Abstract
Background
Early subtle deficits in verbal memory, which may indicate early neural risk, are common in patients with coronary artery disease (CAD). While exercise can improve cognition, cognitive response to exercise is heterogeneous. Sphingolipids have been associated with the development and progression of CAD, and impairments in sphingolipid metabolism may play roles in neurodegeneration, and in the neural adaptation response to exercise. In this study, change in plasma concentrations of sphingolipids were assessed in relation to change in verbal memory performance and in other cognitive domains among CAD subjects undertaking a 6-month cardiac rehabilitation (CR) program.
Methods
Patients with CAD (n=120, mean age=64±6 years, 84% male, years of education=16±3 years) underwent CR with neuropsychological assessments and blood collected at baseline, 3-, and 6-months. Z-scores based on age, gender and education were combined for verbal memory, visuospatial memory, processing speed, executive function and global cognition tasks to calculate cognitive domain Z-scores. Plasma sphingolipid concentrations were measured from fasting blood samples using high performance liquid chromatography coupled electrospray ionization tandem mass spectrometry (LC/MS/MS). Mixed models were used to identify sphingolipids significantly associated with performance in verbal memory and other cognitive domains, adjusting for potential confounders.
Results
A decrease in ceramide C18:0 concentrations was significantly associated with improvement in verbal memory performance (b[SE]=-0.51 [0.25], p=0.04), visuospatial memory (b[SE]=-0.44 [0.22], p=0.05), processing speed (b[SE]=-0.89 [0.32], p=0.007) and global cognition (b[SE]=-1.47 [0.59], p=0.01) over 6 months of CR.
Conclusions
Plasma ceramide C18:0 concentrations may be a sensitive marker of cognitive response to exercise in patients with CAD.
Keywords: ceramides, memory, cognition, coronary artery disease, exercise, sphingolipids
Introduction
Coronary artery disease (CAD) is the leading cause of mortality and morbidity worldwide affecting as many as 1 in 3 individuals before the age of 70[1]. Patients with CAD are a cognitively at-risk population as evidenced by increased brain atrophy[2], white matter lesions[3-7] increased risk of memory impairment, and incipient neurodegenerative diseases that include mild cognitive impairment (MCI)[8-11], vascular dementia[12-15] and Alzheimer's disease (AD)[10, 12, 16, 17]. While CAD patients show disruptions in multiple cognitive domains[9, 18], subtle changes in verbal memory performance have been associated with mortality[19], physical disability[20], progression to dementia,[21] and interference with secondary prevention[22], suggesting that verbal memory may be a key marker of poor outcomes in patients with CAD.
Exercise is increasingly recognized as an effective intervention to improve cardiac outcomes[23] and has also been shown to delay cognitive decline[24-27]. Regular physical activity in healthy elderly individuals has been associated with reduced risk of MCI, AD, and other dementias[28-30]. Exercise interventions have also been associated with increasing brain volumes[31] and improved memory performance in older adults[26, 27]. However, the cognitive response to exercise can be heterogeneous[32] indicating a need to explore mechanisms that may hinder the cognitive benefits of exercise.
Alterations in circulating lipids are common features associated with the clinical presentations of CAD[33-37], and it has become increasingly recognized that similar perturbations in blood lipid composition are associated with neurodegenerative conditions[38-40] suggesting that dysregulations of lipid metabolic pathways may be fundamental mechanisms underlying cognitive risk in CAD. Sphingolipids have recently emerged as an especially promising class of lipids that have been repeatedly identified as prognostic and associative indicators of cognitive decline[41-47]. Higher peripheral blood sphingolipid concentrations including sphingomyelins, ceramides and lactosylceramides were significantly associated with greater declines in verbal memory, speed of processing, executive function and hippocampal volume loss in subjects with MCI[47], increased risk of AD[44], and faster cognitive progression among AD patients[45]. Recent investigations of post-mortem brain tissue suggest that an increase in ceramides may be a specific indicator of neurodegenerative and pathologic changes relative to other lipid classes[42]. In a targeted pilot study, we have shown preliminary associations between higher plasma ceramide concentrations and less improvement in verbal memory during cardiac rehabilitation (CR)[41]. Conversely, the anti-inflammatory and neuroprotective ceramide metabolite sphingosine-1-phosphate (S1P), has been suggested to promote hippocampal neurogenesis[48, 49] and ameliorate memory deficits in animal studies[50, 51]. Collectively, these findings suggest that circulating sphingolipids may be sensitive markers of early cognitive changes in CAD.
Although there is evidence to suggest that the pro-inflammatory[52] and pro-apoptotic[53] properties of most sphingolipid species and the neuroprotective S1P may have opposing effects on the neural adaptation to exercise and neurodegenerative processes[54], sphingolipids have not been comprehensively assessed as prognostic indicators of the cognitive response to exercise in CAD. In the present study we sought to determine if changes in circulating sphingolipids were associated with change in verbal memory performance in CAD subjects undertaking a 6-month CR program. Associations between sphingolipids and other cognitive domains including visuospatial memory, processing speed, executive function and global cognition were also explored.
Materials and Methods
Participants
This prospective panel study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board and the University Health Network Research Ethics Board. Written informed consent was obtained from all subjects before enrollment in the study. Eligible subjects who consented to participate in the study were assessed for inclusion/exclusion criteria. All participants had evidence of CAD (previous hospitalization for acute myocardial infarction, coronary angiographic evidence of ≥ 50% blockage in one or more major coronary artery or prior revascularization). All participants also had dyslipidemia and were being treated with statins. Subjects were excluded based on previously diagnosed neurodegenerative illnesses, active cancer, surgery planned within 12 months, schizophrenia, bipolar affective disorder and substance abuse. Subjects with probable dementia (Mini Mental Status Examination score<24[55]) were also excluded.
Cardiac rehabilitation (CR) program
The CR program comprised of both aerobic and resistance exercise under the supervision of exercise and medical specialists. Participants attended exercise visits that included an aerobic walk or walk/jog each week for 24 weeks. Participants were also expected to independently exercise 5 out of 7 days of the week. Cardiopulmonary fitness was assessed using a cycle ergometer (Ergoselect 200P, Ergoline, Bitz, Germany) symptom-limited graded exercise test and peak oxygen uptake per minute (VO2peak) was obtained at entry into the program and at 3 and 6 months.
Demographics and clinical characteristics
Demographic and clinical characteristics, as well as a detailed medical history including comorbidities independent of CAD, were collected from patient interviews. Cardiac medical history, concomitant medications, cardiac health indicators (body mass index, waist circumference, hyperlipidemia, hypertension, diabetes, waist circumference) and anthropometrics were obtained from patient charts at the Toronto Rehabilitation Institute –University Health Network. Hemoglobin A1c (HbA1c) and lipid profiles, including total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglyceride concentrations were measured using standard clinical assays. Apolipoprotein E (APOE) polymorphism was determined using restriction fragment length polymorphism polymerase chain reaction[56].
