Abstract
Background and Objective
Patients with pre-existing atherothrombotic disease are prone to cognitive impairment. We tested whether impaired cerebrovascular reactivity (CVR), a marker of cerebral microvascular hemodynamic dysfunction, is associated with poorer cognitive scores among patients with and without carotid large-vessel disease.
Methods
A subgroup of non-demented patients with chronic coronary heart disease followed-up for 15 ± 3 years was assessed for cognitive function (Neurotrax Computerized Cognitive Battery; scaled to an IQ style scale with a mean of 100 and SD of 15) and for CVR using the breath-holding index (BHI) with transcranial Doppler and for carotid plaques using ultrasound. We assessed cognitive scores in specific domains in patients with and without impaired CVR (BHI <0.47; bottom quartile).
Results
Among 415 patients (mean age 71.7 ± 6.2 y) median BHI was 0.73 (25% 0.47, 75% 1.04). Impaired CVR was associated with diabetes and peripheral artery disease. Adjusting for potential confounders, impaired CVR was associated with lower executive function (p = 0.02) and global cognitive scores (p = 0.04). There was an interaction with carotid large-vessel disease for executive function (p < 0.001), memory (p = 0.03), and global cognitive scores (p = 0.02). In the carotid large-vessel disease group there were pronounced differences by CVR status in executive function (p < 0.001), memory (p = 0.02), attention (p < 0.001), and global cognitive scores (p = 0.001).
Conclusion
Impaired CVR, a marker of cerebral microvascular dysfunction, is associated with poorer cognitive functions and in particular executive dysfunction among non-demented patients with concomitant carotid large-vessel disease. These findings emphasize the importance of cerebral hemodynamics in cognitive performance.
Keywords: Cerebrovascular disorders, dementia, hemodynamics, transcranial Doppler sonography, vascular dementia
INTRODUCTION
The role of vascular disease in cognitive impairment and dementia is well established [1]. Many individuals with significant atherosclerotic disease remain, however, asymptomatic in terms of cognitive function. Cerebrovascular reactivity (CVR), a marker of cerebral microvascular function, can be assessed non-invasively using transcranial Doppler (TCD) [2, 3]. It is speculated that cerebral microvascular dysfunction occurs due to atherosclerotic changes and decrease of elasticity of the cerebral small-vessel walls [4, 5]. Extracranial large-vessel stenosis has been documented as an independent risk factor for cognitive impairment [6–8]. Strong relationships were observed between indices of vascular aging and either cognitive impairment or cerebral small-vessel disease, suggesting common pathophysiological mechanisms linking large-artery damage to cerebral small-vessel disease. Furthermore, there may be different lines of cross-talk between macrovascular and microvascular structure and function involved in the process underlying vascular cognitive impairment [9]. Our aim was to determine the association between impaired CVR, carotid large-vessel disease and cognitive function among a cohort of patients with pre-existing atherothrombotic disease. We expected that attentional and executive functioning will be particularly affected since they are salient features of vascular cognitive impairment [10].
MATERIAL AND METHODS
Subjects
Subjects of the current study were from seven medical centers residing in the central region of Israel who had previously participated in a clinical trial of lipid modification (the Bezafibrate Infarction Prevention; BIP trial) and survived by 2008 (n = 942). In brief, BIP was a placebo-controlled randomized clinical trial investigating the efficacy of bezafibrate retard 400 mg daily, a fibric derivative, in secondary prevention among 3090 patients with established stable coronary heart disease recruited from 18 medical centers in Israel between May 1990 and January 1993 [11]. Men and women 45 to 74 years of age with a history of myocardial infarction at least 6 months and not longer than 5 years before enrollment and/or stable angina pectoris during the last 2 years confirmed by coronary angiography, and/or radionuclide studies or standard exercise tests were included. Patients were selected on the basis of a specific lipid profile and main exclusion non-lipid criteria were insulin-dependent diabetes mellitus, hepatic or renal failure, and disabling stroke.
