C57BL/6 mice | Leukocytes | Intrinsic Tryptophan | |
| (Zeng et al., 2014) | zebrafish | WBCs (neutrophils, macrophages) | Intrinsic NADH, GFP, DsRed | |
| Retinal flow cytometer (RFC) | (Alt et al., 2007) | BALB/c mice | Lymphocytes | DiD |
| Computer Vision IVFC (CV-IVFC) | (Markovic et al., 2013; Markovic et al., 2015) | Nu/nu mice | MM | DiD |
| Diffuse Fluorescence Flow Cytometry (DiFC) | (Zettergren et al., 2012b; Zettergren et al., 2012a; Pera et al., 2017) | Nu/nu mice | MM | DiD |
| (Pestana et al., 2013) | Nu/nu mice | MSC | DiD | |
| Hybrid Photoacoustic Fluorescence Flow Cytometry (PA-FFC) | (Nedosekin et al., 2013) | Nu/nu mice | Melanoma (B16F10, C8161), MTLn3, RBC | FITC, GFP, iRFP, ICG |
| (Galanzha and Zharov, 2013) | Nu/nu mouse | Melanoma (B16F10, HTB-65, SK-ML-7), ovarian carcinoma (CI81) | GFP | |
| (Nedosekin et al., 2014) | Nu/nu mice | MTLn3 | Dendra2 | |
| (Juratli et al., 2014) | Nu/nu mice | B16F10, MDA-MB-231 | GFP | |
| (Nolan et al., 2016) | Nu/nu mice | B16F10, MDA-MB-231, MTLn3 | GFP, Dendra2 | |
| (Cai et al., 2016) | C57BL/6 mice | Infected (malaria) RBCs | GFP | |
| (Nedosekin et al., 2017) | Nu/nu mice | MDA-MB-231, breast cancer (ZR-75-1) | GFP, Gold Nanoparticles |
2.1.2 Multi-photon IVFC
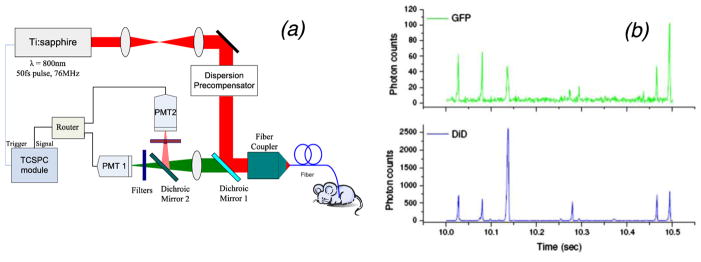
A notable technical variation of IVFC is multi-photon (MP) IVFC. MP-IVFC uses a rapid pulsed (usually femtosecond) laser, either in a slit configuration or in scanning 2-photon microscopy mode. The potential advantages of the multi-photon approach include deeper penetration of the NIR excitation into blood vessels, and the ability to excite multiple fluorophores with a single laser source simultaneously. For example, He et al. (He et al., 2007) and Zhong et al. (Zhong et al., 2008) used a modified two-photon microscope to measure a number of circulating tumor cell types in mice. Rather than use a multi-photon microscope, Chang et al. (Chang et al., 2010) developed a two-photon fiber-optic probe with a ~10 μm excitation volume that could be inserted directly into the bloodstream or a highly perfused organ (such as the liver) in a mouse (figure 2). They demonstrated that it was possible to detect sarcoma cells labeled with green fluorescent protein (GFP) or Vybrant DiD using this method. Other non-linear processes such as third-harmonic generation (THG) have also been implemented. For example, Liu et. al. (Chen and Liu, 2012) imaged circulating blood cells in the capillary bed beneath the skin in humans in vivo.
Figure 2.
(a) Fiber-delivered MP-IVFC system. (b) The fiber probe was inserted into the liver of a mouse for detection of sarcoma cells. Figures reprinted with permission from (Chang et al., 2010)
2.2 IVFC Methods that Sample Larger Circulating Blood Volumes
The sensitivity of IVFC – with respect to the minimum detectable concentration of circulating cells - is a major consideration for applications involving low-abundance cell populations. As we discuss more in section 3.3, this is partially defined by the circulating blood volume that is sampled by the instrument. Microscopy-based IVFC normally probes small blood vessels in the ear with flow rates on the order of 0.1–1 μL of blood per minute. In the case of low-abundance cells (below 1,000 cells per mL of peripheral blood), this may result in unacceptably low count rates or require impractically long acquisition times. Motivated by this, several IVFC instruments have been developed that increases this sampling volume to detect lower-abundance target cell populations.
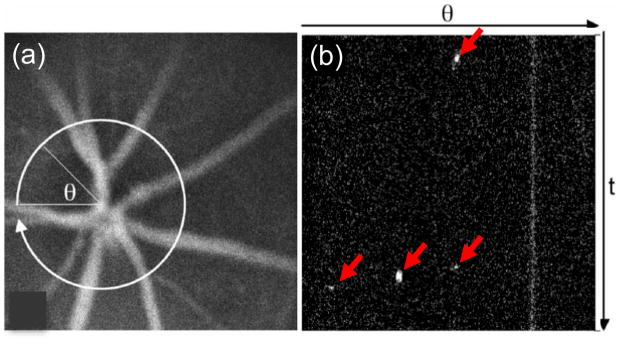
For example, Alt. et. al. (Alt et al., 2007) developed the retinal flow cytometer (RFC), wherein multiple vein-artery pairs in the retina of mouse were radially scanned with a laser (figure 3). This yielded a count rate approximately five times higher than a microscope IVFC instrument in the same animals.
Figure 3.
The RFC uses a radially scanned beam (a) to sample multiple artery vein pairs in the retina. (b) This allows simultaneous detection of circulating cells in approximately 5 times larger circulating blood volume than microscopy IVFC. Figures reprinted with permission from (Alt et al., 2007)
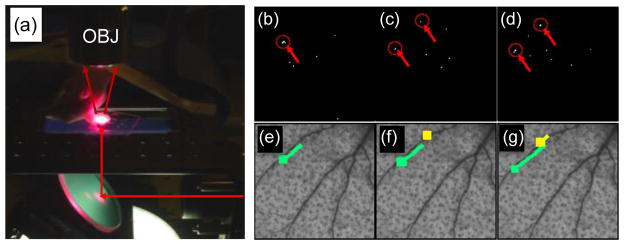
Markovic et al. developed a wide-field fluorescence imaging system - termed ‘computer vision IVFC (CV-IVFC) (Markovic et al., 2013; Markovic et al., 2015) - to image circulating cells in the vasculature in an approximately 5 × 5 mm2 area of the mouse ear (fig. 4a). At such low magnification, individual cells were approximately 1 pixel in size, and significant background noise was observed due to autofluorescence. As such, a computer vision algorithm was concurrently developed to automate detection of cells in low-contrast video sequences (figs. 4b–g). Hence the system allowed quantification of the concentration of circulating cells, but also visualization of cell speed, as well as tracking homing and docking events. It was estimated that the system sampled about 10 μL of blood per minute.
Figure 4.
(a) CV-IVFC used widefield fluorescence imaging of a mouse ear. (b–d) Circulating MM cells were detected in invidual image frames, and then (e–g) merged into trajectories. Figures reprinted with permission from (Markovic et al., 2013).
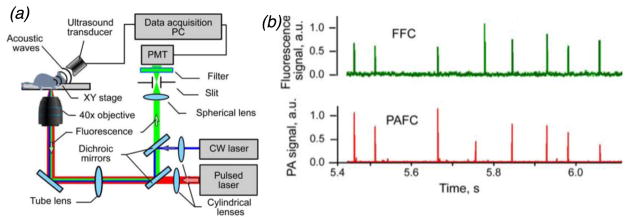
As an alternative to microscopic detection (imaging) of un-scattered light, ‘diffuse in vivo flow cytometry’ (DiFC), uses highly scattered light to interrogate relatively large (several mm) bulk tissue volumes (Zettergren et al., 2012b). This has the advantage of potentially sampling much larger circulating blood volumes in the limbs (for example the hind-leg or tail) of a small animal. In principle, tens to hundreds of μL per minute of blood can be sampled, depending on the limb and enclosed blood vessels. However, the diffusive nature of the light propagation in biological media inherently means that confocal detection of fluorescent light is not possible. As such, a combination of specialized optical, electronic and algorithmic filtering is needed to detect moving cells in a large, non-specific autofluorescence background. Tomographic (Zettergren et al., 2012a; Pera et al., 2013), multispectral (Pestana et al., 2013), and fiber-optic based back-reflectance (figure 5a) (Pera et al., 2017) DiFC designs have been developed. In mice, DiFC has been used to detect multiple myeloma (MM) and mesenchymal stem cells (MSC) in the hind-leg or tail (fig. 5b). Since light is diffuse, the detected ‘spike’ pulsewidth (on the order of 0.1 s depending on flow speed) is significantly wider than microscopy-IVFC designs (1–10 msec). The finite width practically limits the count rate to about 10 counts per second before ‘pulse pile-up’ occurs, meaning that the increased blood sampling volume comes at a cost of lower dynamic range. Moreover, lower cell detection contrast (sensitivity) is expected than, for example, confocal fluorescence microscopy. However, unlike microscopy IVFC where light scatter degrades sensitivity, DiFC actually relies on light scatter and is therefore scalable to larger limbs, species and potentially even humans (see section 4.3 below).