Cognitive testing
Cognitive performance was assessed using the 30-minute standardized battery of tests recommended by the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network (NINDS-CSN)[57] for the investigation of vascular cognitive impairment at baseline, 3 and 6 months. A trained researcher administered the battery at a standardized time (0930 hr ± 30 min) and participants refrained from eating or drinking any caffeine-containing beverages for at least 4 hours before testing. Verbal memory was assessed using the California Verbal Learning Test 2nd Ed. (CVLT-II). The CVLT-II yields multiple measures of verbal memory function including verbal learning (recall of a word list over 5 learning trials), short-delay free recall (recall of a word list after an interfering list) and long-delay free recall (recall of a word list after 20 minutes)[57]. Visuospatial memory was assessed using the Brief Visuospatial Memory Test-Revised (BVMT-R)[58], which yields a measure of visual learning and delayed recall. Speed of processing was assessed using the Trail-Making Test Part A[59] and the Digit Symbol-Coding task, a measure of complex attention and psychomotor speed from the Wechsler Adult Intelligence Scale 3rd Edition[60]. Measures of executive function included the Trail-Making Test Part B[59] and Stroop Color-Word Interference Test. The Montréal Cognitive Assessment (MoCA)[61] was used to assess global cognition. For each cognitive task, a Z-score was determined from age, gender and education matched norms. Z-scores of related tests were summed into composite Z-scores to reflect performance in a cognitive domain and to avoid multiple comparisons[62]. For verbal memory, Z-scores from the three CVLT-II outcomes (verbal learning, short- and long-delay free recall) were summed. For visuospatial memory, Z-scores for the two BVMT-R outcomes (visual learning and delayed recall) were summed. The sum of the Trail Making Test A and Digit Symbol-Coding task Z-scores were used to represent speed of processing while the Z-scores of the Trail Making Test B and Stroop Test were summed for executive function.
Sphingolipid measurements
At baseline, 3 and 6 months, fasting blood was drawn at 0900 h ± 30 min on the same day as the cognitive testing and centrifuged at 1000g for 10 min at 4°C. Plasma was immediately isolated and stored at −80°C until analysis. Quantification of 45 individual sphingolipid species was accomplished by high performance liquid chromatography coupled electrospray ionization tandem mass spectrometry (LC/MS/MS) using multiple reaction monitoring and processed by the Analyst 1.4.2 software package as previously described[63]. Briefly, a crude lipid extraction from plasma was performed using a modified Bligh and Dyer procedure (Avanti Polar Lipids, Alabaster, AL, USA). Plasma extracts were dried and then re-suspended in methanol before analysis. High performance liquid chromatography (PerkinElmer, MA, USA) with a reverse phase C18 column (Phenomenex, Torrance, CA, USA) was used for temporal resolution of compounds. The eluted samples were then injected into an electrospray ion source coupled to a triple-quadrupole mass spectrometer (API3000, AB Sciex Inc, Thornhill, Ontario, Canada) and analyses were conducted by multiple reaction monitoring. Eight-point calibration curves (0.1-1000 ng/mL) were constructed by plotting area under the curve for different sphingolipids, for each calibration standard normalized to the internal standard. Sphingolipid concentrations (ng/mL) were determined by fitting the identified species to standard curves based on acyl chain length. Instrument control and quantification of spectral data were performed using Analyst 1.4.2 and MultiQuant software (AB Sciex Inc, Thornhill, Ontario, Canada).
Statistical Analyses
Plasma sphingolipid measurements were skewed, so they were log-transformed prior to analyses as has been done before[41]. Mixed models were used to examine the time-varying relationship between plasma sphingolipids and cognition over the 6-month CR program. Bivariate associations between patient characteristics (fixed and time-varying) and change in verbal memory and other cognitive domains over CR, were assessed using mixed models. Patient characteristics were included as potential confounders in multivariate models if they were significantly associated with the cognitive domains.
Bivariate mixed models were first used to identify associations between VO2peak, verbal memory and other cognitive domain Z-scores over CR to determine change in cognitive performance with increasing fitness. Bivariate mixed models were also used to identify a panel of sphingolipids associated with verbal memory and other cognitive domains over CR. Because some of the sphingolipids may be correlated, multicollinearity was assessed (tolerance statistic<0.4) before multiple sphingolipid species were included in multivariate mixed models. For lipids that were collinear, only members of a correlated set that maintained the tolerance statistic above 0.4 were included in multivariate analyses. All analyses were finished using the MIXED procedure in SAS University Edition statistical software (SAS Institute Inc., North Carolina, USA) with the significance level set at a two-tailed p≤0.05.
Results
Patient characteristics
Demographics and clinical characteristics of the 120 CAD participants are reported in Table 1.
Table 1. Characteristics of study participants (n=120) over 6 months of cardiac rehabilitation (CR).