New York Heart Association heart failure functional class (NYHA) was assessed at baseline. Blood samples were collected after ≥12 h of fasting using standardized equipment and procedures. Serum analysis was carried out at a central laboratory using standard automated procedures with commercially available diagnostic kits (Boehringer Mannheim, Mannheim, Germany). Accuracy and precision were under periodic surveillance by the Centers for Disease Control/National Heart, Lung, and Blood Institute’s lipids standardization program (Bethesda, Maryland) and by the Wellcome-Murex diagnostic clinical chemistry quality assessment program. Cholesterol levels were determined by the CHOD-PAP (cholesterol oxidase/p-aminophenazone) method, and LDL-C levels were approximated by the formula of Friedewald et al. Glucose concentrations were determined using an enzymatic colometric method (GOD/PAP). Fibrinogen was measured by an automated kinetic method. CRP concentrations were measured in plasma by IMMULITE 2000 analyzer from Diagnostics Products Corporation (DPC) with manufacture’s reagents-solid-phase, chemiluminescent immunometric assay. Glomerular filtration rate (GRF) was estimated using the MDRD equation.
Cognitive and neurovascular evaluation
Patients from seven medical centers in the central region of Israel who survived by 2008 (n = 942) were invited for a medical and cognitive function evaluation. The evaluation was approved by the local institutional review board and informed consent was obtained from patients. This evaluation was performed an average of 15 ± 3 years after the baseline evaluation. All patients were assessed at the Sagol Neuroscience Center.
Cerebrovascular reactivity test
CVR was assessed using the breath-holding test by means of TCD examination, previously described by Muller et al. [12]. All exams were performed by a qualified technician using a Trans-Link 9900 TCD device (Rimed, Raanana, Israel) equipped with a 2 MHz probe. Patients were asked to hold their breath for 30 s or as long as possible. Before proceeding to the definitive recording, subjects were trained to perform the procedure correctly. The duration of apnea was measured in seconds. Mean flow velocity at rest was obtained by continuous recording of a 1-min period of normal breathing. As the CVR measure, the breath-holding index (BHI) in the middle cerebral artery (MCA) was calculated in a standardized manner, as percent increase in MCA mean blood velocity recorded by breath-holding divided by seconds of breath-holding ([Vbh−Vr/Vr] · 100 · s−1), where Vbh is MCA mean blood velocity at the end of breath-holding, Vr the MCA mean blood velocity at rest, and s−1 per second of breath-holding [12]. Subjects were categorized into normal or impaired CVR based on the mean BHI of both MCAs. As there are no reference ranges for CVR in this population, the bottom quartile of CVR (<0.47) was regarded as impaired [13].
Carotid ultrasound examination
Carotid ultrasound examination was performed to identify the presence or absence of carotid plaques, following a standard protocol, [14] using a HDI 5000 SonoCT system device (Philips, Eindhoven, The Netherlands) with a linear array multifrequency transducer (4 to 7 MHz). Presence of bilateral carotid plaque was regarded as evidence of carotid large-vessel disease.
Cognitive assessment
Cognitive function was evaluated through a validated set of computerized cognitive tests (NeuroTrax Corporation, Bellaire, TX). A description of the tests included has been published elsewhere [15]. The cognitive evaluation was designed for use also for the elderly and assessed a wide range of cognitive domains, including memory (verbal and non-verbal), executive function, visual spatial skills, and attention. The tests were interactive and adaptive, adjusting the level of difficulty depending upon performance. All tests were preceded by a short technical practice session in which appropriate guidance and feedback were provided to the subjects. Four index scores were computed summarizing performance in major cognitive domains: memory, executive function, visual spatial processing, and attention. Global cognitive score was computed as a weighted average of all summary scores computed for each domain and served as a measure of overall performance. Cognitive scores were normalized according to age- and education-specific normative data. Normalized scores were then scaled to an IQ-style scale (mean = 100; standard deviation = 15). Cognitive screening was assessed through the Mini-Mental State Examination (MMSE). MMSE scores lower than the corresponding quartile based on age and educational level were considered abnormal.