Figure 5.
In DiFC, (a) diffuse fluorescence light is used to (b) detect circulating cells in large superficial (~1–2 mm deep) blood vessels. Figures reprinted with permission from (Pera et al., 2017).
2.3 Photo-acoustic IVFC and Hybrid Fluorescence Flow Cytometry
Although the focus of this review is fluorescence-contrast based IVFC, we note that a major parallel line of research is ‘photoacoustic in vivo flow cytometry’ (PAFC). PAFC has been reviewed comprehensively in several papers previously (Tuchin et al., 2011; Galanzha and Zharov, 2012) and so is described here only briefly. As a pulsed laser illuminates a blood vessel, circulating pigmented cells absorb light and generate an acoustic pulse that can be detected with an ultrasonic receiver. Therefore, PAFC relies on the intrinsic absorption of target cells, and is well suited to detection of naturally pigmented cells such as melanoma (Hoshimoto et al., 2012). Zharov’s group at the University of Arkansas largely pioneered this technique, and has reported many PAFC technical innovations and applications in oncology and immunology. Wang et. al. recently used PA to both enumerate (image) and kill melanoma cells directly in vivo (He et al., 2016).
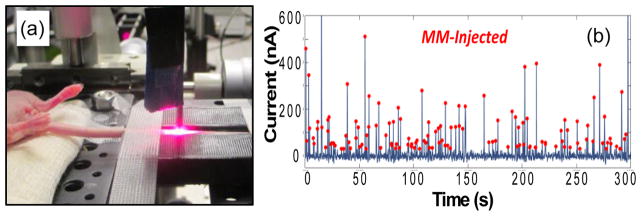
Most relevant to the present review, hybrid photoacoustic and fluorescence flow cytometry (PA-FFC) techniques have also been developed. Nedosekin et al. showed that this multi-modal approach allows simultaneous detection and enumeration of both low absorption and high-absorption fluorescently-labeled circulating tumor cell lines (figure 6) (Nedosekin et al., 2013). Nolan et al. (Nolan et al., 2016) used a similar hybrid method to detect circulating tumor associated exosomes with endogenous (melanin) or nanoparticle enhanced photoacoustic contrast, along with fluorescent proteins for fluorescence contrast.
Figure 6.
(a) PA-FFC is a hybrid IVFC technique that allows enumeration of circulating cells with (b) both fluorescence and photoacoustic modalities. Figures reprinted with permission from (Nedosekin et al., 2013).
3. Cell detection considerations for IVFC
3.1 Contrast mechanisms, labeling and specificity
3.1.1 Intrinsic contrast
In fluorescence IVFC, the target cell line must have sufficient contrast for “detectability” above the non-specific autofluorescence of the surrounding biological material and other circulating cells. A number of fluorescence IVFC techniques have been developed that utilize the endogenous (“intrinsic”) contrast of target cells. Use of intrinsic contrast offers significant advantages, particularly for potential clinical translation since it would avoid the use of administering exogenous contrast agents and the associated regulatory hurdles. Potential disadvantages include the lower specificity of intrinsic contrast for target cells versus exogenous contrast, and in some cases the relatively high laser power associated with multi-photon processes (although these are reported to be well tolerated). For example, Zeng et. al. used two-photon excitation for detection of circulating cells in zebrafish (Zeng et al., 2014). Yelin et. al. used spectrally encoded confocal microscopy to detect flowing red and white blood cells in the blood vessels in a lip in humans (Golan et al., 2012). Li et. al. observed leukocyte trafficking in mice in vivo using two-photon tryptophan contrast (Li et al., 2010). In photoacoustic IVFC, the endogenous absorption of cells is used to generate detectable acoustic signals with a pulsed laser. In this case, there are a number of cell types – such as melanoma CTCs - that are naturally highly pigmented and readily detectable.
3.1.2 Extrinsic fluorescent dyes
In most cases, exogenous (“extrinsic”) fluorophores are used to provide enhanced contrast in fluorescence IVFC. The main methods for IVFC fluorescence cell labeling were described comprehensively previously (Pitsillides et al., 2011), so are reviewed here only in brief:
I) Ex-vivo labeling with fluorescent tracers
Target cells can be brightly labeled in cell culture with a fluorescent molecule, and then re-introduced into the animal by intravenous injection. This method provides very bright labeling for target cells. Cells retain the dye and may stay viable in vivo for weeks with minimal toxicity. Although the labeling intensity decreases by approximately a factor of two for each cell division, the bright initial labeling means that they are detectable even after multiple divisions. As indicated in table 1, there are a number of commercially available dyes in this category that have been used frequently in IVFC, most commonly membrane dyes such as Vybrant DiD and related color variants (Thermofisher Scientific, Waltham, MA). Others include the CellTrace family of dyes (Thermofisher), which bind to amine groups in intra-cellular proteins and brightly label the cell.
II) Fluorescent proteins
Fluorescent proteins are widely used in many areas of biomedical research since the encoding DNA sequence can be stably inserted into the genome of the target cell line (Chudakov et al., 2005). The green fluorescent protein (GFP) was the first to be isolated (Tsien, 1998), but subsequently many variants have been developed that span the visible and near-infrared spectra, from Sirius (which has an excitation maxima at 355 nm) (Tomosugi et al., 2009) to the infrared fluorescent protein (iRFP) family (which has an excitation maxima at 700 nm) (Filonov et al., 2011). These are well suited to IVFC, because cells tagged with fluorescent protein vector stably produce their own fluorescent contrast, and daughter cells retain the gene. For example, in cancer metastasis models, circulating tumor cell burden can be monitored as the disease progresses without the need to administer additional fluorescent dyes as cells replicate.
Despite the apparent advantages of red-shifted fluorescent proteins for in vivo optical imaging (reduced autofluorescence and optical attenuate of biological tissue at longer wavelengths), GFP is still the most commonly used fluorescent protein in IVFC research (table 1). There are several reasons for this, including, a) convenience, since large numbers of GFP-transfected cell lines are already in routine use in research labs throughout the world, b) GFP labeling usually yields brightly labeled cells, and, c) the use of green light produces acceptable contrast for microscopy-IVFC systems. Red-shifted fluorescent proteins (such iRFP) have also been used (Nedosekin et al., 2013), and multi-color instruments (Boutrus et al., 2007; Fan et al., 2010; Hwu et al., 2011; Suo et al., 2015) allow multiplexed detection of multiple cell populations concurrently.
III) Direct labeling of cells in vivo using targeted injectable fluorescent probes
Circulating cells can also be labeled using fluorophores conjugated with antibodies or antibody fragments that target specific cell surface receptors. The specificity of labeling relies on the unique expression of particular cell surface receptors on the cell population of interest, as well as efficient binding of the fluorescent probe to the receptor. This general receptor-targeted approach is widely used in fluorescence molecular imaging, and has been also applied to IVFC. For example, Lin et al. have demonstrated targeting of CD3, CD4, and CD45 receptors on immune cells (Novak et al., 2004), and He et al. (He et al., 2007) demonstrated labeling of folate receptors on cancer cells. Other molecular targets include the sca-1 marker (Pitsillides et al., 2011) and annexin-V for detection of circulating apoptotic cells (Wei et al., 2005). In practical use, experimental parameters such as probe concentration, clearance kinetics, fluorophore loading, binding kinetics and specificity must be carefully established to ensure sufficient labeling and contrast of target cell types.
3.2 Detection sensitivity in IVFC
3.2.1 Cell labeling – quantitative estimates
Unlike conventional flow cytometry where cells are measured in clear sheath fluid, in IVFC cells must be detected within biological tissue that generates non-specific background autofluorescence and attenuates light. In microscopy-IVFC, excitation and emission light must penetrate tens or hundreds of μm of tissue, whereas in DiFC light may penetrate several mm. As such, an evident question is “how much fluorophore labeling does a cell require for detection in IVFC?” Although in practice the answer depends on several factors, it is useful to first quantify the fluorescence labeling associated with the methods outlined in section 3.1.2.
In the biomedical research literature fluorescence labeling is generally reported in qualitative terms such as “bright”, “dim”, or in arbitrary units where the relative intensity of sub-populations are compared. For cell labeling protocols, the incubation dye concentration is usually reported, but not the actual molecular uptake into cells. In the flow cytometry community, labeling is sometimes (although not frequently) reported in units of ‘molecules of equivalent soluble fluorophores’ (MESF), or ‘equivalent reference fluorophores’ (ERF) (Gaigalas et al., 2005). Both metrics are related to the number of fluorescently active (non-aggregated or quenched) molecules per cell, and involve the use of specialized protocols and reference standards for accurate quantification. It is also important to note that different fluorescent molecules vary widely in brightness since they have different extinction coefficients and quantum yields (the ‘brightness’ of a fluorophore in M−1cm−1 is defined as the product of extinction coefficient and quantum yield divided by 1000). The effective measured fluorescence intensity also depends on matching of the IVFC instrument excitation source and detection filters to the absorption and emission spectra of the fluorophore.
