Abstract
Purpose/Aim of the study
To assess the ability of optical coherence tomographic angiography (OCTA) to visualize the normal iris vasculature as well as neovascularization of the iris (NVI).
Materials and Methods
Study participants with healthy eyes, patients at risk of NVI development and patients with active or regressed NVI were consecutively included in this cross-sectional observational study. Imaging was performed using a commercially available OCTA system (RTVue- XR Avanti, Optovue Inc., Fremont, CA, USA). Abnormal iris vessels were graded on OCTA according to a modified clinical staging system and compared to slitlamp and gonioscopic findings.
Results
Fifty eyes of 26 study participants (16 healthy eyes, 19 eyes at risk, 15 eyes with different stages of NVI) were imaged using OCTA. In 11 out of 16 healthy eyes (69%) with light or moderately dark iris pigmentation, we observed physiological, radially aligned iris vasculature on OCTA imaging, which could not be visualized in five eyes (31%) with darkly pigmented irides. One eye in the “eyes at risk” group was diagnosed with NVI based on OCTA, which was not observed clinically. Fifteen eyes with clinically active or regressed NVI were imaged. Different stages of NVI could be differentiated by OCTA, corresponding well to an established clinical grading system. Four eyes showed regressed NVI by OCTA, not seen clinically, and were graded as a newly defined stage 4.
Conclusions
This pilot clinical study showed that OCTA for imaging of the iris vasculature in health and disease is highly dependent on iris pigmentation. Fine, clinically invisible iris vessels can be visualized by OCTA in the very early stages as well as in the regressed stage of NVI.
Keywords: Anterior segment imaging, neovascularization, optical coherence tomography, optical coherence tomography angiography, rubeosis iridis
Introduction
Neovascularization of the iris (NVI), also referred to as rubeosis iridis, is a sight-threatening complication that develops secondary to a number of ocular conditions including retinal vein occlusion, diabetic retinopathy (DR), ocular ischemic syndrome, and uveitis.1–3 New vessels start to grow independently around the pupillary border and at the root of the iris, in later stages penetrating the anterior surface of the iris and merging, thereby giving the iris the red flush that led to the name “rubeosis iridis”.2 Iris neovascularization may result in rubeotic glaucoma and visual loss. The early diagnosis of NVI is critical, as prompt and aggressive treatment can result in better outcomes.3
Optical coherence tomographic angiography (OCTA), a functional extension of conventional optical coherence tomography (OCT), has been shown to be a valuable tool for the detection and management of vascular abnormalities of the retina and choroid.4–6 With OCTA, physicians now have the opportunity of noninvasive detection of blood flow and rendering of vascular structures the size of only a few micrometers such as small capillaries or microaneurysms. The segmentation of the vasculature is based on the detection of signal intensity decorrelation between two or more consecutive OCT B-scans taken at the same position with milliseconds delay. This decorrelation is caused by movement of small particles such as cells in blood vessels and stands in contrast to surrounding static retinal tissue. This contrast can be exploited for segmentation of the vasculature.7 Recently, OCTA has also been applied to the cornea and provided high-quality images of corneal neovascularization with good inter-observer agreement for vasculature measurements.8–10
The iris, being an essentially flat and relatively thin tissue, readily visible and accessible behind the clear cornea, in theory provides an ideal ocular structure for en face imaging. Therefore, we hypothesized that OCTA imaging of the iris would be able to noninvasively detect subtle vessel abnormalities, allowing early detection of NVI. The purpose of this pilot study was to evaluate the utility of OCTA in identifying and evaluating the vasculature of healthy iris as well as distinguishing NVI in diseased eyes.
Materials and methods
Study participants and clinical protocols
The study protocol and procedures adhered to the ethics tenets of the Declaration of Helsinki. Institutional Review Board approval was obtained and the study was HIPAA-compliant. Informed consent was obtained from each participant before inclusion in this observational study. We included three groups of study participants: (1) healthy participants with different degrees of iris pigmentation, (2) patients diagnosed with retinal disease such as DR, central retinal vein occlusion (CRVO) or a history of complicated retinal detachment repair surgery, carrying an increased risk of NVI development, and (3) patients with manifest or regressed NVI in one or both eyes. All participants were imaged at the Department of Ophthalmology of the Feinberg School of Medicine at Northwestern University, Chicago, IL, USA.
The diagnosis of NVI was based on clinical examination including slit lamp examination and gonioscopy. Regressed NVI was diagnosed in cases with previously active NVI no longer clinically visible after treatment of the underlying disease. Eyes with corneal opacification precluding a detailed analysis of the iris were not included. Eyes with OCTA scans of poor quality or severe image distortion, which would interfere with identification of individual iris vessels, were excluded from the analysis.