Additional assessments
Data were systematically collected regarding new co-morbidities and hospitalizations, medication use, and smoking status. In addition, systolic blood pressure (SBP), diastolic blood pressure (DBP), and body mass index (BMI) were measured. Incident stroke during follow-up was assessed by reviewing records from hospital or emergency department discharge, primary care physician, or neurologist. Depressive symptoms were assessed by the Geriatric Depression Scale (GDS) using a cutoff of ≥6 [16]. Dementia and incident stroke were determined by an adjudication committee composed of three investigators (G.W., O.M., D.T.), two of which are experienced board certified neurologists. Dementia was determined based on the sum of cognitive evaluation, clinical interview, and data collected and in accordance with the Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV) criteria [17] and stroke according to World Health Organization criteria [18]. For the purpose of the current analysis patients with dementia (n = 49) were excluded, in-order to assure compliance with and reliable measurement of CVR.
Statistical analysis
Normal distribution of cognitive scores was validated using the Kolmogorov–Smirnov test. Univariate associations between CVR groups, characteristics, and cognitive scores were evaluated through Chi-square tests for categorical variables, Student’s t-test for normally distributed continuous variables and Mann Whitney otherwise. The association of large-vessel disease on CVR and cognitive scores was examined using two-way ANOVA tests. In order to detect interactions, the means of cognitive scores were plotted. Linear regression models adjusting for age, education, previous stroke, diabetes, hypertension, and depressive symptoms were used to examine the association between CVR groups and computerized cognitive scores. Transformation of power of 4 was used for the attention cognitive domain to approximate normality. Hence, for attention cognitive domain unadjusted means are not presented and only p-values are tabulated for linear regression models. Data analysis was performed using R statistical free software, version 2.8.1 (R Foundation).
RESULTS
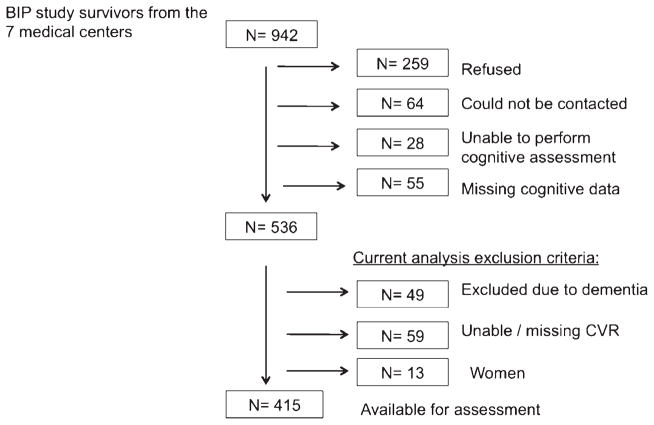
Among 942 patients, 259 refused to participate, 64 could not be contacted, 28 were unable to participate due to language incompatibility, vision or hearing difficulties, or physical condition, and 55 had missing computerized cognitive assessment. Of the remaining 536 patients, 108 were excluded from the present analyses because of dementia (n = 49) or no adequate assessment of CVR (n = 59), remaining with 428 subjects. Women (n = 13) were excluded from the analysis due to small numbers and evidence of sex–related differences in CVR [19] (Fig. 1). Compared to participants who were excluded (n = 121) from the current analysis, those included were younger, had a higher educational level, a lower proportion of smokers and hypertensive patients, and had less depressive symptoms and history of stroke (Supplementary Table 1).
Fig. 1.
Flow diagram of study participants.
Among the 415 male patients included in the analysis, the median (interquartile range) CVR was 0.74 (0.48–1.03). Fifty five percent (n = 229) of the cohort had bilateral carotid large-vessel disease and the median CVR in these subjects was 0.72 (0.47–0.98). In those without large-vessel disease the median CVR was 0.75 (0.48–1.09). Table 1 shows the characteristics of the participants, categorized according to their CVR status. Subjects with impaired CVR had higher prevalence of diabetes (36.7 versus 25.2%, p = 0.03) and peripheral artery disease (6.2 versus 1.6%; p = 0.01). No significant differences were observed in age or others vascular risk factors and co-morbidities.