| Characteristic | CAD (n=120) Mean±SD or n (%) |
|---|---|
| Sociodemographics | |
| Age, years | 64±6 |
| Sex, male | 101 (84) |
| Ethnicity, Caucasian | 99 (83) |
| Marital status, married | 93 (78) |
| Years of education, years | 16±3 |
| Employed | 66 (55) |
| Smoking history, smoker or quit smoking | 74 (62) |
| APOE4 allele carrier | 33 (28) |
| Lipid Profile and HbA1c | |
| Low density lipoprotein (LDL; mmol/L) | |
| Baseline | 1.60±0.61 |
| 3 months | 1.72±0.66 |
| 6 months | 1.66±0.63 |
| High density lipoprotein (HDL; mmol/L) | |
| Baseline | 1.29±0.34 |
| 3 months | 1.33±0.34 |
| 6 months | 1.36±0.39 |
| Total cholesterol (mmol/L) | |
| Baseline | 3.49±0.83 |
| 3 months | 3.16±1.14 |
| 6 months | 3.56±0.81 |
| Triglycerides (mmol/L) | |
| Baseline | 1.31±0.74 |
| 3 months | 1.66±1.12 |
| 6 months | 1.31±1.47 |
| Hemoglobin A1c (HbA1c) | |
| Baseline | 0.059±0.007 |
| 3 months | 0.063±0.053 |
| 6 months | 0.067±0.081 |
| Body Composition | |
| Body mass index (kg/m2) | |
| Baseline | 29.2±5.1 |
| 3 months | 28.9±4.8 |
| 6 months | 28.3±5.1 |
| Body fat percentage | |
| Baseline | 31.7±10.5 |
| 3 months | 30.3±8.5 |
| 6 months | 28.5±8.3 |
| Body mass (kg) | |
| Baseline | 86.4±16.6 |
| 3 months | 85.0±16.3 |
| 6 months | 83.8±17.6 |
| Waist circumference (cm) | |
| Baseline | 99.2±12.2 |
| 3 months | 99.0±14.6 |
| 6 months | 96.9±12.8 |
| CAD Severity | |
| Cumulative stenosis, percent | 150.2±67.2 |
| Number of vessels stenosed | 2±1 |
| Time since acute coronary event, weeks | 22.3±43.4 |
| Cardiac History | |
| Myocardial infarction (MI) | 58 (48) |
| Coronary artery bypass graft surgery (CABG) | 39 (33) |
| Stent | 77 (64) |
| Angina | 9 (8) |
| Hypertension | 112 (93) |
| Comorbidities | |
| Diabetes | 20 (17) |
| Depression | 18 (15) |
| Hypercholesterolemia | 120 (100) |
| Cardiopulmonary Fitness Parameters | |
| Maximum heart rate (bpm) | |
| Baseline | 121.9±20.3 |
| 3 months | 128.6±20.1 |
| 6 months | 133.0±23.9 |
| Maximum systolic blood pressure (mm Hg) | |
| Baseline | 170.1±23.9 |
| 3 months | 171.7±23.7 |
| 6 months | 175.8±21.9 |
| Maximum diastolic blood pressure (mm Hg) | |
| Baseline | 78.4±11.0 |
| 3 months | 76.7±9.9 |
| 6 months | 77.9±9.4 |
| Peak oxygen consumption (VO2 peak; mL/kg/min) | |
| Baseline | 20.9±5.6 |
| 3 months | 24.5±6.6 |
| 6 months | 26.8±7.2 |
| Medications | |
| β-adrenergic receptor blockers | 96 (80) |
| Diuretics | 19 (16) |
| Anti-hypertensive | |
| Angiotensin-converting enzyme inhibitors | 63 (53) |
| Angiotensin II receptor blockers | 23 (19) |
| Calcium channel blocker | 16 (13) |
| Antidiabetic | 16 (13) |
| Antioxidants | 17 (14) |
| Platelet inhibitors | 116 (97) |
| Statins | |
| High dose1 | 74 (63) |
| Low dose | 43 (37) |
High statin dose was defined as atorvastatin, pravastatin, or fluvastatin 40–80 mg/day and rosuvastatin or simvastatin 20–40 mg/day. Low dose statin was defined as 10–20 mg/day and 5–10 mg/day of those medications, respectively.
Bivariate associations between clinical characteristics and verbal memory
A higher level of education (b=0.09, p<0.001) and reduction in waist circumference (b=-0.01, p=0.02) over CR were associated with better verbal memory performance over time. Absence of the APOE4 allele (b=0.26, p=0.04) and not taking antiplatelet drugs (b=0.64, p=0.04) were also associated with better verbal memory performance over CR. There were no other significant associations between change in verbal memory and other sociodemographic characteristics, cardiac risk factors, CAD severity, medical comorbidities, cardiopulmonary fitness parameters or concomitant medications used.
Bivariate associations between clinical characteristics and other cognitive domains
Improvement in visuospatial memory was associated with a higher level of education (b=0.05, p=0.003) and high dose statin therapy (b=0.25, p=0.03). Unmarried participants (b=0.31, p=0.02) also showed improvements in visuospatial memory over CR while participants who did not have a stent worsened in visuospatial memory over CR (b=-0.34, p=0.004). Participants with longer time since the acute coronary event at time of assessment also exhibited worse visuospatial memory over CR (b=-0.003, p=0.02). Improvement in processing speed was associated with a higher level of education (b=0.06, p<0.001), absence of diabetes (b=0.5, p=0.001), use of antidiabetic agent (b=0.44, p=0.01) and not being employed (b=0.25, p=0.03). Participants not taking antioxidants (b=0.38, p=0.01) nor platelet inhibitors (b=1.01, p=0.001) also showed improvements in processing speed over CR. Participants who were not Caucasian (b=-0.66, p<0.001) and those who did not have a stent (b=-0.38, p=0.001) showed worsening in processing speed over CR. Improvement in executive function was associated with a higher level of education (b=0.08, p<0.001), absence of both diabetes (b=0.30, p=0.05) and hypertension (b=0.66, p=0.005), not being married (b=0.40, p=0.004), not being employed (b=0.36, p=0.001) and higher serum HDL concentrations (b=0.26, p=0.03). Participants who were not taking antiplatelet drugs (b=0.86, p=0.005) also showed improvement in executive function over CR while non-Caucasian participants (b=-0.67, p<0.001), those with no angina (b=-0.57, p=0.005) and those not taking diuretics (b=-0.36, p=0.02) showed worsening executive function over CR. A higher level of education (b=0.20, p<0.001) and an increase in weight over CR (b=0.02, p=0.03) was associated with improvement in global cognition as measured by MoCA score. Participants on high dose statin therapy (b=0.67, p=0.03) and those not taking β-adrenergic receptor blockers (b=0.75, p=0.04) also showed improvement in MoCA score over CR while non-Caucasian participants (b=-2.39, p<0.001) and those with a higher number of stenosed vessels (b=-0.35, p=0.05) showed a decline in the MoCA score over CR.
Association between cardiopulmonary fitness and cognitive performance over CR
An increase in VO2peak over CR was significantly associated with improvements in verbal memory performance (b=0.02, p=0.05), processing speed (b=0.03, p=0.001) and executive function (b=0.02, p=0.05) but not in visual memory (b=0.01, p=0.37) nor in global cognition as measured by the MoCA score (b=0.03, p=0.16) in bivariate associations. In multivariate models, increasing fitness was only associated with improved processing speed (b(SE) = 0.02 (0.009), p=0.04).
Identification of a sphingolipid profile associated with cognitive response to CR
As shown in Figure 1, bivariate associations using mixed models identified 9 species that were significantly associated with change in verbal memory performance over CR. These species included sphingomyelins C16:0 (b=1.53, p=0.05), C20:1 (b=1.22, p=0.01), and C24:1 (b=1.41, p=0.02), dihydrosphingomyelins C16:0 (b=-0.88, p=0.03) and C20:0 (b=-1.39, p=0.007), ceramide C18:0 (b=-0.67, p=0.002), lactosylceramides C18:1 (b=1.06, p=0.002) and C24:1 (b=0.70, p=0.02) and S1P (b=-0.37, p=0.02). Figure 1 also shows sphingolipids associated with changes in visuospatial memory, processing speed, executive function and global cognition over CR. Notably, of all the sphingolipids, ceramide C18:0 was most robustly associated with changes in all the cognitive domains over CR.
Figure 1.