Optical coherence tomographic angiography protocol
For OCTA imaging and evaluation, a commercial SD-OCT device (RTVue- XR Avanti; Optovue, Inc., Fremont, CA) was used. The OCT uses a light beam with a center wavelength of 840 nm and a full-width half maximum of 45 nm. The axial scan rate is 70.000 A-scans per second. OCTA volume scans of the anterior segment measuring 304 × 304 B-scans were acquired in 2.9 seconds. For detection of blood flow, the device acquires two consecutive B-scans at the same position in order to differentiate between static tissue and particles with high fluctuation of signal intensity. The machine uses split-spectrum amplitude-decorrelation angiography (SSADA) to improve the performance of the vessel segmentation algorithm.7
Imaging
For the purpose of this study, the anterior segment lens was used in combination with the Angiovue setting of the OCTA device in order to detect vasculature within the iris tissue. We used the retinal angiography (6 × 6 mm) mode and manually adjusted the OCT settings to position the iris close to the zero delay line in order to achieve the best possible image quality. Iris scans centered on the pupil as well as multiple scans centered on the anterior chamber angle at different positions along the limbus (temporally, inferiorly, nasally, and superiorly) were obtained. Since the superior limbus was covered by eyelashes and the upper eyelid in most study participants and manual retraction of the upper eyelid by the operator resulted in poor image quality, we chose not to include images of the superior anterior chamber angle in our analyses. For imaging of iris root vessels, the image was flipped over the zero delay line in order to position the anterior chamber angle closest to the point of highest signal. Furthermore, this method helped avoid artifact signal originating from limbal-corneal blood vessels, which projected onto the iris in standard imaging. The signal strength index (SSI), a measure automatically displayed with every volume scan, was assessed as well.
Evaluation of images and staging of NVI
As no specific algorithm exists for the segmentation of iris vessels, a slab was chosen that included the whole iris thickness. Eyes presenting with NVI were classified into three stages (active NVI) according to the clinical staging published by Gartner and Henkind (Table 1).2 The classification in our study was based on en face OCTA as well as cross-sectional OCTA imaging. As regressed NVI after treatment had not been incorporated in the classic staging method, we introduced a fourth stage for cases with regressed NVI remnants after treatment, which were not clinically visible but were visualized by OCTA. Two ophthalmologists experienced with OCTA imaging (PR, AAF) independently classified NVI into these four stages. If disagreement occurred, open adjudication between the two graders was performed to reach consensus. To test for reliability of our clinical staging system between the two graders, we calculated the intraclass correlation coefficient (ICC).
Table 1.
Staging of NVI by OCTA imaging (modified from the established clinical staging system by Gartner and Henkind).
| Stage | Clinical and OCTA characteristics |
|---|---|
| 1 | Fine, thin-walled irregular vessels at the pupillary border and at the iris root. |
| 2 | Thin-walled vessels located around the pupillary border and at the iris root enlarge and penetrate to the anterior iris surface. New vessels also develop in the iris stroma. |
| 3 | Merging of the two sets of NVI (peripupillary and anterior chamber angle). NVI with connective tissue support covers parts of the anterior surface of the iris. Peripheral anterior synechiae can develop. |
| 4 | Regressed NVI after treatment. Clinically invisible, fine, irregular vessels detected by OCTA. |
Abbreviations. NVI = neovascularization of the iris; OCTA = optical coherence tomographic angiography.
Results
Fifty eyes of 26 study participants (14 female, 12 male) with a mean (±SD) age of 50 (±18) years were prospectively included in this observational case series. Six participants were healthy controls, 10 participants had 1 or 2 eyes with increased risk of NVI development, and 10 participants had 1 or 2 eyes with a history of NVI. Sixteen eyes (healthy control participants and fellow eyes of patients with NVI or eyes at risk) had no increased risk of NVI, 19 eyes were carrying an increased risk of NVI (15 eyes with DR, 2 eyes with a history of complicated retinal detachment repair, 1 eye with CRVO and 1 eye with HLA-B27 associated uveitis), and 15 eyes had a diagnosis of active (6 eyes) or regressed NVI (9 eyes). A detailed description of patients with NVI is reported in Table 2. The mean (±SD) SSI of the acquired images was 36 ± 14.
Table 2.