Table 1.
Characteristics by CVR status
| Normal CVR | Impaired CVR | p value | |
|---|---|---|---|
| No. of subjects | 317 | 98 | |
| Age (years) | 71.6 ± 5.9 | 72 ± 6.9 | 0.53 |
| Educational level | |||
| Primary | 57 (18) | 18 (18.4) | 0.31 |
| Secondary school | 150 (47.5) | 54 (55.1) | |
| Intermediary or University | 109 (34.5) | 26 (26.5) | |
| Smoking status | |||
| Current smoker | 34 (10.7) | 11 (11.2) | 0.46 |
| Past smoker | 205 (64.7) | 57 (58.2) | |
| Never smoker | 78 (24.6) | 30 (30.6) | |
| Systolic BP (mm Hg) | 136.5 ± 19.7 | 138.7 ± 23.4 | 0.37 |
| Diastolic BP (mm Hg) | 76 ± 9.8 | 75 ± 9.8 | 0.37 |
| Body mass index (kg/m2) | 25.9 (24.5–28) | 26.3 (24.7–28.1) | 0.42 |
| Peripheral artery disease | 5 (1.6) | 6 (6.2) | 0.01 |
| Baseline NYHA class ≥2 | 51 (16.3) | 15 (15.5) | 0.85 |
| Baseline values | |||
| Fasting glucose (mg/dL) | 94.1 (87.9–102.9) | 98.5 (88.6–107) | 0.07 |
| Total cholesterol (mg/dL) | 212 ± 17.5 | 214.6 ± 17.4 | 0.21 |
| LDL- C (mg/dL) | 147.8 ± 16.6 | 151 ± 16.7 | 0.10 |
| HDL- C (mg/dL) | 34.9 ± 5.4 | 35.4 ± 5.1 | 0.45 |
| Triglycerides (mg/dL) | 134.7 (104.5–180.5) | 137.8 (103.1–174.4) | 0.60 |
| Estimated GFR (mL/min/1.73 m2) | 70 ± 9.2 | 71.6 ± 10 | 0.15 |
| Fibrinogen (mg/dL) | 329.9 (290.1–372) | 328 (284.9–366.2) | 0.72 |
| White blood count (10∧9/L) | 6.3 (5.4–7.3) | 6.3 (5.6–7.4) | 0.30 |
| C reactive protein (mg/L) | 2.4 (1.5–4.5) | 2.6 (1.5–4.5) | 0.79 |
| Late life evaluation | |||
| Depressive symptoms | 50 (15.8) | 17 (17.3) | 0.71 |
| Prevalent diabetes | 80 (25.2) | 36 (36.7) | 0.03 |
| Prevalent stroke | 23 (7.3) | 11 (11.2) | 0.21 |
| Prevalent hypertension | 134 (42.3) | 41 (41.8) | 0.94 |
Values are n (%) for categorical variables; mean ± SD for normally distributed continuous variables and median (IQR) otherwise.
APOA1, Apolipoprotein A-I; APOB, Apolipoprotein B; BP, Blood pressure; GRF, Glomerular Filtration Rate; HDL, High-density lipoprotein; LDL, low density lipoprotein; NYHA, New York Heart Association.
Overall, presence of impaired CVR was associated with lower global cognitive scores, as well as lower scores in the executive function. After adjustment for potential confounders, the impaired CVR group exhibited worse cognitive performance with lower global cognitive score than the normal CVR group (B coefficient ± SE, −2.43 ± 1.16, p = 0.04), also the executive function domain score was lower in the impaired CVR group (−3.21 ± 1.34; p = 0.02). A similar relationship was observed for attention score (p = 0.002; Table 2). There was no significant difference in the MMSE score between the two groups.