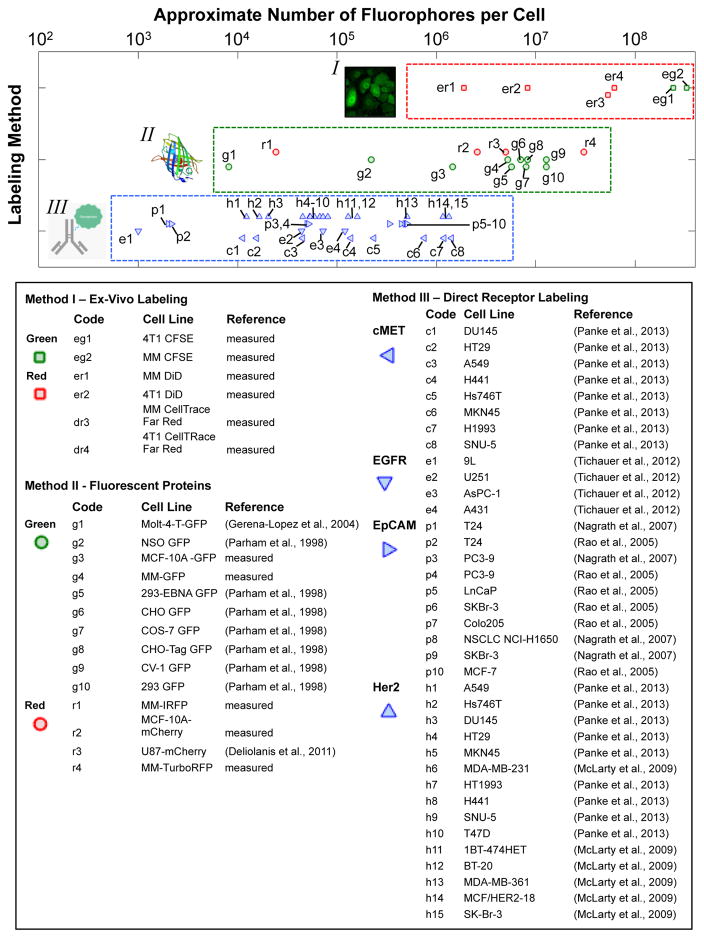
With these issues in mind, it is nevertheless useful for instrument design and experiment planning purposes to review the reported range of fluorescence labeling. The available data (as determined by a literature search) are summarized in figure 7. The numbers shown here should be interpreted with some caution due to the wide variety of fluorophores, wavelength ranges, labeling methods, and quantification methods used (Parham et al., 1998; Deliolanis et al., 2011; Gerena-Lopez et al., 2004). We have chosen to use the metric “approximate number of molecules per cell” in an attempt to standardize between methods. In addition, for the direct labeling method (antibody labeling) we report the cell surface receptor density for each cell line (McLarty et al., 2009; Rao et al., 2005; Nagrath et al., 2007; Tichauer et al., 2012; Panke et al., 2013). Actual cell labeling will therefore depend on the probe design, binding efficiency and the number of fluorescent molecules (loading) per probe. Each data point represents a number reported in the literature, the sources of which are summarized in the accompanying legend.
Figure 7.
Approximate fluorescent labeling that can be achieved with ex-vivo, fluorescent protein, and direct receptor targeted fluorescence-cell labeling methods. Please see text for details.
Since this issue is of significant interest in our own research, we also experimentally tested the labeling of cells with a number of ex-vivo labeled cells against commercially available reference microspheres. We tested multiple myeloma (MM.1s) and murine breast cancer (4T1) cells labeled with a membrane dye (DiD) and CellTrace Far Red and green (CFSE) dyes. We also tested several GFP, TurboRFP, mCherry and iRFP labeled cell lines against the reference calibration beads. Our measurements are indicated in open points (the details of our experiments are given in appendix A). As indicated, these generally agree well with literature values.
Overall, several orders-of-magnitude range of labeling is observed using the methods. Ex-vivo labeling (method-I) produces very brightly-labeled cells, and fluorescent protein expression (method-II) and direct antibody labeling (method-III) may produce several orders of magnitude variation depending on factors such as cell line, transfection efficiency, cell cycle stage, receptor density, probe binding efficiency. However, for IVFC instrument design and planning purposes, 105–107 molecules (1–100 attomole) per cell are certainly achievable and may be used as reasonable values for calculations pertaining to estimation of detection sensitivity.
3.2.2 Cell labeling and lowest detectable fluorophore concentration
Assuming the quantitative fluorescence labeling of cells is known, it is possible to estimate the lower bounds of ‘detectability’ in IVFC. For most IVFC methods, “spikes” are detected as fluorescently-labeled circulating cells pass through the instrument field-of-view. In this case, it is straightforward to use the standard definition of the signal-to-noise ratio (SNR) of 20log10(I/σ), where I is the spike amplitude and σ is the standard deviation of background noise. The background noise may arise from a number of sources, including detector noise on the background autofluorescence signal, and motion artifacts related to breathing. The cell labeling method used may introduce other sources of noise, including non-specific uptake of targeted dyes, or scavenging of free dye or cell fragments by non-target cells.
In order to enumerate cells, a minimum detection threshold must be selected. The threshold is chosen to maximize the number of peaks counted while maintaining an acceptably low false alarm rate (FAR), which is the expected count rate for an animal with no circulating cells (control animal). In practice, this is typically about 10–14 dB, or a spike height 3–5 times the noise level. Therefore, one potentially useful metric for characterizing the detection sensitivity of an IVFC system is the cell labeling at the minimum threshold. This can be achieved by measuring the cell brightness in culture (using a flow cytometer or fluorescence microscope) against a known calibration standard. Reference fluorescent micro-spheres with calibrated intensities (for example, relative brightness or calibrated units of MESF) are used routinely in flow cytometry and are sold by a number of vendors. The average measured spike height in vivo can be compared to the average brightness measured ex-vivo, and the minimum detectable labeling estimated. In practical terms, this also means that, depending on cell labeling a fraction target cell population may not be detectable for a given cell type, labeling method and IVFC system.
In practice this varies significantly amongst the fluorescence-based instruments described in the literature (section 2). For microscopy-IVFC, Lin et. al. previously computed a lower-limit of detectability for confocal detection of Cy5.5-conjugated antibody labeled cells of approximately 103 molecules of fluorophore per cell (Runnels et al., 2006). For wide-field and diffuse methods the threshold may be several orders of magnitude higher.
3.3 Count rate and lowest detectable cell concentration
Another important issue with respect to IVFC sensitivity is the lowest detectable concentration of circulating cells. This is particularly important in applications involving rare circulating cells, such as CTCs, where clinically relevant CTC burdens are as low as 1–100 cells per mL of peripheral blood. The expected cell count rate measured with an IVFC system in a blood vessel dNc/dt at a given time t is given by,
| (1) |
where Qs is the circulating blood volume sampled by the instrument per unit time, Φd is the fraction of target circulating cells that are detectable by the instrument, Cc(t) is the concentration of target cells per unit volume, which may change over time t. The total number of cells counted Nc in an IVFC scan is the time-integral of equation 1 over the acquisition time.
The blood sampling volume Qs varies significantly by IVFC method. For microscopy IVFC, this is about 0.1–1 μL/minute, for RFC or CV-IVFC about 1–10 μL/minute, and for DiFC about 10–100 μL/minute. Considering a low abundance target cell population of Cc(t) = 100 cells/mL, if all cells were detected then, the expected count rate dNc/dt is 0.1, 1 and 10 cells per minute, respectively. However in general a certain fraction of cells may be below the detection threshold of the system (section 3.2), so that Φd < 1. For example, for a weakly labeled cell population in a hypothetical IVFC system, an improvement in Qs may be outweighed by a reduction in Φd.
For low-count rate measurements (rare cells), the time required to count a statistically significant number of cells may be impractically long. Assuming Poisson count statistics, the standard deviation of the measurement is √Nc, so that a minimum of 100 cell counts are required for a measurement uncertainty of ±10%. This may be infeasible if the target circulating cell concentration Cc(t) is expected to change significantly in the acquisition period, or if it is undesirable to hold animals under anesthetic for long acquisition periods. Likewise, if the FAR of the instrument exceeds or is comparable to dNc/dt, long acquisition periods may be needed to establish statistical significance compared to control measurements.