Characteristics of study participants with neovascularization of the iris.
| Patient | Gender | Age (years) |
Underlying disease | Ethnicity | Iris color | Previous treatment | NVI activity (clinical diagnosis) |
NVI identified clinically |
NVI identified by OCTA |
NVI stage by OCTA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 63 | Retinal vasculitis OU | Caucasian | Blue | PRP OU | Active OU | OD: yes | OD: yes | OD: 3 |
| 2× PST triamcinolone acetonide/intravitreal Avastin injections OS | OS: yes | OS: yes | OS: 2 | |||||||
| 2 | Female | 66 | Proliferative diabetic retinopathy OU | Northern African | Brown | PRP OU | Active OS | OD no | OD yes | OD: 1 |
| Glaucoma surgery OS | OS yes | OS yes | OS: 3 | |||||||
| 3 | Female | 62 | Central retinal vein occlusion OD | African American | Brown | Multiple anti-VEGF injections | Active OD | OD yes | OD yes | OD: 3 |
| OS no | OS no | OS: - | ||||||||
| 4 | Female | 53 | Proliferative diabetic retinopathy OU | Caucasian | Blue | PRP OU, retinal detachment repair surgery OD (PPV + endolaser + gas) | Active OD | OD yes | OD yes | OD: 3 |
| OS no | OS no | OS: - | ||||||||
| 5 | Female | 34 | HLA-B27 positive uveitis OU | Caucasian | Brown | 2× PST triamcinolone acetonide injections OS, oral Prednisone 10mg | Active OS | OD: no | OD: no | OD: - |
| OS: yes | OS: yes | OS: 3 | ||||||||
| 6 | Female | 57 | Sarcoidosis-associated panuveitis OU | African American | Brown | Mycophenolate mofetil 1.5g | Regressed OU | OD no | OD no | OD: - |
| BID, oral Prednisone, multiple PST triamcinolone acetonide injections OU | OS no | OS yes | OS: 4 | |||||||
| 7 | Female | 72 | Proliferative diabetic retinopathy OU | African American | Brown | PRP OU | Regressed OU | OD: no | OD: no | OD: – |
| OS: no | OS: yes | OS: 4 | ||||||||
| 8 | Female | 52 | Proliferative diabetic retinopathy OU | African American | Brown | PRP OU | Regressed OU | OD: no | OD: yes | OD: 4 |
| OS: no | OS: yes | OS: 4 | ||||||||
| 9 | Female | 33 | Proliferative diabetic retinopathy OU | Caucasian | Blue | PRP OU, | Regressed OU | OD: no | OD: no | OD: - |
| 1× intravitreal Avastin injection OU | OS: no | OS: no | OS: - | |||||||
| 10 | Female | 39 | Sickle beta thalassemia-associated retinopathy OU | African American | Brown | Retinal detachment repair surgery OU (PPV + endolaser + gas) | Regressed OD | OD: no | OD: no | OD: - |
| OS: no | OS: no | OS: - |
Abbreviations: NVI = neovascularization of the iris, OD = right eye, OS = left eye, OU = both eyes, PRP = panretinal photocoagulation, PST triamcinolone acetate = posterior subtenon injection of triamcinolone acetate.
Healthy vasculature and influence of iris pigmentation
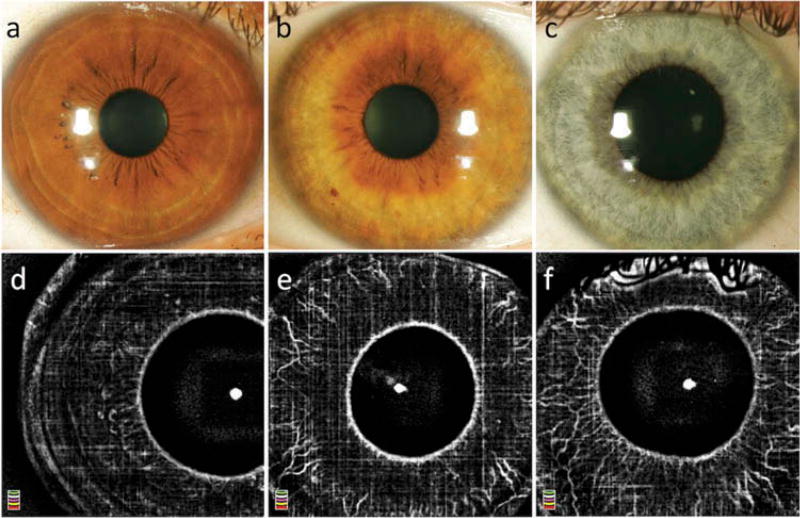
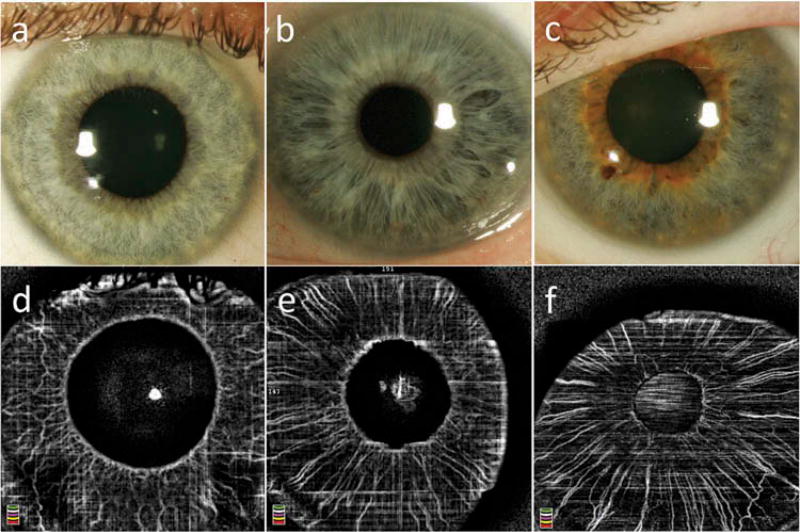