Table 2.
Differences in cognitive scores between patients exhibiting impaired versus normal CVR
| Cognitive domain | B coefficient ± SE | p |
|---|---|---|
| Executive function | −3.21 ± 1.34 | 0.02 |
| Memory | −1.57 ± 1.71 | 0.36 |
| Visual Spatial | −1.02 ± 1.78 | 0.57 |
| Attention | † | ≤0.001 |
| Global cognitive score | −2.43 ± 1.16 | 0.04 |
|
| ||
| OR (95% CI)‡ | ||
|
| ||
| Stratified MMSE cutoff | 1.41 (0.87–2.29) | 0.16 |
Linear regression adjusted for age, education, previous stroke, diabetes, hypertension and depressive symptoms.
Transformation of power of 4 was used for the attention cognitive domain to approximate normality (hence, only p values are tabulated).
Logistic regression model adjusted for age, education, previous stroke, diabetes, hypertension and depressive symptoms.
MMSE, Mini-Mental State Examination.
In sensitivity analyses, excluding subjects with prevalent stroke (n = 34) (data not tabulated), the associations between impaired CVR and cognitive performance were attenuated: for executive function domain −2.61 ± 1.36; (p = 0.057), memory domain − .19 ± 1.8 (p = 0.51), and global cognitive score − 1.88 ± 1.18 (p = 0.11).
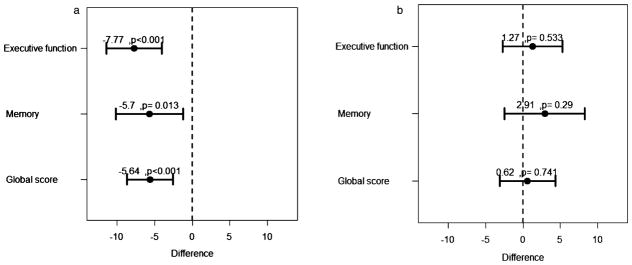
We looked additionally for several pre-defined interactions (Table 3 and Fig. 2). We found a significant interaction with carotid large-vessel disease for the relation between impaired CVR and cognitive performance. Differences in cognitive scores by CVR status were evident and pronounced in the group with carotid large-vessel disease. Significant interactions were observed for executive function (p < 0.001), memory (p = 0.03), and global cognitive score (p = 0.02). In the carotid large-vessel disease group there were significant differences in the executive function (−6.92 ± 1.81; p < 0.001), memory (−5.23 ± 2.21; p = 0.02), attention (p < 0.001), and global cognitive score (−4.97 ± 1.5; p = 0.001) by CVR status. In contrast, in the group without carotid large-vessel disease none of the cognitive scores showed to be significantly different between patients with impaired versus normal CVR.
Table 3.
Linear regression models for computerized cognitive scores in patients with impaired versus normal CVR – subgroup analyses
| Subgroup | B coefficient ± SE* | p | B coefficient ± SE* | p | p for interaction |
|---|---|---|---|---|---|
| Carotid large-vessel disease | Yes (n = 229) | No (n = 183) | |||
| Executive function | −6.92 ± 1.81 | <0.001 | 1.06 ± 1.98 | 0.59 | <0.001 |
| Memory | −5.23 ± 2.21 | 0.02 | 3.36 ± 2.68 | 0.21 | 0.03 |
| Visual Spatial | −1.97 ± 2.35 | 0.40 | 0.38 ± 2.79 | 0.89 | 0.48 |
| Attention | † | <0.001 | † | 0.40 | 0.09 |
| Global cognitive score | −4.97 ± 1.50 | <0.001 | 0.67 ± 1.84 | 0.72 | 0.02 |
| Age (years) | >60 (n = 130) | ≤60 (n = 285) | |||
| Executive function | −5.86 ± 2.81 | 0.04 | −2.06 ± 1.5 | 0.17 | 0.23 |
| Memory | −1.50 ± 2.79 | 0.59 | −1.41 ± 2.15 | 0.51 | 0.99 |
| Visual Spatial | −0.87 ± 3.45 | 0.80 | −1.03 ± 2.11 | 0.63 | 0.87 |
| Attention | † | 0.11 | † | 0.01 | 0.78 |
| Global cognitive score | −2.99 ± 2.36 | 0.21 | −2.19 ± 1.34 | 0.10 | 0.87 |
| Diabetes | Yes (n = 116) | No (n = 229) | |||
| Executive function | −5.10 ± 2.35 | 0.03 | −2.35 ± 1.66 | 0.13 | 0.33 |
| Memory | −1.01 ± 2.86 | 0.73 | −1.90 ± 2.14 | 0.38 | 0.82 |
| Visual Spatial | −1.21 ± 3.02 | 0.69 | −1.13 ± 2.21 | 0.61 | 0.95 |
| Attention | † | 0.06 | † | 0.02 | 1.00 |
| Global cognitive score | −2.80 ± 1.90 | 0.14 | −2.30 ± 1.47 | 0.12 | 0.82 |
Adjusted for age, education, previous stroke, diabetes, hypertension and depressive symptoms.