In general, microscopy-IVFC, multi-photon IVFC and RFC are capable of detecting more weakly labeled cells, in exchange for lower blood sampling volume, whereas CV-IVFC or DiFC require more brightly-labeled cells, in exchange for higher blood sampling volume. As we discuss further in the next section, we suggest that enhanced standardization of system capabilities by the IVFC communities could further stimulate growth in the field and adoption of IVFC by prospective users in the general biomedical research communities.
4. Future prospects for IVFC
4.1 IVFC in biomedical research
While we have focused this review on technological considerations, in this section we discuss two example areas of biomedical research where IVFC can uniquely yield important insights.
4.1.1 Immunology
The immune system responds to local infection or injury by recruiting leukocytes from distant locations such as lymph nodes, bone marrow, and the spleen. Leukocytes released from these lymphoid organs traffic to their destination via the circulation. Thus IVFC is an ideal tool to monitor the immune response noninvasively, by “spying” on these cellular armies as they make their way to the battlefield. For example, we have investigated the T cell response following allogeneic islet transplantation (Fan et al., 2010), an experimental treatment for type-1 diabetes that aims to replenish the insulin-producing beta cells lost to the autoimmune destruction with transplanted pancreatic islets from healthy donors. The challenge is that unless the donor is genetically matched to the recipient (e.g. identical twins), the transplanted tissue will be recognized by the patient’s immune system as foreign and be rejected. The goal in transplantation immunology is to induce a state of tolerance, where the immune system is educated to accept tissue from the donor, while retaining the full capacity to mount an immune response against other invading organisms. Thus tolerance induction is different from immune suppression, which will render the patient susceptible to infection and even cancer.
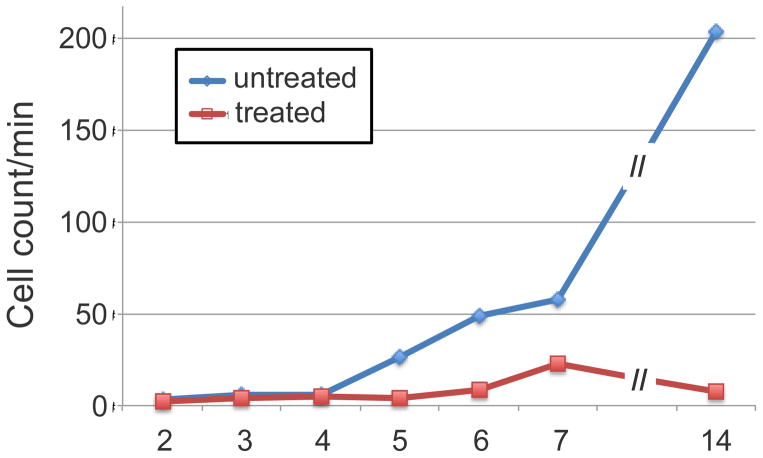
In the mouse model of pancreatic islet transplantation, allogeneic (foreign) islets were placed under the kidney capsule, a standard location for transplantation studies. Without tolerance induction, the islets were rejected within 2 weeks and the mice all became diabetic, whereas with tolerance induction (CD154 plus rapamycin), the mice maintained normal glucose levels for the duration of the experiment (>1 month), indicating donor islets were functional in insulin production. However difference in the kinetics of T cell response in these two cases was not known. We used IVFC to monitor the number of circulating T cells (figure 8) and detected a marked increase (reaching >100-fold) in the number of DsRed+ CD4 T cells during the two week period prior to islet rejection in mice that did not receive tolerance induction (Fan et al., 2010). In contrast, in mice receiving tolerance induction, the number of CD4 T cells only showed a moderate and transient increase but the absolute number was approximately a factor of 10 lower than the rejecting cohort. Using multi-color IVFC, we also monitored the number of GFP+ regulatory T cells hypothesized to be important for tolerance induction. Contrary to expectation, an increase in regulatory T cells was not detected, suggesting that while this T cell subset might have been important functionally, they were not released into the circulation and instead exerted their regulatory function in the lymph nodes. These findings were born out by intravital imaging and quantification of the T cells that have reached their destination (graft site under the kidney capsule). However, intravital imaging was invasive where as IVFC was completely noninvasive, making it possible to perform serial measurements of T cell numbers circulating through the same blood vessel day after day. Because of the massive T cell expansion during the immune response, the experiment was only possible using T cells expressing fluorescent proteins that are not diluted by the proliferation.
Figure 8.
Number of circulating CD4+ effector T cells in the peripheral circulation of mice receiving allogeneic islet transplantation together with or without tolerance induction (anti-CD154 + rapamycin treatment). Figure reprinted with permission from (Fan et al., 2010)
4.1.2 Cancer Research and Oncology
Metastasis is responsible for >90% of cancer related deaths, and one of the common metastatic pathways is via circulating tumor cells (CTCs) through the blood. CTC burden has been shown to correlate with disease progression and response to treatments both pre-clinically and clinically (Cristofanilli et al., 2004; Bidard et al.). In small animal models, it has been shown that CTC dissemination occurs before distant metastases are detectable (Hwu et al., 2011). Therefore, CTCs represent a promising potential new biomarker for both treatment monitoring and early detection of metastatic progression. Ideally, CTCs could help guide clinical treatment in the future.
This has driven interest in new technologies for study of CTCs; in 2016, there were nearly 1500 journal papers published (Pubmed database) related to CTCs, with approximately 20% related to small animal pre-clinical research. CTCs are difficult to study in part because they are rare; CTC burden of 1–100 cells per mL are clinically significant. Therefore, isolation and purification of CTCs is a major problem. Although this is a very active area of research, currently CellSearch is the only FDA approved method for CTC enumeration, and is based on the presence of cells with epithelial CTC markers in blood samples.
Due to the low abundance of CTCs, in small animal experiments it may be necessary to euthanize the animal and analyze the entire peripheral blood volume. In addition to known problems associated with sample preparation and analysis, this also eliminates the possibility of serial study of the same animal over time. This specific problem continues to be a major driver for development of new IVFC techniques capable of sensitive detection of rare cells (such as RFC, CV-IVFC, and DiFC). As noted, cell lines labeled with fluorescent protein(s) are well suited to this application, since CTCs remain brightly and specifically labeled during disease progression without the need for administering additional dyes. IVFC has already been applied in CTC research in monitoring disease progression and response to treatment in various small animal models of hepatocellular cancer (Fan et al., 2012), lung cancer (He et al., 2007), breast cancer (Hwu et al., 2011), and multiple myeloma (Runnels et al., 2011).
4.2 Prospects for broader adoption of IVFC in pre-clinical research
IVFC has enjoyed significant growth over the last decade. In this section, we discuss prospects for further growth and broader adoption of IVFC by a wider group of users in the future.
4.2.1 Technical capabilities
As we have demonstrated in this review, there has been continuous push to improve the technical capabilities of IVFC. Improved instrument sensitivity is a critical area of research, both with respect to the minimum detectable cell concentration and fluorophore concentration. Identification of novel contrast agents and intrinsic contrast mechanisms for sensitive and specific detection of target circulating cell types is also an active area of work. Moreover, depending on the specific application, ‘enumeration’ (counting) of one specific cell population may be inadequate. In vivo molecular characterization and phenotyping through concurrent measurement of a set of biomarkers is therefore advantageous. For example, in the case of CTCs, it may be desirable to distinguish between epithelial or mesenchymal cell subpopulations. Likewise, it may be necessary to characterize the response of specific clonal sub-populations to a new treatment.
4.2.2. Matching users with instrument capabilities
The two example applications discussed in section 4.1 are illustrative because they involve very different circulating cell populations, concentrations and labeling methods. As such, the most appropriate IVFC methods may be different: an application involving an abundant cell population, but with low expected cell brightness may be best suited to microscopy IVFC, whereas a lower abundance cell population that is brightly labeled with a constitutively expressed fluorescent protein may be better suited toward RFC, CF-IVFC, or DiFC. However to a potential end-user in the research community, matching their application to the technical capabilities of the instrument may be difficult. To this end, quantitative and qualitative reporting of IVFC instrumentation capabilities – such as advantages and disadvantages for specific applications - may serve to address this and foster further collaborations. Specifically, researchers may characterize and report the following where possible: i) the minimum detectable cell labeling (e.g. relative to a reference particle or in MESF), ii) the average measured signal to noise ratio SNR for a cell labeled at a given level, iii) the instrument false-alarm rate (FAR), and, iv) the expected count rate (dNc/dt) at a given cell concentration (Cc(t)) for a fixed cell labeling level (in MESF) or reference brightness.
4.2.3 Availability and cost
At the time of writing of this review, there is not yet a commercial IVFC system available for end-user purchase. Because current generations of IVFC may require advanced training in biomedical optics (e.g. for alignment of the illumination and detection optics, instrument calibration, and analysis of data), broader adoption would be enabled by more emphasis on developing IVFC instrumentation that can be operated by trained non-specialist users. In terms of cost, the optical components for IVFC are generally comparable to fluorescence microscopy and are therefore not prohibitive for most research labs. Microscopy IVFC instrumentation includes light sources, microscope objective(s), mirrors, laser scanning mirrors, filters, optical detectors and computer hardware. Multi-photon IVFC requires specialized pulsed lasers, which currently implies a more expensive instrument, although this may change with the dissemination of lower cost mode-locked pulsed fiber lasers. DiFC uses a relatively simple optical implementation, although it may require specialized optical fiber bundles. The general characteristics of existing fluorescence IVFC systems are summarized in table 2. Last, an alternative approach to developing a specialized IVFC system is to use the line scan mode of a commercial confocal or 2 photon microscope (He et al., 2007).