Seven of the healthy control eyes had lightly pigmented irides (blue), 4 eyes had medium pigmentation (hazel), and 5 eyes had darkly pigmented irides (dark brown). Normal iris vasculature was observed in en face OCTA scans in 11 out of 16 healthy eyes (69%); all of those with light or medium iris pigmentation, but none with dark pigmentation (Figure 1). In eyes where physiological iris vasculature was visualized, we observed variation in vessel morphology between study participants (Figure 2), but intra-individual symmetry between the right and left eyes.
Figure 1.
Slit lamp photography (a–c) and optical coherence tomography angiography (d–f) of eyes without NVI with dark (a,d), medium (b,e), and light (c,f) iris pigmentation. Note the influence of pigment density on the detection of the physiological iris vasculature. In the iris with dense pigmentation (= brown iris; a,d) few blood vessels are visualized. In the medium pigmented iris (= hazel; b,e), the vessels are observed; however, a larger number of vessels and more details of the healthy vasculature are visualized in lightly pigmented iris (= blue; c,f).
Figure 2.
Physiological iris vasculature in eyes without NVI with light (blue) iris color. Images a and d, b, and e, c, and f were taken from the same eyes, respectively. Inter-individual differences can be observed, particularly in regards to interconnecting circular vessels.
Stages and patterns of abnormal iris vascularization
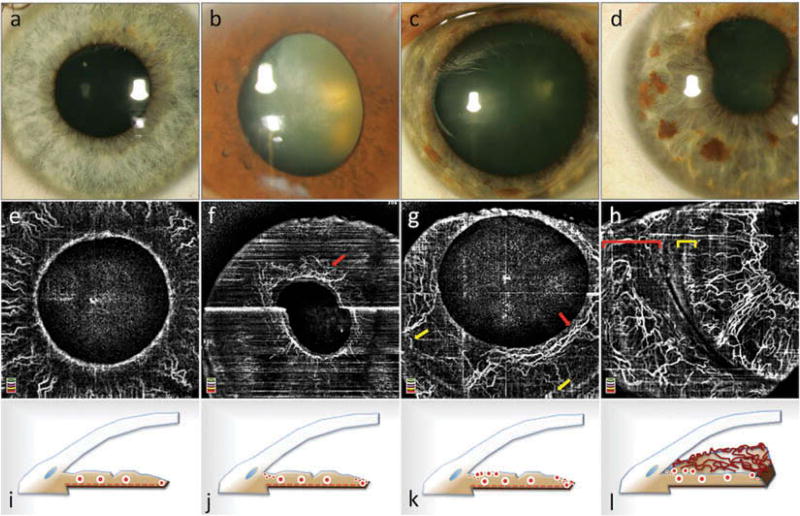
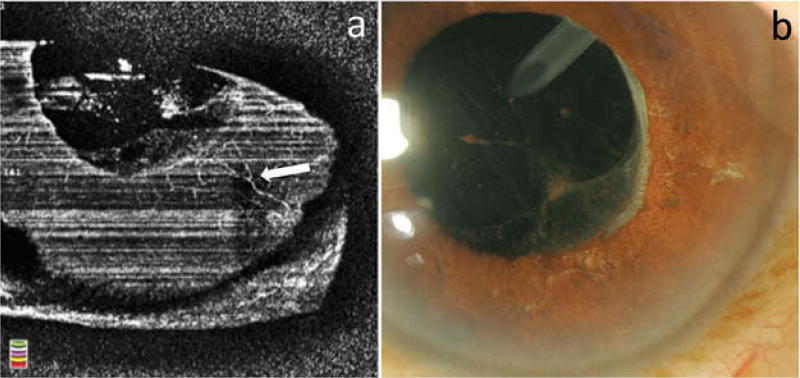
The stages and patterns of NVI, based on previous clinical and histopathological examination, were nicely visualized by OCTA imaging (Figure 3).2 The reliability analysis comparing the two graders showed excellent agreement for clinical staging, with ICC = 0.875 (95% confidence interval = 0.670 – 0.953). In the “eyes at risk” group, 18 out of 19 eyes (95%) did not show iris vessel abnormality. In one eye in the “eyes at risk group” with proliferative DR and no prior history of NVI, we observed fine capillaries arranged circularly around the pupil on OCTA, consistent with stage 1 NVI. In this eye, the vessels were clinically invisible and were only diagnosed by OCTA (Figure 3f). More tortuous vessels of larger caliber located around the pupil and at the iris root with only little communication between these two networks, most consistent with stage 2 NVI, were observed in one eye. The vessels were abnormally tortuous and did not follow the radial pattern of the physiological iris vasculature (Figure 3g). These vessels were also seen clinically. Florid, clinically detectable rubeosis with large-caliber, telangiectatic blood vessels was observed in five eyes (Figures 3h and 4). NVI root, defined as a network of irregular and tortuous vessels at the iris root, was also observed in these five eyes. OCTA showed vasculature at the iris root not following a physiological vascular pattern (Figure 3h).