Transformation of power of 4 was used for attention cognitive domains to approximate normality (hence, only p values are tabulated).
Fig. 2.
Linear regression coefficients (95% CI) adjusted to age, sex, education, C reactive protein, previous stroke, diabetes, hypertension, and depressive symptoms for mean differences of each cognitive score between the two CVR groups, in subjects with (A) and without bilateral carotid plaque (B).
DISCUSSION
Preservation of function of the cerebral microvasculature is essential for the maintenance of cognitive function, as cognitive health depends considerably on cerebrovascular wellness. There is a growing body of evidence supporting the role of vascular risk factors and microvascular cerebral disease in cognitive impairment and dementia [1]. We have found that impaired CVR in adults with concomitant carotid large-vessel disease is associated with lower cognitive scores, in particular in the executive function domain. These findings emphasize the importance of cerebral hemodynamics in cognitive performance.
Results are in agreement with small TCD-based studies [20, 21]. Findings suggest that impaired CVR and cerebral autoregulation might be one of the mediators of vascular cognitive impairment [5, 22]. Previous studies have examined the association between impaired CVR and cognitive function in patients with Alzheimer’s disease [23, 24], and lend further support for a strong link between vascular disease and cognitive impairment [2, 20]. Altered CVR in dementia has also been observed with other techniques such as functional neuroimaging studies with PET and SPECT [25].
An intriguing finding of our study was the interaction effect of carotid large-vessel disease on the relation between impaired CVR and cognitive performance. The mechanisms underlying this finding are not clear. An adjuvant effect of macro- and micro-vascular disease, reflecting the overall effect of lifetime exposure to vascular risk factors, might lead to cognitive impairment. Structural damage at the molecular and cellular level, occurring both in the wall of large and small arteries, may also promote cerebral ischemia and cognitive impairment. Important interactions exist between the macro and micro-circulation. Balestrini et al. found that severe carotid stenosis and reduced CVR ipsilateral to the carotidstenosis significantly influenced the occurrence of cognitive decline [26]. Indeed altered cerebral hemodynamics might favor the progression of cognitive impairment by producing a detrimental effect on the neurovascular unit. A dysregulation of the neurovascular unit could reduce the clearance of products of inflammation, oxidative stress, and amyloid-β, promoting neurodegeneration [27]. Large-artery stiffening is an important feature of arterial aging, increasing white matter hyperintensity burden and associated with increased risk of cognitive impairment [28]. Since the brain is continually and passively perfused at high-volume flow, small cerebral vessels are exposed to highly pulsatile pressure and flow, leading to hypertrophic remodeling and rarefaction of small arteries. Impaired CVR is indeed associated with increased arterial stiffness [2]. Increase in vasomotor tone of small arteries and capillary rarefaction could in turn promote large-artery stiffening leading to a vicious circle.