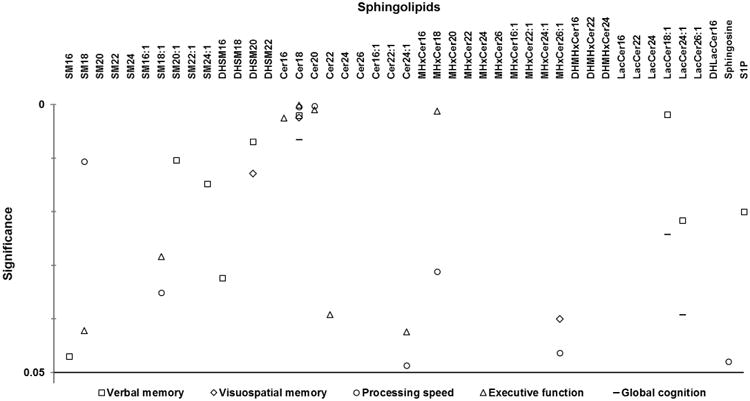
Sphingolipids associated with change in cognitive domains over 6 months of cardiac rehabilitation in patients with coronary artery disease. Symbols denote p-values < 0.05 from bivariate associations using mixed models between cognitive domain Z-scores and log sphingolipid concentrations over cardiac rehabilitation; all identified species were included in multivariate mixed models. For Cer18, overlapping symbols in the top cluster include a circle representing processing speed and a triangle representing executive function, and the two overlapping symbols in the bottom cluster include a square representing verbal memory and a diamond representing visuospatial memory. For Cer20, overlapping symbols include a circle representing processing speed and a triangle representing executive function. Abbreviations: SM, sphingomyelin; DHSM, dihydrosphingomyelin; Cer, ceramide; MHxCer, monohexylceramide; DHMHxCer, dihydromonohexylceramide; LacCer, lactosylceramide; DHLacCer, dihyrdolactosylceramide; S1P, sphingosine-1-phosphate.
Multivariate models predicting cognitive performance over CR
We focused on sphingolipid species significantly associated with change in memory and other cognitive domains over CR, which were not collinear in multivariate analyses. Over the 6-month CR program, in a model including sphingomyelin C16:0, sphingomyelin C20:1, sphingomyelin C24:1, dihydrosphingomyelin C20:0, lactosylceramide C18:1, lactosylceramide C24:1, total years of education, waist circumference, presence of APOE4 allele and VO2peak, each log-unit decrease in ceramide C18:0 was associated with a 0.51 standard deviation improvement in verbal memory performance (b[SE]=-0.51 [0.25], p=0.04) and each log-unit decrease in S1P was associated with a 0.53 standard deviation improvement in verbal memory performance (b[SE]=-0.53 [0.17], p=0.002) (Table 2). As shown in Table 3, a decrease in ceramide C18:0 over the 6-month CR was associated with improvements in visuospatial memory (b[SE]=-0.44[0.22], p=0.05), processing speed (b[SE]=-0.89[0.32], p=0.007) and MoCA score (b[SE]=-1.47[0.59], p=0.01). There was also a trend towards a decrease in ceramide C18:0 and improvement in executive function over CR (b[SE]=-0.59[0.31], p=0.06). Ceramide C18:0 (b=-0.006, p=0.005) levels were inversely associated with increases in VO2peak over CR in bivariate analyses (Supplementary Table 1).
Table 2. Multivariate mixed model showing the association between change in sphingolipids of interest and change in verbal memory domain Z-score over 6 months of cardiac rehabilitation (CR) in patients with coronary artery disease (CAD).
| Variable | b (SE) | p-value (p≤0.05)* |
|---|---|---|
| Years of education | 0.08 (0.02) | <0.0001* |
| Waist circumference | 0.0005 (0.005) | 0.91 |
| APOE4 allele | 0.19 (0.14) | 0.06 |
| VO2peak | 0.003 (0.009) | 0.76 |
| Sphingomyelin C16:0 | 0.76 (1.14) | 0.51 |
| Sphingomyelin C20:1 | 0.95 (0.65) | 0.07 |
| Sphingomyelin C24:1 | -0.46 (0.83) | 0.58 |
| Dihydrosphingomyelin C20:0 | -1.13 (0.63) | 0.07 |
| Ceramide C18:0 | -0.51 (0.25) | 0.04* |
| Lactosylceramide C18:1 | 0.006 (0.48) | 0.99 |
| Lactosylceramide C24:1 | 0.85 (0.45) | 0.06 |
| Sphingosine-1-phosphate | -0.53 (0.17) | 0.002* |
Table 3. Multivariate mixed models showing associations between change in ceramide C18:0 and change in 4 cognitive domain Z-scores over 6 months of cardiac rehabilitation (CR) in patients with coronary artery disease (CAD).
| Cognitive domain (outcome variable) | Ceramide C18:0 | |
|---|---|---|
| b (SE) | p-value (p≤0.05)* | |
| Visuospatial memory2 | -0.44 (0.22) | 0.05* |
| Processing speed3 | -0.89 (0.32) | 0.007* |
| Executive function4 | -0.59 (0.31) | 0.06 |
| Global cognition (MoCA score)5 | -1.47 (0.59) | 0.01* |
model also included years of education, stent procedure, statin dose, dihydrosphingomyelin C20:0 and monohexylceramide C26:1
model also included years of education, ethnicity, stent procedure, diabetes, VO2peak, sphingomyelin C18:1, ceramide C22:0, ceramide C24:1, monohexylceramide C18:0, monohexylceramide C26:1 and sphingosine
model also included years of education, ethnicity, diabetes, VO2peak, serum HDL concentration, sphingomyelin C18:1, ceramide C22:0, ceramide C24:1 and monohexylceramide C18:0
model also included years of education, ethnicity, weight, β-adrenergic receptor blocker use, statin dose, lactosylceramide C18:1and lactosylceramide C24:1
Discussion
This study assessed the relationships between plasma sphingolipids and cognition over a 6-month CR program among patients with CAD. Decreasing plasma ceramide C18:0 concentration was consistently associated with improved performance in verbal memory, visuospatial memory, processing speed and global cognition as well as an increase in VO2peak over CR, suggesting that plasma ceramide C18:0 may be a surrogate measure of the cognitive response to exercise.
Overall, participants experienced a subtle yet significant improvement in processing speed performance with increasing fitness over the course of the 6-month CR program. Previous findings suggest that certain cognitive domains, such as processing speed and attention[64, 65], may be more amenable to exercise effects; however, effects of exercise on cognition have not been consistent[66]. While adaptations to exercise are incompletely understood, they are associated with markers of neurogenesis and angiogenesis in patients with CAD[67]. These processes may underlie increases in gray and white matter volumes[31, 68] as well as cerebral blood flow[69, 70], which may partly mediate exercise-induced cognitive improvement[71, 72].