Table 2.
General characteristics of IVFC methods
| Sensitivity (labeling) | Sensitivity (sampling volume) | Instrument Cost | |
|---|---|---|---|
| Microscopy IVFC | +++ | + | $$ |
| Multi-Photon IVFC | +++ | + | $$$ |
| RFC | +++ | ++ | $$ |
| CV-IVFC | ++ | ++ | $$ |
| DiFC | + | +++ | $ |
4.3 Towards clinical IVFC
Finally, we discuss prospects for translation of IVFC to humans clinically. Analogous to the basic biomedical research discussed in section 4.1, there are a number of important clinical problems where the capabilities of IVFC would have high potential value. Enumeration of CTCs would be valuable in staging, early-detection and treatment response monitoring in metastatic cancer. Quantifying the number of circulating leukocytes can provide a diagnostic marker for infection or inflammation.
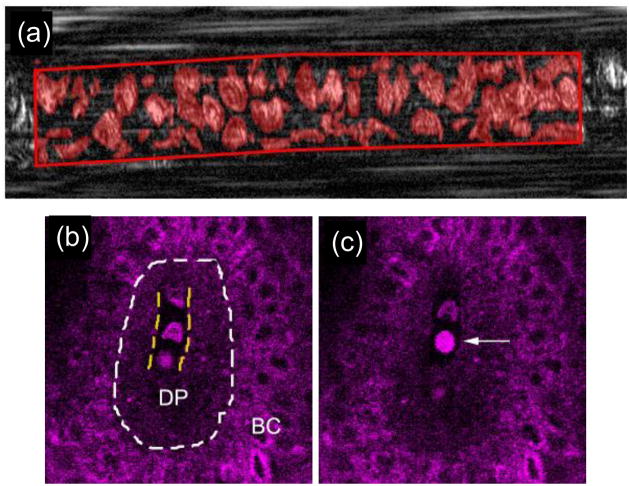
In principle, IVFC technology can be translated clinically to humans, as has already been demonstrated by imaging blood cells in the lower lip (Golan et al., 2012) (figure 9a). Chen and Liu demonstrated imaging of blood cells in superficial capillary beds in the arm using third harmonic generation microscopy (Chen and Liu, 2012) (fig. 9b,c). Likewise, reflectance confocal microscopy with intrinsic contrast is already widely studied in humans in dermatological applications, e.g. (Rajadhyaksha et al., 1995; Rajadhyaksha et al., 2017; Stevenson et al., 2013; Longo et al., 2013). Other candidate anatomical locations include the capillary bed in the earlobe or retina of a human. DiFC is scalable to larger limbs and could theoretically be used to probe, for example, superficial blood vessels in the forearm or wrist of an adult human.
Figure 9.
Example of imaging of circulating cells in humans with intrinsic contrast. Circulating RBCs (a) in the lower lip by spectrally encoded flow cytometry, figure reprinted with permission from (Golan et al., 2012). Third harmonic generation imaging (b,c) of circulating blood cells (likely WBCs) in the skin. Figures reprinted with permission from (Chen and Liu, 2012). The dermal papilla (DP) and basal cells (BC) are indicated.
As such, the largest barrier to clinical translation of fluorescence-based IVFC is imaging contrast of target cells. In principle, it would be possible to use antibody (or anti-body fragment) targeted fluorescent probes (He et al., 2007; Wei et al., 2005), analogous to those used in fluorescence-guided surgery (“molecular imaging”) (Vahrmeijer et al., 2013; Harlaar et al., 2016). To achieve the required specificity, this could involve the use of activatable probes (Kobayashi and Choyke, 2011), or multiplexed combinations of specific and non-specific probes (Tichauer et al., 2012). In practice, the potential regulatory hurdles associated with using contrast agents (with potential side-effects) in humans may outweigh the benefits of performing an in vivo measurement, especially in light of the fact that blood can readily be drawn from the patient and analyzed. There are also currently only a small number of fluorescent molecules that are approved for use in humans including indocyanine green (ICG) (Marshall et al., 2010) and 5-amininolevulinic acid (ALA) induced protoporphyrin IX (PPIX) (Guyotat et al., 2016). However, there are a number of ongoing clinical trials involving the use of new, targeted contrast agents in fluorescence-guided surgery (Rosenthal et al., 2015).
Use of intrinsic cellular contrast would therefore be preferable, since it could represent an easier path to clinical translation. In this case, the primary challenge is identification of a sufficiently sensitive and specific optical marker or set of markers. As we have noted, a number of groups are actively working on this problem. In addition to fluorescence contrast, use of photoacoustic contrast (PAFC) for detection of highly pigmented melanoma cells is also a promising strategy.
5. Conclusions
In summary, IVFC has grown significantly in the past decade as a unique method for detecting, enumerating and characterizing single circulating cells directly in the bloodstream. IVFC has already been applied to many important pre-clinical research applications. Continued growth of the IVFC field will be facilitated by continued technical improvements in the area of instrument detection sensitivity, multiplexing capabilities, and ease of use, and through better matching of IVFC technologies with potential applications. Despite present technical and regulatory hurdles, IVFC may represent an important new clinical tool for oncology and immunology in the future.
Acknowledgments
This work was funded in part by the National Institutes of Health (R01HL124315; NHLBI). The authors thank Prof. Theodore Norris of the University of Michigan, Prof. Tzu-Ming Liu of the National Taiwan University, Professor Vladimir Zharov of the University of Arkansas Medical Sciences, Prof. Phillip S. Low of Purdue University, Prof. Dvir Yelin of Technion-Isreal Institute of Technology and colleagues for kindly allowing us to use their images in this paper. We also thank Prof. Yuji Mishima and Prof. Irene Ghobrial of the Dana Farber Cancer Institute, as well as Prof. Anand Asthigari of Northeastern University for donating fluorescent protein labeled cell lines.
Appendix A. Supplemental Methods
Vybrant DiD cell labeling
Multiple myeloma (MM.1s) and 4T1 cells were harvested using a cell scraper, spun down at 400 g, and then re-suspended in RPMI with 0.1% bovine serum albumin (BSA) at a concentration of 2 × 106 cells / mL. Cells were dyed using a final concentration of 1 μmol / L of Vybrant-DiD (Thermofisher Scientific, Waltham, MA) and incubated for 20 min at 37 °C. At the end of the incubation process, FBS was added (2% of total volume) to prevent cell clumping during centrifuging. Cells were centrifuged as before and washed, once with PBS with FBS to remove any free DiD in suspension. Last, 106 labeled cells were resuspended in 1 mL of RPMI media for flow cytometry.
CellTrace Far Red and CFSE cell labeling
MM and 4T1 cells were suspended at 2 × 106 cells/mL in PBS. Labeling solution from either CellTrace FarRed (Cat. C34564) or CFSE (CellTrace CSFE (Cat. C34553) proliferation kits (Thermofisher) were added to a final concentration of 2 μM and incubated for 30 min at 37o C. After the labeling period, 5 times the original staining volume of phenol free RPMI 1640 with 2 % FBS were added. Cells were centrifuged at 1000rpm for 5 mins and then re-suspended in 2 × 106 cells/ml in phenol free RPMI 1640. Cells were incubated for an additional 30 min at 37°C. 10ml of phenol free RPMI 1640 with 10% FBS was then added to remove any free dye. Last, 106 labeled cells were centrifuged and re-suspended in 1 mL of RPMI media for flow cytometry.
Fluorescent protein labeled cell lines
MM.1s-iRFP702 and MM.1s-TurboRFP were provided by Prof. Yuji Mishima and Prof. Irene Ghobrial of Dana Farber Cancer Institute, Boston, MA. MCF-10A-GFP-mCherry cells were provided by Prof. Anand Asthigari, Northeastern University, Department of Bioengineering, Boston, MA.
Reference beads
MESF reference beads were purchased from Bang’s Laboratories, Inc. (Fishers, IN). We used the following fluorescence quantification kits for these experiments: red – Quantum Alexa Fluor (AF) 647 MESF (Cat. 647), Yellow – Quantum R-PE MESF (Cat 827). Green – Quantum Alexa Fluor (AF) 488 MESF (Cat 488).
Flow cytometry
Cell brightness (peak height) was measured using a Cytek DxP11 Analyzer (Fremont, CA), located at the Brigham and Women’s Hospital in Boston, MA. Cells were suspended at a concentration of 106 cells per mL. At least 10,000 events were recorded for each measurement. The following laser filter combinations were used for measurements: Red: Excitation = 637 nm, emission = 660/16 nm, Yellow: Excitation = 561 nm, emission = 590/20 nm, Blue: Excitation = 488 nm, emission = 505/10 nm.