Strengths of our study include the relatively large cohort of extensively studied subjects and the use of validated assessment of cognitive function scores, carotid plaque and CVR. Associations were predominantly with executive functions in line with a large body of evidence related to cerebral microvascular damage [1]. Limitations included first, the cross-sectional design of the current analyses prohibiting the possibility to assess causality. We have excluded patients with dementia, since CVR assessment may be technically compromised in these patients due to their difficulty to follow instructions and perform breath holding. Second, systematic structural brain imaging data is lacking. Brain imaging studies have shown that the impact of ischemic brain injury extends much beyond that of acute clinical events such as stroke [29]. In sensitivity analyses, excluding subjects with prevalent stroke the associations between impaired CVR and cognitive performance were attenuated, suggesting that the underlying mechanism may be mediated in-part by brain infarcts. Third, we have not quantified systematically the degree of carotid stenosis, yet only a small minority had hemodynamically significant carotid stenosis. Nevertheless, our findings emphasize the importance of cerebral hemodynamics in cognitive performance. Finally, the characteristics of this cohort of men with dyslipidemia and pre-existing atherothrombotic disease limit generalization of these findings.
Among non-demented patients with pre-existing atherothrombotic disease, we found a robust association between impaired CVR and poorer executive function cognitive scores and global cognitive performance among patients with carotid large-vessel disease. These findings emphasize the importance of cerebral hemodynamics in cognitive performance. The implications of these findings are that strategies may be thought to promote vascular cognitive health at a preclinical stage by identifying persons with cerebral microvascular and macrovascular structural or functional impairment.
Supplementary Material
Acknowledgments
The Computerized Cognitive Battery was kindly provided by NeuroTrax Corporation.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0052).
Footnotes
The supplementary table is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150052.
References
- 1.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S American Heart Association Stroke Council, Council on Epidemiology, Prevention Council on Cardiovascular Nursing, Council on Cardiovascular Radiology, Intervention Council on Cardiovascular Surgery, Anesthesia. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zavoreo I, Demarin V. Breath holding index and arterial stiffness as markers of vascular aging. Curr Aging Sci. 2010;3:67–70. doi: 10.2174/1874609811003010067. [DOI] [PubMed] [Google Scholar]
- 3.Silvestrini M, Troisi E, Matteis M, Cupini LM, Caltagirone C. Transcranial Doppler assessment of cerebrovascular reactivity in symptomatic and asymptomatic severe carotid stenosis. Stroke. 1996;27:1970–1973. doi: 10.1161/01.str.27.11.1970. [DOI] [PubMed] [Google Scholar]
- 4.Birns J, Jarosz J, Markus HS, Kalra L. Cerebrovascular reactivity and dynamic autoregulation in ischaemic subcortical white matter disease. J Neurol Neurosurg Psychiatr. 2009;80:1093–1098. doi: 10.1136/jnnp.2009.174607. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer I. Cognitive impairment of vascular origin: Neuropathology of cognitive impairment of vascular origin. J Neurol Sci. 2010;299:139–149. doi: 10.1016/j.jns.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 6.O’Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, Bommer W, Price TR, Gardin JM, Savage PJ. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23:1752–1760. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 7.Zavoreo I, Basic Kes V, Lisak M, Marsic N, Ciliga D, Trost Bobic T. Cognitive decline and cerebral vasoreactivity in asymptomatic patients with severe internal carotid artery stenosis. Acta Neurol Belg. 2013;113:453–458. doi: 10.1007/s13760-013-0196-4. [DOI] [PubMed] [Google Scholar]