We hypothesized that sphingolipids may be interfering with exercise-induced improvements in cognitive performance due to their role in inducing inflammatory signals and damaging neural progenitor cells directly by activating apoptotic cascades[73] in patients with CAD. In the present study, bivariate analyses identified associations between several sphingolipid species and cognitive performance in CAD patients undergoing an exercise intervention (Figure 1). While mechanisms underlying the physiological effects of sphingolipids remain unclear, several species identified in this study have been consistently associated with metabolic disease[74-76] and neurodegenerative disorders[40, 44, 47]. Glycosphingolipids, including monohexylceramides and lactosylceramides have been previously associated with atherosclerosis and arterial stiffness in animal studies[74]. In particular, lactosylceramides have been associated with increased risk of AD[44]. Long-chain (C16:0 and C18:0) and very long-chain (C24:1) ceramides are higher in obese individuals and patients with diabetes[75, 77, 78] and accumulation of these species in the skeletal muscle is inversely associated with insulin sensitivity[75] which plays an important role in the development of AD[79]. High HbA1c levels consistent with a diagnosis of prediabetes and diabetes in the present study suggest ceramide-mediated insulin insensitivity as an important mechanism underlying cognitive decline in CAD. Long-chain and very long-chain ceramides have also been linked to systemic metabolic health (C16:0, C18:0)[76], depression (C16:0, C18:0, C20:0, C24:1)[80], cognitive impairment in Parkinson's disease (C16:0, C18:0, C20:0, C22:0, C24:1)[40], hippocampal volume loss in MCI (C22:0)[47] and increased risk of AD (C16:0)[44]. Preliminary findings from a pilot study also showed an association between higher ceramide C22:0 and C24:0 and less improvement in verbal memory in CAD patients undertaking CR[41]. These published data combined with the findings from this study suggest that long-chain ceramides, very long-chain ceramides and glycosphingolipids of certain chain lengths may be especially relevant to cognitive changes in CAD.
Our results suggest that ceramide C18:0 is most significantly and robustly associated with the cognitive response to exercise in CAD patients. These results are consistent with other studies suggesting that ceramide C18:0 may be of etiopathological importance in AD[81]. Recently, ceramide C18:0 was shown to be positively associated with amyloid-β and tau in cognitively normal individuals with a confirmed parental history of AD[46]. Those associations were stronger in individuals aged 54 years or older, consistent with the age range in this study of another at-risk population.
A significant association between decreasing ceramide C18:0 and increasing fitness over CR suggests that ceramide metabolism may be responsive to exercise. Previously, better cardiopulmonary fitness was associated with lower plasma ceramide concentrations in healthy elderly subjects[82] and muscle ceramide concentrations were decreased following chronic aerobic exercise[83, 84]. The present study shows similar effect of exercise in CAD, a disease characterized by aberrant ceramide metabolism. Plasma ceramides were also shown to be reduced following 12 weeks of aerobic exercise training in obese individuals and those with diabetes[85]. A decrease in ceramide concentrations imply increased insulin sensitivity[78], which may partly contribute to the cognitive benefits of exercise.
Exercise may influence ceramide production through various mechanisms. Exercise training has been shown to reduce plasma inflammatory markers including tumor necrosis factor[78], a key inducer of ceramide synthesis[86]. Reduced inflammatory signaling with exercise has been associated with decreased de novo synthesis of ceramides[87]. Lipid utilization during exercise has also been hypothesized to reduce ceramide production by reducing the availability of substrates needed for ceramide synthesis[83, 84]. In addition, exercise may induce ceramide degradation and clearance by increasing the expression of genes responsible for ceramide clearance, including acidic and alkaline ceramidase 1 and 3, glucosylceramide synthase, and sphingosine kinase 1[78].
Exercise also induces the release of S1P[88]. S1P concentrations increase in blood as a result of release from erythrocytes, muscular tissue and vascular endothelium following exercise[89], inducing VEGF receptor activation[90], angiogenesis, neural progenitor cell proliferation and morphogenesis and long term potentiation[73]. However, change in S1P was not associated with cardiopulmonary fitness and improvement in verbal memory was associated with a decrease in S1P over CR. It is possible that plasma S1P concentrations are inversely related to cerebrospinal fluid concentrations as previously suggested for other sphingolipids[47]. Decreasing plasma concentrations over the course of CR may be indicative of higher S1P concentrations in the brain, which has been previously associated with hippocampal neurogenesis and memory improvement[48-51]. Future studies should examine plasma- cerebrospinal fluid correlations to further understand the utility of plasma S1P concentrations as markers of cognitive response to exercise.
This study was strengthened by a methodologically robust analysis of sphingolipids to determine species associated with cognitive response to exercise. The use of a rigorous statistical pipeline including use of composite Z-scores representing important cognitive domains and use of mixed models that allowed for modeling of the complex temporal relationships between cognitive outcomes, sphingolipids, effects of exercise and important clinical confounders also strengthened the findings. The use of an objective measurement of fitness was also a strength of this study.
This study was limited by the lack of a control group; as such, independent influence of CAD and exercise on cognitive outcomes and sphingolipids could not be determined. However, CAD patients who have aberrant lipid metabolism and who are as of yet free of overt cognitive impairment but show early subtle changes in cognitive function[22, 91] are an ideal population to study these associations as they may be representative of a preclinical stage. Future randomized prospective trials are needed to clarify the direction of cause and effects of these results. Practice effects may have contributed to the overall improvement in verbal memory and other cognitive outcomes; however, a 3-month interval between testing would be expected to minimize such effects on tests of verbal memory[92]. The study sample included participants who were referred to and agreed to enter CR, which may have introduced selection bias. In addition, the study sample was predominantly Caucasian, male and highly educated, which may not be representative of all CAD patients. However, the study population was representative of CR participants[93-96] and CR is a standard of care, which may contribute to the generalizability of the results to CAD patients undertaking CR.
Future work should look at associations between S1P and cognition in this population to further elucidate mechanisms underlying exercise-induced neural adaptation and to better develop blood sphingolipids as clinically useful markers of early cognitive changes.
Conclusions
In CAD patients undertaking CR, lower plasma ceramide C18:0 was associated with improvements in verbal memory, visuospatial memory, processing speed and global cognition. Bivariate analysis showed that ceramide C18:0 decreased with increasing fitness over CR. These findings suggest that ceramide C18:0 (and possibly other related sphingolipids) may be modulated by lifestyle modifications such as exercise, and underscores the importance of exercise in preserving cognitive function in an at-risk population such as individuals with CAD.
Supplementary Material
Supplementary Table 1. Bivariate mixed models showing associations between peak oxygen consumption (VO2peak) and log plasma concentrations of sphingolipids associated with change in cognitive performance over 6 months of cardiac rehabilitation (CR) in patients with coronary artery disease (CAD)
Acknowledgments
This study was supported by a research grant from the Canadian Institutes of Health Research (LanctotMOP-114913). MS was supported by a doctoral grant from the Alzheimer's Society of Canada. MM was supported by grants from the National Institute on Aging (U01 AG37526, R01 AG49704). NJH is supported by the National Institutes of Health (MH105280, MH075673, DA040390, MH096630 and MH110246).