MESF quantification
The intensity recorded for each cell line was compared against appropriate MESF calibration beads (red, yellow or green) as per the manufacturer’s instructions. The measurements were corrected for the relative molar extinction coefficents, quantum yields, spectral absorption and emission of the fluorophores relative to the reference beads for GFP (Patterson et al., 1997; Patterson et al., 2001), mCherry (Shaner et al., 2008), AF-488, R-PE, AF-647 (ThermoFisher, 2017; ChromaTechnology, 2017), TurboRFP (Evrogen, 2017). Extinction coefficients, quantum yields, absorption and emission spectra for CFSE, CTFR and DiD were obtained from the manufacturer (ThermoFisher, 2017).
References
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–22. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt C, Veilleux I, Lee H, Pitsillides CM, Cote D, Lin CP. Retinal flow cytometer. Opt Lett. 2007;32:3450–2. doi: 10.1364/ol.32.003450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreopoulou E, Yang LY, Rangel KM, Reuben JM, Hsu L, Krishnamurthy S, Valero V, Fritsche HA, Cristofanilli M. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect versus Veridex CellSearch system. Int J Cancer. 2012;130:1590–7. doi: 10.1002/ijc.26111. [DOI] [PubMed] [Google Scholar]
- Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, Thompson B, Maiso P, Sun JD, Hart CP, Roccaro AM, Sacco A, Ngo HT, Lin CP, Kung AL, Carrasco RD, Vanderkerken K, Ghobrial IM. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119:5782–94. doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidard F-C, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J, Caldas C, Gazzaniga P, Manso L, Zamarchi R, de Lascoiti AF, De Mattos-Arruda L, Ignatiadis M, Lebofsky R, van Laere SJ, Meier-Stiegen F, Sandri M-T, Vidal-Martinez J, Politaki E, Consoli F, Bottini A, Diaz-Rubio E, Krell J, Dawson S-J, Raimondi C, Rutten A, Janni W, Munzone E, Carañana V, Agelaki S, Almici C, Dirix L, Solomayer E-F, Zorzino L, Johannes H, Reis-Filho JS, Pantel K, Pierga J-Y, Michiels S. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. The Lancet Oncology. 15:406–14. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- Biris AS, Galanzha EI, Li Z, Mahmood M, Xu Y, Zharov VP. In vivo Raman flow cytometry for real-time detection of carbon nanotube kinetics in lymph, blood, and tissues. J Biomed Opt. 2009;14:021006. doi: 10.1117/1.3119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrus S, Greiner C, Hwu D, Chan M, Kuperwasser C, Lin CP, Georgakoudi I. Portable two-color in vivo flow cytometer for real-time detection of fluorescently-labeled circulating cells. J Biomed Opt. 2007;12:020507. doi: 10.1117/1.2722733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Carey KA, Nedosekin DA, Menyaev YA, Sarimollaoglu M, Galanzha EI, Stumhofer JS, Zharov VP. In vivo photoacoustic flow cytometry for early malaria diagnosis. Cytometry A. 2016;89:531–42. doi: 10.1002/cyto.a.22854. [DOI] [PubMed] [Google Scholar]
- Calcaterra F, Taddeo A, Colombo E, Cappelletti M, Martinelli A, Calabrese S, Mavilio D, Cetin I, Della Bella S. Reduction of maternal circulating endothelial progenitor cells in human pregnancies with intrauterine growth restriction. Placenta. 2014;35:431–6. doi: 10.1016/j.placenta.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Chang YC, Ye JY, Thomas TP, Cao Z, Kotlyar A, Tkaczyk ER, Baker JR, Jr, Norris TB. Fiber-optic multiphoton flow cytometry in whole blood and in vivo. J Biomed Opt. 2010;15:047004. doi: 10.1117/1.3463481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Liu TM. Imaging morphodynamics of human blood cells in vivo with video-rate third harmonic generation microscopy. Biomed Opt Express. 2012;3:2860–5. doi: 10.1364/BOE.3.002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudakov DM, Lukyanov S, Lukyanov KA. Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 2005;23:605–13. doi: 10.1016/j.tibtech.2005.10.005. [DOI] [PubMed] [Google Scholar]
- ChromaTechnology. 2017 http://www.chroma.com.
- Costiniuk CT, Hibbert BM, Simard T, Ghazawi FM, Angel JB, O’Brien ER. Circulating endothelial progenitor cells in HIV infection: a systematic review. Trends Cardiovasc Med. 2013;23:192–200. doi: 10.1016/j.tcm.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- Danila DC, Fleisher M, Scher HI. Circulating tumor cells as biomarkers in prostate cancer. Clin Cancer Res. 2011;17:3903–12. doi: 10.1158/1078-0432.CCR-10-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahaye M, Lawrence K, Ward SJ, Hoare M. An ultra scale-down analysis of the recovery by dead-end centrifugation of human cells for therapy. Biotechnol Bioeng. 2015;112:997–1011. doi: 10.1002/bit.25519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliolanis NC, Wurdinger T, Pike L, Tannous BA, Breakefield XO, Weissleder R, Ntziachristos V. In vivo tomographic imaging of red-shifted fluorescent proteins. Biomed Opt Express. 2011;2:887–900. doi: 10.1364/BOE.2.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco GS, Rustemeyer P, Brand M, Koch R, Kentrup D, Grabner A, Greve B, Wittkowski W, Pavenstadt H, Hausberg M, Reuter S, Lang D. Circulating endothelial progenitor cells in kidney transplant patients. PLoS One. 2011;6:e24046. doi: 10.1371/journal.pone.0024046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CG, Caporali F, Capecchi PL, Lazzerini PE, Pasini FL, Sorrentino V. Levels of circulating CXCR4-positive cells are decreased and negatively correlated with risk factors in cardiac transplant recipients. Heart Vessels. 2011;26:258–66. doi: 10.1007/s00380-010-0053-9. [DOI] [PubMed] [Google Scholar]
- Evrogen. 2017 http://www.everogen.com.
- Fan Z, Spencer JA, Lu Y, Pitsillides CM, Singh G, Kim P, Yun SH, Toxavidis V, Strom TB, Lin CP, Koulmanda M. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat Med. 2010;16:718–22. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZC, Yan J, Liu GD, Tan XY, Weng XF, Wu WZ, Zhou J, Wei XB. Real-time monitoring of rare circulating hepatocellular carcinoma cells in an orthotopic model by in vivo flow cytometry assesses resection on metastasis. Cancer Res. 2012;72:2683–91. doi: 10.1158/0008-5472.CAN-11-3733. [DOI] [PubMed] [Google Scholar]
- Filonov GS, Piatkevich KD, Ting LM, Zhang J, Kim K, Verkhusha VV. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat Biotechnol. 2011;29:757–61. doi: 10.1038/nbt.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaigalas AK, Wang L, Schwartz A, Marti GE, Vogt RF., Jr Quantitating Fluorescence Intensity From Fluorophore: Assignment of MESF Values. J Res Natl Inst Stand Technol. 2005;110:101–14. doi: 10.6028/jres.110.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanzha EI, Zharov VP. Photoacoustic flow cytometry. Methods. 2012;57:280–96. doi: 10.1016/j.ymeth.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanzha EI, Zharov VP. Circulating Tumor Cell Detection and Capture by Photoacoustic Flow Cytometry in Vivo and ex Vivo. Cancers (Basel) 2013;5:1691–738. doi: 10.3390/cancers5041691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakoudi I, Solban N, Novak J, Rice WL, Wei X, Hasan T, Lin CP. In vivo flow cytometry: a new method for enumerating circulating cancer cells. Cancer Res. 2004;64:5044–7. doi: 10.1158/0008-5472.CAN-04-1058. [DOI] [PubMed] [Google Scholar]
- Gerena-Lopez Y, Nolan J, Wang L, Gaigalas A, Schwartz A, Fernandez-Repollet E. Quantification of EGFP expression on Molt-4 T cells using calibration standards. Cytometry A. 2004;60:21–8. doi: 10.1002/cyto.a.20019. [DOI] [PubMed] [Google Scholar]
- Golan L, Yeheskely-Hayon D, Minai L, Dann EJ, Yelin D. Noninvasive imaging of flowing blood cells using label-free spectrally encoded flow cytometry. Biomed Opt Express. 2012;3:1455–64. doi: 10.1364/BOE.3.001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett DR, Weaver WM, Mach AJ, Hur SC, Tse HT, Lee W, Amini H, Di Carlo D. Label-free cell separation and sorting in microfluidic systems. Anal Bioanal Chem. 2010;397:3249–67. doi: 10.1007/s00216-010-3721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyotat J, Pallud J, Armoiry X, Pavlov V, Metellus P. 5-Aminolevulinic Acid-Protoporphyrin IX Fluorescence-Guided Surgery of High-Grade Gliomas: A Systematic Review. Adv Tech Stand Neurosurg. 2016:61–90. doi: 10.1007/978-3-319-21359-0_3. [DOI] [PubMed] [Google Scholar]