Figure 3.
Slit lamp photographs (a–d) and optical coherence tomography angiographic (OCTA) imaging of neovascularization of the iris (NVI) (e–h) and corresponding schematic cross-sections (i–l) based on the clinical stages described by Gartner and Henkind.2 Healthy iris vasculature (a, e) with physiological radial vessel configuration of large-caliber stromal vessels (i). Stage 1 NVI: a ring of fine blood vessels around the pupillary border, which was clinically invisible (b), can be observed in en face OCTA (red arrow in f). The corresponding schematic (j) illustrates the independent growth of fine new blood vessels at the pupillary border and at the iris root. Vessel growth at the iris root was not observed in this eye. Stage 2 NVI: blood vessels around the pupil (red arrow in g) and at the root of the iris (yellow arrows in g) are thicker and more tortuous (c, k); however, there are hardly any connections between the two neovascular networks. Image (c) illustrates the clinical appearance. Stage 3 NVI: connections between the peripupillary NVI network and the iris root network have formed (d, h, l). The vessels are very tortuous and show the same thickness as physiological iris vasculature. A network of neovascularization of the iris root can be observed (yellow bracket in h). Conjunctival vessels are highlighted by a red bracket in (h). The bright white horizontal line in (f) is caused by motion artifact.
Figure 4.
Optical coherence tomography angiography (OCTA) en face images of a patient with unilateral stage 3 NVI in the left eye (b, c). The right eye (a) shows no obvious pathologic vasculature on exam or on en face OCTA. In the left eye, a dense network of small-caliber vessels can be observed surrounding the pupil inferiorly, nasally and superiorly. Temporally, big vessels are present. Slit lamp photography is presented for comparison (c). While large-caliber vessels are easily appreciated on slit lamp photography, detection of smaller capillaries can be challenging (c).
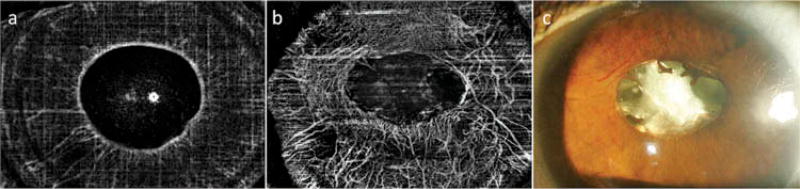
Clinically invisible, regressed NVI was detected on OCTA in four eyes, showing fine vessels, less tortuous than vessels in eyes with clinically active NVI, termed stage 4 NVI (Figure 5). In the other five eyes with clinically regressed NVI, no abnormal vessels were detected by OCTA.
Figure 5.
En face optical coherence tomographic angiography (OCTA) (a) and slit lamp photography (b) of an eye with stage 4 neovascularization of the iris (NVI). Fine remnants of the NVI are detected by OCTA imaging (white arrow in a), but are not seen clinically (b).