Footnotes
Conflict of Interest: The authors have no conflict of interest to report.
References
- 1.The Stationary Office (UK Government) London: 1998. [Google Scholar]
- 2.Koschack J, Irle E. Small hippocampal size in cognitively normal subjects with coronary artery disease. Neurobiol Aging. 2005;26:865–871. doi: 10.1016/j.neurobiolaging.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Andrell P, Jensen C, Norrsell H, Ekre O, Ekholm S, Norrsell U, Eliasson T, Mannheimer C, Blomstrand C. White matter disease in magnetic resonance imaging predicts cerebral complications after coronary artery bypass grafting. Ann Thorac Surg. 2005;79:74–79. doi: 10.1016/j.athoracsur.2004.06.085. discussion 79-80. [DOI] [PubMed] [Google Scholar]
- 4.Emmrich P, Hahn J, Ogunlade V, Geiger K, Schober R, Mohr FW. Neuropathological findings after cardiac surgery-retrospective study over 6 years. Z Kardiol. 2003;92:925–937. doi: 10.1007/s00392-003-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim BJ, Lee SH, Kim CK, Ryu WS, Kwon HM, Choi SY, Yoon BW. Advanced coronary artery calcification and cerebral small vessel diseases in the healthy elderly. Circ J. 2011;75:451–456. doi: 10.1253/circj.cj-10-0762. [DOI] [PubMed] [Google Scholar]
- 6.Rapp MA, Rieckmann N, Lessman DA, Tang CY, Paulino R, Burg MM, Davidson KW. Persistent depressive symptoms after acute coronary syndrome are associated with compromised white matter integrity in the anterior cingulate: a pilot study. Psychother Psychosom. 2010;79:149–155. doi: 10.1159/000286959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geerlings MI, Appelman AP, Vincken KL, Algra A, Witkamp TD, Mali WP, van der Graaf Y. Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis. 2010;210:130–136. doi: 10.1016/j.atherosclerosis.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinkers DJ, Stek ML, van der Mast RC, de Craen AJ, Le Cessie S, Jolles J, Westendorp RG, Gussekloo J. Generalized atherosclerosis, cognitive decline, and depressive symptoms in old age. Neurology. 2005;65:107–112. doi: 10.1212/01.wnl.0000167544.54228.95. [DOI] [PubMed] [Google Scholar]
- 10.Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. Bmj. 1994;308:1604–1608. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima LM, Carvalho M, Ferreira CN, Fernandes AP, Neto CP, Garcia JC, Reis HJ, Janka Z, Palotas A, Sousa M. Atheromatosis extent in coronary artery disease is not correlated with apolipoprotein-E polymorphism and its plasma levels, but associated with cognitive decline. Curr Alzheimer Res. 2010;7:556–563. doi: 10.2174/156720510792231711. [DOI] [PubMed] [Google Scholar]
- 12.Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 13.Haratz S, Weinstein G, Molshazki N, Beeri MS, Ravona-Springer R, Marzeliak O, Goldbourt U, Tanne D. Impaired Cerebral Hemodynamics and Cognitive Performance in Patients with Atherothrombotic Disease. J Alzheimers Dis. 2015 doi: 10.3233/JAD-150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santiago C, Herrmann N, Swardfager W, Saleem M, Oh PI, Black SE, Lanctot KL. White Matter Microstructural Integrity Is Associated with Executive Function and Processing Speed in Older Adults with Coronary Artery Disease. Am J Geriatr Psychiatry. 2014 doi: 10.1016/j.jagp.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Wardlaw JM, Allerhand M, Doubal FN, Valdes Hernandez M, Morris Z, Gow AJ, Bastin M, Starr JM, Dennis MS, Deary IJ. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology. 2014;82:1331–1338. doi: 10.1212/WNL.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61:403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 17.Justin BN, Turek M, Hakim AM. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135–145. doi: 10.2147/CLEP.S30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxton J, Ratcliff G, Newman A, Belle S, Fried L, Yee J, Kuller L. Cognitive test performance and presence of subclinical cardiovascular disease in the cardiovascular health study. Neuroepidemiology. 2000;19:312–319. doi: 10.1159/000026270. [DOI] [PubMed] [Google Scholar]
- 19.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- 20.Elias MF, Dore GA, Davey A, Robbins MA, Elias PK. From blood pressure to physical disability: the role of cognition. Hypertension. 2010;55:1360–1365. doi: 10.1161/HYPERTENSIONAHA.110.149823. [DOI] [PubMed] [Google Scholar]
- 21.Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, Michel B, Puel M, Volteau M, Touchon J, Verny M, Dubois B. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69:1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 22.Swardfager W, Herrmann N, Marzolini S, Oh PI, Saleem M, Shammi P, Kiss A, Cappell J, Lanctot KL. Verbal Memory Performance and Completion of Cardiac Rehabilitation in Patients With Coronary Artery Disease. Psychosom Med. 2011 doi: 10.1097/PSY.0b013e318227fff9. [DOI] [PubMed] [Google Scholar]
- 23.Giallauria F, Lucci R, D'Agostino M, Vitelli A, Maresca L, Mancini M, Aurino M, Del Forno D, Giannuzzi P, Vigorito C. Two-year multicomprehensive secondary prevention program: favorable effects on cardiovascular functional capacity and coronary risk profile after acute myocardial infarction. J Cardiovasc Med (Hagerstown) 2009;10:772–780. doi: 10.2459/JCM.0b013e32832d55fe. [DOI] [PubMed] [Google Scholar]
- 24.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanek KM, Gunstad J, Spitznagel MB, Waechter D, Hughes JW, Luyster F, Josephson R, Rosneck J. Improvements in cognitive function following cardiac rehabilitation for older adults with cardiovascular disease. Int J Neurosci. 2011;121:86–93. doi: 10.3109/00207454.2010.531893. [DOI] [PubMed] [Google Scholar]
- 26.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 28.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 29.Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, Harris TB. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72:2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middleton LE, Manini TM, Simonsick EM, Harris TB, Barnes DE, Tylavsky F, Brach JS, Everhart JE, Yaffe K. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171:1251–1257. doi: 10.1001/archinternmed.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 32.Swardfager W, Herrmann N, Marzolini S, Saleem M, Kiss A, Shammi P, Oh PI, Lanctot KL. Cardiopulmonary Fitness Is Associated with Cognitive Performance in Patients with Coronary Artery Disease. J Am Geriatr Soc. 2010 doi: 10.1111/j.1532-5415.2010.02966.x. [DOI] [PubMed] [Google Scholar]