- Harlaar NJ, Koller M, de Jongh SJ, van Leeuwen BL, Hemmer PH, Kruijff S, van Ginkel RJ, Been LB, de Jong JS, Kats-Ugurlu G, Linssen MD, Jorritsma-Smit A, van Oosten M, Nagengast WB, Ntziachristos V, van Dam GM. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: a single-centre feasibility study. Lancet Gastroenterol Hepatol. 2016;1:283–90. doi: 10.1016/S2468-1253(16)30082-6. [DOI] [PubMed] [Google Scholar]
- He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci U S A. 2007;104:11760–5. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang L, Shi J, Yao J, Li L, Zhang R, Huang CH, Zou J, Wang LV. In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells. Sci Rep. 2016;6:39616. doi: 10.1038/srep39616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Fan H, Li JQ, Qi HZ. Decreased circulating CD4+CD25highFoxp3+ T cells during acute rejection in liver transplant patients. Transplant Proc. 2011;43:1696–700. doi: 10.1016/j.transproceed.2011.03.084. [DOI] [PubMed] [Google Scholar]
- Hoff J. Methods of blood collection in the lab mouse. Lab Animal. 2000;29:49–53. [Google Scholar]
- Hoshimoto S, Shingai T, Morton DL, Kuo C, Faries MB, Chong K, Elashoff D, Wang HJ, Elashoff RM, Hoon DS. Association between circulating tumor cells and prognosis in patients with stage III melanoma with sentinel lymph node metastasis in a phase III international multicenter trial. J Clin Oncol. 2012;30:3819–26. doi: 10.1200/JCO.2011.40.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui YY, Su LJ, Chen OY, Chen YT, Liu TM, Chang HC. Wide-field imaging and flow cytometric analysis of cancer cells in blood by fluorescent nanodiamond labeling and time gating. Sci Rep. 2014;4:5574. doi: 10.1038/srep05574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu D, Boutrus S, Greiner C, DiMeo T, Kuperwasser C, Georgakoudi I. Assessment of the role of circulating breast cancer cells in tumor formation and metastatic potential using in vivo flow cytometry. J Biomed Opt. 2011;16:040501. doi: 10.1117/1.3560624. [DOI] [PubMed] [Google Scholar]
- Juratli MA, Sarimollaoglu M, Siegel ER, Nedosekin DA, Galanzha EI, Suen JY, Zharov VP. Real-time monitoring of circulating tumor cell release during tumor manipulation using in vivo photoacoustic and fluorescent flow cytometry. Head Neck. 2014;36:1207–15. doi: 10.1002/hed.23439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabacak NM, Spuhler PS, Fachin F, Lim EJ, Pai V, Ozkumur E, Martel JM, Kojic N, Smith K, Chen PI, Yang J, Hwang H, Morgan B, Trautwein J, Barber TA, Stott SL, Maheswaran S, Kapur R, Haber DA, Toner M. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694–710. doi: 10.1038/nprot.2014.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Choyke PL. Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc Chem Res. 2011;44:83–90. doi: 10.1021/ar1000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Huff TB, Cheng JX. Coherent anti-Stokes Raman scattering imaging of lipids in cancer metastasis. BMC Cancer. 2009;9:42. doi: 10.1186/1471-2407-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Alt C, Pitsillides CM, Puoris’haag M, Lin CP. In vivo imaging flow cytometer. Opt Express. 2006;14:7789–800. doi: 10.1364/oe.14.007789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Pastila RK, Pitsillides C, Runnels JM, Puoris’haag M, Cote D, Lin CP. Imaging leukocyte trafficking in vivo with two-photon-excited endogenous tryptophan fluorescence. Opt Express. 2010;18:988–99. doi: 10.1364/OE.18.000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Guo J, Wang C, Fan Z, Liu G, Wang C, Gu Z, Damm D, Mosig A, Wei X. Circulation times of prostate cancer and hepatocellular carcinoma cells by in vivo flow cytometry. Cytometry A. 2011;79:848–54. doi: 10.1002/cyto.a.21134. [DOI] [PubMed] [Google Scholar]
- Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, Rao SB, Eng-Wong J, Seillier-Moiseiwitsch F, Noone AM, Isaacs C. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27:5153–9. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo C, Casari A, Beretti F, Cesinaro AM, Pellacani G. Skin aging: in vivo microscopic assessment of epidermal and dermal changes by means of confocal microscopy. J Am Acad Dermatol. 2013;68:e73–82. doi: 10.1016/j.jaad.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Lopez M, San Roman J, Estrada V, Vispo E, Blanco F, Soriano V. Endothelial dysfunction in HIV infection--the role of circulating endothelial cells, microparticles, endothelial progenitor cells and macrophages. AIDS Rev. 2012;14:223–30. [PubMed] [Google Scholar]
- Markovic S, Li B, Pera V, Sznaier M, Camps O, Niedre M. A computer vision approach to rare cell in vivo fluorescence flow cytometry. Cytometry A. 2013;83:1113–23. doi: 10.1002/cyto.a.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic S, Li S, Niedre M. Performance of computer vision in vivo flow cytometry with low fluorescence contrast. J Biomed Opt. 2015;20:035005. doi: 10.1117/1.JBO.20.3.035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MV, Rasmussen JC, Tan IC, Aldrich MB, Adams KE, Wang X, Fife CE, Maus EA, Smith LA, Sevick-Muraca EM. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open Surg Oncol J. 2010;2:12–25. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen RR, Borgen E, Renolen A, Lokkevik E, Nesland JM, Anker G, Ostenstad B, Lundgren S, Risberg T, Mjaaland I, Kvalheim G, Lonning PE, Naume B. Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res. 2012;14:R117. doi: 10.1186/bcr3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarty K, Cornelissen B, Scollard DA, Done SJ, Chun K, Reilly RM. Associations between the uptake of 111In-DTPA-trastuzumab, HER2 density and response to trastuzumab (Herceptin) in athymic mice bearing subcutaneous human tumour xenografts. Eur J Nucl Med Mol Imaging. 2009;36:81–93. doi: 10.1007/s00259-008-0923-x. [DOI] [PubMed] [Google Scholar]
- Miller MC, Doyle GV, Terstappen LW. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol. 2010;20:10 617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedosekin DA, Fahmi T, Nima ZA, Nolan J, Cai C, Sarimollaoglu M, Dervishi E, Basnakian A, Biris AS, Zharov VP. Photoacoustic in vitro flow cytometry for nanomaterial research. Photoacoustics. 2017;6:16–25. doi: 10.1016/j.pacs.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedosekin DA, Sarimollaoglu M, Galanzha EI, Sawant R, Torchilin VP, Verkhusha VV, Ma J, Frank MH, Biris AS, Zharov VP. Synergy of photoacoustic and fluorescence flow cytometry of circulating cells with negative and positive contrasts. J Biophotonics. 2013;6:425–34. doi: 10.1002/jbio.201200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedosekin DA, Verkhusha VV, Melerzanov AV, Zharov VP, Galanzha EI. In vivo photoswitchable flow cytometry for direct tracking of single circulating tumor cells. Chem Biol. 2014;21:792–801. doi: 10.1016/j.chembiol.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan J, Sarimollaoglu M, Nedosekin DA, Jamshidi-Parsian A, Galanzha EI, Kore RA, Griffin RJ, Zharov VP. In Vivo Flow Cytometry of Circulating Tumor-Associated Exosomes. Anal Cell Pathol (Amst) 2016:1628057. doi: 10.1155/2016/1628057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, Georgakoudi I, Wei X, Prossin A, Lin CP. In vivo flow cytometer for real-time detection and quantification of circulating cells. Opt Lett. 2004;29:77–9. doi: 10.1364/ol.29.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, Puoris’haag M. Two-color, double-slit in vivo flow cytometer. Opt Lett. 2007;32:2993–5. doi: 10.1364/ol.32.002993. [DOI] [PubMed] [Google Scholar]
- Panke C, Weininger D, Haas A, Schelter F, Schlothauer T, Bader S, Sircar R, Josel HP, Baer U, Burtscher H, Mundigl O, Grote M, Brinkmann U, Sustmann C. Quantification of cell surface proteins with bispecific antibodies. Protein Eng Des Sel. 2013;26:645–54. doi: 10.1093/protein/gzt035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham JH, Iannone MA, Overton LK, Hutchins JT. Optimization of transient gene expression in mammalian cells and potential for scale-up using flow electroporation. Cytotechnology. 1998;28:147–55. doi: 10.1023/A:1008046101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–72. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson G, Day RN, Piston D. Fluorescent protein spectra. J Cell Sci. 2001;114:837–8. doi: 10.1242/jcs.114.5.837. [DOI] [PubMed] [Google Scholar]