The pigment-dependent quality of blood vessel imaging was also observed in the study participants with NVI. In patients with dark iris pigmentation, the physiological iris vasculature was not visualized by OCTA, whereas NVI was detected even in cases where vessels were not seen clinically, indicative of a more superficial location of the pathologic vessels (Figure 3). In patients with light colored irides, a differentiation between healthy iris vessels and NVI was more challenging due to the overlap between physiological and pathological iris vessels detected by OCTA (Figure 3h). In these eyes, graders incorporated information about the known anatomical iris networks to make decisions about NVI.
Discussion
This pilot study describes the ability of OCTA to image iris vessels and, in particular, NVI. Ang et al. recently showed that OCTA provides good repeatability in visualization of the corneal and limbal vasculature.8,9 Using a similar method for imaging the anterior segment with a commercially available OCTA system, we show its utility for visualization of the iris vasculature. As proof of principle, six healthy participants with normal slit lamp examinations, as well as 10 patients at risk of NVI development and 10 patients with active and regressed NVI were studied to show the overall performance of iris OCTA for detection and classification of NVI.
Current screening for NVI in patients at risk involves slit lamp biomicroscopy, gonioscopy, and for detection of subclinical stages of NVI may also include fluorescein angiography (FA) or indocyanine green angiography (ICGA).3,11,12 While gonioscopy has the advantage of being a dynamic examination that can be easily performed at the slit lamp, it requires some experience to perform an adequate examination, and fine NVI may be missed. Iris FA can be helpful in these cases, showing leakage from these new vessels.11–13 Anterior segment ICGA has the advantage of displaying the vasculature in more detail, because ICG molecules are primarily protein bound and mostly remain within blood vessels. Additionally, ICGA is performed using near-infrared wavelengths allowing better penetration through pigment.12 Therefore, for the purpose of structural imaging of the blood vessels, ICGA may be superior to FA as it shows less leakage but instead highlights vascular microstructure. Ang et al. recently showed that corneal OCTA is comparable to corneal ICGA for the measurement of corneal vascularization.14 Although dye-based iris angiography has been shown to be superior to biomicroscopy in the detection of NVI, the imaging may be cumbersome to perform and carries slight, but real risks of complications from the administration of fluorescein.11 Furthermore, as dye-based iris angiography has to be performed with a nondilated pupil, it is not feasible to perform FA/ICGA of the iris and retina at the same visit, which might be desirable since most cases of NVI are caused by an underlying ischemic retinopathy.3 An advantage over iris FA/ICGA, other than the noninvasiveness of OCTA, is that there is no dye leakage, which might hamper detailed analysis of vessel anatomy.
Numerous studies have shown that OCTA is a valuable tool in patients with choroidal neovascularization (CNV) for diagnosis and evaluation of treatment response.4,5,15 Recently, a similar application of OCTA has been demonstrated for serial imaging of corneal neovascularization before and after treatment.16 Our pilot clinical study demonstrates that a similar approach for iris neovascularization could be feasible, provided further research and improvement of software algorithms. Also, application of OCTA to iris vascularization could theoretically allow for a fast and noninvasive screening of patients at risk of NVI development. However, current technological issues, which may be overcome by software development, include reliable image registration for follow-up imaging as well as a quantitative and objective evaluation method for iris vasculature.
We observed variation in the morphology of the iris vasculature in eyes without NVI between study participants; however, for an individual subject, there was excellent symmetry in the vascular arrangement comparing the right and left eyes. In particular, we found significant variability between individuals in the morphology of the incomplete minor arterial circle, a circular vascular structure of anastomosing arteries, and veins located between the iris root and the pupil. These vessels are often helical and tortuous to adapt to changes in the iris shape when the pupil size changes (Figure 2).17 In eyes with NVI, the abnormal blood vessels were characterized by random orientation of the vessels, significant tortuosity, and a smaller caliber of newly-formed capillaries as compared to healthy, radially aligned blood vessels.