- 33.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 34.Ichi I, Nakahara K, Miyashita Y, Hidaka A, Kutsukake S, Inoue K, Maruyama T, Miwa Y, Harada-Shiba M, Tsushima M, Kojo S, Kisei Cohort Study G. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859–863. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- 35.Kinnunen PK, Holopainen JM. Sphingomyelinase activity of LDL: a link between atherosclerosis, ceramide, and apoptosis? Trends Cardiovasc Med. 2002;12:37–42. doi: 10.1016/s1050-1738(01)00143-8. [DOI] [PubMed] [Google Scholar]
- 36.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 37.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 38.Vasantharekha R, Priyanka HP, Swarnalingam T, Srinivasan AV, ThyagaRajan S. Interrelationship between Mini-Mental State Examination scores and biochemical parameters in patients with mild cognitive impairment and Alzheimer's disease. Geriatr Gerontol Int. 2016 doi: 10.1111/ggi.12957. [DOI] [PubMed] [Google Scholar]
- 39.Anstey KJ, Ashby-Mitchell K, Peters R. J Alzheimers Dis. 2016. Updating the Evidence on the Association between Serum Cholesterol and Risk of Late-Life Dementia: Review and Meta-Analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mielke MM, Maetzler W, Haughey NJ, Bandaru VV, Savica R, Deuschle C, Gasser T, Hauser AK, Graber-Sultan S, Schleicher E, Berg D, Liepelt-Scarfone I. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson's disease and associated with cognitive impairment: a pilot study. PLoS One. 2013;8:e73094. doi: 10.1371/journal.pone.0073094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleem M, Ratnam Bandaru VV, Herrmann N, Swardfager W, Mielke MM, Oh PI, Shammi P, Kiss A, Haughey NJ, Rovinski R, Lanctot KL. Ceramides predict verbal memory performance in coronary artery disease patients undertaking exercise: a prospective cohort pilot study. BMC Geriatr. 2013;13:135. doi: 10.1186/1471-2318-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han X. Multi-dimensional mass spectrometry-based shotgun lipidomics and the altered lipids at the mild cognitive impairment stage of Alzheimer's disease. Biochim Biophys Acta. 2010;1801:774–783. doi: 10.1016/j.bbalip.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010;31:17–24. doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mielke MM, Bandaru VV, Haughey NJ, Xia J, Fried LP, Yasar S, Albert M, Varma V, Harris G, Schneider EB, Rabins PV, Bandeen-Roche K, Lyketsos CG, Carlson MC. Serum ceramides increase the risk of Alzheimer disease: The Women's Health and Aging Study II. Neurology. 2012 doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mielke MM, Haughey NJ, Bandaru VV, Weinberg DD, Darby E, Zaidi N, Pavlik V, Doody RS, Lyketsos CG. Plasma sphingomyelins are associated with cognitive progression in Alzheimer's disease. J Alzheimers Dis. 2011;27:259–269. doi: 10.3233/JAD-2011-110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mielke MM, Haughey NJ, Bandaru VV, Zetterberg H, Blennow K, Andreasson U, Johnson SC, Gleason CE, Blazel HM, Puglielli L, Sager MA, Asthana S, Carlsson CM. Cerebrospinal fluid sphingolipids, beta-amyloid, and tau in adults at risk for Alzheimer's disease. Neurobiol Aging. 2014;35:2486–2494. doi: 10.1016/j.neurobiolaging.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mielke MM, Haughey NJ, Ratnam Bandaru VV, Schech S, Carrick R, Carlson MC, Mori S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimers Dement. 2010;6:378–385. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Y, Hong F, Zhang L, Feng L. The sphingosine-1-phosphate analogue, FTY-720, promotes the proliferation of embryonic neural stem cells, enhances hippocampal neurogenesis and learning and memory abilities in adult mice. Br J Pharmacol. 2016;173:2793–2807. doi: 10.1111/bph.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Efstathopoulos P, Kourgiantaki A, Karali K, Sidiropoulou K, Margioris AN, Gravanis A, Charalampopoulos I. Fingolimod induces neurogenesis in adult mouse hippocampus and improves contextual fear memory. Transl Psychiatry. 2015;5:e685. doi: 10.1038/tp.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asle-Rousta M, Oryan S, Ahmadiani A, Rahnema M. Activation of sphingosine 1-phosphate receptor-1 by SEW2871 improves cognitive function in Alzheimer's disease model rats. EXCLI J. 2013;12:449–461. [PMC free article] [PubMed] [Google Scholar]
- 51.Hemmati F, Dargahi L, Nasoohi S, Omidbakhsh R, Mohamed Z, Chik Z, Naidu M, Ahmadiani A. Neurorestorative effect of FTY720 in a rat model of Alzheimer's disease: comparison with memantine. Behav Brain Res. 2013;252:415–421. doi: 10.1016/j.bbr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Kim MY, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991;266:484–489. [PubMed] [Google Scholar]
- 53.Arboleda G, Morales LC, Benitez B, Arboleda H. Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev. 2009;59:333–346. doi: 10.1016/j.brainresrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Xing HR, Kolesnick R. Kinase suppressor of Ras signals through Thr269 of c-Raf-1. J Biol Chem. 2001;276:9733–9741. doi: 10.1074/jbc.M008096200. [DOI] [PubMed] [Google Scholar]
- 55.Cacciatore F, Abete P, Maggi S, Luchetti G, Calabrese C, Viati L, Leosco D, Ferrara N, Vitale DF, Rengo F. Disability and 6-year mortality in elderly population. Role of visual impairment. Aging Clin Exp Res. 2004;16:382–388. doi: 10.1007/BF03324568. [DOI] [PubMed] [Google Scholar]
- 56.Zivelin A, Rosenberg N, Peretz H, Amit Y, Kornbrot N, Seligsohn U. Improved method for genotyping apolipoprotein E polymorphisms by a PCR-based assay simultaneously utilizing two distinct restriction enzymes. Clin Chem. 1997;43:1657–1659. [PubMed] [Google Scholar]
- 57.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 58.Benedict RH, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological Assessment. 1996;8:9. [Google Scholar]
- 59.Gaudino EA, Geisler MW, Squires NK. Construct validity in the Trail Making Test: what makes Part B harder? J Clin Exp Neuropsychol. 1995;17:529–535. doi: 10.1080/01688639508405143. [DOI] [PubMed] [Google Scholar]
- 60.Joy S, Kaplan E, Fein D. Speed and memory in the WAIS-III Digit Symbol--Coding subtest across the adult lifespan. Arch Clin Neuropsychol. 2004;19:759–767. doi: 10.1016/j.acn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 62.Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64:1323–1329. doi: 10.1001/archneur.64.9.1323. [DOI] [PubMed] [Google Scholar]
- 63.Mielke MM, Bandaru VV, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ. Factors affecting longitudinal trajectories of plasma sphingomyelins: the Baltimore Longitudinal Study of Aging. Aging Cell. 2015;14:112–121. doi: 10.1111/acel.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Bherer L. Cognitive plasticity in older adults: effects of cognitive training and physical exercise. Ann N Y Acad Sci. 2015;1337:1–6. doi: 10.1111/nyas.12682. [DOI] [PubMed] [Google Scholar]