- Patterson GH, Knobel SM, Sharif WD, Kain SR, Piston DW. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys J. 1997;73:2782–90. doi: 10.1016/S0006-3495(97)78307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera V, Tan X, Runnels J, Sardesai N, Lin CP, Niedre M. Diffuse fluorescence fiber probe for in vivo detection of circulating cells. J Biomed Opt. 2017;22:37004. doi: 10.1117/1.JBO.22.3.037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera V, Zettergren E, Brooks DH, Niedre M. Maximum likelihood tomographic reconstruction of extremely sparse solutions in diffuse fluorescence flow cytometry. Opt Lett. 2013;38:2357–9. doi: 10.1364/OL.38.002357. [DOI] [PubMed] [Google Scholar]
- Pestana N, Mortensen LJ, Runnels JP, Vickers D, Murthy SK, Lin CP, Niedre M. Improved diffuse fluorescence flow cytometer prototype for high sensitivity detection of rare circulating cells in vivo. J Biomed Opt. 2013;18:077002. doi: 10.1117/1.JBO.18.7.077002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsillides CM, Runnels JM, Spencer JA, Zhi L, Wu MX, Lin CP. Cell labeling approaches for fluorescence-based in vivo flow cytometry. Cytometry A. 2011;79:758–65. doi: 10.1002/cyto.a.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, Zwingers T, Friedl TW, Lorenz R, Tesch H, Fasching PA, Fehm T, Schneeweiss A, Lichtenegger W, Beckmann MW, Friese K, Pantel K, Janni W, Group SS. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946–52. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: From bench to bedside. Lasers Surg Med. 2017;49:7–19. doi: 10.1002/lsm.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CG, Chianese D, Doyle GV, Miller MC, Russell T, Sanders RA, Jr, Terstappen LW. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int J Oncol. 2005;27:49–57. [PubMed] [Google Scholar]
- Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, Brandwein-Gensler M, Strong TV, Schmalbach CE, Morlandt AB, Agarwal G, Hartman YE, Carroll WR, Richman JS, Clemons LK, Nabell LM, Zinn KR. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res. 2015;21:3658–66. doi: 10.1158/1078-0432.CCR-14-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels JM, Carlson AL, Pitsillides C, Thompson B, Wu J, Spencer JA, Kohler JM, Azab A, Moreau AS, Rodig SJ, Kung AL, Anderson KC, Ghobrial IM, Lin CP. Optical techniques for tracking multiple myeloma engraftment, growth, and response to therapy. J Biomed Opt. 2011;16:011006. doi: 10.1117/1.3520571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels JM, Zamiri P, Spencer JA, Veilleux I, Wei X, Bogdanov A, Lin CP. Imaging molecular expression on vascular endothelial cells by in vivo immunofluorescence microscopy. Mol Imaging. 2006;5:31–40. [PMC free article] [PubMed] [Google Scholar]
- Shaner NC, Lin MZ, McKeown MR, Steinbach PA, Hazelwood KL, Davidson MW, Tsien RY. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat Methods. 2008;5:545–51. doi: 10.1038/nmeth.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H. Practical Flow Cytometry. 3. New York: Wiley-Liss; 1995. [Google Scholar]
- Shields CWt, Reyes CD, Lopez GP. Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip. 2015;15:1230–49. doi: 10.1039/c4lc01246a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–73. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5:206–19. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson AD, Mickan S, Mallett S, Ayya M. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19–27. doi: 10.5826/dpc.0304a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo Y, Liu T, Xie C, Wei D, Tan X, Wu L, Wang X, He H, Shi G, Wei X, Shi C. Near infrared in vivo flow cytometry for tracking fluorescent circulating cells. Cytometry A. 2015;87:878–84. doi: 10.1002/cyto.a.22711. [DOI] [PubMed] [Google Scholar]
- Suo Y, Xie C, Zhu X, Fan Z, Yang Z, He H, Wei X. Proportion of circulating tumor cell clusters increases during cancer metastasis. Cytometry A. 2017;91:250–3. doi: 10.1002/cyto.a.23037. [DOI] [PubMed] [Google Scholar]
- ThermoFisher. 2017 http://www.thermofisher.com/us/en/home.
- Tichauer KM, Samkoe KS, Sexton KJ, Hextrum SK, Yang HH, Klubben WS, Gunn JR, Hasan T, Pogue BW. In vivo quantification of tumor receptor binding potential with dual-reporter molecular imaging. Mol Imaging Biol. 2012;14:584–92. doi: 10.1007/s11307-011-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkaczyk ER, Tkaczyk AH. Multiphoton flow cytometry strategies and applications. Cytometry A. 2011;79:775–88. doi: 10.1002/cyto.a.21110. [DOI] [PubMed] [Google Scholar]
- Tkaczyk ER, Zhong CF, Ye JY, Myc A, Thomas T, Cao Z, Duran-Struuck R, Luker KE, Luker GD, Norris TB, Baker JR. In Vivo Monitoring of Multiple Circulating Cell Populations Using Two-photon Flow Cytometry. Opt Commun. 2008;281:888–94. doi: 10.1016/j.optcom.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomosugi W, Matsuda T, Tani T, Nemoto T, Kotera I, Saito K, Horikawa K, Nagai T. An ultramarine fluorescent protein with increased photostability and pH insensitivity. Nat Methods. 2009;6:351–3. doi: 10.1038/nmeth.1317. [DOI] [PubMed] [Google Scholar]
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–44. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- Tuchin VV, Tarnok A, Zharov VP. In vivo flow cytometry: a horizon of opportunities. Cytometry A. 2011;79:737–45. doi: 10.1002/cyto.a.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507–18. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein PN, Sirota RL. Identification errors in pathology and laboratory medicine. Clin Lab Med. 2004;24:979–96. vii. doi: 10.1016/j.cll.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Wagar EA, Stankovic AK, Raab S, Nakhleh RE, Walsh MK. Specimen labeling errors: a Q-probes analysis of 147 clinical laboratories. Arch Pathol Lab Med. 2008;132:1617–22. doi: 10.5858/2008-132-1617-SLEAQA. [DOI] [PubMed] [Google Scholar]
- Wei X, Sipkins DA, Pitsillides CM, Novak J, Georgakoudi I, Lin CP. Real-time detection of circulating apoptotic cells by in vivo flow cytometry. Mol Imaging. 2005;4:415–6. doi: 10.2310/7290.2005.05148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Chen J, Ji SM, Cheng D, Liu ZH. Evaluation of vascular lesions using circulating endothelial cells in renal transplant patients. Clin Transplant. 2012;26:E344–50. doi: 10.1111/j.1399-0012.2012.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KH, Sandlin RD, Carey TR, Miller KL, Shank AT, Oklu R, Maheswaran S, Haber DA, Irimia D, Stott SL, Toner M. The Role of Physical Stabilization in Whole Blood Preservation. Sci Rep. 2016;6:21023. doi: 10.1038/srep21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Fan Z, Wu X, Xu M, Jiang J, Tan C, Wu W, Wei X, Zhou J. Circulating tumor cells are correlated with disease progression and treatment response in an orthotopic hepatocellular carcinoma model. Cytometry A. 2015;87:1020–8. doi: 10.1002/cyto.a.22782. [DOI] [PubMed] [Google Scholar]
- Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192:373–82. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zborowski M, Chalmers JJ. Rare cell separation and analysis by magnetic sorting. Anal Chem. 2011;83:8050–6. doi: 10.1021/ac200550d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Yan B, Sun Q, He S, Jiang J, Wen Z, Qu JY. In vivo micro-vascular imaging and flow cytometry in zebrafish using two-photon excited endogenous fluorescence. Biomed Opt Express. 2014;5:653–63. doi: 10.1364/BOE.5.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettergren E, Swamy T, Runnels J, Lin CP, Niedre M. Tomographic sensing and localization of fluorescently labeled circulating cells in mice in vivo. Phys Med Biol. 2012a;57:4627–41. doi: 10.1088/0031-9155/57/14/4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettergren E, Vickers D, Runnels J, Murthy SK, Lin CP, Niedre M. Instrument for fluorescence sensing of circulating cells with diffuse light in mice in vivo. J Biomed Opt. 2012b;17:037001. doi: 10.1117/1.JBO.17.3.037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharov VP, Galanzha EI, Tuchin VV. Integrated photothermal flow cytometry in vivo. J Biomed Opt. 2005a;10:051502. doi: 10.1117/1.2070167. [DOI] [PubMed] [Google Scholar]
- Zharov VP, Galanzha EI, Tuchin VV. Photothermal image flow cytometry in vivo. Opt Lett. 2005b;30:628–30. doi: 10.1364/ol.30.000628. [DOI] [PubMed] [Google Scholar]
- Zhong CF, Tkaczyk ER, Thomas T, Ye JY, Myc A, Bielinska AU, Cao Z, Majoros I, Keszler B, Baker JR, Norris TB. Quantitative two-photon flow cytometry--in vitro and in vivo. J Biomed Opt. 2008;13:034008. doi: 10.1117/1.2931077. [DOI] [PubMed] [Google Scholar]