The dependence of visibility of iris vasculature on iris pigmentation is well known and has been described previously in regards to clinical examination as well as iris FA.2,11 In patients with dark iris color, only superficial vessels are visualized, whereas in eyes with light iris color or patients with albinism, even the deep iris vessels are visualized in great detail (Figures 1 and 2).11 In healthy eyes, the iris blood vessels are located in the stroma, covered by the anterior border layer, which, depending on its pigment, determines the iris color.17 In eyes with a blue iris, there is only little pigment in the anterior border layer allowing visibility of underlying stromal vessels whereas in a brown iris, the presence of dense pigment in the anterior border layer obscures the normal stromal vessels. In our study, this pigment-dependence of vessel imaging was also shown to be the case in OCTA imaging. Iris pigmentation also had a big impact on detection of NVI. Based on the preliminary results of our study, distinction of NVI by OCTA might be easier in eyes with darker iris color than in eyes with light iris color. This is related to the fact that NVI develops on the surface of the iris, and in light irides the normal iris vasculature is not obscured by pigment. Differentiation between the prominently visualized physiological iris vasculature and NVI in light irides therefore requires careful evaluation of en face angiographic images. In contrast, in darkly pigmented irides, with obscuration of the deeper located, normal iris vasculature, NVI is more clearly visualized. Figure 3 illustrates this observation: Grade 1 NVI is easily identifiable around the pupillary border in a darkly pigmented iris (Figure 3f), whereas the differentiation of grade 2 and 3 NVI from healthy physiological vasculature is challenging in lightly colored irides (Figures 3g and 3h), even though the abnormal vessels are more numerous and of larger caliber. In these controversial cases, iris FA may be superior to iris OCTA by showing leakage if NVI is present. Staging of NVI by OCTA in this series showed good comparability to the clinical staging and histopathological data published previously.2 En face OCTA imaging showed different stages of NVI in vivo without the need for invasive procedures and only little adjustments of the OCTA device.
We introduced a new stage for clinically invisible, regressed NVI, which is detectable by OCTA. We hypothesize that even after successful treatment of the underlying disease, the new blood vessels do not completely regress, but, similar to treated CNV, these vessels become clinically invisible without signs of activity such as ocular hypertension or hyphema. Interestingly, Ishibashi et al. recently showed that after intravitreal bevacizumab treatment for NVI and neovascularization of the anterior chamber angle, newly formed blood vessels regress clinically but are still visible on iris ICGA.18 Follow-up in their study was, however, limited, with only 4–6 days after the intravitreal injection. In our study, en face OCTA noninvasively showed persistent abnormal vessels after treatment, which we graded as stage 4 NVI. OCTA helps in visualizing the persistence of these iris neovascular channels by imaging blood flow, but similar to the situation in CNV, OCTA may not be able to help clinicians discern their clinical activity. It will be important in future studies to follow these vessels longitudinally before and after therapy to identify potential markers of regression on OCTA. In the regressed NVI cases not visualized by OCTA, the flow may have decreased below the detectable threshold.
Interpretation of cross-sectional images required some background knowledge about OCTA and the most common artifacts such as projection artifacts. The iris pigment epithelium, similar to the retinal pigment epithelium is a bright, hyper-reflective structure on OCT imaging and, despite being avascular, reflects flow signal originating from overlying blood vessels.19 When evaluating OCTA B-scans of the iris, this potential artifact should be taken into account.
The authors are aware of a number of limitations of this study. Of these, the small number of patients needs to be mentioned. Iris FA/ICGA, which we did not include in our study, could have detected subclinical NVI that may have been missed on OCTA. However, we do not advocate invasive procedures such as iris FA/ICGA for screening of patients at risk of NVI, whereas noninvasive OCTA bears no risk for the patient. Visual acuity was severely decreased in all patients resulting in difficulty with fixation and motion artifacts in a majority of the volume scans. Incorporation of an eye-tracker in the future OCTA system could resolve this issue. The limited field of view and the signal roll-off in SD-OCT based OCTA did not allow for simultaneous high-quality visualization of both the peripupillary part of the iris and the iris root in our case series. Swept-source OCT based OCTA systems operating at longer wavelengths with a wide field of view and reduced signal roll-off might be a way to overcome this issue. The mean SSI (36 ± 14SD) in our study was comparable to previous anterior segment OCTA studies investigating the cornea.8,9 The OCTA device used for this study does not provide anterior segment angiography software; we therefore used the retina angiography, software, which was not conceived for anterior segment imaging, together with the anterior segment lens to acquire the iris OCTA scans. Specific anterior segment angiography modules with automated adjustment of imaging parameters such as focus and scaling could likely improve the scan quality. Furthermore, segmentation algorithms, specifically designed for differentiating pathologic from healthy iris vasculature, could greatly facilitate visualization and quantification of pathologic iris vasculature. We inverted the B-scan, which facilitated imaging of neovascularization at the iris root by removing the confounding projection artifacts originating from the cornea and limbus. The imaging method presented in this study offers a fast and noninvasive tool for anterior segment and, specifically, iris angiographic imaging. The technique may allow assessment of microstructural changes of NVI in response to different treatments such as retinal laser therapy or intravitreal anti-vascular endothelial growth factor therapy.
In conclusion, our study demonstrates the ability of OCTA to image both normal and pathological iris vessels. The noninvasiveness of OCTA is a great advantage of this imaging technique over conventional dye-based angiography. OCTA may be a promising addition to our armamentarium of anterior segment imaging modalities, particularly for detection and management of iris neovascularization. Further larger-scale studies are needed in order to validate the clinical application proposed in our study.