- 66.Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015:CD005381. doi: 10.1002/14651858.CD005381.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swardfager W, Herrmann N, Marzolini S, Saleem M, Shammi P, Oh PI, Albert PR, Daigle M, Kiss A, Lanctot KL. Brain derived neurotrophic factor, cardiopulmonary fitness and cognition in patients with coronary artery disease. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, Volker K, Ho HV, Mooren F, Knecht S, Floel A. Physical activity and memory functions: An interventional study. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, Rejeski WJ. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 72.Luk TH, Dai YL, Siu CW, Yiu KH, Li SW, Fong B, Wong WK, Tam S, Tse HF. Association of Lower Habitual Physical Activity Level With Mitochondrial and Endothelial Dysfunction in Patients With Stable Coronary Artery Disease. Circ J. 2012 doi: 10.1253/circj.cj-12-0364. [DOI] [PubMed] [Google Scholar]
- 73.Kanno T, Nishizaki T, Proia RL, Kajimoto T, Jahangeer S, Okada T, Nakamura S. Regulation of synaptic strength by sphingosine 1-phosphate in the hippocampus. Neuroscience. 2010;171:973–980. doi: 10.1016/j.neuroscience.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 74.Chatterjee S, Bedja D, Mishra S, Amuzie C, Avolio A, Kass DA, Berkowitz D, Renehan M. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E-/- mice and rabbits fed a high-fat and -cholesterol diet. Circulation. 2014;129:2403–2413. doi: 10.1161/CIRCULATIONAHA.113.007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, Sifuentes AJ, McDonald JG, Gordillo R, Scherer PE. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab. 2015;22:266–278. doi: 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Kuo MS, Perreault L. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab. 2015;309:E398–408. doi: 10.1152/ajpendo.00134.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Wei T, Thomas MK, Kuo MS, Perreault L. Muscle sphingolipids during rest and exercise: a C18:0 signature for insulin resistance in humans. Diabetologia. 2016;59:785–798. doi: 10.1007/s00125-015-3850-y. [DOI] [PubMed] [Google Scholar]
- 79.Arrieta-Cruz I, Gutierrez-Juarez R. The Role of Insulin Resistance and Glucose Metabolism Dysregulation in the Development of Alzheimer s Disease. Rev Invest Clin. 2016;68:53–58. [PubMed] [Google Scholar]
- 80.Gracia-Garcia P, Rao V, Haughey NJ, Ratnam Banduru VV, Smith G, Rosenberg PB, Lobo A, Lyketsos CG, Mielke MM. Elevated plasma ceramides in depression. J Neuropsychiatry Clin Neurosci. 2011;23:215–218. doi: 10.1176/appi.neuropsych.23.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Filippov V, Song MA, Zhang K, Vinters HV, Tung S, Kirsch WM, Yang J, Duerksen-Hughes PJ. Increased ceramide in brains with Alzheimer's and other neurodegenerative diseases. J Alzheimers Dis. 2012;29:537–547. doi: 10.3233/JAD-2011-111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fabbri E, Yang A, Simonsick EM, Chia CW, Zoli M, Haughey NJ, Mielke MM, Ferrucci L, Coen PM. Circulating ceramides are inversely associated with cardiorespiratory fitness in participants aged 54-96 years from the Baltimore Longitudinal Study of Aging. Aging Cell. 2016;15:825–831. doi: 10.1111/acel.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJ, Dyck DJ. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291:E99–E107. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- 84.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kasumov T, Solomon TP, Hwang C, Huang H, Haus JM, Zhang R, Kirwan JP. Improved insulin sensitivity after exercise training is linked to reduced plasma C14:0 ceramide in obesity and type 2 diabetes. Obesity (Silver Spring) 2015;23:1414–1421. doi: 10.1002/oby.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alessenko AV, Shupik MA, Bugrova AE, Dudnik LB, Shingarova LN, Mikoyan A, Vanin AF. The relation between sphingomyelinase activity, lipid peroxide oxidation and NO-releasing in mice liver and brain. FEBS Lett. 2005;579:5571–5576. doi: 10.1016/j.febslet.2005.08.085. [DOI] [PubMed] [Google Scholar]
- 87.Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology. 2004;145:4592–4602. doi: 10.1210/en.2003-1749. [DOI] [PubMed] [Google Scholar]
- 88.Baranowski M, Blachnio-Zabielska AU, Charmas M, Helge JW, Dela F, Ksiazek M, Dlugolecka B, Klusiewicz A, Chabowski A, Gorski J. Exercise increases sphingoid base-1-phosphate levels in human blood and skeletal muscle in a time- and intensity-dependent manner. Eur J Appl Physiol. 2015;115:993–1003. doi: 10.1007/s00421-014-3080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baranowski M, Charmas M, Dlugolecka B, Gorski J. Exercise increases plasma levels of sphingoid base-1 phosphates in humans. Acta Physiol (Oxf) 2011;203:373–380. doi: 10.1111/j.1748-1716.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- 90.Harada J, Foley M, Moskowitz MA, Waeber C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem. 2004;88:1026–1039. doi: 10.1046/j.1471-4159.2003.02219.x. [DOI] [PubMed] [Google Scholar]
- 91.Cohen RA, Moser DJ, Clark MM, Aloia MS, Cargill BR, Stefanik S, Albrecht A, Tilkemeier P, Forman DE. Neurocognitive functioning and improvement in quality of life following participation in cardiac rehabilitation. Am J Cardiol. 1999;83:1374–1378. doi: 10.1016/s0002-9149(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 92.Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010;11:118. doi: 10.1186/1471-2202-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swardfager W, Herrmann N, Marzolini S, Saleem M, Farber SB, Kiss A, Oh PI, Lanctot KL. Major depressive disorder predicts completion, adherence, and outcomes in cardiac rehabilitation: a prospective cohort study of 195 patients with coronary artery disease. J Clin Psychiatry. 2010 doi: 10.4088/JCP.09m05810blu. [DOI] [PubMed] [Google Scholar]
- 94.Grace SL, Turk-Adawi K, Santiago de Araujo Pio C, Alter DA. Ensuring Cardiac Rehabilitation Access for the Majority of Those in Need: A Call to Action for Canada. Can J Cardiol. 2016;32:S358–S364. doi: 10.1016/j.cjca.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Mead H, Ramos C, Grantham SC. Drivers of Racial and Ethnic Disparities in Cardiac Rehabilitation Use: Patient and Provider Perspectives. Med Care Res Rev. 2016;73:251–282. doi: 10.1177/1077558715606261. [DOI] [PubMed] [Google Scholar]
- 96.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Bivariate mixed models showing associations between peak oxygen consumption (VO2peak) and log plasma concentrations of sphingolipids associated with change in cognitive performance over 6 months of cardiac rehabilitation (CR) in patients with coronary artery disease (CAD)