- 8.Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bonaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis: The Tromso Study. Neurology. 2004;62:695–701. doi: 10.1212/01.wnl.0000113759.80877.1f. [DOI] [PubMed] [Google Scholar]
- 9.Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: Pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 11.Bezafibrate Infarction Prevention s. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Muller M, Voges M, Piepgras U, Schimrigk K. Assessment of cerebral vasomotor reactivity by transcranial Doppler ultrasound and breath-holding. A comparison with acetazolamide as vasodilatory stimulus. Stroke. 1995;26:96–100. doi: 10.1161/01.str.26.1.96. [DOI] [PubMed] [Google Scholar]
- 13.Markus HS, Harrison MJ. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke. 1992;23:668–673. doi: 10.1161/01.str.23.5.668. [DOI] [PubMed] [Google Scholar]
- 14.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg BS, Katarick S, Needleman L, Pellerito J, Polak JF, Rholl KS, Wooster DL, Zierler E Society of Radiologists in U. Carotid artery stenosis: Grayscale and Doppler ultrasound diagnosis–Society of Radiologists in Ultrasound consensus conference. Ultrasound Q. 2003;19:190–198. doi: 10.1097/00013644-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Dwolatzky T, Whitehead V, Doniger GM, Simon ES, Schweiger A, Jaffe D, Chertkow H. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatr. 2003;3:4. doi: 10.1186/1471-2318-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. In Clinical Gerontology: A Guide to Assessment and Intervention. Haworth Press; New York: 1986. p. ix.p. 517. [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- 18.Hatano S. Experience from a multicentre stroke register: A preliminary report. Bull World Health Organ. 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 19.Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M. Age and sex differences in cerebral hemodynamics: A transcranial Doppler study. Stroke. 1998;29:963–967. doi: 10.1161/01.str.29.5.963. [DOI] [PubMed] [Google Scholar]
- 20.Zavoreo I, Kes VB, Morovic S, Seric V, Demarin V. Breath holding index in detection of early cognitive decline. J Neurol Sci. 2010;299:116–119. doi: 10.1016/j.jns.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 21.Vicenzini E, Ricciardi MC, Altieri M, Puccinelli F, Bonaffini N, Di Piero V, Lenzi GL. Cerebrovascular reactivity in degenerative and vascular dementia: A transcranial Doppler study. Eur Neurol. 2007;58:84–89. doi: 10.1159/000103642. [DOI] [PubMed] [Google Scholar]
- 22.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefani A, Sancesario G, Pierantozzi M, Leone G, Galati S, Hainsworth AH, Diomedi M. CSF biomarkers, impairment of cerebral hemodynamics and degree of cognitive decline in Alzheimer’s and mixed dementia. J Neurol Sci. 2009;283:109–115. doi: 10.1016/j.jns.2009.02.343. [DOI] [PubMed] [Google Scholar]
- 24.Likitjaroen Y, Suwanwela NC, Phanthumchinda K. Vasoreactivity induced by acetazolamide in patients with vascular dementia versus Alzheimer’s disease. J Neurol Sci. 2009;283:32–35. doi: 10.1016/j.jns.2009.02.363. [DOI] [PubMed] [Google Scholar]
- 25.De Reuck J, Decoo D, Hasenbroekx MC, Lamont B, Santens P, Goethals P, Strijckmans K, Lemahieu I. Acetazolamide vasoreactivity in vascular dementia: A positron emission tomographic study. Eur Neurol. 1999;41:31–36. doi: 10.1159/000007995. [DOI] [PubMed] [Google Scholar]
- 26.Balestrini S, Perozzi C, Altamura C, Vernieri F, Luzzi S, Bartolini M, Provinciali L, Silvestrini M. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology. 2013;80:2145–2150. doi: 10.1212/WNL.0b013e318295d71a. [DOI] [PubMed] [Google Scholar]
- 27.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 28.Brisset M, Boutouyrie P, Pico F, Zhu Y, Zureik M, Schilling S, Dufouil C, Mazoyer B, Laurent S, Tzourio C, Debette S. Large-vessel correlates of cerebral small-vessel disease. Neurology. 2013;80:662–669. doi: 10.1212/WNL.0b013e318281ccc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanne D, Levine SR. Capturing the scope of stroke: Silent, whispering, and overt. Arch Neurol. 2009;66:819–820. doi: 10.1001/archneurol.2009.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.