Acknowledgments
Funding This work was partly supported by NIH DP3DK108248 (AAF). Research instrument support for this work was provided by OptoVue, Inc. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/icey.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Hayreh SS. Neovascular glaucoma. Prog Retin Eye Res. 2007;26:470–485. doi: 10.1016/j.preteyeres.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gartner S, Henkind P. Neovascularization of the iris (rubeosis iridis) Surv Ophthalmol. 1978;22:291–312. doi: 10.1016/0039-6257(78)90175-3. [DOI] [PubMed] [Google Scholar]
- 3.Sivak-Callcott JA, O’Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108:1767–1776. doi: 10.1016/s0161-6420(01)00775-8. [DOI] [PubMed] [Google Scholar]
- 4.Huang D, Jia Y, Rispoli M, Tan O, Lumbroso B. Optical coherence tomography angiography of time course of choroidal neovascularization in response to anti-angiogenic treatment. Retina. 2015;35:2260–2264. doi: 10.1097/IAE.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia Y, Bailey ST, Hwang TS, McClintic SM, Gao SS, Pennesi ME, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci USA. 2015;112:E2395–E2402. doi: 10.1073/pnas.1500185112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palejwala NV, Jia Y, Gao SS, Liu L, Flaxel CJ, Hwang TS, et al. Detection of nonexudative choroidal neovascularization in age-related macular degeneration with optical coherence tomography angiography. Retina. 2015;35:2204–2211. doi: 10.1097/IAE.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang M, Sim DA, Keane PA, Sng CC, Egan CA, Tufail A, et al. Optical coherence tomography angiography for anterior segment vasculature imaging. Ophthalmology. 2015;122:1740–1747. doi: 10.1016/j.ophtha.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Ang M, Cai Y, Shahipasand S, Sim DA, Keane PA, Sng CC, et al. En face optical coherence tomography angiography for corneal neovascularisation. Br J Ophthalmol. 2016;100:616–621. doi: 10.1136/bjophthalmol-2015-307338. [DOI] [PubMed] [Google Scholar]
- 10.Ang M, Cai Y, Tan AC. Swept source optical coherence tomography angiography for contact lens-related corneal vascularization. J Ophthalmol. 2016;2016:9685297. doi: 10.1155/2016/9685297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brancato R, Bandello F, Lattanzio R. Iris fluorescein angiography in clinical practice. Surv Ophthalmol. 1997;42:41–70. doi: 10.1016/s0039-6257(97)84042-8. [DOI] [PubMed] [Google Scholar]
- 12.Parodi MB, Bondel E, Russo D, Ravalico G. Iris indocyanine green videoangiography in diabetic iridopathy. Br J Ophthalmol. 1996;80:416–419. doi: 10.1136/bjo.80.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li ZQ, Zhou XX, Lin S, Li JL, Wu JG. Angiography reveals early hiding iris neovascularization after ischemic CRVO. Int J Ophthalmol. 2013;6:253–254. doi: 10.3980/j.issn.2222-3959.2013.02.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang M, Cai Y, MacPhee B, Sim DA, Keane PA, Sng CC, et al. Optical coherence tomography angiography and indocyanine green angiography for corneal vascularisation. Br J Ophthalmol. 2016 Jan 28; doi: 10.1136/bjophthalmol-2015-307706. [DOI] [PubMed] [Google Scholar]
- 15.Lumbroso B, Rispoli M, Savastano MC. Longitudinal optical coherence tomography-angiography study of type 2 naive choroidal neovascularization early response after treatment. Retina. 2015;35:2242–2251. doi: 10.1097/IAE.0000000000000879. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y, Alio Del Barrio JL, Wilkins MR, Ang M. Serial optical coherence tomography angiography for corneal vascularization. Graefes Arch Clin Exp Ophthalmol. 2016;255:135–139. doi: 10.1007/s00417-016-3505-9. [DOI] [PubMed] [Google Scholar]
- 17.Standring S. Gray’s anatomy: the anatomical basis of clinical practice. Amsterdam NX: Elsevier; 2016. [Google Scholar]
- 18.Ishibashi S, Tawara A, Sohma R, Kubota T, Toh N. Angiographic changes in iris and iridocorneal angle neovascularization after intravitreal bevacizumab injection. Arch Ophthalmol. 2010;128:1539–1545. doi: 10.1001/archophthalmol.2010.282. [DOI] [PubMed] [Google Scholar]
- 19.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35:2163–2180. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]